Morpho-Anatomical and Physiological Assessments of Cryo-Derived Pineapple Plants (Ananas comosus var. comosus) after Acclimatization
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Morphological Variables
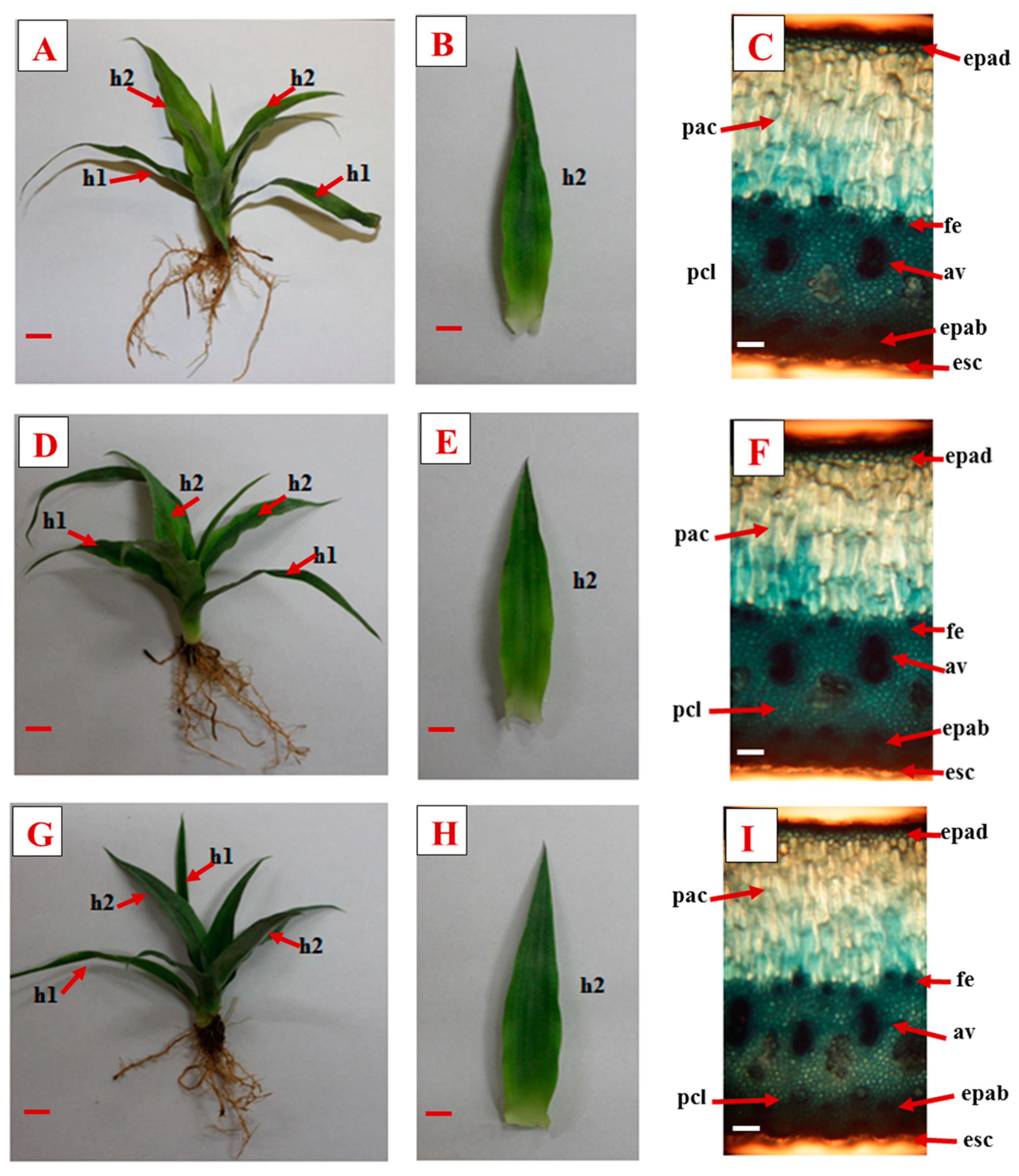
3.2. Morpho-Anatomical Characteristics
3.3. Physiological Indicators
3.4. Gas Exchange Rate and Organic Acid Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiroutová, P.; Sedlák, J. Cryobiotechnology of plants: A hot topic not only for gene banks. Appl. Sci. 2020, 10, 4677. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Mathew, L.; Pathirana, R.; Wiedow, C.; Hunter, D.A.; McLachlan, A. Eradication of Potato Virus S, Potato Virus A, and Potato Virus M from infected in vitro-grown potato shoots using in vitro therapies. Front. Plant Sci. 2022, 13, 1431. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, M.-R.; Li, Z.; Panis, B.; Bettoni, J.C.; Vollmer, R. Overcoming challenges for shoot tip cryopreservation of root and tuber crops. Agronomy 2023, 13, 219. [Google Scholar] [CrossRef]
- Martinez-Montero, M.E.; Gonzalez-Arnao, M.T.; Engelmann, F. Cryopreservation of tropical plant germplasm with vegetative propagation-review of sugarcane (Saccharum spp.) and pineapple (Ananas comusus (L.) Merrill) cases. In Current Frontiers in Cryopreservation; Katkov, I., Ed.; IntechOpen: London, UK, 2012; pp. 360–387. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Gonzalez-Arnao, M.T.; Ravelo, M.M.; Urra-Villavicencio, C.; Martinez-Montero, M.M.; Engelmann, F. Cryopreservation of pineapple (Ananas comosus) apices. CryoLetters 1998, 19, 375–382. [Google Scholar]
- Gamez-Pastrana, R.; Martinez-Ocampo, Y.; Beristain, C.; Gonzalez-Arnao, M. An improved cryopreservation protocol for pineapple apices using encapsulation-vitrification. CryoLetters 2004, 25, 405–414. [Google Scholar]
- Martinez-Montero, M.E.; Martinez, J.; Engelmann, F.; Gonzalez-Arnao, M. Cryopreservation of pineapple (Ananas comosus (L.) Merr) apices and calluses. Acta Hortic. 2005, 666, 127–131. [Google Scholar] [CrossRef]
- Souza, F.V.D.; Kaya, E.; de Jesus Vieira, L.; de Souza, E.H.; de Oliveira Amorim, V.B.; Skogerboe, D. Droplet-vitrification and morphohistological studies of cryopreserved shoot tips of cultivated and wild pineapple genotypes. Plant Cell Tissue Organ Cult. 2016, 124, 351–360. [Google Scholar] [CrossRef]
- Souza, F.V.D.; de Souza, E.H.; Kaya, E.; de Jesus Vieira, L.; da Silva, R.L. Cryopreservation of pineapple shoot tips by the droplet vitrification technique. Plant Cell Cult. Protoc. 2018, 1815, 269–377. [Google Scholar] [CrossRef]
- Villalobos-Olivera, A.; Rodríguez, J.M.; Bernabé, N.Q.; Souza, F.V.D.; Olmedo, J.G.; Martinez-Montero, M.E. Effect of temperature on pre-conditioning pineapple in vitro donor plants for cryopreservation protocol of shoot apices. Acta Hortic. 2019, 1239, 113–120. [Google Scholar] [CrossRef]
- Villalobos-Olivera, A.; García-Brizuela, J.; Olaru, S.; Rodriguez-Ayerbe, P.; Martinez-Montero, M. A dynamical systems approach for pineapple cryopreservation analysis. IFAC-PapersOnLine 2019, 52, 263–268. [Google Scholar] [CrossRef]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. CryoLetters 2007, 28, 151–172. [Google Scholar] [PubMed]
- Panis, B.; Piette, B.; Swennen, R. Droplet vitrification of apical meristems: A cryopreservation protocol applicable to all Musaceae. Plant Sci. 2005, 168, 45–55. [Google Scholar] [CrossRef]
- Thalip, A.A.; Tong, P.S.; Ng, C. The MD2 “Super Sweet” pineapple (Ananas comosus). UTAR Agric. Sci. J. 2015, 1, 14–17. [Google Scholar]
- Lakho, M.A.; Jatoi, M.A.; Solangi, N.; Abul-Soad, A.A.; Qazi, M.A.; Abdi, G. Optimizing in vitro nutrient and ex vitro soil mediums-driven responses for multiplication, rooting, and acclimatization of pineapple. Sci. Rep. 2023, 13, 1275. [Google Scholar] [CrossRef]
- Villalobo, A.; González, J.; Santos, R.; Rodríguez, R. Morpho-physiological changes in pineapple plantlets [Ananas comosus (L.) Merr.] during acclimatization. Ciência Agrotecnol. 2012, 36, 624–630. [Google Scholar] [CrossRef]
- Harding, K. Genetic integrity of cryopreserved plant cells: A review. CryoLetters 2004, 25, 3–22. [Google Scholar]
- Martinez-Montero, M.E.; Harding, K. Cryobionomics: Evaluating the concept in plant cryopreservation. In Plant Omics: The Omics of Plant Science; Barh, D., Khan, M., Davies, E., Eds.; Springer: New Delhi, India, 2015; pp. 655–682. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.W.; Zhang, Z.B.; Wang, R.R.; Ma, Y.L.; Blystad, D.R. Three vitrification-based cryopreservation procedures cause different cryo-injuries to potato shoot tips while all maintain genetic integrity in regenerants. J. Biotechnol. 2014, 184, 47–55. [Google Scholar] [CrossRef]
- Czégény, G.; Mátai, A.; Hideg, É. UV-B effects on leaves—Oxidative stress and acclimation in controlled environments. Plant Sci. 2016, 248, 57–63. [Google Scholar] [CrossRef]
- Karim, M.F.; Johnson, G.N. Acclimation of photosynthesis to changes in the environment results in decreases of oxidative stress in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 683968. [Google Scholar] [CrossRef]
- Wang, M.R.; Bi, W.; Shukla, M.R.; Ren, L.; Hamborg, Z.; Blystad, D.R. Epigenetic and genetic integrity, metabolic stability, and field performance of cryopreserved plants. Plants 2021, 10, 1889. [Google Scholar] [CrossRef]
- Ren, L.; Wang, M.-R.; Wang, Q.-C. ROS-induced oxidative stress in plant cryopreservation: Occurrence and alleviation. Planta 2021, 124, 254. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Olivera, A.; Entensa, Y.; Martínez, J.; Escalante, D.; Quintana, N.; Souza, F.V. Storage of pineapple shoot tips in liquid nitrogen for three years does not modify field performance and fruit quality of recovered plants. Acta Physiol. Plant. 2022, 44, 65. [Google Scholar] [CrossRef]
- Villalobos-Olivera, A.; Ferreira, C.F.; Yanes-Paz, E.; Lorente, G.Y.; Souza, F.V.; Engelmann, F.; Martínez-Montero, M.E.; Lorenzo, J.C. Inter simple sequence repeat (ISSR) markers reveal DNA stability in pineapple plantlets after shoot tip cryopreservation. Vegetos 2022, 35, 360–366. [Google Scholar] [CrossRef]
- Villalobos-Olivera, A.; Nápoles, L.; Mendoza, J.R.; Escalante, D.; Martínez, J.; Concepción, O.; Zevallos, B.E.; Martínez-Montero, M.E.; Cejas, I.; Engelmann, F.; et al. Exposure of pineapple shoot tips to liquid nitrogen and cryostorage do not affect the histological status of regenerated plantlets. Rom. Biotechnol. Lett. 2019, 24, 1061–1066. [Google Scholar] [CrossRef]
- Rodríguez-Escriba, R.C.; Rodríguez, R.; López, D.; Lorente, G.Y.; Pino, Y.; Aragón, C.E. High light intensity increases CAM expression in MD-2 micro-propagated pineapple plants at the end of acclimatization stage. Am. J. Plant Sci. 2015, 6, 3109–3125. [Google Scholar] [CrossRef]
- Aragón, C.; Pascual, P.; González, J.; Escalona, M.; Carvalho, L.; Amancio, S. The physiology of ex vitro pineapple (Ananas comosus L. Merr. var MD-2) as CAM or C3 is regulated by the environmental conditions: Proteomic and transcriptomic profiles. Plant Cell Rep. 2013, 32, 1807–1818. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nápoles, L.; Cid, M.; Hernández, L.; Alvares, Y.; Zamora, V.; Lorente, G.L.; Rodríguez, R.; Concepción, O. Scale up of in vitro plant production of ‘MD2’ pineapple free of Pineapple mealybug wilt-associated virus-1, -2 and -3 for introduction to productive scale in Cuba. Acta Hortic. 2019, 1039, 121–128. [Google Scholar] [CrossRef]
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef]
- Pino, Y.; Concepción, O.; Santos, R.; González, J.; Rodríguez, R. Effect of previcur (r) energy fungicide on MD-2 pineapple (Ananas comosus var. comosus) plantlets during the acclimatization phase. Pineapple News 2014, 21, 24–26. [Google Scholar]
- Ebel, A.I.; Itati Giménez, L.; González, A.M.; Alayón Luaces, P. Evaluación morfoanatómica de hojas “D” de piña (Ananas comosus (L.) Merr. var. comosus) en respuesta a la implantación de dos sistemas de cultivo en Corrientes, Argentina. Acta Agronómica 2016, 65, 390–397. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Fazio, G.; Carvalho Costa, L.; Hurtado-Gonzales, O.P.; Rwahnih, M.A.; Nedrow, A.; Volk, G.M. Thermotherapy followed by shoot tip cryotherapy eradicates latent viruses and apple hammerhead viroid from in vitro apple rootstocks. Plants 2022, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.A.; de Souza, E.H.; de Andrade, E.C.; Max, D.D.A.S.; de Oliveira, R.S.; Souza, F.V.D. Comparison of shoot tip culture and cryotherapy for eradication of ampeloviruses associated with Pineapple mealybug wilt in wild varieties. In Vitro Cell. Dev. Biol.-Plant 2020, 56, 903–910. [Google Scholar] [CrossRef]
- Wang, M.R.; Bi, W.L.; Bettoni, J.C.; Zhang, D.; Volk, G.M.; Wang, Q.C. Shoot tip cryotherapy for plant pathogen eradication. Plant Pathol. 2022, 71, 1241–1254. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Marković, Z.; Bi, W.; Volk, G.M.; Matsumoto, T.; Wang, Q.-C. Grapevine shoot tip cryopreservation and cryotherapy: Secure storage of disease-free plants. Plants 2021, 10, 2190. [Google Scholar] [CrossRef]
- Wang, M.R.; Hamborg, Z.; Slimestad, R.; Elameen, A.; Blystad, D.R.; Haugslien, S. Assessments of rooting, vegetative growth, bulb production, genetic integrity and biochemical compounds in cryopreserved plants of shallot. Plant Cell Tissue Organ Cult. 2021, 144, 123–131. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Souza, J.A.; Volk, G.M.; Dalla Costa, M.; da Silva, F.N.; Kretzschmar, A.A. Eradication of latent viruses from apple cultivar ‘Monalisa’ shoot tips using droplet-vitrification cryotherapy. Sci. Hortic. 2019, 250, 12–18. [Google Scholar] [CrossRef]
- Tavazza, R.; Lucioli, A.; Benelli, C.; Giorgi, D.; D’aloisio, E.; Papacchioli, V. Cryopreservation in artichoke: Towards a phytosanitary qualified germplasm collection. Ann. Appl. Biol. 2013, 163, 231–241. [Google Scholar] [CrossRef]
- Wang, Q.; Mawassi, M.; Li, P.; Gafny, R.; Sela, I.; Tanne, E. Elimination of grapevine virus A (GVA) by cryopreservation of in vitro-grown shoot tips of Vitis vinifera L. Plant Sci. 2003, 165, 321–327. [Google Scholar] [CrossRef]
- Van Huylenbroeck, J.; Piqueras, A.; Debergh, P. Photosynthesis and carbon metabolism in leaves formed prior and during ex vitro acclimatization of micropropagated plants. Plant Sci. 1998, 134, 21–30. [Google Scholar] [CrossRef]
- González, G.Y.L.; Rodríguez-Escriba, R.C.; Martínez, R.E.I.; Pérez, L.S.; Borroto, Y.L.D.; Sánchez, R.R. Response of ‘MD-2’ pineapple plantlets (Ananas comosus var. comosus) to a controlled release fertilizer during the acclimatization stage. Pineapple News 2017, 23, 37–40. [Google Scholar]
- Hu, W.H.; Liu, S.F.; Liaw, S.I. Long-term preconditioning of plantlets: A practical method for enhancing survival of pineapple (Ananas comosus (L.) Merr.) shoot tips cryopreserved using vitrification. CryoLetters 2015, 36, 226–236. [Google Scholar]
- Hamid, N.S.; Tan, B.C.; Khalid, N.; Osman, N.; Jalil, M. Adaptive features of in vitro-derived plantlets of MD2 pineapple during acclimatization process. Indian J. Hortic. 2020, 77, 595–602. [Google Scholar] [CrossRef]
- Agbidinoukoun, A.; Ouikoun, G.C.; Mikpon, T.; Kamade, G.T.; Badou, B.T.; Aïsso, R.C. Influence of watering solution and phenotype on the growth of in vitro propagated pineapple (Smooth Cayenne Cultivar) plantlets during acclimatization. Agric. Sci. 2021, 12, 1215–1230. [Google Scholar] [CrossRef]
- Souza, J.A.; Bettoni, J.C.; Dalla Costa, M.; Baldissera, T.C.; dos Passos, J.F.M.; Primieri, S. In vitro rooting and acclimatization of ‘Marubakaido’ apple rootstock using indole-3-acetic acid from rhizobacteria. Commun. Plant Sci. 2022, 12, 16–23. [Google Scholar] [CrossRef]
- Mežaka, I.; Kļaviņa, D.; Kaļāne, L.; Kronberga, A. Large-Scale In Vitro Propagation and Ex Vitro Adaptation of the Endangered Medicinal Plant Eryngium maritimum L. Horticulturae 2023, 9, 271. [Google Scholar] [CrossRef]
- Bouzo, C.A.; Favaro, J.C. Container size effect on the plant production and precocity in tomato (Solanum lycopersicum L.). Bulg. J. Agric. Sci. 2015, 21, 325–332. [Google Scholar]
- Durand, M.; Mainson, D.; Porcheron, B.; Maurousset, L.; Lemoine, R.; Pourtau, N. Carbon source–sink relationship in Arabidopsis thaliana: The role of sucrose transporters. Planta 2018, 247, 587–611. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source–sink perspective on growth rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Shawon, M.R.A.; An, J.H.; Yun, Y.J.; Park, S.J.; Na, J.K.; Choi, K.Y. Influence of substrate composition and container size on the growth of tissue culture propagated apple rootstock plants. Agronomy 2021, 11, 2450. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Dalla Costa, M.; Gardin, J.P.P.; Kretzchmar, A.A.; Souza, J.A. In vitro propagation of grapevine cultivars with potential for South of Brazil. Am. J. Plant Sci. 2015, 6, 1806–1815. [Google Scholar] [CrossRef]
- Demarco, P.; Gómez Herrera, M.D.; Gonzalez, A.; Alayón Luaces, P. Effects of foliar versus soil water application on ecophysiology, leaf anatomy and growth of pineapple. Fruits 2020, 75, 44–51. [Google Scholar] [CrossRef]
- de Paula Alves, J.; Pinheiro, M.V.M.; Corrêa, T.R.; Alves, G.L.; dos Santos Marinho, T.R.; Batista, D.S. Morphophysiology of Ananas comosus L. Merr during in vitro photomixotrophic growth and ex vitro acclimatization. In Vitro Cell. Dev. Biol.-Plant 2023, 59, 106–120. [Google Scholar] [CrossRef]
- Barberis, I.M.; Cárcamo, J.M.; Cárcamo, J.I.; Albertengo, J. Phenotypic plasticity in Bromelia serra Griseb.: Morphological variations due to plant size and habitats with contrasting light availability. Rev. Bras. Biociências 2017, 15, 3–10. [Google Scholar]
- Couto, T.R.; Silva, J.R.; Moraes, C.R.O.; Ribeiro, M.S.; Netto, A.T.; Carvalho, V.S. Photosynthetic metabolism and growth of pineapple (Ananas comosus L. Merr.) cultivated ex vitro. Theor. Exp. Plant Physiol. 2016, 3, 333–339. [Google Scholar] [CrossRef]
- Gotoh, E.; Oiwamoto, K.; Inoue, S.I.; Shimazaki, K.-I.; Doi, M. Stomatal response to blue light in crassulacean acid metabolism plants Kalanchoe pinnata and Kalanchoe daigremontiana. J. Exp. Bot. 2019, 70, 1367–1374. [Google Scholar] [CrossRef]
- Kitajima, K.; Hogan, K.P. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef]
- Mitchell, A.; Arnott, J. Effects of shade on the morphology and physiology of amabilis fir and western hemlock seedlings. New For. 1995, 10, 79–98. [Google Scholar] [CrossRef]
- Males, J.; Griffiths, H. Stomatal biology of CAM plants. Plant Physiol. 2017, 174, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, S.; Wang, S.; Li, W.; Huang, W. Morphological, photosynthetic, and CAM physiological responses of the submerged macrophyte Ottelia alismoides to light quality. Environ. Exp. Bot. 2022, 202, 105002. [Google Scholar] [CrossRef]
- Ceusters, N.; Borland, A.M.; Ceusters, J. How to resolve the enigma of diurnal malate remobilisation from the vacuole in plants with crassulacean acid metabolism? New Phytol. 2021, 229, 3116–3124. [Google Scholar] [CrossRef]
- Ceusters, N.; Valcke, R.; Frans, M.; Claes, J.E.; Van den Ende, W.; Ceusters, J. Performance index and PSII connectivity under drought and contrasting light regimes in the CAM orchid Phalaenopsis. Front. Plant Sci. 2019, 10, 1012. [Google Scholar] [CrossRef]
- Marques, A.R.; Duarte, A.A.; de Souza, F.A.; de Lemos-Filho, J.P. Does seasonal drought affect C3 and CAM tank-bromeliads from campo rupestre differently? Flora 2021, 282, 151886. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.Q.; Zeng, Z.L.; Yu, H.; Huang, W. Photosynthesis under fluctuating light in the CAM plant Vanilla planifolia. Plant Sci. 2022, 317, 111207. [Google Scholar] [CrossRef]
- Qiu, S.; Xia, K.; Yang, Y.; Wu, Q.; Zhao, Z. Mechanisms Underlying the C3–CAM Photosynthetic Shift in Facultative CAM Plants. Horticulturae 2023, 9, 398. [Google Scholar] [CrossRef]
- Heyduk, K. Evolution of Crassulacean acid metabolism in response to the environment: Past, present, and future. Plant Physiol. 2022, 190, 19–30. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, J.; Jawdy, S.; Sreedasyam, A.; Lipzen, A.; Wang, M.; Ng, V.; Daum, C.; Keymanesh, K.; Liu, D.; et al. Comparative genomics analysis of drought response between obligate CAM and C3 photosynthesis plants. J. Plant Physiol. 2022, 277, 153791. [Google Scholar] [CrossRef]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid responses of plants to temperature changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef] [PubMed]
- Baťková, P.; Pospíšilová, J.; Synková, H. Production of reactive oxygen species and development of antioxidative systems during in vitro growth and ex vitro transfer. Biol. Plant. 2008, 52, 413–422. [Google Scholar] [CrossRef]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
| Components | CaO | K2O | P2O5 | OM | EC | pH |
|---|---|---|---|---|---|---|
| mg∙L−1 | % | mS∙cm−1 | ||||
| Soil + filter cake | 211.32 | 108.86 | 1107.9 | 31.4 | 1.07 | 7.10 |
| Variables | Plastic Trays | Black Polyethylene Bags | Average | SE | ||||
|---|---|---|---|---|---|---|---|---|
| Microp | Non-Cryo | Cryo | Microp | Non-Cryo | Cryo | |||
| Plant height (cm) | 10.48 b | 10.52 b | 10.53 b | 11.98 a | 11.97 a | 11.99 a | 11.24 | ±0.04 |
| Number of leaves | 8.26 b | 8.24 b | 8.21 b | 9.20 a | 9.23 a | 9.21 a | 8.65 | ±0.04 |
| D leaf length (cm) | 9.11 a | 9.16 a | 9.16 a | 9.14 a | 9.13 a | 9.12 a | 9.14 | ±0.05 |
| D leaf width (cm) | 1.55 b | 1.42 b | 1.54 b | 1.60 a | 1.63 a | 1.62 a | 1.56 | ±0.08 |
| D leaf area (cm2) | 6.88 b | 6.82 b | 6.75 b | 7.02 a | 7.01 a | 7.03 a | 6.94 | ±0.07 |
| Diameter of stem base (cm) | 1.32 a | 1.29 a | 1.28 a | 1.36 a | 1.35 a | 1.36 a | 1.32 | ±0.04 |
| Number of roots | 10.88 b | 11.02 b | 10.96 b | 12.24 a | 12.17 a | 12.31 a | 11.59 | ±0.14 |
| Plant fresh weight (g) | 10.48 b | 10.52 b | 10.46 b | 10.66 a | 10.62 a | 10.66 a | 10.48 | ±0.06 |
| Plant dry weight (g) | 1.83 b | 1.82 b | 1.80 b | 1.86 a | 1.86 a | 1.86 a | 1.83 | ±0.03 |
| Evaluation Time (Days) | D Leaf Water Content (g H2O cm−2) | Chlorophyll a (µg Chlorophylls cm−2 of D Leaf) | Chlorophyll b (µg Chlorophylls cm−2 of D Leaf) | Chlorophyll (a + b) (µg Chlorophylls cm−2 of D Leaf) | Chlorophylls a/b | Mesophilic SUCULENCE Index (g H2O mg−1 Chlorophylls) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microp | Non-Cryo | Cryo | Microp | Non-Cryo | Cryo | Microp | Non-Cryo | Cryo | Microp | Non-Cryo | Cryo | Microp | Non-Cryo | Cryo | Microp | Non-Cryo | Cryo | |
| 0 | 0.053 b | 0.052 b | 0.052 b | 30.97 b | 30.38 b | 30.92 b | 16.23 b | 16.38 b | 16.15 b | 47.27 c | 47.12 c | 47.21 c | 1.89 a | 1.87 a | 1.90 a | 0.88 c | 0.90 c | 0.89 c |
| 15 | 0.045 c | 0.044 c | 0.044 c | 20.77 c | 21.02 c | 20.98 c | 19.14 ab | 19.27 ab | 19.12 ab | 39.91 b | 39.78 b | 39.88 b | 1.08 b | 1.07 b | 1.06 b | 1.88 a | 1.89 a | 1.87 a |
| 30 | 0.048 b | 0.049 b | 0.047 b | 21.14 c | 21.12 c | 21.19 c | 20.18 b | 19.88 b | 19.95 b | 40.99 b | 40.20 b | 40.52 b | 1.09 b | 1.10 b | 1.08 b | 1.54 b | 1.52 b | 1.53 b |
| 45 | 0.081 a | 0.080 a | 0.080 a | 33.47 a | 33.56 a | 33.54 a | 24.27 a | 24.14 a | 24.19 a | 58.05 a | 58.02 a | 57.98 a | 1.88 a | 1.89 a | 1.87 a | 1.44 b | 1.42 b | 1.42 b |
| SE | ±0.012 | ±0.12 | ±0.15 | ±0.78 | ±0.02 | ±0.032 | ||||||||||||
| Indicator | Microp | Non-Cryo | Cryo | ||||
|---|---|---|---|---|---|---|---|
| 12:00 a.m. | 12:00 p.m. | 12:00 a.m. | 12:00 p.m. | 12:00 a.m. | 12:00 p.m. | SE | |
| D leaf transpiration rate (µmol H2O m−2 s−1) | 1.97 a | 0.02 b | 1.96 a | 0.03 b | 1.95 a | 0.04 b | ±0.01 |
| D leaf stomatal conductance (µmol H2O m−2s−1) | 0.10 b | 58.22 a | 0.26 b | 58.57 a | 0.25 b | 58.56 a | ±0.25 |
| D leaf CO2 assimilation (µmol CO2 m−2 s−1) | 8.26 a | 0.01 b | 8.20 a | 0.07 b | 8.21 a | 0.06 b | ±0.06 |
| D leaf CO2 assimilation percentage (%) | 99.87 a | 0.12 b | 99.15 a | 0.84 b | 99.27 a | 0.72 b | ±0.71 |
| D leaf water use efficiency (µmol CO2 µmol−1 H2O) | 4.19 a | 0.14 b | 4.18 a | 0.13 b | 4.20 a | 0.15 b | ±0.01 |
| D leaf organic acid levels (µmol H+ g−1 fresh weight) | 52.58 a | 6.22 b | 52.46 a | 6.34 b | 52.47 a | 6.33 b | ±0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos-Olivera, A.; Lorenzo-Feijoo, J.C.; Quintana-Bernabé, N.; Leiva-Mora, M.; Bettoni, J.C.; Martínez-Montero, M.E. Morpho-Anatomical and Physiological Assessments of Cryo-Derived Pineapple Plants (Ananas comosus var. comosus) after Acclimatization. Horticulturae 2023, 9, 841. https://doi.org/10.3390/horticulturae9070841
Villalobos-Olivera A, Lorenzo-Feijoo JC, Quintana-Bernabé N, Leiva-Mora M, Bettoni JC, Martínez-Montero ME. Morpho-Anatomical and Physiological Assessments of Cryo-Derived Pineapple Plants (Ananas comosus var. comosus) after Acclimatization. Horticulturae. 2023; 9(7):841. https://doi.org/10.3390/horticulturae9070841
Chicago/Turabian StyleVillalobos-Olivera, Ariel, José Carlos Lorenzo-Feijoo, Nicolás Quintana-Bernabé, Michel Leiva-Mora, Jean Carlos Bettoni, and Marcos Edel Martínez-Montero. 2023. "Morpho-Anatomical and Physiological Assessments of Cryo-Derived Pineapple Plants (Ananas comosus var. comosus) after Acclimatization" Horticulturae 9, no. 7: 841. https://doi.org/10.3390/horticulturae9070841
APA StyleVillalobos-Olivera, A., Lorenzo-Feijoo, J. C., Quintana-Bernabé, N., Leiva-Mora, M., Bettoni, J. C., & Martínez-Montero, M. E. (2023). Morpho-Anatomical and Physiological Assessments of Cryo-Derived Pineapple Plants (Ananas comosus var. comosus) after Acclimatization. Horticulturae, 9(7), 841. https://doi.org/10.3390/horticulturae9070841







