Residual Effects of Phosphorus and Micronutrients in Vegetable Growing Areas under Different Organomineral Fertilizer Doses
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area
2.2. Experimental Design
2.3. Complementary Information
2.4. Assessments
2.5. Statistical Analyses
3. Results
3.1. Fresh and Dry Weight Production
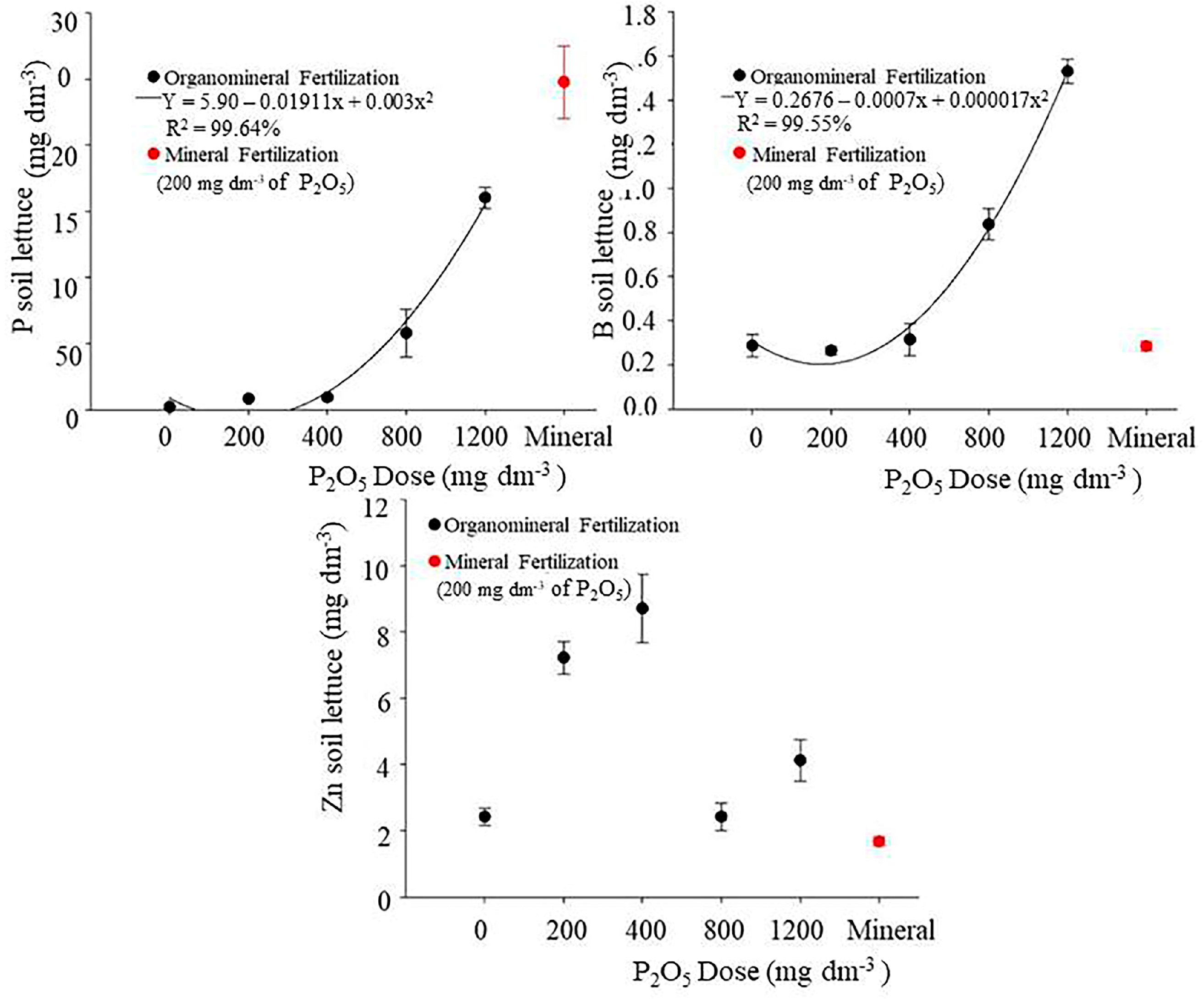
3.2. Influence of Mineral and Organomineral Fertilizers on Some Soil Chemical Properties
3.3. Assessment of Plant Nutritional Status
4. Discussion
4.1. Fresh and Dry Weight Production
4.2. Influence of Mineral and Organomineral Fertilizers on Some Soil Chemical Properties
4.3. Assessment of Plant Nutritional Status
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magela, M.L.M.; Camargo, R.; Lana, R.M.Q.; Carvalho, M.M.C. Application of organomineral fertilizers sourced from filter cake and sewage sludge can affect nutrients and heavy metals in soil during early development of maize. Aust. J. Crop Sci. 2019, 13, 863–873. [Google Scholar] [CrossRef]
- Vieira, D.M.S.; Camargo, R.; Torres, J.L.R.; Silva, A.A.; Lana, R.M.Q.; Carvalho, F.J. Growing vegetables in succession in different soils and doses of phosphorus in an organomineral fertilizer. Rev. Bras. Eng. Agric. Ambient. 2020, 24, 806–813. [Google Scholar] [CrossRef]
- Smith, W.B.; Wilson, M.; Pagliari, P. Organomineral fertilizers and their application to field crops. In Animal Manure—Production, Characteristics, Environmental Concerns, and Management; Waldrip, H.M., Pagliari, P.H., He, Z., Eds.; ASA and SSSA: Madison, WI, USA, 2020; Volume 67, pp. 229–243. [Google Scholar] [CrossRef]
- Silva, L.G.; Reginaldo, C.; Lana, R.M.Q.; Delvaux, J.C.; Fagan, E.B.; Machado, V.J. Chemical changes and development of soybean with use of pelletized organominerals fertilizer based of sewage sludge and filter cake. Acta Sci. Agron. 2020, 42, e44249. [Google Scholar] [CrossRef]
- Ferreira, J.B.N.; Gabetto, P.; Araujo, A.C.M.; Dias, R.C.; Sobrinho, N.M.B.; Zonta, E. Combining biosolid and mineral sources of phosphorus and potassium in organomineral fertilizers influences the dynamics and efficiency of nutrient release. Environ. Geo. Health 2023. [Google Scholar] [CrossRef]
- Queiroz, A.A.; Cruvinel, V.B.; Figueiredo, K.M.E. Produção de alface americana em função da fertilização com organomineral. Enc. Bios. 2017, 14, 1053–1063. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; Torres, J.L.R.; Orioli Junior, V.; Charlo, H.C.O.; Vieira, D.M.S. Growth analysis of green-leaf lettuce under different sources and doses of organic and mineral fertilization. Rev. Col. Cienc. Hortic. 2019, 13, 237–247. [Google Scholar] [CrossRef]
- Castoldi, R.; Charlo, H.C.O.; Vargas, P.F.; Braz, L.T. Crescimento, acúmulo de nutrientes e produtividade da cultura da couve-flor. Hortic. Bras. 2009, 27, 438–446. [Google Scholar] [CrossRef]
- Grangeiro, L.C.; Costa, K.R.; Medeiros, M.A.; Salviano, A.M.; Negreiros, M.Z.; Bezerra Neto, F.; Oliveira, S.L. Acúmulo de nutrientes por três cultivares de alface cultivadas em condições do semiárido. Hortic. Bras. 2006, 24, 190–194. [Google Scholar] [CrossRef]
- Torres, J.L.R.; Silva, G.G.; Charlo, H.C.O.; Loss, A.; Lemes, E.M.; Vieira, D.M.S. Lettuce crop fertilized with organomineral source of phosphorus and micronutrients. Hortic. Bras. 2022, 40, 402–403. [Google Scholar] [CrossRef]
- Guppy, C.N.; Menzies, N.W.; Blamey, F.P.C.; Moody, P.W. Do decomposing organic matter residues reduce phosphorus sorption in highly weathered soils? Soil Sci. Soc. Am. J. 2005, 69, 1405–1411. [Google Scholar] [CrossRef]
- Hansel, F.D.; Amado, T.J.C.; Bortolotto, R.P.; Trindade, B.S.; Hansel, D.S.S. Influence of different phosphorus sources on fertilization efficiency. Appl. Res. Agrotechnol. 2014, 7, 103–111. [Google Scholar] [CrossRef]
- Borges, B.M.M.N.; Abdala, D.B.; Souza, M.F.; Viglio, L.M.; Coelho, M.J.A.; Pavinato, P.S.; Franco, H.C.J. Organomineral phosphate fertilizer from sugarcane byproduct and its effects on soil phosphorus availability and sugarcane yield. Geoderma 2019, 339, 20–30. [Google Scholar] [CrossRef]
- Yuri, J.E.; Resende, G.M.; Mota, J.H.; Rodrigues Júnior, J.C.; Souza, R.J.; Carvalho, J.G. Comportamento da alface americana em função do uso de doses e épocas de aplicação de boro em cultivo de inverno. Hortic. Bras. 2004, 22, 593–596. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: London, UK, 2012; 643p. [Google Scholar]
- Pol, C.H.; Nogaroli, J.A. Omissão de nutrientes na cultura da alface (Lactuca sativa L.). Rev. Tuiuti Ciência Cult. 2020, 6, 68–87. [Google Scholar] [CrossRef]
- Rodrigues, M.; Pavinato, P.S.; Withers, P.J.A.; Teles, A.P.B.; Herrera, W.F.B. Legacy phosphorus and no tillage agriculture in tropical oxisols of the Brazilian savanna. Sci. Total Environ. 2016, 542, 1050–1061. [Google Scholar] [CrossRef]
- Alvarez, V.H.V.; Novais, R.F.; Barros, N.F.; Canturutti, R.B.; Lopes, A.S. Interpretação dos resultados das análises de solos. In Recomendações para Uso de Corretivos e Fertilizantes em Minas Gerais: 5ª Aproximação; Ribeiro, A.C., Guimarães, P.T.G., Alvarez, V.H.V., Eds.; Comissão de Fertilidade do solo do Estado de Minas Gerais: Viçosa, Brazil, 1999; pp. 25–32. [Google Scholar]
- Bergamin, L.G.; Cruz, M.C.P.; Ferreira, M.E.; Barbosa, J.C. Produção de repolho em função da aplicação de boro associada a adubo orgânico. Hortic. Bras. 2005, 23, 311–315. [Google Scholar] [CrossRef]
- Kiehl, E.J. Fertilizantes Organominerais, 4th ed.; Degaspari: Piracicaba, Brazil, 2008; 160p. [Google Scholar]
- Beck, H.; Zimmermann, N.; Mcvicar, T.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Koppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- INMET. Gráficos. Available online: http://www.inmet.gov.br/portal/index.php?r=tempo/graficos (accessed on 5 April 2019).
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture-Natural Resources Conservation Service: Washington, DC, USA, 2014; 360p.
- Ribeiro, A.C.; Guimarães, P.T.G.; Alvarez, V.V.H. Recomendações para o Uso de Corretivos e Fertilizantes em Minas Gerais: 5ª Aproximação; UFV: Viçosa, Brazil, 1999; 359p. [Google Scholar]
- Hargreaves, G.H.; Samani, Z.A. Estimating potential evapotranspiration. J. Irrig. Drain. Div. 1982, 108, 225–230. [Google Scholar] [CrossRef]
- Martinez, H.E.P.; Carvalho, J.G.; Souza, R.B. Diagnose foliar. In Recomendações para o Uso de Corretivos e Fertilizantes em Minas Gerais: 5ª. Aproximação; Ribeiro, A.C., Guimarães, P.T.G., Alvarez, V.V.H., Eds.; Universidade Federal de Viçosa: Viçosa, Brazil, 1999; pp. 143–168. [Google Scholar]
- Silva, F.C. (Ed.) Manual de Análises Químicas de Solos, Plantas e Fertilizantes, 2nd ed.; Embrapa Informação Tecnológica: Brasília, Brazil, 2009; 627p. [Google Scholar]
- Bataglia, O.C.; Furlani, A.M.C.; Teixeira, J.P.F.; Furlani, P.R.; Gallo, J.R. Métodos de análise química de plantas; Boletim técnico n. 78; Instituto Agronômico: Campinas, Brazil, 1983; 48p.
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do estado nutricional das plantas: Princípios e aplicações; POTAFOS: Piracicaba, Brazil, 1997; 201p. [Google Scholar]
- Ferreira Junior, J.J.; Torres, J.L.R.; Charlo, H.C.O.; Orioli Júnior, V.; Loss, A.; Barreto, A.C. No-till broccoli production using different cover crop residues and nitrogen doses. Hortic. Bras. 2023, 41, e2479. [Google Scholar] [CrossRef]
- Silva, L.O.D. Influência de doses e modos de aplicação de fósforo e determinação da curva de acúmulo de nutrientes na cultura do repolho. Master’s Dissertation, Universidade Federal de Viçosa, Viçosa, Brazil, 2016; 53p. [Google Scholar]
- Trentini, H.; Hojo, E.T.D. Uso de adubação orgânica e mineral na produtividade de alface americana cv. Amélia. Cult. Sab. 2019, 1, 83–90. [Google Scholar]
- Rodrigues, A.R.S.P. Viabilidade do uso de aditivos organominerais: Ênfase na produção sustentável de alimentos. Rev. Int. Saú. Hum. Tecn. 2023, 11, 1635–1642. [Google Scholar] [CrossRef]
- Lana, R.M.Q.; Zanão Júnior, L.A.; Luz, J.M.Q.; Silva, J.C. Produção da alface em função do uso de diferentes fontes de fósforo em solo de Cerrado. Hortic. Bras. 2004, 22, 525–528. [Google Scholar] [CrossRef]
- Oliveira Júnior, P.P.; Ferreira, R.L.F.; Araújo Neto, S.E.; Souza, L.G.S. Rendimento de alface em ambientes de cultivo utilizando mudas produzidas com volume crescente de substrato. Sci. Nat. 2020, 2, 499–507. [Google Scholar]
- Borges, E.C.; Schmid, D.M.; Saul, L.T.; Sordi, A. Produtividade de cultivares de alface (Lactuca sativa L.) em função de tipos de telas de sombreamento no município de Maravilha, SC. An. Pesq. Ext. Uno. São Mig. do Oeste 2020, 5, 1–9. [Google Scholar]
- Novais, R.F.; Smyth, T.J. Fósforo em solo e Planta em Condições Tropicais; Universidade Federal de Viçosa: Viçosa, Brazil, 1999; 399p. [Google Scholar]
- Trani, P.E.; Raij, B. Hortaliças. In Recomendações de adubação e calagem para o estado de São Paulo; Raij, B., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Boletim técnico, 100; IAC: Campinas, Brazil, 1997; p. 163. [Google Scholar]
- Lana, A.M.Q.; Lana, R.M.Q.; Gozuen, C.F.; Bonotto, I.; Trevisan, L.R. Aplicação de reguladores de crescimento na cultura do feijoeiro. Biosc. J. 2009, 25, 13–20. [Google Scholar]
- Resende, A.V. Adubação Com Micronutrientes no Cerrado; Boletim Técnico; Embrapa Cerrado: Planaltina, Brazil, 2003; 41p. [Google Scholar]
- Araújo, A.P.; Machado, C.T.T. Fósforo. In Nutrição Mineral de Plantas; Fernandes, M.S., Ed.; SBCS: Viçosa, Brazil, 2006; pp. 253–280. [Google Scholar]
- Zanão Júnior, L.A.; Lana, R.M.Q.; Guimarães, E.C. Variabilidade espacial do pH, teores de matéria orgânica e micronutrientes em profundidade em um Latossolo Vermelho sob semeadura direta. Cienc. Rural 2007, 37, 1000–1007. [Google Scholar] [CrossRef]
- Silva, M.G.S.; Trevisan, A.R. Interações iônicas e seus efeitos na nutrição das plantas. Inf. Agron. 2015, 1, 10–16. [Google Scholar]
- Olsen, S.R. Micronutrients Interactions. In Micronutrients in Agriculture; Mortvedt, J.J., Giordano, P.M., Lindsay, W.L., Eds.; Soil Science Society of America: Madison, WI, USA, 1972; pp. 243–264. [Google Scholar]
- Loneragan, J.F.; Webb, M.J. Interactions between zinc and other nutrients affecting the growth of plants. In Zinc in Soil and Plants; Robson, A.D., Ed.; Kluwer Academic: Madison, WI, USA, 1993; pp. 119–134. [Google Scholar]
- Broadley, M.R.; Lochlainn, S.O.; Hammond, J.P.; Bowen, H.C.; Cakmak, I.; Eker, S.; Erdem, H.; King, G.J.; White, P.J. Shoot zinc (Zn) concentration varies widely within Brassica oleracea L.and is affected by soil Zn and phosphorus (P) levels. J. Hor. Sci. Biot. 2010, 85, 375–380. [Google Scholar] [CrossRef]
- Sinha, P.; Dube, B.K.; Chatterjee, C. Phosphorus stress alters boron metabolism of mustard. Commun. Soil Sci. Plant Anal. 2003, 34, 315–326. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Dikilitas, M.; Ashraf, M.; Koskeroglu, S.; Guneri, M. Supplementary phosfhorus can alleviate boron toxicity in tomato. Sci. Hortic. 2009, 121, 284–288. [Google Scholar] [CrossRef]
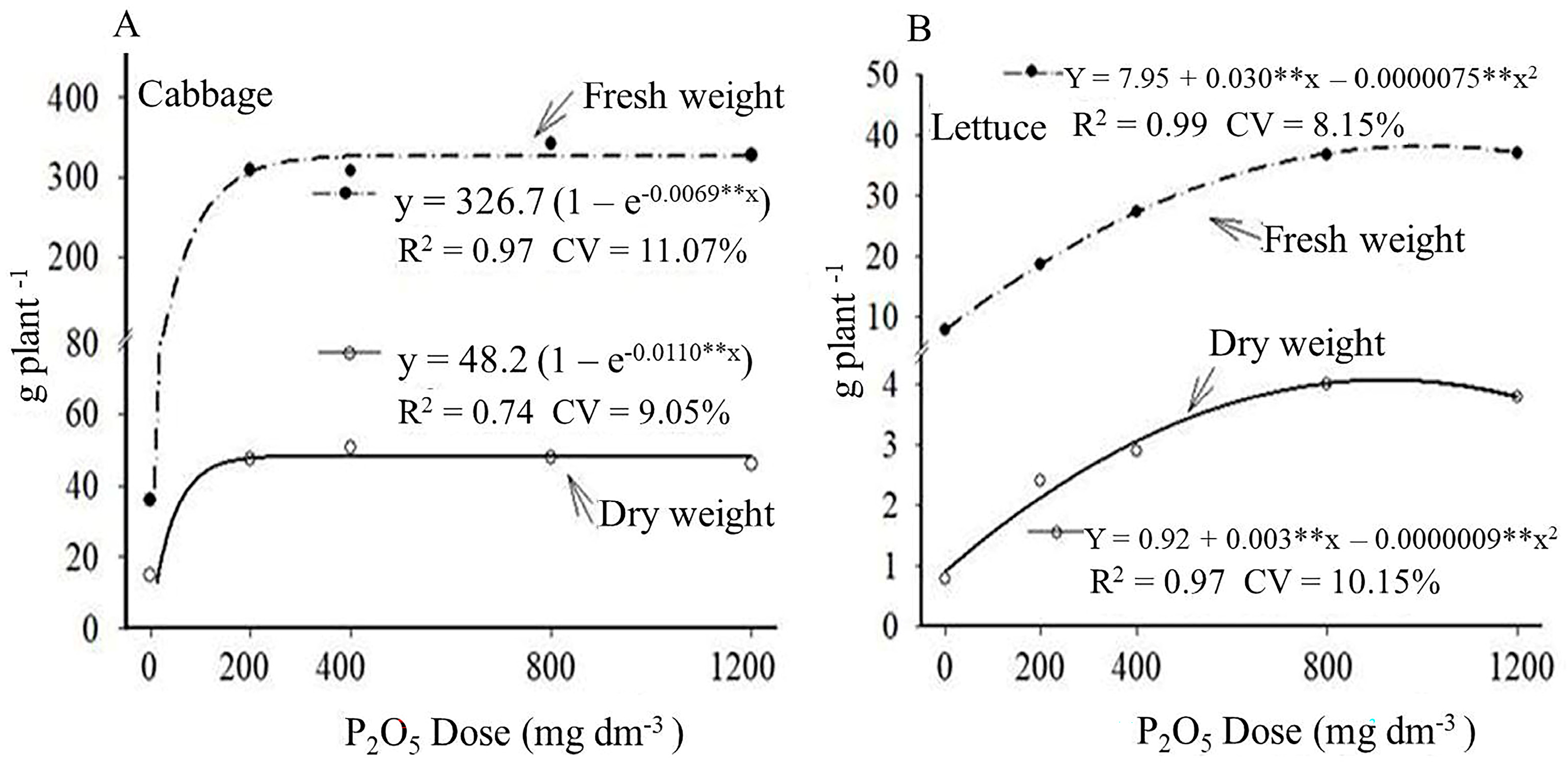



| Dose | Cabbage | Lettuce | ||
|---|---|---|---|---|
| P2O5 | FW | DW | FW | DW |
| (mg dm−3) | (g Planta−1) | (g Planta−1) | (g Planta−1) | (g Planta−1) |
| 0 (No P) | 35.8 b* | 14.8 b* | 7.9 e* | 0.8 d* |
| 200—OF | 308.0 a | 47.5 a | 18.8 d* | 2.4 c* |
| 400—OF | 308.3 a | 47.9 a | 27.4 c* | 2.9 c* |
| 800—OF | 341.8 a | 50.5 a | 37.1 b* | 3.8 b* |
| 1200—OF | 327.3 a | 46.1 a | 36.8 b* | 4.0 b* |
| 200—MF | 281.2 a | 44.3 a | 45.1 a | 5.9 a |
| CV% | 18.2 | 12.7 | 20.7 | 21.0 |
| Dose P2O5 | Soil pH | P | B | Zn |
|---|---|---|---|---|
| (mg dm−3) | (H2O) | (mg dm−3) | (mg dm−3) | (mg dm−3) |
| Cabbage | ||||
| 0 (No P) | 6.80 a* | 4.37 g* | 0.26 b+ | 5.92 b+ |
| 200—OF | 6.70 b* | 12.40 e* | 0.30 a+ | 13.45 a+ |
| 400—OF | 6.67 b* | 36.60 d*+ | 0.40 a+ | 14.20 a+ |
| 800—OF | 6.65 b* | 58.62 c*+ | 0.44 a*+ | 10.45 a+ |
| 1200—OF | 6.45 b*+ | 152.50 b*+ | 0.44 a*+ | 10.50 a+ |
| 200—MF | 6.17 c+ | 266.65 a | 0.27 b+ | 13.37 a+ |
| Initial | 6.80 a* | 8.50 f* | 0.12 c* | 0.47 c* |
| CV% | 1.66 | 17.83 | 22.38 | 21.06 |
| Lettuce | ||||
| 0 (No P) | 6.67 b* | 2.22 d* | 0.26 c | 2.42 c |
| 200—OF | 6.50 b* | 8.45 d* | 0.30 c | 8.22 a* |
| 400—OF | 6.30 c+ | 9.48 d* | 0.31 c | 8.92 a+* |
| 800—OF | 6.27 c+ | 58.05 c* | 0.84 b+* | 12.42 a+ |
| 1200—OF | 5.97 d+ | 160.45 b+* | 1.67 a+* | 4.12 b+ |
| 200—MF | 6.07 d+ | 247.77 a+ | 0.28 c | 1.67 c |
| Initial | 6.80 a* | 8.50 d* | 0.12 c | 0.47 d |
| CV% | 12.80 | 26.32 | 27.59 | 34.62 |
| Dose | Cabbage | Lettuce | ||||
|---|---|---|---|---|---|---|
| P2O5 | P | B | Zn | P | B | Zn |
| (mg dm−3) | (g kg−1) | (mg kg−1) | (mg kg−1) | (g kg−1) | (mg kg−1) | (mg kg−1) |
| 0 (No P) | 2.94 b | 103.17 c | 93.05 a* | 2.40 | 42.55 c* | 68.90 a* |
| 200—OF | 2.99 b | 114.27 c | 76.62 b | 2.55 | 43.07 c* | 46.55 b |
| 400—OF | 3.37 b | 141.70 c | 90.40 a* | 2.69 | 52.37 b* | 45.90 b |
| 800—OF | 3.59 a | 365.22 a* | 51.85 c* | 2.84 | 91.25 a* | 38.40 c* |
| 1200—OF | 4.61 a* | 228.02 b* | 27.80 d* | 2.70 | 92.15 a* | 34.65 d* |
| 200—MF | 3.37 b | 92.92 c | 71.07 b | 2.37 | 31.67 d | 52.30 b |
| CV% | 15.39 | 16.39 | 16.38 | 10.11 | 5.54 | 8.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, D.M.d.S.; Torres, J.L.R.; Camargo, R.d.; Silva, A.d.A.; Lana, R.M.Q.; Charlo, H.C.d.O.; Lemes, E.M.; Carvalho, É.R. Residual Effects of Phosphorus and Micronutrients in Vegetable Growing Areas under Different Organomineral Fertilizer Doses. Horticulturae 2023, 9, 761. https://doi.org/10.3390/horticulturae9070761
Vieira DMdS, Torres JLR, Camargo Rd, Silva AdA, Lana RMQ, Charlo HCdO, Lemes EM, Carvalho ÉR. Residual Effects of Phosphorus and Micronutrients in Vegetable Growing Areas under Different Organomineral Fertilizer Doses. Horticulturae. 2023; 9(7):761. https://doi.org/10.3390/horticulturae9070761
Chicago/Turabian StyleVieira, Dinamar Márcia da Silva, José Luiz Rodrigues Torres, Reginaldo de Camargo, Adriane de Andrade Silva, Regina Maria Quintão Lana, Hamilton César de Oliveira Charlo, Ernane Miranda Lemes, and Érica Reis Carvalho. 2023. "Residual Effects of Phosphorus and Micronutrients in Vegetable Growing Areas under Different Organomineral Fertilizer Doses" Horticulturae 9, no. 7: 761. https://doi.org/10.3390/horticulturae9070761
APA StyleVieira, D. M. d. S., Torres, J. L. R., Camargo, R. d., Silva, A. d. A., Lana, R. M. Q., Charlo, H. C. d. O., Lemes, E. M., & Carvalho, É. R. (2023). Residual Effects of Phosphorus and Micronutrients in Vegetable Growing Areas under Different Organomineral Fertilizer Doses. Horticulturae, 9(7), 761. https://doi.org/10.3390/horticulturae9070761





