Abstract
Root restriction is suitable for horticultural soilless cultivation characterized by high efficiency and quality in the case of high density and low node order pinching. However, little research is available on the mechanism of root restriction improving the flavor and nutritional quality of tomatoes. We investigated the effects of Extreme Root Restriction (ERR, 750 mL/plant) on the content of metabolites, activity of enzymes, and gene expression level involving sucrose metabolism in different clusters of two tomato types. The fruit diameter and single fruit weight of common tomato at CIII were reduced by 5.6% and 14% under ERR, as a result, the fruit uniformity throughout the whole plant was improved. The ERR enhanced the accumulation of metabolites in tomato fruits, such as soluble sugars, amino acids, vitamin C, lycopene, and polyphenol, which was caused by ‘concentration effect’ that occurred with a reduction of fruit size. The activities of enzymes (SS, SPS, NI, AI) at CIII and CIV of cherry tomatoes increased by 3–4 folds under ERR. ERR enhanced accumulation of sucrose, glucose, and fructose in tomato fruits not only by modulating activities of metabolizing enzymes but also by inducing the expression of sucrose metabolism genes, including sucrose synthase genes (SS1, SS3–6) in common tomato, fructokinase genes (FKs), hexokinase genes (HKs), and sucrose phosphate synthase genes (SPSs), in cherry tomato. The above results are expected to provide a theoretical basis for root restriction cultivation techniques and practical guidance for high-quality tomato production in industrialized cultivation.
1. Introduction
Tomato (Solanum lycopersicum L.) is the second most important vegetable crop next to potato globally, with approximately 182.3 million tons each year [1]. China is the highest tomato-producing country in the world, accounting for about 30% of the global output [1]. In China, tomato has become the largest vegetable cultivated in protected horticulture, and the output has been able to meet people’s daily constant demand due to the stable production scale [2]. Tomato is one of the highest-value fruit and vegetable crops worldwide and makes a substantial nutritional contribution to the human diet [3] as it is rich in vitamins, dietary fibers, minerals, amino acids, carotenoids, and phenolic compounds [4]. The demand for fresh tomatoes of high flavor, nutritional and functional quality is on the rise [5,6]. Vegetative characteristics are affected by various factors, such as variety, climate, soil characteristics, cultivation processes, while cultivation technique is the key factor to affect the quality of tomato quality in the case of a given variety [7]. The flavor and nutrients in tomato fruit can be effectively regulated by reasonable environmental control and appropriate agronomic techniques [5].
Root restriction is a resource-saving, innovative, and stress-inducing technique in agricultural production [8]. This technique has been widely used in fruit trees [6]. The mechanism of root restriction inhibited growth has already been clarified in many fruit trees, such as the reduction of nitrate and nitrite reductase activities, decreased total amount of N in grape leaves and berry flesh [9], down-regulation of gene expression in nitrogen metabolism in leaves of grapes [10], low sink demand from the roots of chili [11], low O2 availability within the root of tomatoes [12], etc. Moreover, the effects of root restriction on fruit quality improvement were reported in grape [13], chili [11], and sweet cherry [14], but little research has been done on vegetables, especially tomatoes.
Root restriction is suitable for high-density, high-efficiency, high-yield, and quality cultivation for plants [6,8]. In Japan, tomatoes grown in extremely low-volume substrate (ELVS, 250 mL/plant) displayed high total soluble solid content and flavor as well as high fruit production quality (35 t/year) in the case of low-node-order pinching and high-density planting, which could reach up to 3.5 crops per year and realize annual sustainable production [15]. Root restriction is important for high-quality tomato production in industrialized cultivation. We previously reported the management techniques of nutrient solution and proposed a modified nutrient strategy for improving the quality of tomatoes grown in the ELVS [16,17]. Moreover, we enhanced the quality of tomatoes by reducing the drainage rate, increasing electricity conductivity, or adding NaCl under root restricted conditions (0.75 L/plant, [18]). Previous studies were mainly focused on the effects of nutrient solution management mode on fruit production and fruit quality [16,17,18]. The regulation mechanism of sucrose metabolism as well as its metabolites by root restriction is still unknown.
In the present study, two types of common tomato and cherry tomato widely cultivated in south China are used, which are grown in cocopeat strip (control, 24 L/3 plants) and cocopeat pot (Extreme Root Restriction, ERR, 0.75 L/plant) system. The content of metabolites, including soluble sugars, organic acids, amino acids, Vc, lycopene, polyphenol, and the activity of enzymes and sucrose metabolism-associated gene expression in different clusters were investigated.
2. Materials and Methods
2.1. Plant Materials and Culture Conditions
The experiment was conducted in an experimental greenhouse (28/18 °C in day/night with relative humidity within 65–70%), at the College of Horticulture, South China Agricultural University (23.15868 N, 113.34462 E) during the autumn-winter cropping season (5 September 2019 to 23 January 2020). The air temperature and relative humidity were monitored by a detector produced by IOT Ltd. (Model IOT-WD20, Guangzhou, China). Tomato grafting seedlings were provided by Hua You seedlings factory (Guangdong, China), specifically root stock was eggplant (Solanum torvum), the scion tomatoes (Solanum Lycopersicum) were common (Hong Tao K) and cherry (Qian Xi) types, both were widely cultivated in Guangdong province.
The treatments included two different root zone cultivation containers, one was a widely used cocopeat strip (control, every three plants were cultivated in one strip, 3 plants/24 L), the other was an extreme low-volume substrate, as discussed in our previous research [18] (plastic pot filled with cocopeat (ERR, Extreme Root Restriction), one plant cultivated in one pot (0.75 L/plant). Thus, the two types of root volume containers were filled with just pure cocopeat.
All plants were supplied with the “Enshi formula” nutrient solution through drip irrigation system, as described in our previous research [16], from transplanting to the end of the experiment. The fertigation frequency was controlled by a timer, which was between 4 (cloudy)~12 (sunny) times per day, and the fertigation amount was based on the drainage rate of 20–30%, pump flow rate was 15 mL·min−1. Periodic operations of binding, lateral bud, and basal leaf pruning were carried out in a timely manner. Plants were pruned with a single stem and pinched at cluster IV in common tomato and cluster VII in cherry tomato. Four replicates for each treatment were designed, each replicate containing 12 plants. Ten full ripening fruits in each replicate at the same cluster were randomly selected, each sample including 4 biological replicates, the pericarp and flesh of fruits were frozen in liquid nitrogen then stored at −80 °C until the analysis of metabolite analysis, enzyme activities, and gene expression.
2.2. Measurement of Plant Growth and Fruit Production
Plant height, leaf area, and stem diameter below cluster I and III were dynamically measured from 3 to 7 weeks after treatment. Plant height from basal part to apical point was measured by a meter ruler. Length and width of leaf under cluster II were measured by a ruler and calculated the leaf area by an equation (S = 0.6393 × length × width). Stem diameter below cluster I and III, and fruit diameter in cluster II, III, and IV were measured by a digital caliper. Fruit weight was recorded using an electronic balance during fruit ripening stage. Fruit setting rate was the ratio of the number of fruits to the number of flowers.
2.3. Analysis of Metabolites in Tomato Fruits
Sugar content was measured by the method of previous research [19] with some modifications. Namely, 0.5 g frozen-dried samples were homogenized in 5 mL 90% ethanol, then they were placed in water bath at 80 °C for 25 min. The mixture was centrifuged at 7000 rpm for 5 min, the supernatant was transferred to a glass tube, and the above extraction was repeated twice. The supernatant was then dried under pressure and pure water was added. Samples (1 mL) of the resulting solution were filtered through a 0.45 µm MilliporeTM filter then analyzed by angilent 1200 HPLC system (Agilent technologies, Waldbronn, Germany). Chromatographic column: Coregel 87 C (Transgenomic CHO-99-58600) column was used with pure water as the mobile phase at a flow rate of 0.6 mL/min, and the column temperature was 80 °C.
Organic acids were measured by the procedure of previous research [19] with some modifications. Namely, 0.5 g frozen-dried samples in 5 mL 0.01 mM KH2PO4 (pH = 2.55) and the mixture was ice bath ultrasound for 40 min and then centrifuged at 20,000 rpm for 20 min at 4 °C. The extract was filtered through a 0.22 µm MilliporeTM filter, and the filtrate was then analyzed by high-performance liquid chromatography (HPLC, Shimadzu, Japan). Detection of organic acids was performed at 210 nm using a photodiode array detector. Test conditions: C18 column (InertSustain ® 250 mm × 4.6 mm), the mobile phase was 0.01 mol/L potassium dihydrogen phosphate (pH = 2.55, adjusted with phosphoric acid): Methanol = 97:3, the column temperature was 30 °C, the detection wavelength was 210 nm, and the flow rate was 0.5 mL/min.
The total soluble solid content (TSSC) of fruit juice in each cluster squeezed by hand with a cheesecloth was investigated by a hand refractometer (Model FR-100; Atago Co., Ltd., Tokyo, Japan) as described in our previous research [16]. The vitamin C and polyphenol content were measured according to the procedure described in our previous research [20]. Lycopene content was measured according to our previous research [18]. 0.5 g frozen-dried samples were extracted with 30 mL mixed solution (hexane: acetone: absolute ethanol = 1:1:1 (v/v/v)), shaken for 30 min, and then 10 mL ultra-pure water was added. After centrifugation at 2000 rpm for 10 min, the absorbance value was measured by UV spectrophotometer at 502 nm.
Amino acid composition and content were analyzed and referred to the JY-T0194-1996 General Rules. Namely, 0.1 g frozen-dried samples were homogenized in 2 mL 6% sulfosalicylic acid for 1 min. After 20 min of ultrasound with ultrasonic cleaning, the mixtures were centrifuged at 12,000 rpm at 4 °C for 10 min. The supernatant was filtered through a 0.22 µm MilliporeTM filter, then 0.2 mL filtrate was added to 0.8 mL ultrapure water analyzed by L-8900 Amino Acid Analyzer. Test conditions: Hitachi type 855–4507 column, the column reaction temperature was 135 °C, the pH buffer of citric acid (lithium) was eluted in gradient, and the detection wavelength was 570 + 440 nm. The pump flow rate was 0.35 mL/min, and the derivative pump flow rate was 0.30 mL/min, and the analysis time was 148 min.
2.4. Analysis of Enzyme Activity
Frozen-dried fruit samples were ground into powder for enzyme activity analysis. The activity of four enzymes was determined with a sucrose phosphate synthase kit (SPS), a soluble acid convertase kit (S-AI), a neutral translate kit (NI), and a sucrose synthetase kit (SS-I) by microassay determination method. All the kits were produced by Suzhou Keming Biotechnology Co., Ltd., Suzhou, China.
2.5. Quantitative Real-Time PCR Analysis
Expression of genes involved in the sucrose metabolism in tomato fruits was evaluated by performing quantitative real-time PCR (qRT-PCR), as described in our previous research [21,22]. Total RNA was isolated from the samples by using RNAprep Pure Plant Kit (Tiangen Biotech Co., Ltd., Beijing, China). The cDNA was synthesized from 1 µg of total RNA by using the PrimeScriptTM RT reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa Bio, Inc., Shiga, Japan). The qRT-PCR was conducted using the Light Cycler 480 Real-Time PCR system (Roche, Basel, Switzerland) with one-step SYBR Premix Ex Taq TM (Takara, Dalian, China). The relative expression was normalized with the results of the mean values of Sl-UBI using the 2−ΔΔCt method with IQ5 software (BIO-RAD, Hercules, CA, USA). Genes were designed on the NCBI primer blast website (https://www.ncbi.nlm.nih.gov/tools/primer-blast/ accessed on 26 September 2021). The primer quality and efficiency were checked by qPCR on a series of diluting cDNA. The primer sequences are listed in the Supplementary Materials, Table S1.
2.6. Statistical Analysis
All values are shown as the mean ± standard error. Significant differences among the clusters were determined by analysis of variance (ANOVA), followed by Duncan’s multiple range tests of SPSS 22.0 at p ≤ 0.05. Statistical differences between ERR and control treatment were evaluated with t-test at * p < 0.05; and ** p < 0.01 levels. Heat maps were generated using TBtools (http://cj-chen.github.io/TBtools/, accessed on 23 February 2023).
3. Results
3.1. Plant Growth and Fruit Production
Although two tomato types were planted at five fully expanded leaves simultaneously, common tomato displayed higher plant height (Figure 1A and Figure S1), leaf area (Figure 1B), and stem diameter below CI (Figure 1C) than in cherry tomato after three weeks of transplanting. In this study, the cocopeat strip was used as the control. Plant growth during three to seven weeks after treatment in two types was markedly inhibited by ERR treatment, which was more severe in cherry tomato than in common tomato (Figure 1). Firstly, the plant height in ERR-cultivated common tomato was significantly lower than that in the control from the three to five weeks after treatment, while the cherry tomato was from three to seven weeks after treatment (Figure 1A). Secondly, ERR-cultivated common tomato showed significantly decreased leaf area from five to seven weeks after treatment, but cherry tomato was from three to seven weeks (Figure 1B). Thirdly, we measured stem diameter below CI and CIII, the inhibition effects of ERR on stem diameter were more severe on cherry tomato than on common tomato as compared with the control (Figure 1C,D). Meanwhile, the magnitude of reduction was larger in CIII than in CI (Figure 1C,D).
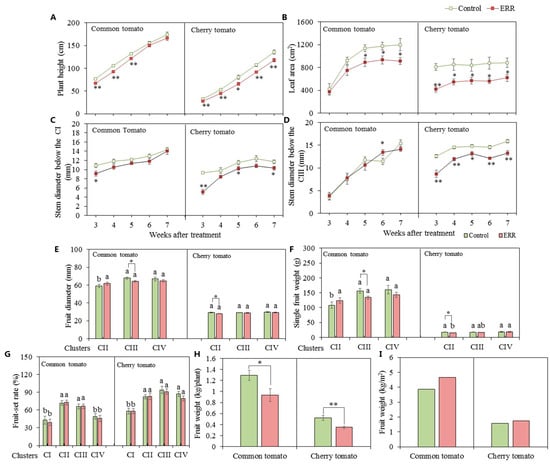
Figure 1.
Plant growth parameters and fruit production of common tomato and cherry tomato grown in coco coir strip (control) and coco coir pot (Extreme Root Restriction, ERR). (A) Plant height. (B) Leaf area. (C) Stem diameter below cluster I (CI). (D) Stem diameter below cluster III (CIII). (E) Fruit diameter. (F) Single fruit weight. (G) Fruit set rate in different clusters. (H) Fruit weight. (I) Fruit weight per unit area. CII, cluster II; CIII, cluster III; CIV, cluster IV. The data represent the mean ± SE (n = 4). Different lowercase letters indicate significant differences among three clusters (p ≤ 0.05). The t-test was applied to analyze the difference between the ERR and control at * p ≤ 0.05 and ** p ≤ 0.01 levels.
The fruit diameter, single fruit weight, and rate of fruit set were investigated at the different clusters (Figure 1E–I). The ERR-cultivated two types exhibited significantly decreased fruit diameter and single fruit weight, common tomato was observed at CIII, cherry tomato was at CII (Figure 1E,F). As compared with the control, fruit diameter and single fruit weight of common tomato at CIII were reduced by 5.6% and 14% under ERR treatment (Figure 1E,F), therefore, there were no significant differences in fruit diameter and single fruit weight among three clusters under ERR, which increased with cluster under the control (Figure 1E,F). Fruit size was more uniform among clusters of common tomato grown under ERR than the control.
The fruit set rate was not influenced by ERR, the highest level was concentrated in the central cluster (Figure 1G). To compare with the control, fruit weight per plant was reduced by 28% and 33%, respectively, in common and cherry tomatoes under ERR (Figure 1H). Because the planting density was five plants/m2 in ERR and three plants/m2 in control, the yield per square meter was increased by ERR (Figure 1I).
3.2. Nutritional and Flavor Quality of Tomatoes
The two tomato types varied in terms of soluble sugars, cherry tomato produced more soluble sugars (sucrose, glucose, and fructose) than common tomato (Figure 2A–C). Fructose and glucose are the main accumulated sugars in two types. The effects of ERR on the accumulation of soluble sugars and organic acids were different between types and among clusters (Figure 2). In the case of common tomato, the content of sucrose and glucose increased significantly with clusters under ERR, whereas this phenomenon was not observed under the control (Figure 2A,B). The ERR created a significant increase of sucrose, mainly in CIII and CIV (Figure 2A), glucose was in all clusters (Figure 2B), and fructose was in CII and CIV (Figure 2C). The sucrose content was three-fold higher in ERR than in the control (Figure 2A). To compare with the control, the content of fructose and glucose increased by 24% and 20% under ERR in CIV, respectively (Figure 2B,C). In the case of cherry tomato, the highest content of soluble sugars in cherry tomato was observed in CIII. To compare with the control, the ERR-cultivated cherry tomato showed a significantly higher content of sucrose in CII and CIV, glucose in CIII and CIV, and fructose in three clusters (Figure 2A–C). The sucrose content was two-fold higher in ERR than in the control in CIV (Figure 2A). To compare with the control, content of fructose and glucose increased by 23% and 23% under ERR in CII, respectively (Figure 2B,C).
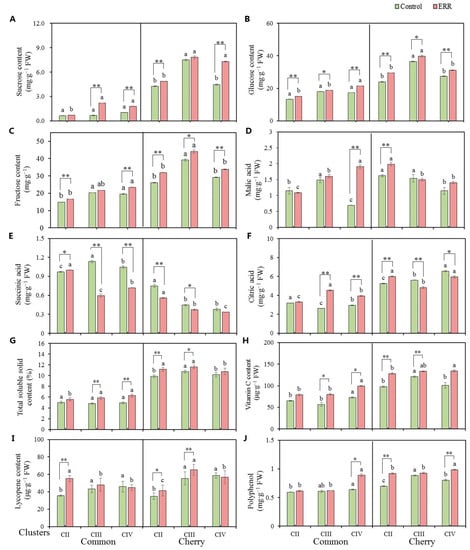
Figure 2.
Content of metabolites in fruits of common and cherry tomato grown in coco coir strip (control) and coco coir pot (Extreme Root Restriction, ERR). (A) Sucrose. (B) Glucose. (C) Fructose. (D) Malic acid. (E) Succinic acid. (F) Citric acid. (G) Total soluble solid content. (H) Vitamin C. (I) Lycopene. (J) Polyphenol. The data represent the mean ± SE (n = 4). The t-test was applied to analyze the difference between the ERR and control at * p ≤ 0.05 and ** p ≤ 0.01 levels. Different lowercase letters indicate significant differences among three clusters (p ≤ 0.05).
Citric acid is the main accumulated organic acid in both types. The content of citric acid in CIII and CIV of common tomato was significantly increased by the ERR treatment, which was the opposite in cherry tomato (Figure 2F). CII showed significantly higher citric acid and malic acid in ERR treatment than the control in cherry tomato (Figure 2D,F). Except for CII in common tomato, other clusters exhibited a significantly lower content of succinic acid in ERR treatment than the control (Figure 2E).
The cherry tomato contained higher total soluble solids (TSS), vitamin C, and polyphenol than common tomato (Figure 2G). For common tomato, the content of TSS in CIII and CIV was significantly higher under ERR treatment as compared with the control, resulting in the content of TSS increasing with clusters, which was not observed in the control (Figure 2G). Furthermore, compared with the control, vitamin C and polyphenol in common tomato grown under ERR were significantly higher in CIV (Figure 2H,J). In the case of cherry tomato, compared with the control, the contents of TSS (Figure 2G) and lycopene (Figure 2I) were significantly higher in CII and CIII, the vitamin C (Figure 2H) and polyphenol (Figure 2J) were significantly higher in CII and CIV. In general, the content of total TSS, vitamin C, lycopene and polyphenol was increased by the ERR treatment, mainly reflected in the upper cluster of common tomato.
A total of 17 amino acids were detected in this study. To evaluate the contribution of amino acids to tomato fruit flavor and nutritional value, we classified them into four groups, including officinal, tasty, sweet, and aromatic, respectively (Figure 3). In the case of common tomato, both CIII and CIV contained significantly higher or extremely significantly higher levels of amino acids under the ERR treatment than the control. The level of sweet amino acids (Ser and Pro) was significantly higher under the ERR than the control in CIII and CIV. The level of tasty amino acids (Asp and Glu) was significantly higher under the ERR than the control from CII to CIV. In the case of cherry tomato, the promotion effects of ERR on amino acid accumulation were mainly in CII, for example, most of the amino acid content was higher under the ERR than the control in CII, while only the Glu and Pro were higher under the ERR than the control in CIII, no different amino acids were observed in CIV. CII of cherry tomato showed extremely higher sweet amino acids (Ser, Pro, and Ala), tasty amino acids (Asp and Glu), and officinal amino acid (GABA) under the ERR treatment than the control.
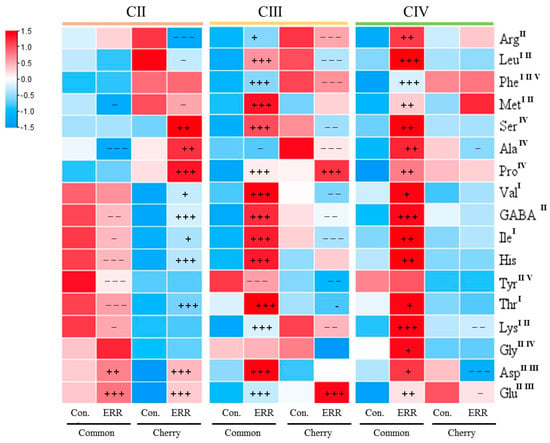
Figure 3.
Amino acid composition and content in fruits of common tomato and cherry tomato grown in cocopeat strip (control) and cocopeat pot (Extreme Root Restriction ERR). I, Essential amino acids. II, Officinal amino acids. III, Tasty amino acids. IV, Sweet amino acids. V, Aromatic amino acids. The plus/minus +/−, ++/−− and +++/−−− indicate a significantly higher/lower in ERR than in control at p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001.
3.3. Activity of Sucrose Metabolism Associated Enzymes
The changes in soluble sugars in fruits could be attributed to the differences in enzyme activity. As shown in Figure 4, the influence of ERR on the activity of enzymes involved in soluble sugar metabolism was different between types and among clusters. The enhancement effect of ERR on enzyme activity in cherry tomato (3–4 folds) was stronger than that in common tomato. As compared with the control, activity of SS and NI (Figure 4A,D) in ERR-treated cherry tomato was extremely significantly higher from CII to CIV, while activity of SPS and AI (Figure 4B,C) was significantly increased in CIII and CIV. Therefore, activity of enzymes involved in sucrose metabolism was enhanced by ERR, mainly in CIII and CIV (Figure 4). However, in the case of common tomato, the activity of SS, AI, and NI was reduced by ERR in CII and CIV, which was enhanced in CIII (Figure 4A–D).
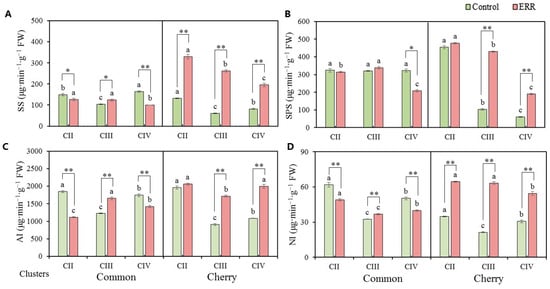
Figure 4.
Enzyme activity of sucrose metabolism in fruits of common tomato and cherry tomato grown in coco coir strip (control) and coco coir pot (Extreme Root Restriction, ERR). (A) Sucrose synthase (SS). (B) Sucrose phosphate synthase (SPS). (C) Acid invertase (AI). (D) Neutral invertase (NI). Error bars represent standard deviations of the means of three independent replicates. The data represent the mean ± SE (n = 4). Different lowercase letters indicate significant differences among three clusters (p ≤ 0.05). The t-test was applied to analyze the difference between the ERR and control at * p ≤ 0.05 and ** p ≤ 0.01 levels.
3.4. Genes Expression Level
To determine whether the sucrose metabolism responds to ERR differentially between types and among clusters, we examined the expression level of sucrose metabolism genes in both types and three clusters by using RT-PCR-based expression analysis. A total of 27 genes encoding invertase (LIN5, LIN6, LIN7, VI, NI1, NI3, NI4, NI5), invertase inhibitor (VIF and CIF2), sucrose synthase (SS1, SS3, SS4, SS5 and SS6), sucrose phosphate synthase (SPSA1 and SPSB), hexokinase (HK1, HK2, HK3, HK4, HK6), and fructokinase (FK1, FK2, FK3, FKL1 and FKL2) were identified (Figure 5A,B). The responses of sucrose metabolism genes to ERR were different between types. The differentially expressed genes between CII and CIV were consistent, in which the expression of SS1, SS3, SS4, SS5, and SS6 was up-regulated by the ERR in common tomato, whereas in cherry tomato, the expression of SPSB, HK1, HK4, HK6, FK1, and FKL2 was up-regulated by the ERR (Figure 5B). In consideration of the inhibited genes, the expression of Lin5, Lin6, HK3, and VI was down-regulated by the ERR in common tomato, but only CIF2 was down-regulated by the ERR in cherry tomato. In general, ERR induced expression of genes involved in the sucrose degradation and synthesis by sucrose synthase in common tomatoes. However, in cherry tomato, the expression of genes involved in the phosphorylation of fructose and glucose, as well as sucrose phosphate synthase, was enhanced by ERR.
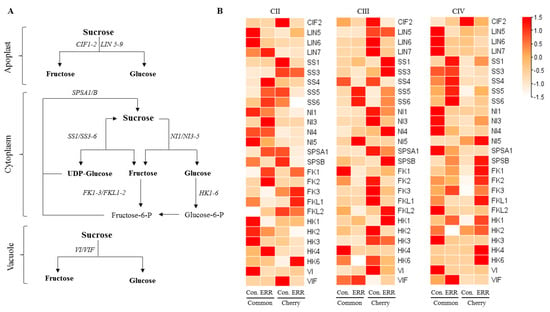
Figure 5.
The expression of a number of genes involved in sucrose metabolism in fruits of common tomato and cherry tomato grown in cocopeat strip (control) and cocopeat pot (Extreme Root Restriction, ERR). (A) The sucrose metabolism pathway. (B) The expression profiles of sucrose metabolism genes in various clusters.
PCA was used to visualize the effects of ERR on the plant growth, fruit production, and quality of two tomato types. Overall, PC1 and PC2 explained 67.7% and 16.6% of the total variance (Figure 6) in both tomato types. The cherry and common were divided into two clear groups in the PCA scatter plot. Cherry tomato in the upper quadrants (ERR treatment) was characterized by higher fruit metabolites and activities of sucrose metabolism enzymes. Common tomato in the ERR treatment was characterized by inhibited plant growth.
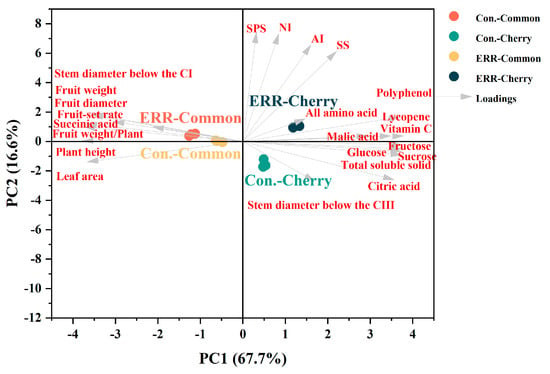
Figure 6.
Principle component analyses showing the differences in the investigated parameters in common tomato and cherry tomato grown in cocopeat strip (Con.) and cocopeat pot (Extreme Root Restriction, ERR).
4. Discussion
4.1. ERR Suppressed Plant Growth and Fruit Size, but Improved Fruit Uniformity
Root restriction was considered to be an effective means to regulate the balance between vegetative growth and reproductive development [8]. Root restriction decelerated vegetative growth but enhanced the reproductive growth in different fruits, such as grapes [23], sweet cherry [14], chili [11], and tomato [6]. In this study, although the nutrient solution was fertigated to the tomato plants at a ‘low concentration and high frequency’ principle to accommodate the low preservation ability of water and nutrition under ERR, the growth parameters of both types, including plant height, leaf area, stem diameter were reduced by ERR at different extents (Figure 1A–D). The suppressed growth under ERR coincided with the above-mentioned previous research.
In response to the ERR, both the period and extant of growth restriction last longer and are more severe in cherry tomato than in common tomato (Figure 1A,B,D). Correspondingly, both single fruit weight and size were significantly reduced in the upper clusters by ERR, there were no significant differences in fruit size among clusters and single fruit weight of common tomato (Figure 1E,F), which means the fruit size was more uniform in common tomato grown in ERR. This phenomenon was not observed in cherry tomato. Because common tomato displayed higher fruit load than cherry tomato, the inhibition effect of ERR on fruit enlargement mainly reflected during the full fruit load period as the crop cycle processed, especially in the higher fruit load cultivar. The fruit set was not affected by the ERR treatment (Figure 1H). The fruit weight per plant was reduced by 28% and 33%, respectively, in common and cherry tomatoes under ERR (Figure 1H). However, the yield per unit area was increased (Figure 1I) due to the higher planting density in ERR than in the control, the planting density was about three plants/m2 under control and five plants/m2 under ERR. Previous research indicated that the number of tomato fruits was decreased by root restriction [24]. Contrarily, the yield of tomato increased with the extent of root restriction when the yield was recorded by per unit area [6]. Tomatoes displayed high TSSC and flavor as well as high fruit production (35 t/year/10a) in the case of high density [15]. These results suggested that although ERR suppressed plant growth and reduced fruit size, it improved uniformity of fruit size among different clusters as well as fruit production in the case of high-density planting.
4.2. ERR Improved Nutritional and Flavor Quality of Tomatoes
Root restriction is considered an abiotic stress cultivation technique, such as a limited supply of water [25], nutrients [11], salinity [26] for roots, etc. Plants respond to environmental stress by accumulating sugars, which are precursors of metabolites [27]. In the present study, the effects of ERR on tomato fruit quality were evaluated by the accumulation of soluble sugars, organic acids, vitamin C, lycopene, polyphenol, and amino acid (Figure 2 and Figure 3). In general, the contents of soluble sugars were increased by ERR in which sucrose and glucose increased significantly with cluster under ERR in the case of common tomato, whereas this phenomenon was not observed in the control (Figure 2A,B). Regarding sugars, the levels of organic acids, total soluble solids, vitamin C, polyphenol (Figure 2), and amino acids (Figure 3) were also increased under the ERR, mainly in the upper cluster of common tomato and lower cluster of cherry tomato. The changes in metabolite content are consistent with the decreased fruit size in the upper clusters of common tomato and lower clusters in cherry tomato (Figure 1E,F).
Previous research indicated that the enhancement of metabolites in tomato fruits exposed to salt stress is explained as a ‘concentration effect’ that occurs with a decrease in fruit water and size [28]. A moderate water deficit increases the ratio of sugars to acids in tomato fruit [4]. Root restriction combined with salinity stress increased the total soluble solid content in tomatoes [28]. In the present study, a higher level of metabolites was mainly attributed to the ‘concentration effect’, together with reduced fruit size caused by water or salinity stress. Moreover, ERR regulates the metabolic module from the glycolytic pathway to the tricarboxylic acid cycle and then to the biosynthesis of amino acid, vitamin C, lycopene, and polyphenol. The higher level of metabolites improved the quality of tomatoes. Abiotic stresses can negatively affect crop yield, but they can enhance fruit quality attributes by using grafting plants [29], such as tomato fruit quality and dry matter content, which are positively affected by the rootstock [30]. Some rootstocks are able to increase agronomic water use efficiency (WUE) by reducing leaf growth and water use but maintaining fruit yield [31]. Two root-derived hormones are positive (ABA) or negative (ACC) factors controlling the productivity parameters of tomatoes [32]. ACC was considered to be an important root-derived hormonal signal that negatively affects leaf growth but does not limit fruit yield, thereby increasing water use efficiency [31]. In conclusion, there was probably hormone signaling transduction under the ERR in the present research. It is necessary to further clarify the regulatory modules of root hormone signal transduction.
4.3. ERR Enhanced Enzyme Activity Involved Sucrose Metabolism in Tomato Fruits
Fruits are the main sink of assimilates during the reproductive stage, depending on the size and activity of sink [33]. The regulation of assimilate metabolism in tomato fruit mainly depends on the activities of SS, SPS, AI, and NI [34]. In the present study, the sink strength has been firstly reduced by ERR in both types due to decreased fruit size (Figure 1E,F). However, the activity of enzymes (SS, SPS, AI, NI) involved in sucrose metabolism was generally enhanced by ERR in all clusters in the case of cherry tomato, but common tomato just showed in CIII (Figure 4). It suggests that the effects of ERR on enzyme activity depended on the type of fruits. In this study, to compare with common tomato, cherry tomato exhibited relatively low level of plant growth, fruit production (Figure 1), and enzyme activity of sucrose metabolism (Figure 4), but produced higher sugar level, meanwhile ERR has a stronger effect of reduction on its growth and fruit production, but enhancement of enzyme activity as well as sugar accumulation (Figure 2). Cherry tomato itself exhibited higher productivity and quality than common tomato [35]. Thus, the typical characteristics of cherry tomato determine the different response to ERR. PCA analyses indicated cherry tomato was different from common tomato in response to ERR (Figure 6). Cherry tomato in the upper quadrants (ERR treatment) was characterized by higher fruit metabolites and activities of sucrose metabolism enzymes. Common tomato in the ERR treatment was characterized by inhibited plant growth (Figure 6).
Water stress can enhance the activities of AI and SS in tomato fruit, which leads to higher levels of soluble sugars [36]. The AI is known as a key enzyme in root restriction induction, resulting in the higher sugar content in grape fruits under root restriction [23]. Similarly in our study, the AI activity kept the highest level, followed by SPS and SS, the last was NI in both types. The effect of ERR on enzyme activity was different between types and clusters. In the case of common tomato, activities of SS, AI and NI were significantly enhanced by ERR in CIII (Figure 4), which was consistent with higher levels of sucrose and glucose (Figure 2) and decreased fruit size and single fruit weight in CIII (Figure 1). However, this relationship was not observed in the other clusters. This suggests that increased sugar content in CIII was not only related to fruit size but also to enzyme activity in CIII. In the case of cherry tomato, activities of SS, SPS, AI, and NI were all extremely significantly increased by ERR in the clusters of CIII and CIV (Figure 4), in which SPS level was about three to four-fold higher than that in the control (Figure 4B). This is consistent with increased content of sucrose (Figure 2A). The SPS is known as the key enzyme for sucrose synthesis, higher SPS activity resulting in the accumulation of sucrose in tomatoes [34]. In addition, SPS activity was positively correlated with sucrose accumulation in the late period of tomato fruit development [37]. The above results suggest that ERR can enhance the sucrose-metabolizing enzyme activities, which leads to a higher content of soluble sugars in the upper clusters.
4.4. ERR Induced the Expression of Sucrose Metabolism Genes in Tomato Fruits
Root restriction has induced genes involved in nitrogen metabolism [10], anthocyanin biosynthesis [38], phytohormone biosynthesis, and the signal transduction of grapes [26]. The only observation worthy of mention related to sugar metabolism is that the sugar transporter gene VvSWEET15 in grape was significantly up-regulated under root restriction [39].
The VI, VIF, SS, HK, FK, and SPS gene families is known to regulate the degradation and re-synthesis of sucrose in tomatoes [40]. In the present study, a total of 27 genes encoding invertase, invertase inhibitor, sucrose synthase, sucrose phosphate synthase, hexokinase, and fructokinase were identified (Figure 5A,B). The differentially expressed genes between CII and CIV were consistent, in which the expression of SS1, SS3, SS4, SS5, and SS6 was up-regulated by the ERR in common tomato (Figure 5B), which is consistent with the increased content of fructose and glucose (Figure 2B,C). Overexpression of SS3 in tobacco resulted in fructose accumulation and sucrose hydrolysis [41]. Thus, the induced SSs genes by ERR may be one of the reasons for the increased content of fructose and glucose in the common type. It is necessary to clarify regulation mechanisms in further research.
For the cherry tomato, the expression of SPSB, HK1, HK4, HK6, FK1, and FKL2 was up-regulated by the ERR (Figure 5B). The expression of SPS gene was up-regulated at tomato fruit ripening, which promoted sucrose synthesis and increased the sweetness of fruit [42]. Research indicates that water stress on rice [43] enhances SPS activity or upregulates SPSs gene expression. However, the effects of water stress on SPS gene expression are weak in tomatoes [36]. In the present study on cherry tomato, the expression of SPSB was up-regulated by the ERR (Figure 5B), which coincided with significantly increased SPS enzyme activity (Figure 4B) and sucrose sugar content in CIV (Figure 2A). FK and HK play important roles in hexose phosphorylation, sensing, and signaling transduction [44]. In the present study, the expression of HK1, HK4, HK6, FK1, and FKL2 was also up-regulated by the ERR, suggesting that phosphorylation of fructose and glucose was enhanced by ERR in cherry tomato [45]. The above results suggest that ERR treatment enhanced the accumulation of sugars by regulating the expression of sucrose metabolism gene, specifically SS1, SS3, SS4, SS5, and SS6 in common tomato and FK, HK and SPS in cherry tomato, which led to the improvement of quality in tomato.
5. Conclusions
The flavor and nutritional quality of both tomato types were enhanced by Extreme Root Restriction caused by the reduction of fruit size. Meanwhile, Extreme Root Restriction enhanced enzyme activity and genes expression involved in sucrose metabolism. Together, these results indicate a typical characteristic of tomatoes grown in Extreme Root Restriction (Figure 7).
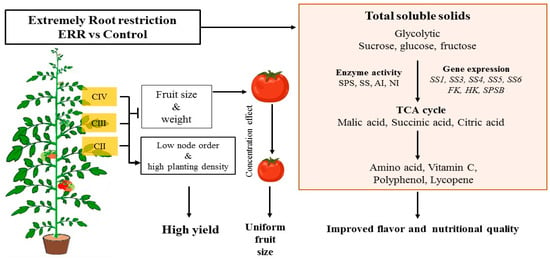
Figure 7.
ERR regulation of plant growth, fruit production, sucrose metabolism, and accumulation of nutritional and flavor compounds in tomatoes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9070813/s1, Figure S1: Plants appearance of common tomato and cherry tomato grown in coco coir strip (control) and coco coir pot (Extreme Root Restriction, ERR). Flowering stage of common tomato grown in control (A) and ERR (B). Flowering stage of cherry tomato grown in control (C) and ERR (D). Fruit ripening stage of common tomato grown in control (E) and ERR (F). Fruit ripening stage of cherry tomato grown in control (G) and ERR (H). Figure S2: Fruits appearance of common tomato and cherry tomato grown in coco coir strip (control) and coco coir pot (Extreme Root Restriction, ERR). Table S1: Gene-specific primers used for quantitative real-time PCR analysis.
Author Contributions
D.L. and J.C. performed the experiments and statistical analysis, Y.H. contributed to manuscript revision, X.Y. contributed to manuscript revision, R.C. contributed to manuscript revision and approved the submitted version of the manuscript, Y.Z. conceived and designed the experiments, analyzed the results, and drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (32102461), Guangdong Province Office of Education foundation (2022KTSCX014), Huangpu Innovation Research Institute, South China Agricultural University, and the China Agriculture Research System (CARS-25-C-04).
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#home (accessed on 3 July 2023).
- Li, T.L.; Xu, Y.; Zhang, J. Current situation and trend of industry development of facilities vegetable, western melon and edible fungus. China Veg. 2019, 11, 6–9. [Google Scholar]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the fruit metabolome in tomato breeding. Cell 2018, 172, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.; Urban, L.; Brunel, B.; Bertin, N. Water deficit effects on tomato quality depend on fruit developmental stage and genotype. J. Plant Physiol. 2016, 190, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Ayarna, A.W.; Tsukagoshi, S.; Nkansah, G.O. Effect of root restriction on the performance of three-truss-cultivated tomato in the low node pinching order at high-density cultivation system. Horticulturae 2021, 7, 60. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.; Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Zahid, M.S.; Hussain, M.; Song, Y.; Li, J.J.; Guo, D.H.; Li, X.Y.; Song, S.R.; Wang, L.; Xu, W.P.; Wang, S.P. Root-zone restriction regulates soil factors and bacterial community assembly of grapevine. Int. J. Mol. Sci. 2022, 23, 15628. [Google Scholar] [CrossRef]
- Yu, X.M.; Wang, B.; Zhang, C.X.; Xu, W.P.; He, J.J.; Zhu, L.; Wang, S.P. Effect of root restriction on nitrogen levels and glutamine synthetase activity in ‘Kyoho’ grapevines. Sci. Hortic. 2012, 137, 156–163. [Google Scholar] [CrossRef]
- Yu, X.M.; Li, J.F.; Zhu, L.N.; Wang, B.; Wang, L.; Bai, Y.; Zhang, C.X.; Xu, W.P.; Wang, S.P. Effects of root restriction on nitrogen and gene expression levels in nitrogen metabolism in ‘jumeigui’ grapevines (Vitis vinifera L. × Vitis labrusca L.). J. Integr. Agric. 2015, 14, 67–79. [Google Scholar] [CrossRef]
- Zakaria, N.I.; Ismail, M.R.; Awang, Y.; Megat Wahab, P.E.; Berahim, Z. Effect of root restriction on the growth, photosynthesis rate, and source and sink relationship of chilli (Capsicum annuum L.) grown in soilless culture. BioMed. Res. Int. 2020, 2020, 2706937. [Google Scholar] [CrossRef]
- Shi, K.; Fu, L.J.; Dong, D.K.; Zhou, Y.H.; Yu, J.Q. Decreased energy synthesis is partially compensated by a switch to sucrose synthase pathway of sucrose degradation in restricted root of tomato plants. Plant Physiol. Biochem. 2008, 46, 1040–1044. [Google Scholar] [CrossRef]
- Li, X.J.; Han, Z.; Li, C.; Xie, Z.S.; Wang, S.P.; Li, B. Effects of root restriction on soluble sugar contents and ultrastructure of phloem tissues in ‘kyoho’ grape berry. Plant Physiol. 2016, 52, 1546–1554. [Google Scholar]
- White, M.D.; Tustin, D.S.; Foote, K.F.; Campbell, J.M. Growth of young sweet cherry trees in response to root restriction using root control bags. Acta Hortic. 2001, 557, 391–398. [Google Scholar] [CrossRef]
- Tamai, D. The practical cultivation and technologist training in tomato low node order pinching and high density planting cultivation. Shisetsu Engei 2014, 165, 62–65. (In Japanese) [Google Scholar]
- Zhang, Y.T.; Kiriiwa, Y.; Nukaya, A. Influence of nutrient concentration and composition on the growth, uptake patterns of nutrient elements and fruit coloring disorder for tomatoes grown in extremely low-volume substrate. Hortic. J. 2015, 84, 37–45. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Suzuki, K.; Liu, H.C.; Nukaya, A.; Kiriiwa, Y. Fruit yellow-shoulder disorder as related to mineral element uptake of tomatoes grown in high temperature. Sci. Hortic. 2018, 242, 25–29. [Google Scholar] [CrossRef]
- Liu, A.L.; Liu, D.X.; Yin, D.S.; Lian, H.; Zhang, Y.T.; Chen, R.Y. Effects of minimal drainage and nutrient concentration regulation on tomato fruit quality. China Veg. 2021, 10, 66–78, (In Chinese with English Abstract). [Google Scholar]
- Wang, H.C.; Huang, H.B.; Huang, X.M.; Hu, Z.Q. Sugar and acid compositions in the arils of litchi chinensis sonn.: Cultivar differences and evidence for the absence of succinic acid. J. Hortic. Sci. Biotechnol. 2006, 81, 57–62. [Google Scholar] [CrossRef]
- He, R.; Wei, J.J.; Zhang, J.Y.; Tan, X.; Li, Y.M.; Gao, M.F.; Liu, H.C. Supplemental blue light frequencies improve ripening and nutritional qualities of tomato fruits. Front. Plant Sci. 2022, 13, 888976. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.X.; Qin, M.; Xie, Z.B.; Chen, R.Y.; Zhang, Y.T. Effects of supplemental lighting on potassium transport and fruit coloring of tomatoes grown in hydroponics. Int. J. Mol. Sci. 2021, 22, 2687. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Liu, A.L.; Hao, Y.W.; Su, W.; Sun, G.W.; Song, S.W.; Liu, H.C.; Chen, R.Y. Nitric oxide is essential for melatonin to enhance nitrate tolerance of cucumber seedlings. Molecules 2022, 27, 5806. [Google Scholar] [CrossRef]
- Xie, Z.S.; Li, B.; Forney, C.F.; Xu, W.P.; Wang, S.P. Changes in sugar content and relative enzyme activity in grape berry in response to root restriction. Sci. Hortic. 2009, 123, 39–45. [Google Scholar] [CrossRef]
- Saito, T.; Fukuda, N.; Iikubo, T.; Inai, S.; Fujii, T.; Konishi, C.; Eaura, H. Effects of root-volume restriction and salinity on the fruit yield and quality of processing tomato. J. Jap. Soc. Hortic. Sci. 2008, 77, 165–172. [Google Scholar] [CrossRef]
- Goto, T.; Matsuno, T.; Yoshida, Y.; Kageyama, Y. Photosynthetic, evapotranspiratory and leaf morphological properties of chrysanthemum grown under root restriction as affected by fertigation frequency. Engei Gakkai Zasshi 2002, 71, 277–283. [Google Scholar] [CrossRef]
- Leng, F.; Lin, Q.; Wu, D.; Wang, S.P.; Wang, D.L.; Sun, C.D. Comparative transcriptomic analysis of grape berry in response to root restriction during developmental stages. Molecules 2016, 21, 1431. [Google Scholar] [CrossRef]
- Zhu, L.C.; Li, B.Y.; Wu, L.M.; Li, H.X.; Wang, Z.Y.; Wei, X.Y.; Ma, B.Q.; Zhang, Y.F.; Ma, F.W.; Ruan, Y.L.; et al. MdERDL6-mediated glucose efflux to the cytosol promotes sugar accumulation in the vacuole through up-regulating TSTs in apple and tomato. Proc. Natl. Acad. Sci. USA 2021, 118, e2022788118. [Google Scholar] [CrossRef]
- Saito, T.; Matsukura, C.; Ban, Y.; Shoji, K.; Sugiyama, M.; Fukuda, N.; Nishimura, S. Salinity stress affects assimilate metabolism at the gene-expression level during fruit development and improves fruit quality in tomato. J. Jap. Soc. Hortic. Sci. 2008, 77, 61–68. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, P.; Chaudhari, S.; Edelstein, M. Tomato grafting: A global perspective. Hortic. Sci. 2017, 52, 1328–1336. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Effect of nickel and grafting combination on yield, fruit quality, antioxidative enzyme activities, lipid peroxidation, and mineral composition of tomato. J. Plant Nutr. Soil Sci. 2015, 178, 848–860. [Google Scholar] [CrossRef]
- Cantero-Navarro, E.; Romero-Aranda, R.; Fernández-Muñoz, R.; Martínez-Andújar, C.; Pérez-Alfocea, F.; Albacete, A. Improving agronomic water use efficiency in tomato by rootstock-mediated hormonal regulation of leaf biomass. Plant Sci. 2016, 251, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Martínez-Andújar, C.; Ghanem, M.E.; Acosta, M.; Sánchez-Bravo, J.; Asins, M.J.; Cuartero, J.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence, and increased leaf area and crop productivity in salinized tomato. Plant Cell Environ. 2009, 32, 928–938. [Google Scholar] [CrossRef]
- Kanayama, Y. Sugar metabolism and fruit development in the tomato. Hortic. J. 2017, 86, 417–425. [Google Scholar] [CrossRef]
- Nguyen-Quoc, B.; Foyer, C.H. A Role for ‘futile cycles’ involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. J. Expt. Bot. 2001, 52, 881–889. [Google Scholar] [CrossRef]
- Figás, M.R.; Prohens, J.; Raigón, M.D.; Fita, A.; García-Martínez, M.D.; Casanova, C.; Borras, D.; Plazas, M.; Andújar, I.; Soler, S. Characterization of composition traits related to organoleptic and functional quality for the differentiation, selection and enhancement of local varieties of tomato from different cultivar groups. Food Chem. 2015, 187, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.W.; Li, T.L.; Jiang, J. Tomato key sucrose metabolizing enzyme activities and gene expression under NaCl and PEG iso-osmotic stresses. Agric. Sci. China 2009, 8, 1046–1052. [Google Scholar] [CrossRef]
- Miron, D.; Schaffer, A.A. Sucrose phosphate synthase sucrose synthase, and invertase activities in developing fruit of lycopersicon esculentum mill. and the sucrose accumulating lycopersicon hirsutum humb. and Bonpl. Plant Physiol. 1991, 95, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, J.J.; Duan, C.Q.; Yu, X.M.; Zhu, L.N.; Xie, Z.S.; Zhang, C.X.; Xu, W.P.; Wang, S.P. Root restriction affects anthocyanin accumulation and composition in berry skin of “kyoho” grape (Vitis vinifera L. × Vitis labrusca L.) during ripening. Sci. Hortic. 2012, 137, 20–28. [Google Scholar] [CrossRef]
- Li, D.M.; Liu, B.Y.; Wang, Z.P.; Li, X.Y.; Sun, S.J.; Ma, C.; Wang, L.; Wang, S.P. Sugar accumulation may be regulated by a transcriptional cascade of ABA-VvGRIP55-VvMYB15-VvSWEET15 in grape berries under root restriction. Plant Sci. 2022, 322, 111288. [Google Scholar] [CrossRef]
- Carrari, F.; Fernie, A.R. Metabolic regulation underlying tomato fruit development. J. Exp. Bot. 2006, 57, 1883–1897. [Google Scholar] [CrossRef]
- Daloso, D.M.; Williams, T.C.R.; Antunes, W.C.; Antunes, W.C.; Pinheiro, D.P.; Muller, C.; Loureiro, M.E.; Fernie, A.R. Guard cell-specific upregulation of sucrose synthase 3 reveals that the role of sucrose in stomatal function is primarily energetic. New Phytol. 2016, 209, 1470–1483. [Google Scholar] [CrossRef]
- Su, J.Y.; Yao, Y.; Liu, Y.H.; Han, Q.Y.; Zhang, W.L. Function, structure and catalytic mechanism of sucrose phosphate synthase, a review. Chin. J. Biotech. 2021, 37, 1858–1868. [Google Scholar]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Zhu, Q.S. Activities of starch hydrolytic enzymes and sucrose-phosphate synthase in the stems of rice subjected to water stress during grain filling. J. Exp. Bot. 2001, 52, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Claeyssen, E.; Rivoal, J. Isozymes of plant hexokinase: Occurrence, properties and functions. Phytochemistry 2007, 68, 709–731. [Google Scholar] [CrossRef] [PubMed]
- Eveland, A.L.; Jackson, D.P. Sugars, signalling, and plant development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).