Effects of Endogenous Melatonin Deficiency on the Growth, Productivity, and Fruit Quality Properties of Tomato Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Assessment of Fruit Hardness
2.2.2. Determination of Tomato Lycopene and Carotenoid Content
2.2.3. Determination of Ascorbic Acid Content
2.2.4. Determination of Soluble Solid Content, Titratable Acid Content, and Sugar-to-Acid Ratio
2.2.5. Analysis of Tomato Agronomic Traits
2.2.6. Determination of Endogenous Melatonin Content
2.2.7. RNA Isolation and Quantitative Real-Time PCR
2.3. Data Processing and Analysis
3. Results
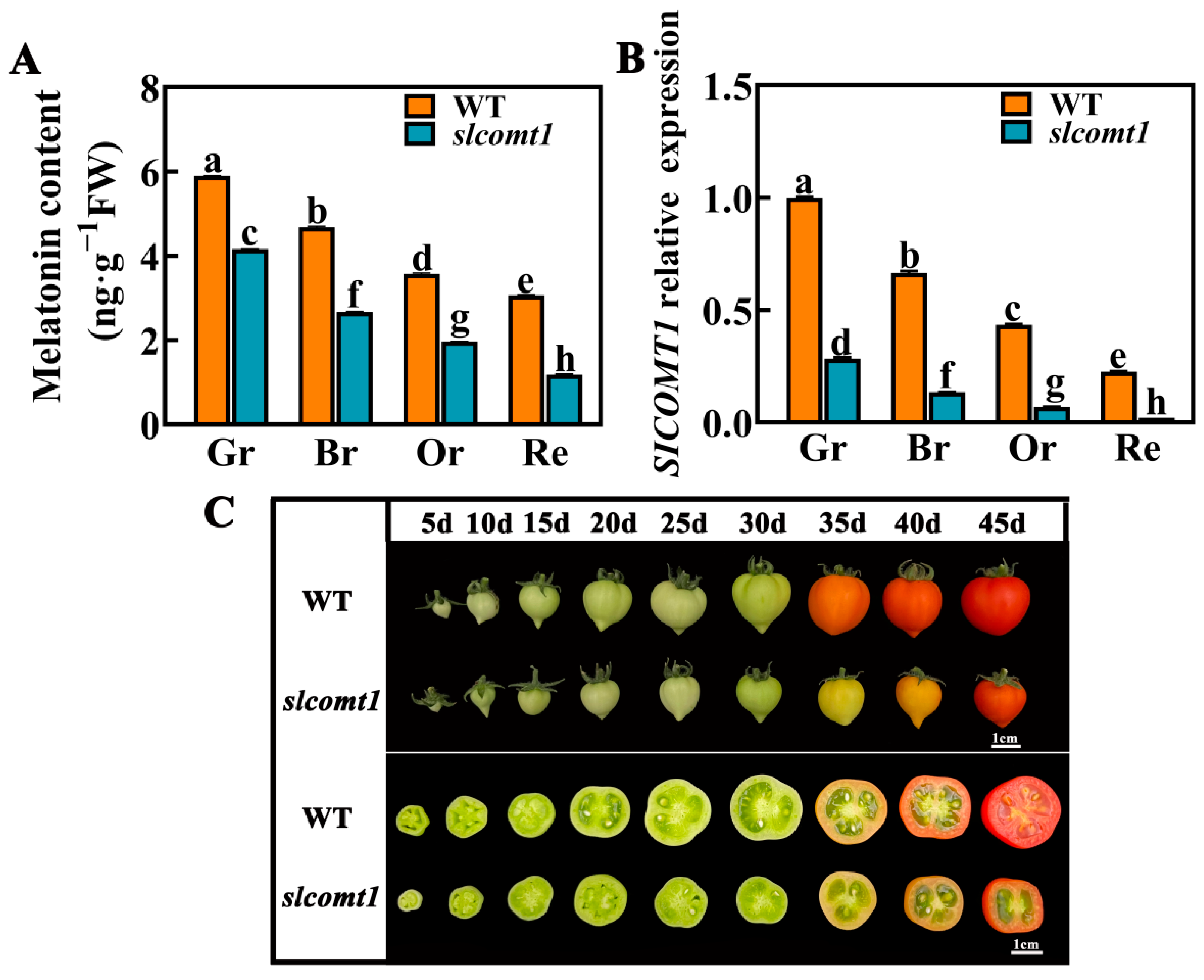
3.1. The Effects of SlCOMT1 Gene Deficiency on Endogenous Melatonin Content and Fruit Phenotype and Ventricle
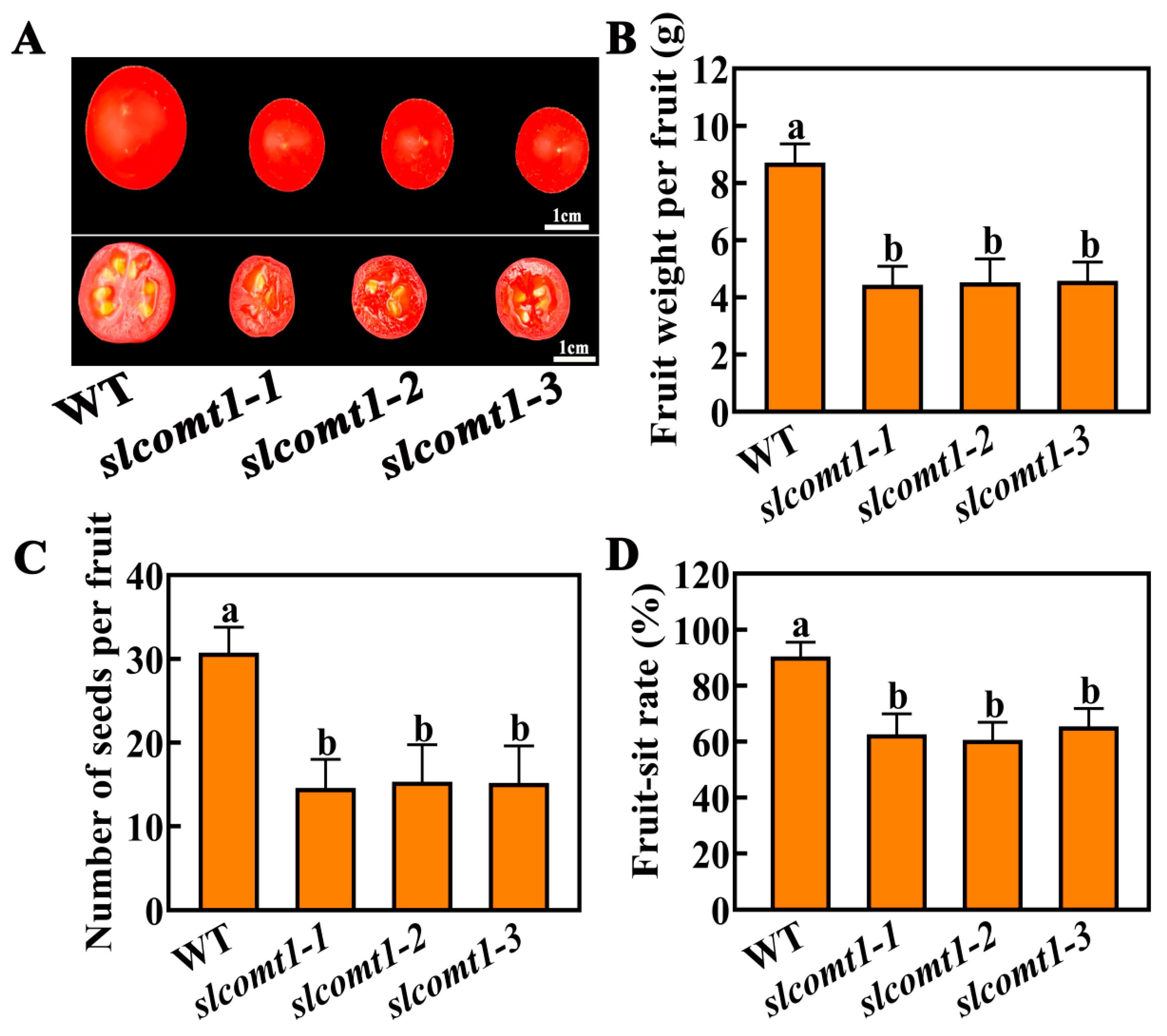
3.2. Impact of SlCOMT1 Gene Deficiency on Fruit Development
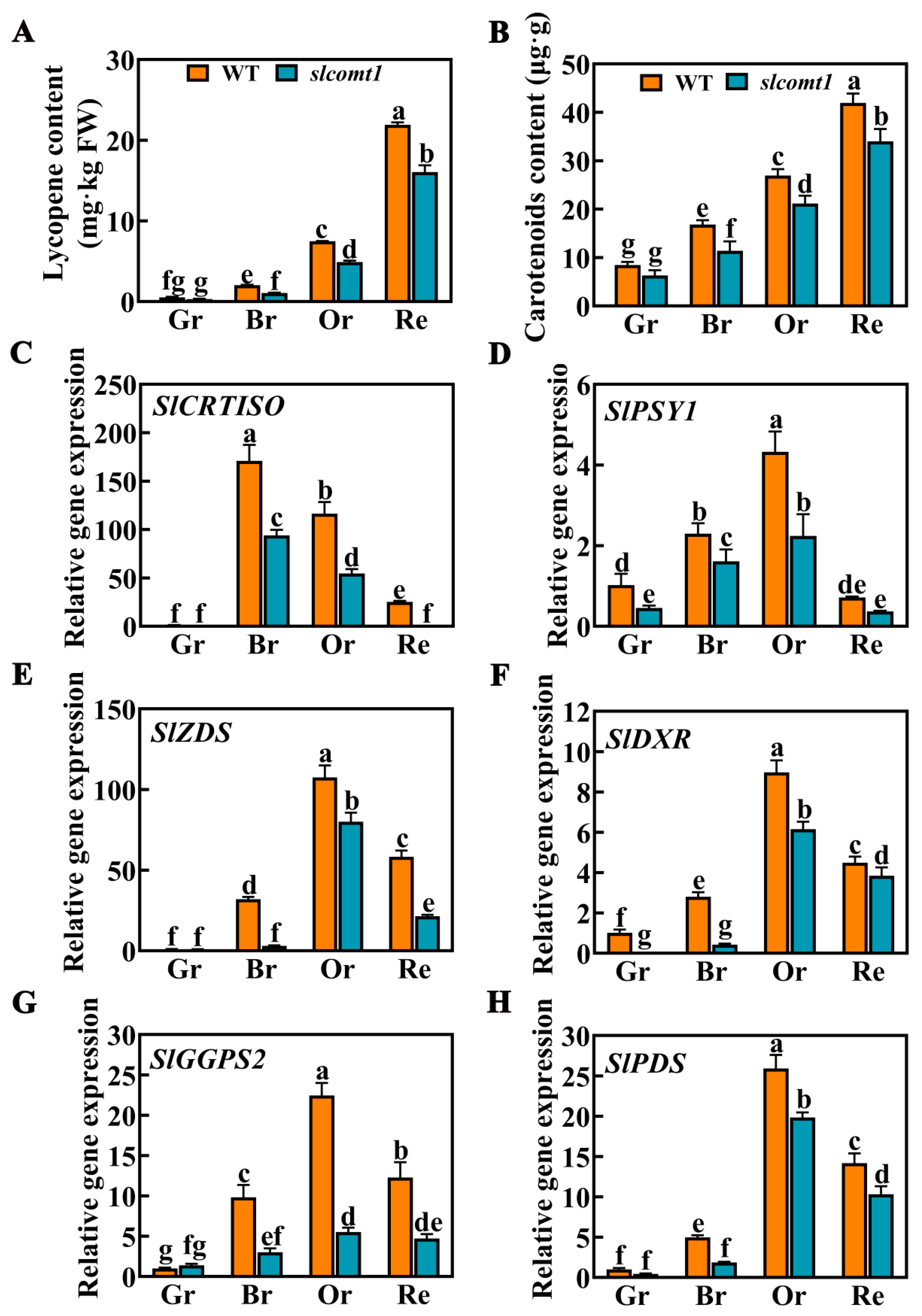
3.3. Impact of SlCOMT1 Gene Deficiency on Fruit Color Formation
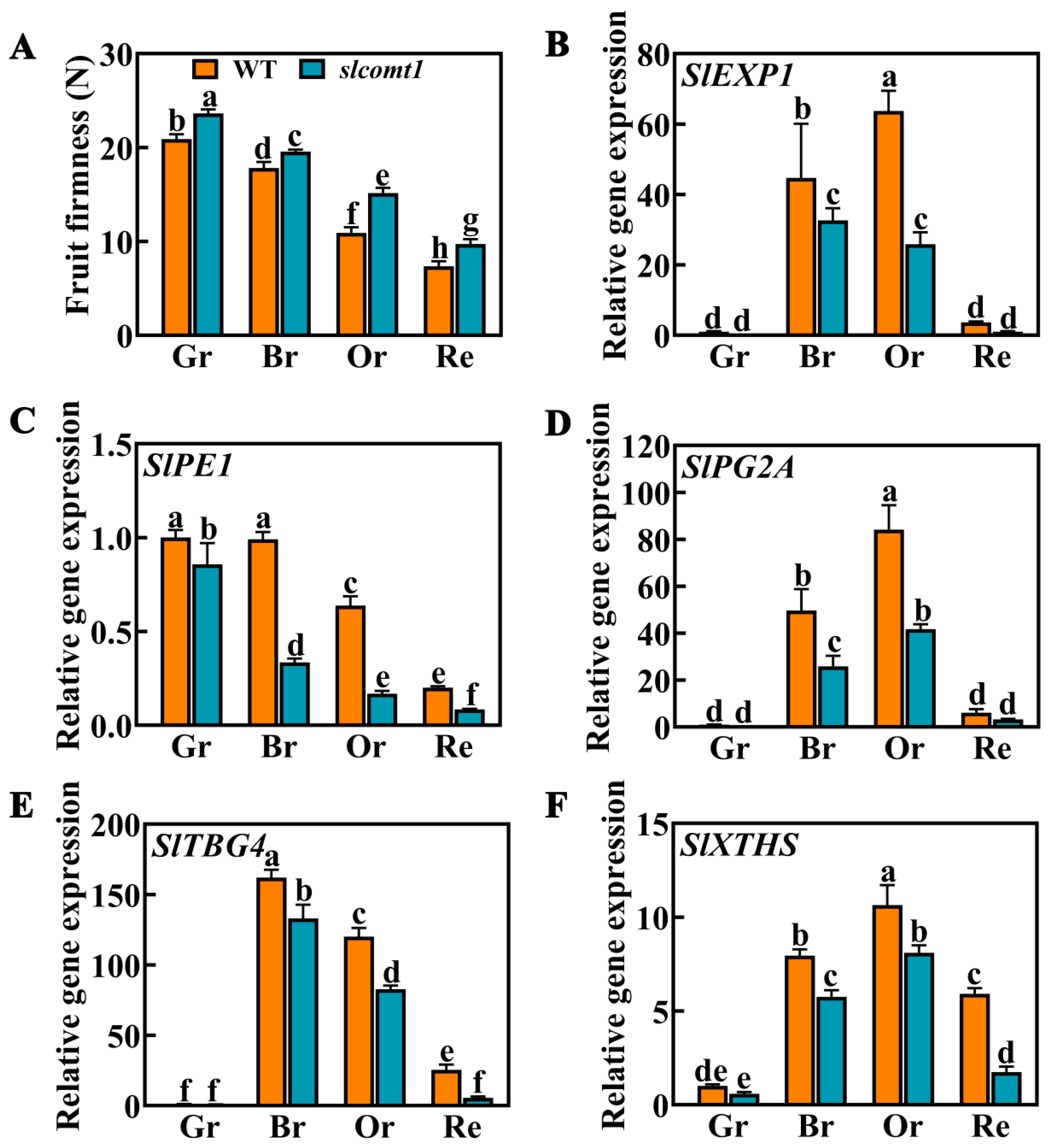
3.4. Impact of SlCOMT1 Gene Deficiency on Fruit Texture
3.5. Impact of SlCOMT1 Gene Deficiency on Fruit Flavor
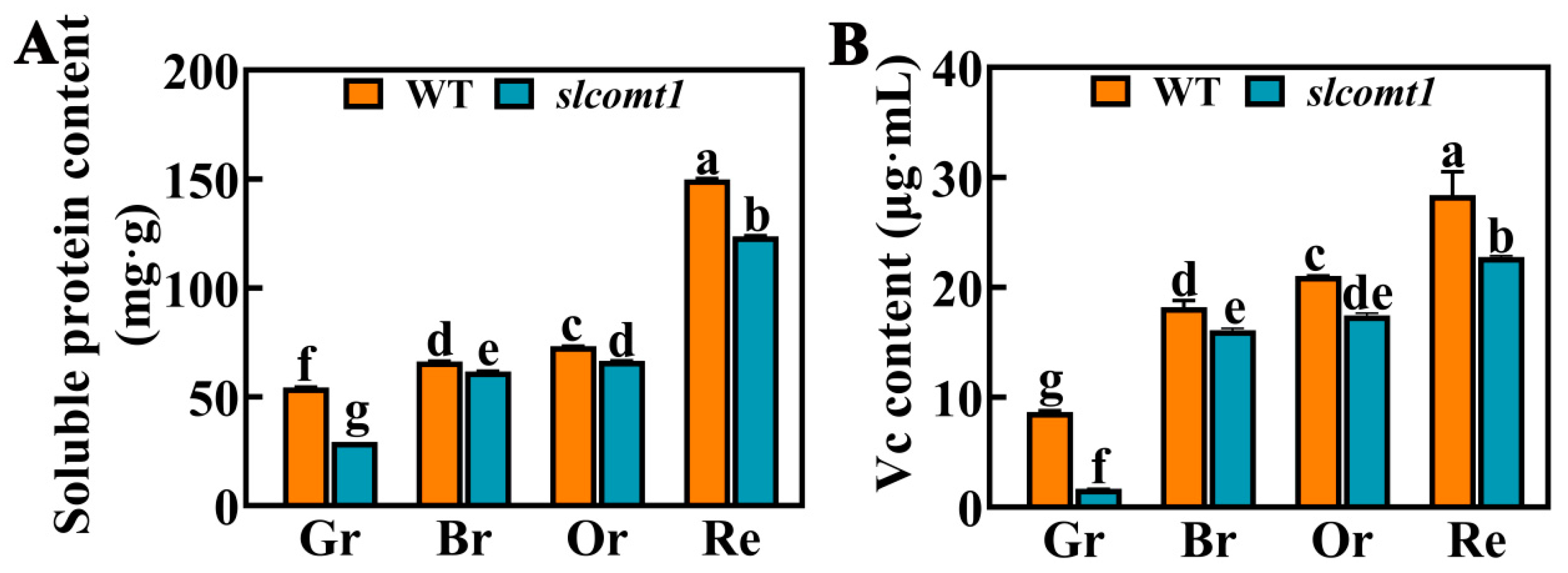
3.6. Impact of SlCOMT1 Gene Deficiency on Fruit Nutrient Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, T.L.; Ye, H.X.; Zheng, J.R.; Li, M. Research progress on major flavor compounds in tomato fruits. J. Zhejiang Agric. Sci. 2020, 32, 1513–1522. [Google Scholar] [CrossRef]
- Maul, F. Flavor of Fresh Market Tomato (Lycopersicon esculentum mill.) As Influenced by Harvest Maturity and Storage Temperature; University of Florida: Gainesville, FL, USA, 1999; pp. 35–38. [Google Scholar]
- Tian, Y.Q.; Gao, L.H. Theory and technology of high-quality cultivation of greenhouse tomatoes. China Veg. 2021, 384, 30–40. [Google Scholar] [CrossRef]
- Wang, Y.G.; Bai, Y.B. Nutritional value and growth requirements of common vegetables. China Fruits Veg. 2019, 39, 73–76. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.Y.; Zhu, H.; Yue, L.M.; Song, Z.Y.; Wu, R.H.; Liu, G.H. Research progress on biological functions of tomato lycopene. Food Res. Dev. 2020, 41, 202–207. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, a pineal factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Kaneko, R.O.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by High-performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Badria, F.A. Melatonin, serotonin, and tryptamine in some Egyptian food and medicinal plants. J. Med. Food. 2002, 5, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Huo, Y.S.; Tan, D.X.; Liang, Z.; Zhang, W.B.; Zhang, Y.K. Melatonin in Chinese medicinal herbs. Life Sci. 2003, 73, 19–26. [Google Scholar] [CrossRef]
- Wang, Y.P.; Reiter, R.J.; Chan, Z.L. Phytomelatonin: A universal abiotic stress regulator. J. Exp. Bot. 2018, 69, 963–974. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.X.; Rutto, L.; Katuuramu, D. Melatonin acts synergistically with auxin to promote lateral root development through fine tuning auxin transport in Arabidopsis thaliana. PLoS ONE 2019, 14, e0221687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Melatonin modulates photosynthesis, redox status, and elemental composition to promote growth of Brassica juncea-a dose-dependent effect. Protoplasma 2020, 257, 1685–1700. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, Q.; Wang, Y.P.; Chan, Z.L. Global transcriptomic network of melatonin regulated root growth in Arabidopsis. Gene 2021, 764, 145082. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.Y.; Sun, L.Y.; Wang, T.Y.; Miao, P.; Zhu, X.C.; Liu, S.Q.; Song, F.B.; Mao, H.P.; Li, X.N. Melatonin improves the photosynthetic carbon assimilation and antioxi-dant capacity in wheat exposed to nano-ZnO stress. Molecules 2017, 22, 1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.Q.; Zhang, Z.W.; Chen, Y.E.; Ding, C.B.; Yuan, S.; Reiter, R.J.; Yuan, M. Melatonin: A potential agent in delaying leaf senescence. Crit. Rev. Plant Sci. 2021, 40, 1–22. [Google Scholar] [CrossRef]
- Liang, D.; Shen, Y.Q.; Ni, Z.Y.; Wang, Q.; Lei, Z.; Xu, N.Q.; Deng, Q.X.; Liu, L.; Wang, J.; Lv, X.L.; et al. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.L.; Lan, Z.X.; Xu, K.; Chang, J.J.; Ahammed, G.J.; Ma, J.X.; Wei, C.H.; Zhang, X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.Y.; Chen, W.W.; Guo, Q.G.; Xia, Y.; Wu, D.; Jing, D.L.; Liang, G.L. Melatonin treatment maintains quality and delays lignification in loquat fruit during cold storage. Sci. Hortic. 2021, 284, 110126. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xu, W.; Liu, A.R.; Chen, S.C. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 303–311. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.M.; Tao, M.Q.; Sun, J.; Guo, S.R. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, Q.Y.; Chen, W.W.; Guo, Q.G.; Xia, Y.; Wang, S.M.; Jing, D.L.; Liang, G.L. Physiological and transcription analyses reveal the regulatory mechanism of melatonin in inducing drought resist-ance in loquat (Eriobotrya japonica Lindl.) seedlings. Environ. Exp. Bot. 2021, 181, 104291. [Google Scholar] [CrossRef]
- Wang, T.; Song, J.X.; Liu, Z.; Liu, Z.L.; Cui, J. Melatonin alleviates cadmium toxicity by reducing nitric oxide accumulation and IRT1 expression in Chinese cabbage seedlings. Environ. Sci. Pollut. Res. 2020, 28, 15394–15405. [Google Scholar] [CrossRef]
- Dai, L.L.; Li, J.; Harmens, H.; Zheng, X.D.; Zhang, C.L. Melatonin enhances drought resistance by regulating leaf stomatal behaviour, root growth and catalase activity in two contrasting rapeseed (Brassica napus L.) genotypes. Plant Physiol. Biochem. 2020, 149, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Liu, J.; Zhu, T.T.; Zhao, C.; Li, L.Y.; Chen, M. The role of melatonin in salt stress responses. Int. J. Mol. Sci. 2019, 20, 1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.G.; Xu, Y.; Bai, L.K.; Zhang, S.Y.; Wang, Y. Melatonin enhances thermotolerance of maize seedlings (Zea mays L.) by modulating antioxidant defense, methylglyoxal detoxification, and osmoregulation systems. Protoplasma 2019, 256, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.J.; Yang, X.Y.; Liu, Y.; Tang, H.M.; Wang, Q.F.; Chu, S.P.; Hu, J.X.; Zhang, N.; Shi, Q.H. Improvement of Seed Germination under Salt Stress via Overexpressing Caffeic Acid O-methyltransferase 1 (SlCOMT1) in Solanum lycopersicum L. Int. J. Mol. Sci. 2023, 24, 734. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.S.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S.Y. Melatonin-induced water stress tolerance in plants: Recent advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef]
- Zheng, X.D.; Zhou, J.Z.; Tan, D.X.; Wang, N.; Wang, L.; Shan, D.Q.; Kong, J. Melatonin improves waterlogging tolerance of Malus baccata (Linn.) Borkh. seedlings by maintaining aerobic respiration, photosynthesis and ROS migration. Front. Plant Sci. 2017, 8, 483. [Google Scholar] [CrossRef] [Green Version]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Altaf, M.M.; Jahan, M.S.; Khan, L.U. Melatonin mitigates nickel toxicity by improving nutrient uptake fluxes, root architecture system, photosynthesis, and antioxidant potential in tomato seedling. J. Soil. Sci. Plant Nutr. 2021, 21, 1842–1855. [Google Scholar] [CrossRef]
- He, J.L.; Zhuang, X.L.; Zhou, J.T.; Sun, L.Y.; Wan, H.X.; Li, H.F.; Lyu, D.G. Exogenous melatonin alleviates cadmium uptake and toxicity in apple root-stocks. Tree Physiol. 2020, 40, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Kan, L.U.; Altaf, M.M.; Jahan, M.S.; Nawaz, M.A.; Naz, S.; Shahid, S.; Lal, M.K.; et al. Protective mechanisms of melatonin against vanadium phytotoxicity in tomato seedlings:insights into nutritional status, photosynthesis, root architecture system, and antioxidant machinery. J. Plant Growth Regul. 2021, 41, 3300–3316. [Google Scholar] [CrossRef]
- Liu, C.X.; Chen, L.L.; Zhao, R.R.; Li, R.; Zhang, S.J.; Yu, W.Q.; Sheng, J.P.; Shen, L. Melatonin induces disease resistance to Botrytis cinerea in tomato fruit by activating jasmonic acid signaling pathway. J. Agric. Food Chem. 2019, 67, 6116–6124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, M.J.; Song, J.B.; Zhang, S.Y.; Guo, R.; Hou, D.R.; Hao, C.Y.; An, H.L.; Huang, X. Roles of endogenous melatonin in resistance to Botrytis cinerea infection in an Arabidopsis model. Front. Plant Sci. 2021, 12, 683228. [Google Scholar] [CrossRef]
- Murch, S.J.; Campbell, S.S.B.; Saxena, P.K. The role of serotonin and melatonin in plant morphogenesis: Regulation of auxin-induced root organogenesis in in vitro-cultured explants of St. John’s wort (Hypericum perforatum L.). In Vitro Cell. Dev. Biol.-Plant 2001, 37, 786–793. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Lee, K.; Back, K. Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. J. Pineal Res. 2014, 57, 219–227. [Google Scholar] [CrossRef]
- Ye, X.Y. The Role of Caffeic Acid O-Methyltransferase Gene SlCOMT1 in Regulating Leaf Senescence in Tomato. Master’s Thesis, Guizhou University, Guiyang, China, 2022. [Google Scholar]
- Sun, Q.Q.; Zhang, N.; Wang, J.F.; Zhang, H.J.; Li, D.B.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.X.; et al. Melatonin promotes ripening and improves quality of tomato fruit druing postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, L.; Popko, J.L.; Umezawa, T.; Chiang, V.L. 5-hydroxyconiferyl aldehyde modulates enzymatic methylation for syringyl monolignol formation, a new view of monolignol biosynthesis in angiosperms. J. Biol. Chem. 2000, 275, 6537–6545. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Lee, H.Y.; Back, K. Rice histone deacetylase 10 and Arabidopsis histone deacetylase 14 genes encode N-acetylserotonin deacetylase, which catalyzes conversion of N-acetylserotonin into serotonin, a reverse reaction for melatonin biosynthesis in plants. J. Pineal Res. 2018, 64, e12460. [Google Scholar] [CrossRef]
- DellaPenna, D.; Pogon, B.J. Vitamin synthesis in plants: Tocopherols and carotenoids. Ann. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klee, H.J.; Giovannoni, J.J. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, H. Chromoplast biogenesis and carotenoid accumulation. Arch. Biochem. Biophys. 2013, 539, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, T.; Ohad, I.; Beyer, P.; Hirschberg, J. Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants. Plant Physiol. 2004, 136, 4246–4255. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, C.J.; Korban, S.S.; Chen, K.S. Regulatory Mechanisms of Textural Changes in Ripening Fruits. Crit. Rev. Plant Sci. 2010, 29, 222–243. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–340. [Google Scholar] [CrossRef]
- Ei-Naby, S.K.M.A.; Mohamed, A.A.A.; Ei-Naggar, Y.I.M. Effect of melatonin, GA3 and NAA on vegetative growth, yield and quality of ‘Canino’ apricot fruits. Acta Sci. Pol.-Hortorum Cultus 2019, 18, 167–174. [Google Scholar] [CrossRef]
- Guo, G.Z.; Wu, Y.; Guo, J.; Zhuang, X.; Yang, J. Cultivation techniques of yellow tomato in Sunlight Greenhouse. Inn. Mong. Agric. Sci. Technol. 2014, 1, 110. [Google Scholar]
- Tan, Q.M. Vegetable Breeding, 1st ed.; Agricultural Publishers: Beijing, China, 1984. [Google Scholar]
- Zhu, L. Research progress and future directions of major vegetable varieties breeding in China. China Veg. 1996, 1, 1–4. [Google Scholar]
- Miranda, S.; Vilches, P.; Suazo, M.; Pavez, L.; Garcia, K.; Mendez, M.A.; Gonzalez, M.; Meisal, L.A.; Defilippi, B.G.; Pozo, T.D. Melatonin triggers metabolic and gene expression changes leading to improved quality traits of two sweet cherry cultivars during cold storage. Food Chem. 2020, 319, 126360. [Google Scholar] [CrossRef]
- Chen, Z.; Gallie, D.R. The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 2004, 16, 1143–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemavathi; Upadhyaya, C.; Akula, N.; Kim, H.S.; Jeon, J.H.; Ho, O.M.; Chun, S.C.; Kim, D.H.; Park, S.W. Biochemical analysis of enhanced tolerance in transgenic potato plants overexpressing D-galacturonic acid reductase gene in response to various abiotic stresses. Mol. Breed. 2011, 28, 105–115. [Google Scholar] [CrossRef]
- Gallie, D.R. L-ascorbic acid: A multifunctional molecule supporting plant growth and development. Scientifica 2013, 2013, 795964–795988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, K.; Suzuki, K.; Kitamura, S. Characterization of a GDP-D-mannose 3″,5″-epimerase from rice. Phytochemistry 2006, 67, 338–346. [Google Scholar] [CrossRef]
- Liu, J.L. Effects of Exogenous Melatonin on the Antioxidant System, Yield, and Fruit Quality of Tomatoes under Drought Stress. Master’s Thesis, Northwest A&F University, Xianyang, China, 2015. [Google Scholar]
- Liu, J.L.; Zhang, R.M.; Sun, Y.K.; Liu, Z.Y.; Wen, J.; Yan, S. The beneficial effects of exogenous melatonin on tomato fruit properties. Sci. Hortic. 2016, 207, 14–20. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Wen, C.; Xu, W. Effects of Endogenous Melatonin Deficiency on the Growth, Productivity, and Fruit Quality Properties of Tomato Plants. Horticulturae 2023, 9, 851. https://doi.org/10.3390/horticulturae9080851
He Z, Wen C, Xu W. Effects of Endogenous Melatonin Deficiency on the Growth, Productivity, and Fruit Quality Properties of Tomato Plants. Horticulturae. 2023; 9(8):851. https://doi.org/10.3390/horticulturae9080851
Chicago/Turabian StyleHe, Zhuo, Cen Wen, and Wen Xu. 2023. "Effects of Endogenous Melatonin Deficiency on the Growth, Productivity, and Fruit Quality Properties of Tomato Plants" Horticulturae 9, no. 8: 851. https://doi.org/10.3390/horticulturae9080851
APA StyleHe, Z., Wen, C., & Xu, W. (2023). Effects of Endogenous Melatonin Deficiency on the Growth, Productivity, and Fruit Quality Properties of Tomato Plants. Horticulturae, 9(8), 851. https://doi.org/10.3390/horticulturae9080851



