Abstract
Citriculture is considered the most important fruit industry and involves the cultivation of several fruit varieties, which are susceptible to many plant pathogens. In this sense, soil-borne pathogenic fungi, such Rosellinia necatrix, threaten citrus fruit production worldwide because they can cause fruit loss. Therefore, we assayed the physiological reaction of novel citrus rootstocks against white root rot disease during long-term management. Data from above-ground symptoms and chlorophyll content were periodically obtained during the experimental process. In addition, plant leaf area and percentage of biomass reduction were determined for each rootstock when the experiment finished. The behavior of the inoculated tolerant rootstocks was as follows: the lowest symptom rate of SAUDPC was achieved by B11R5T25 and N40R3T25; AMB+CZO manifested the highest disease incidence; B11R5T25 and A+VOLK × Orange 19-11-8 displayed the highest and the lowest chlorophyll content, respectively; AMB+CZO and A+VOLK × Orange 19-11-8 showed the highest biomass reduction, and the lowest was detected in B11R5T25 and N40R2T19; concerning the leaf area, N40R1T18, N40R3T25 and N40R2T19 showed the lowest response, and 2247 × 2075-01-2 achieved the highest rate. In summary, B11R5T25 and N40R3T25 displayed the lowest disease rate.
1. Introduction
Biotic factors such as plant pathogens are limiting agents that have reduced crop production since the beginning of agriculture. Depending on the crop type, environmental conditions and location, biotic factors and plant pathogens can cause losses ranging between 11 to 59% and 3 to 24%, respectively [1]. In this sense, Citrus (Rutaceae family) are also affected by plant pathogens causing pre-harvest diseases as well as crop losses.
Rosellinia necatrix (Prill.) is a soil-borne phytopathogenic ascomycete described in all continents except Antarctica [2]. This phytopathogen is very polyphagous due to its ability to infect more than 340 plant species [3], whereby it induces white root rot disease (WRR) and results in important economic losses, especially in woody crops as major hosts, including citrus [2,4,5,6]. Contrarily to Phytophthora citrus diseases, the control strategies for WRR are not fully effective because R. necatrix has a long latency in soil, is an extensive host species, and can tolerate many chemical compounds [2]. Some control tools developed in other crops include irrigation management [7], soil solarization [8,9,10], antagonistic fungi [8,11,12,13,14,15,16], antagonistic rhizobacteria [17,18,19], mycoviruses [20,21,22], antagonistic bacteria, antagonistic fungi plus a fungicide [23,24,25], and different synthetic fungicides as chemical controls [26,27,28,29,30]. Nevertheless, most of these chemical compounds for controlling R. necatrix are banned for agricultural use in the European Union (EU) [31], and all of them are banned in Spanish citrus orchards [32]. Additionally, EU authorities are constantly reducing the list of permitted pesticides in agriculture, as described in Directive 2009/128/CE and “farm to fork” strategy (European Green Deal), the latter of which aims to reduce 50% of hazardous pesticides by 2030 [33].
Traditionally, the cultivation of tolerant citrus rootstocks has helped to reduce the incidence of phytopathogenic diseases caused by Phytophthora spp., nematodes and citrus tristeza virus (CTV) [34]. Nevertheless, little information is available relating to citrus plant material and WRR tolerance. Two previous works assessed the incidence of R. necatrix in different rootstocks, in which P. trifoliata and two new candidates (P. trifoliata descendants) showed the highest levels of tolerance [5,35]. In the case of other fruit trees (apple, avocado, grapevine and persimmon), breeding researchers have obtained tolerant rootstocks against R. necatrix [36,37,38,39,40,41,42]. As a consequence, the use of tolerant plant material can be positioned and integrated as an optimal control strategy in woody orchards with WRR problems.
Citrus crops are notably grown in the Mediterranean and regions with subtropical climates, where they constitute one of the most relevant fruit sectors. In 2021, Spain was the fifth highest producer of total citrus crops, producing 6.7 million tons, and it was the first fresh fruit exporter, exporting 3.5 million tons worldwide [43]. The Valencian and Andalusian regions were responsible for more than 80% of the production [44]. In this country, R. necatrix is reported as an endemic phytopathogen located in different regions, including Valencia and Andalusia, and targets several fruit crops [45,46,47,48,49]. Furthermore, citrus-producing countries in the Mediterranean basin face the threat of emerging diseases, including Huanglongbing or citrus greening disease (HLB). HLB is the most destructive disease in citriculture worldwide [50], and it is caused by three species of bacteria from the genus Candidatus Liberibacter [51,52], which are vectored by psyllid insects, principally Trioza erytreae and Diaphorina citri [53,54,55,56]. To date, the non-causal agent of HLB has been reported in the Mediterranean basin region [57,58]. However, T. erytreae has been spreading along the Atlantic coastal area of Iberian mainland from the north to the south since 2014 [59,60,61], and D. citri was first found in mandarin and orange trees from Israel in 2021 [57].
Currently, the main breeding programs from around the world have incorporated, among others, conventional biotic stresses (Phytophthora, nematodes or CTV) into the evaluation of new citrus rootstocks facing HLB [34,62], but R. necatrix disease has not been considered. Therefore, the purpose of this research was to determine the susceptibility of novel HLB-tolerant citrus rootstocks from the breeding program of the Citrus Research and Education Center (CREC), University of Florida, to WRR.
2. Materials and Methods
2.1. Plant Material and Experimental Design
In total, 198 plants from 11 different citrus rootstocks were assessed in this study. Ten of these rootstocks (5 diploids and 5 tetraploids) were recently obtained from the breeding program of CREC, whereas Carrizo citrange (diploid) was used as comparative candidate (Table 1). This rootstock was available from the Spanish office of plant varieties under the registered number 16690003 [63]. All plants were first grown from in vitro culture, cultivated in 3 litre plastic pots containing coconut fiber (80%) and peat (20%) substrate, and enriched with 5 g of Osmocote ® Pro (16 + 11 + 10 + 2 MgO + trace elements, longevity 12–14 months) per litre of substrate and supplied by Agromillora Group nursery (Subirats, Barcelona, Spain) at the age of three. When the plants were received, they were separated into two lots for each R. necatrix effect (inoculated and control (non-inoculated)) and distributed into random conditions within a greenhouse. The experiment was carried out during the spring-summer-autumn seasons of 2022 under controlled conditions (24.5 °C and relative humidity of 69.3%) in a greenhouse located in “Las Torres” Center. Each R. necatrix effect and rootstock comprised nine replicates (n = 9); thus, a total of 18 plants were used per rootstock in both R. necatrix effects.

Table 1.
HLB-tolerant citrus rootstocks assayed against R. necatrix infection in this study.
2.2. Fungal Isolate
The fungal isolate of R. necatrix (Rn452), with high pathogenicity, was provided from the fungal collection at the Institute for Sustainable Agriculture (IAS). The species was morphologically identified by the in vitro growth of white and cottony mycelium, and the microscopic presence of pear-shaped swellings in the hypha close to the septum. Later, this visual identification was molecularly confirmed following the methodology of Arjona-López [72]. Thus, the sequence showed 100% identity to the corresponding region of R. necatrix and was uploaded to the GenBank database (accession number: OP482261).
2.3. Plant Inoculation
The plant inoculation process was performed following the methodology of Sztejnberg and Madar [5]. In brief, an R. necatrix isolate from the stock culture was cultured on 15 mL of potato dextrose agar (15 mL; PDA; Difco Laboratories, Detroit, MI, USA) on Petri plates (90 mm in diameter) for refreshing and kept for 7 days at 25 °C in darkness. Next, wheat seeds were moistened in 1000 mL Teqler flasks for one day and double-sterilized after removing the water. Thus, wheat grains were inoculated with mycelial disks of refreshed Rn452 and incubated for three weeks at 25 °C in darkness until the fungus fully colonized the grains. Finally, the plants from the Rn452 effect were ready for inoculation by depositing the inoculum in each pot next to roots at a proportion of 3.75 g of colonized wheat grains to one litre of substrate.
2.4. Evaluation of Aerial Plant Symptoms
The examination of WRR disease was carried out at the aerial part of the plants in both R. necatrix effects and rootstocks using a symptom scale of 1–5 [35] (Figure 1). Plant symptom evaluations were carried out twice per week for 269 days since the beginning of the experiment (inoculation day) until all plants from one rootstock and with the Rn452 effect died. Symptom values were used to obtain the standardized area under the disease progress curve (SAUDPC), adapted from [73,74]:
where: yi is an assessment of white root rot disease aerial plant symptoms at the corresponding ith evaluation day, ti is day in its corresponding ith evaluation, and n is the number of assessment days.
where: is the total number of evaluation days.

Figure 1.
Disease symptom scale of 1–5: 1, healthy plant; 2, first signs of leaf decline and chlorosis on plant; 3, plant with chlorotic and curly leaves; 4, wilted plant with first symptoms of leaf desiccation; and 5, dead plant.
2.5. Leaf Chlorophyll Content Assessment
The measurement of chlorophyll content was performed in all replicates from all R. necatrix effects and rootstocks by selecting three leaves (sub-samples) per plant. This evaluation process was carried out with an MC-100 chlorophyll meter (µmol of chlorophyll per m2 of leaf; Apogee Instruments Inc., North Logan, UT, USA) once per week (every Wednesday), starting from Rn452 inoculation until all inoculated plants from one rootstock died (269 days in total). Thus, the standardized area under the chlorophyll progress curve (SAUCPC) was calculated from all these values and adapted according to [73,74]:
where: yi is an assessment of chlorophyll content at the corresponding ith evaluation day, ti is the day at the corresponding ith evaluation day, and n is the total number of assessment days.
where: is the total number of assessment days.
2.6. Biomass of Fresh Weight
Nine replicates per R. necatrix effect and rootstock were chosen for harvesting in three pieces (roots, stems and leaves) when the experiment finished. For each plant, stem and leaf sections were hand collected and immediately weighted with a CB-3000C digital scale (g; COBOs precision, L’Hospitalet de Llobregat, Barcelona, Spain). Each root was extracted from the pot, washed with water for removing the substrate, dried over filter paper and weighted in the digital scale. After recording the weight, all stems and roots were removed, and the leaves from each plant were kept in a paper envelope. Finally, percentage of biomass reduction (%; PBR) was calculated with the weight data from each above-ground (stems + leaves) and root section in each plant, using Vincent’s equation [75]:
where: CW is weight (g) of control plants (non-inoculated) averaged across nine replicates from fresh weight per plant section; TW is weight (g) of inoculated plants from fresh weight per plant section.
2.7. Evaluation of Leaf Area
When the experiment finished, each collected leaf sample in the paper envelope was used for measuring the leaf area using an LI-3100C area meter (cm2; LI-COR Biosciences, Lincoln, NE, USA). After this process, all leaves were removed.
2.8. Statistical Analysis
One-way analysis of variance (ANOVA) was performed for SAUDPC and PBR from both groups of plant sections (above ground (leaves + stems) and root sections). Two-way ANOVA was executed for SAUCPC and the leaf area parameters. In both cases, the free software R (version 4.1.2) was utilized [76], and the LSD-Fisher test (p < 0.05) [77] was used for separation of means with the “agricolae” package [78]. Figure 2 was plotted with the “ggplot2” [79] package by the same free software version.
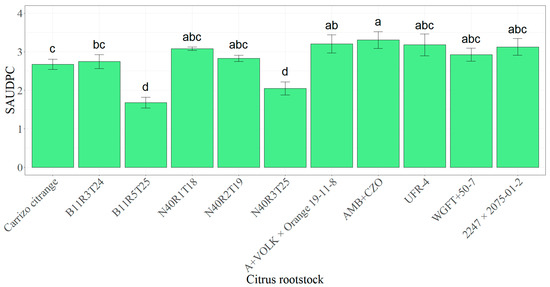
Figure 2.
Mean standardized area under the disease progress curve (SAUDPC) ± standard error (SE) in inoculated plants from 11 citrus rootstocks (Carrizo citrange, B11R3T24, B11R5T25, N40R1T18, N40R2T19, N40R3T25, A+VOLK × Orange 19-11-8, AMB+CZO, UFR-4, WGFT+50-7 and 2247 × 2075-01-2). Values in columns with different letters denote statistical differences among citrus rootstocks assayed by LSD-Fisher’s test (p < 0.05).
3. Results
3.1. Plant Symptom Response
Results from SAUDPC showed significant differences among the assessed rootstocks (F10; 87 = 7.41 and p < 0.001). Any disease symptom was recorded in plants that were not inoculated with R. necatrix. In the case of plants inoculated with R. necatrix, the highest symptom rate (SAUDPC) was found with AMB+CZO (3.30), followed by A+VOLK × Orange 19-11-8 (3.21), UFR-4 (3.18), 2247 × 2075-01-2 (3.12), N40R1T18 (3.08), WGFT+50-7 (2.93) and N40R2T19 (2.83) without statistical differences among them. In contrast, AMB-CZO showed significant differences compared with the remaining citrus rootstocks, as did B11R3T24 (2.75), Carrizo citrange (2.67), N40R3T25 (2.05) and B11R5T25 (1.68). These last two candidates were significantly lower than the other tested rootstocks (Figure 2).
3.2. Chlorophyll Content in the Different Citrus Rootstocks
Statistical differences were detected on plant chlorophyll content under SAUCPC between the effect of R. necatrix inoculation among the citrus rootstocks and in the combination of both (effect of R. necatrix inoculation: F1; 566 = 346.92, and p < 0.001; citrus rootstock: F10; 566 = 68.67, and p < 0.001; effect of R. necatrix inoculation × citrus rootstock: F10; 566 = 6.75, p < 0.001). Additionally, the results of SAUCPC were significantly lower in inoculated plants than control plants for each rootstock, except for N40R3T25. The lowest chlorophyll rate was found in inoculated plants of A+VOLK × Orange 19-11-8 with statistical differences among the citrus rootstocks, except N40R1T18 and UFR-4. On the contrary, B11R5T25 displayed the highest significant SAUCPC among the rootstocks and inoculated plants. The remaining citrus rootstocks showed intermediate response of SAUCPC with different grades of significance among them, and between both R. necatrix effects (Table 2).

Table 2.
Mean standardized area under the chlorophyll progress curve (SAUCPC) ± standard error (SE) due to the effect of R. necatrix infection (inoculated and control (non-inoculated)) on leaf chlorophyll content in plants from 11 citrus rootstocks (Carrizo citrange, B11R3T24, B11R5T25, N40R1T18, N40R2T19, N40R3T25, A+VOLK × Orange 19-11-8, AMB+CZO, UFR-4, WGFT+50-7 and 2247 × 2075-01-2).
3.3. Effect of R. necatrix on the Reduction of Biomass Fresh Weight
Significant differences were obtained in the PBR results for the fresh weight of above-ground sections among the citrus rootstocks evaluated (F10; 87 = 4.86, and p < 0.001). In this sense, the highest PBR was accomplished by AMB+CZO, followed by WGFT+50-7, UFR-4 and A+VOLK × Orange 19-11-8 without statistical differences. Conversely, N40R3T25, followed by B11R5T25 and N49R2T19, displayed the lowest significant reduction of biomass compared with AMB+CZO. In addition, an intermediate PBR response was found for Carrizo citrange, N40R1T18, 2247 × 2075-01-2 and B11R3T24, with statistical differences respecting AMB+CZO and N40R3T25 (Table 3).

Table 3.
Mean percentage of biomass reduction (%) ± standard error (SE) in inoculated plants as fresh weight above ground (leaves + stems) and root sections in plants from 11 citrus rootstocks (Carrizo citrange, B11R3T24, B11R5T25, N40R1T18, N40R2T19, N40R3T25, A+VOLK × Orange 19-11-8, AMB+CZO, UFR-4, WGFT+50-7 and 2247 × 2075-01-2).
Regarding PBR for the fresh weight of root sections, statistical differences were detected among the rootstocks (F10; 87 = 3.68, and p < 0.001). AMB+CZO and A+VOLK × Orange 19-11-8 displayed the highest PBR, followed by WGFT+50-7, UFR-4 and Carrizo citrange without significant differences. On the contrary, the lowest PBR was achieved by N40R2T19 and B11R5T25, followed by N40R3T25, 2247 × 2075-01-2, B11R3T24 and N40R1T18 without significant differences, but were statistically different with the highest response (Table 3).
3.4. Response of Leaf Area in Different Citrus Rootstocks
In the case of leaf area response, statistical differences between the effect of R. necatrix inoculation among the citrus rootstocks and the combination of them were detected (effect of R. necatrix inoculation: F1; 168 = 80.61, and p < 0.001; citrus rootstock: F10; 168 = 17.85, and p < 0.001; effect of R. necatrix inoculation × citrus rootstock: F10; 168 = 2.12, and p = 0.025). The result of leaf area was lower in inoculated plants than control plants, which differed statistically between both effects for each citrus rootstock, except for B11R3T24, N40R1T18, N40R3T25 and 2247 × 2075-01-2. The lowest leaf area rate for inoculated and non-inoculated plants was achieved by N40R1T18, N40R3T25 and N40R2T19, followed by AMB+CZO, WGFT+50-7, B11R5T25 and B11R3T24 without statistical differences among them. On the other hand, 2247 × 2075-01-2 obtained the highest leaf area response among the rootstocks and per each effect of R. necatrix inoculation, which were statistically different compared with the lowest rate. In the control plants, this last result was followed by A+VOLK × Orange 19-11-8, B11R5T25 and Carrizo citrange without significant differences. Furthermore, an intermediate significant result of leaf area was found in A+VOLK × Orange 19-11-8, Carrizo citrange and UFR-4 for the inoculated plants, compared with the highest and lowest response (Table 4).

Table 4.
Mean leaf area response (cm2) ± standard error (SE) due to the effect of R. necatrix infection (inoculated and control (non-inoculated)) in plant leaves from 10 citrus rootstocks (Carrizo citrange, B11R3T24, B11R5T25, N40R1T18, N40R2T19, N40R3T25, A+VOLK × Orange 19-11-8, AMB+CZO, UFR-4, WGFT+50-7 and 2247 × 2075-01-2).
4. Discussion
This study has achieved the physiological response of 11 new and HLB-tolerant citrus rootstocks to WRR after artificial inoculation of R. necatrix. Proper rootstock selection is usually carried out to avoid abiotic (salinity, soil with high pH, flooding, drought and freeze) and biotic (Phytophthora, nematodes and citrus tristeza virus) diseases, as well as horticultural traits (tree size, fruit production and quality). Among these rootstocks, only AMB+CZO, UFR-4 and WGFT+50-7 have been priorly evaluated in term of fruit production and were quality grafted with ‘Hamling’ orange in two locations and during two harvest seasons (2018–19 and 2019–20) [80]. Thus, these three rootstocks displayed high ‘Hamling’ fruit production in one trial location and during both harvest seasons. In the second trial location, AMB+CZO and UFR-4 achieved high and intermediate-low fruit production during the harvested seasons of 2018–19 and 2019–20, respectively. Concerning WGFT+50-7, it showed low and intermediate-low production during 2018–19 and 2019–20, respectively. In addition, AMB+CZO, UFR-4 and WGFT+50-7 rootstocks are currently under field evaluation in two different locations of Florida by another research project [66]. All the 11 citrus rootstocks assessed in this study have been grown recently (2021) and were priorly grafted with ‘Lane Late’ cultivar in a field research plot located at “Las Torres” Center from Andalusian Institute for Agricultural and Fisheries Research and Training and Alcalá del Río municipality (37°30′52.52″ N; 55°7′59.66″ W; Seville, Spain), where agronomic traits are being evaluated, but no data have yet been recorded.
In prior research, Sztejnberg and Madar [5] demonstrated that Poncirus trifoliata displayed the highest tolerance rate under R. necatrix inoculations. In our results, the diploid rootstocks B11R5T25 and N40R3T25 achieved the lowest disease occurrence of symptoms caused by R. nectrix inoculations. Additionally, B11R5T25 showed the highest chlorophyll content in both effects of R. necatrix, and the lowest PBR response for both sections were found in N40R3T25, N40R2T19 and B11R5T25. On the contrary, these three diploid rootstocks obtained low leaf area rates. In this sense, B11R5T25 and N40R3T25 have the same direct parent, which is P. trifoliata. Similarly, a recent study carried out by this group identified two other new HLB-tolerant citrus rootstocks with the slightest incidence of WRR; in addition P. trifoliata constitutes a direct parent in the cross of those both candidates [35].
In Spain, Carrizo citrange is the most cultivated rootstock with a frequency of 61% [81], causing a low diversity rate of plant material among the citrus orchards. Therefore, we include this rootstock as the standard-comparative as it shares a common parent (P. trifoliata) with B11R5T64 and B11R5T60, but it displayed an intermediate response against WRR in this present study. Although Sztejnberg and Madar [5] did not assess Carrizo citrange, they found an intermediate R. necatrix incidence in Troyer citrange (Citrus sinensis ‘Washington’ × P. trifoliata), which is usually described as being exactly alike [64,82]. Otherwise, Carrizo citrange was reported as one of the most susceptible rootstocks to WRR after artificial inoculations were compared with other new HLB-tolerant citrus rootstocks [35]. On the other hand, AMB+CZO and A+VOLK × Orange 19-11-8 accomplished the highest disease incidence in symptoms and the second one displayed the lowest chlorophyll content under the inoculated effect of R. necatrix, and the highest PBR was found in both of these tetraploid rootstocks for root sections, where this pathogen directly infected the plant. Concerning leaf area, the highest rate was achieved by 2247 × 2075-01-2 in plants with both R. necatrix effects.
In previous work related to the susceptibility of citrus rootstocks to R. necatrix disease, Sztenjnberg and Madar [5] found one rootstock from a total of four with the highest tolerance against WRR (25%), and Arjona-López et al. [35] found two from a total of 12 (16.7%). In this current study, we also identified two rootstocks with the highest tolerance to WRR from a total of 11 tested candidates (18.2%); thus, the successes percentage is between those of both prior studies. Regarding other fruit trees, our success percentage (18.2%) is higher than in other investigations, such as apple (2.8%) [36], avocado (0.3% and 1.5%) [38,83] and persimmon rootstock “Diospyros virginiana”, but lower than the persimmon rootstock “D. kaki” [41,42].
5. Conclusions
In our findings, we characterized physiological susceptibility of several new HLB-tolerant citrus rootstocks inoculated by R. necatrix. In total, we found two interesting candidates (B11R5T25 and N40R3T25) with the highest tolerance rate to WRR. Therefore, these two rootstocks could be interesting future choices for growers with R. necatrix problems in their orchards and/or for breeding programs in the development of new technology.
Author Contributions
Conceptualization, J.M.A.-L. and F.J.A.-A.; methodology, J.M.A.-L. and F.J.A.-A.; software, J.M.A.-L., J.L.C.-S. and E.R.-R.; validation, J.M.A.-L. and F.J.A.-A.; formal analysis, J.M.A.-L.; investigation, J.M.A.-L., J.L.C.-S. and E.R.-R.; resources, F.G.G.J., J.W.G., C.J.L.-H. and F.J.A.-A.; data curation, J.M.A.-L., J.L.C.-S. and E.R.-R.; writing—original draft preparation, J.M.A.-L.; writing—review and editing, F.G.G.J., J.W.G., C.J.L.-H. and F.J.A.-A.; visualization, J.M.A.-L. and F.J.A.-A.; supervision, J.M.A.-L. and F.J.A.-A.; project administration, J.M.A.-L. and F.J.A.-A.; funding acquisition, F.J.A.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are highly thankful to Agromillora Group for providing the plant material, including Mariàngela Mestre and Joan Torrent, and to the “Juan de la Cierva-training” postdoctoral grant 2021 (FJC2021-047313-I) from Spanish Ministry of Science and Innovation (Ministerio de Ciencia e Innovación). We are also grateful for the technical assistance of Carlos Casanova (Institute for Sustainable Agriculture, Spanish Research Council), Oliva Inmaculada López Castrillón and José Antonio Monferrer Salinas.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- ten Hoopen, G.M.; Krauss, U. Biology and control of Rosellinia bunodes, Rosellinia necatrix and Rosellinia pepo: A review. Crop Prot. 2006, 25, 89–107. [Google Scholar] [CrossRef]
- Fungal Databases, U.S. National Fungus Collection, United States Department of Agriculture. 2022. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 25 August 2022).
- Sivanesan, A.; Holliday, P. Rosellinia necatrix. CMI Descriptions of Pathogenic Fungi and Bacteria; Commonwealth Mycological Institute, Kew: Surrey, UK, 1972; ISBN 0009-9716. [Google Scholar]
- Sztejnberg, A.; Madar, Z. Host range of Dematophora necatrix, the cause of white root rot disease in fruit trees. Plant Dis. 1980, 64, 662–664. [Google Scholar] [CrossRef]
- Arakawa, M.; Nakamura, H.; Uetake, Y.; Matsumoto, N. Presence and distribution of double-stranded RNA elements in the white root rot fungus Rosellinia necatrix. Mycoscience 2002, 43, 21–26. [Google Scholar] [CrossRef]
- Martínez-Ferri, E.; Moreno-Ortega, G.; van den Berg, N.; Pliego, C. Mild water stress-induced priming enhance tolerance to Rosellinia necatrix in susceptible avocado rootstocks. BMC Plant Biol. 2019, 19, 458. [Google Scholar] [CrossRef]
- Sztejnberg, A.; Freeman, S.; Chet, I.; Katan, J. Control of Rosellinia necatrix in soil and in apple orchard by solarization and Trichoderma harzianum. Plant Dis. 1987, 71, 365–369. [Google Scholar] [CrossRef]
- López-Herrera, C.J.; Pérez-Jiménez, R.M.; Zea-Bonilla, T.; Basallote-Ureba, M.J.; Melero-Vara, J.M. Soil solarization in established avocado trees for control of Dematophora necatrix. Plant Dis. 1998, 82, 1088–1092. [Google Scholar] [CrossRef]
- López-Herrera, C.J.; Pérez-Jiménez, R.M.; Basallote-Ureba, M.J.; Zea-Bonilla, T.; Melero-Vara, J.M. Loss of viability of Dematophora necatrix in solarized soils. Eur. J. Plant Pathol. 1999, 105, 571–576. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; López-Herrera, C.J. Evaluation of Trichoderma spp. as biocontrol agents against avocado white root rot. Biol. Control 2009, 51, 66–71. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Del Moral-Navarrete, L.; Lopez-Herrera, C.J. Selection of Trichoderma spp. isolates antagonistic to Rosellinia necatrix. Span. J. Agric. Res. 2010, 8, 1084–1097. [Google Scholar] [CrossRef]
- Arjona-Girona, I.; López-Herrera, C.J. Study of a new biocontrol fungal agent for avocado white root rot. Biol. Control 2018, 117, 6–12. [Google Scholar] [CrossRef]
- Arjona-López, J.M.; López-Herrera, C.J. Entoleuca sp. infected by mycoviruses as potential biocontrol agents of avocado white root rot. Eur. J. Plant Pathol. 2021, 159, 409–420. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Lopez-Herrera, C.J. Biocontrol de la podredumbre blanca del aguacate con aislados no-patogénicos de Rosellinia necatrix. In Proceedings of the XIII Congreso de la Sociedad Española de Fitopatología, Murcia, Spain, 18–22 September 2006; Montesinos, E., Ed.; Sociedad Española de Fitopatología: Murcia, Spain, 2006; p. 377. [Google Scholar]
- Pal, J.; Sharma, S.K.; Devi, S.; Sharma, R.; Raj, H.; Karn, M.; Verma, S.; Vedukola, P.R.; Sharma, A. Screening, identification, and colonization of fungal root endophytes against Dematophora necatrix: A ubiquitous pathogen of fruit trees. Egypt. J. Biol. Pest Control 2020, 30, 112. [Google Scholar] [CrossRef]
- Cazorla, F.M.; Duckett, S.B.; Bergström, E.T.; Noreen, S.; Odijk, R.; Lugtenberg, B.J.J.; Thomas-Oates, J.E.; Bloemberg, G. V Biocontrol of avocado Dematophora root rot by antagonistic Pseudomonas fluorescens PCL1606 correlates with the production of 2-hexyl 5-propyl resorcinol. Mol. Plant-Microbe Interact. 2006, 19, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Pliego, C.; Ramos, C.; de Vicente, A.; Cazorla, F.M. Screening for candidate bacterial biocontrol agents against soilborne fungal plant pathogens. Plant Soil 2011, 340, 505–520. [Google Scholar] [CrossRef]
- Tienda, S.; Vida, C.; Lagendijk, E.; de Weert, S.; Linares, I.; González-Fernández, J.; Guirado, E.; de Vicente, A.; Cazorla, F.M. Soil application of a formulated biocontrol rhizobacterium, Pseudomonas chlororaphis PCL1606, induces soil suppressiveness by impacting specific microbial communities. Front. Microbiol. 2020, 11, 1874. [Google Scholar] [CrossRef]
- Kanematsu, S.; Arakawa, M.; Oikawa, Y.; Onoue, M.; Osaki, H.; Nakamura, H.; Ikeda, K.; Kuga-Uetake, Y.; Nitta, H.; Sasaki, A.; et al. A Reovirus causes hypovirulence of Rosellinia necatrix. Phytopathology 2004, 94, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Salaipeth, L.; Lin, Y.-H.; Sasaki, A.; Kanematsu, S.; Suzuki, N. A novel bipartite double-stranded RNA mycovirus from the white root rot fungus Rosellinia necatrix: Molecular and biological characterization, taxonomic considerations, and potential for biological control. J. Virol. 2009, 83, 12801–12812. [Google Scholar] [CrossRef] [PubMed]
- Arjona-López, J.M.; Telengech, P.; Suzuki, N.; López-Herrera, C.J. A moderate level of hypovirulence conferred by a hypovirus in the avocado white root rot fungus, Rosellinia necatrix. Fungal Biol. 2021, 125, 69–76. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Arjona-Girona, I.; López-Herrera, C.J. Integrated control of avocado white root rot combining low concentrations of fluazinam and Trichoderma spp. Crop Prot. 2017, 112, 363–370. [Google Scholar] [CrossRef]
- Arjona-López, J.M.; Tienda, S.; Arjona-Girona, I.; Cazorla, F.M.; López-Herrera, C.J. Combination of low concentrations of fluazinam and antagonistic rhizobacteria to control avocado white root rot. Biol. Control 2019, 136, 103996. [Google Scholar] [CrossRef]
- Arjona-López, J.M.; López-Herrera, C.J. Control of avocado white root rot using non-pathogenic Rosellinia necatrix isolates combined with low concentration of fluazinam. BioControl 2020, 65, 247–255. [Google Scholar] [CrossRef]
- Behdad, E. The influence of several new systemic fungicides on Rosellinia necatrix (Hart.) Berl. Iran. J. Pant Pathol. 1976, 12, 40–41. [Google Scholar]
- Kanadani, G.; Date, H.; Nasu, H. Effect of fluazinam soil-drench on white root rot of grapevine. Jpn. J. Phytopathol. 1998, 64, 139–141. [Google Scholar] [CrossRef]
- Nitta, H.; Hatamoto, M.; Kurihisa, H. Control of white root rot on Japanese pear using dazomet micro-granules. Bull. Hiroshima Pref. Agr. Res. Cent. 2002, 72, 25–34. [Google Scholar]
- López-Herrera, C.J.; Zea-Bonilla, T. Effects of benomyl, carbendazim, fluazinam and thiophanate methyl on white root rot of avocado. Crop Prot. 2007, 26, 1186–1192. [Google Scholar] [CrossRef]
- Arjona-López, J.M.; Capote, N.; Melero-Vara, J.M.; López-Herrera, C.J. Control of avocado white root rot by chemical treatments with fluazinam in avocado orchards. Crop Prot. 2020, 131, 105100. [Google Scholar] [CrossRef]
- EU Pesticide Database. 2023. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 8 March 2023).
- Registro de Productos Fitosanitarios, Ministerio de Agricultura, Pesca y Alimentación. 2023. Available online: https://www.mapa.gob.es/es/agricultura/temas/sanidad-vegetal/productos-fitosanitarios/registro-productos/ (accessed on 8 March 2023).
- Farm to Fork Strategy, European Commission. 2023. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 8 March 2023).
- Bowman, K.D.; Joubert, J. Citrus rootstocks. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 105–127. ISBN 9780128121634. [Google Scholar]
- Arjona-López, J.M.; Gmitter, F.G.; Romero-Rodríguez, E.; Grosser, J.W.; Hervalejo, A.; López-Herrera, C.J.; Arenas-Arenas, F.J. Susceptibility of novel promising citrus rootstocks to white root rot. Plants 2022, 11, 3388. [Google Scholar] [CrossRef]
- Lee, S.B.; Ko, K.; Aldwinckle, H.S. Resistance of selected Malus germplasm to Rosellinia necatrix. J. Am. Pomol. Soc. 2000, 54, 219–228. [Google Scholar]
- Choi, B.-H.; Kim, C.-S.; Jeong, Y.-J.; Park, I.-H.; Han, S.-G.; Yoon, T.-M. Resistance evaluation of G, CG, or M series apple rootstocks to soil-borne diseases (Phytophthora root rot, white root rot, and southern blight) and woolly apple aphid. Hortic. Sci. Technol. 2021, 39, 167–174. [Google Scholar] [CrossRef]
- Barceló-Muñoz, A.; Zea-Bonilla, T.; Jurado-Valle, I.; Imbroada-Solano, I.; Vidoy-Mercado, I.; Pliego-Alfaro, F.; López-Herrera, C.J. Programa de selección de portainjertos de aguacate tolerantes a la podredumbre blanca causada por Rosellinia necatrix en el sur de España (1995–2007). In Proceedings of the VI World Avocado Congress (Actas VI Congreso Mundial del Aguacate), Viña del Mar, Chile, 12–16 November 2007; pp. 1–8. [Google Scholar]
- Pérez Jiménez, R.M.; Zea Bonilla, T.; Imbroda Solano, I.; Pliego-Alfaro, F.; López Herrera, C.J.; Barceló-Muñoz, A. Selección de portainjertos de aguacate tolerantes a la podredumbre blanca causada por Rosellinia necatrix. In Proceedings of the V World Avocado Congress (Actas V Congreso Mundial del Aguacate), Granada-Málaga, Spain, 19–24 October 2003; pp. 537–541. [Google Scholar]
- Mansoori, B.; Dorostkar, M. Reactions of some grape cultivars to Dematophora necatrix. Vitis 2008, 47, 231–233. [Google Scholar]
- Sztejnberg, A.; Jabareen, H. Dematophora root rot disease in persimmon and studies on resistance of rootstocks to the disease. Alon Hanotea 1985, 39, 757–762. [Google Scholar]
- Sztejnberg, A.; Jabareen, H. Studies of resistance of persimmon rootstocks to Dematophora root rot. Phytoparasitica 1986, 14, 240. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization (FAO) of the United Nations. 2023. Available online: http://www.fao.org/faostat/es/#home (accessed on 26 January 2023).
- MAPA. Ministerio de Agricultura, Pesca y Alimentación. 2023. Available online: https://www.mapa.gob.es (accessed on 8 March 2023).
- Melgarejo Nárdiz, P.; García-Jiménez, J.; Jordá Gutiérrez, M.C.; López González, M.M.; Andrés Yebes, M.F.; Duran-Vila, N. Patógenos de Plantas Descritos en España, 2nd ed.; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2010. [Google Scholar]
- González-Domínguez, E.; Pérez-Sierra, A.; Álvarez, L.A.; León, M.; Abad-Campos, P.; Armengol, J.; García-Jiménez, J. Agentes fúngicos presentes en plantaciones de nísperos (Eriobotrya japonica Lindl.) con síntomas de decaimiento en la provincia de Alicante. Boletín Sanid. Veg. Plagas 2009, 35, 453–467. [Google Scholar]
- López-Herrera, C.J. Podredumbres radiculares del aguacate en la Costa del Sol. Años 1987–1988. In Estudios Fitopatología; Moral, J., Ed.; SEFDGIEA: Badajoz, Spain, 1989; pp. 172–176. [Google Scholar]
- Arjona-Girona, I.; López-Herrera, C.J. First report of Rosellinia necatrix causing white root rot in mango trees in Spain. Plant Dis. 2018, 102, 2639. [Google Scholar] [CrossRef]
- Armengol, J.; Vicent, A.; León, M.; Berbegal, M.; Abad-Campos, P.; Garcí-a-Jiménez, J. Analysis of population structure of Rosellinia necatrix on Cyperus esculentus by mycelial compatibility and inter-simple sequence repeats (ISSR). Plant Pathol. 2010, 59, 179–185. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Jagoueix, S.; Bove, J.M.; Garnier, M. The phloem-limited bacterium of greening disease of citrus is a member of the α subdivision of the Proteobacteria. Int. J. Syst. Evol. Microbiol. 1994, 44, 379–386. [Google Scholar] [CrossRef]
- Texeira, D.C.; Ayres, J.; Kitajima, E.W.; Danet, L.; Jagoueix-Eveillard, S.; Saillard, C.; Bové, J.M. First report of a Huanglongbing-like disease of citrus in São Paulo State, Brazil and association of a new Liberibacter species, “Candidatus Liberibacter americanus”, with the disease. Plant Dis. 2005, 89, 107. [Google Scholar] [CrossRef]
- McClean, A.P.D.; Oberholzer, P.C.J. Citrus psylla, a vector of the greening disease of sweet orange. S. Afr. J. Agric. Sci. 1965, 8, 297–298. [Google Scholar]
- Capoor, S.P.; Rao, D.G.; Viswanath, S.M. Diaphorina citri Kuway., a vector of the greening disease of citrus in India. Indian J. Agric. Sci. 1967, 37, 572–579. [Google Scholar]
- Yamamoto, P.T.; Felippe, M.R.; Garbim, L.F.; Coelho, J.H.C.; Ximenes, N.L.; Martins, E.C.; Leite, A.P.R.; Sousa, M.C.; Abrahão, D.P.; Braz, J.D. Diaphorina citri (Kuwayama) (Hemiptera: Psyllidae): Vector of the bacterium Candidatus Liberibacter americanus. In Proceedings of the Huanglongbing-Greening International Workshop, Ribeirão Prêto, Brazil, 16–20 July 2006; Pietersen, G., Le Roux, H.F., Eds.; Citrus Research International: Ribeiro Preto, Brazil, 2006; p. 96. [Google Scholar]
- Ajene, I.J.; Khamis, F.; Mohammed, S.; Rasowo, B.; Ombura, F.L.; Pietersen, G.; van Asch, B.; Ekesi, S. First report of field population of Trioza erytreae carrying the Huanglongbing-associated pathogen, Candidatus Liberibacter asiaticus, in Ethiopia. Plant Dis. 2019, 103, 1766. [Google Scholar] [CrossRef]
- EPPO Global Database. 2023. Available online: https://gd.eppo.int/ (accessed on 8 March 2023).
- Siverio, F.; Marco-Noales, E.; Bertolini, E.; Teresani, G.R.; Peñalver, J.; Mansilla, P.; Aguín, O.; Pérez-Otero, R.; Abelleira, A.; Guerra-García, J.A.; et al. Survey of huanglongbing associated with “Candidatus Liberibacter” species in Spain: Analyses of citrus plants and Trioza erytreae. Phytopathol. Mediterr. 2017, 56, 98–110. [Google Scholar] [CrossRef]
- Pérez-Otero, R.; Mansilla, J.P.; Del Estal, P. Detección de la psila africana de los cítricos, Trioza erytreae (Del Guercio, 1918) (Hemiptera: Psylloidea: Triozidae), en la Península Ibérica. Arq. Entomolóxicos 2015, 13, 119–122. [Google Scholar]
- Arenas-Arenas, F.J.; Duran-Vila, N.; Quinto, J.; Hervalejo, Á. Is the presence of Trioza erytreae, vector of huanglongbing disease, endangering the Mediterranean citrus industry? Survey of its population density and geographical spread over the last years. J. Plant Pathol. 2018, 100, 567–574. [Google Scholar] [CrossRef]
- Arenas-Arenas, F.J.; Duran-Vila, N.; Quinto, J.; Hervalejo, Á. Geographic spread and inter-annual evolution of populations of Trioza erytreae in the Iberian Peninsula. J. Plant Pathol. 2019, 101, 1151–1157. [Google Scholar] [CrossRef]
- Florida Citrus Rootstock Selection Guide, 4th ed. 2023. Available online: https://crec.ifas.ufl.edu/extension/citrus_rootstock/tables.html (accessed on 20 March 2023).
- Oficina Española de Variedades Vegetales, Registro de Variedades, Ministerio de Agricultura, Pesca y Alimentación. 2023. Available online: https://www.mapa.gob.es/es/agricultura/temas/medios-de-produccion/semillas-y-plantas-de-vivero/registro-de-variedades/ (accessed on 29 August 2022).
- Savage, E.M.; Gardner, F.E. The Troyer and Carrizo citranges. Calif. Citrogr. 1965, 50, 112–116. [Google Scholar]
- Aparicio-Durán, L.; Arjona-López, J.M.; Hervalejo, A.; Calero-Velázquez, R.; Arenas-Arenas, F.J. Preliminary findings of new Citrus rootstocks potentially tolerant to foot rot caused by Phytophthora. Horticulturae 2021, 7, 389. [Google Scholar] [CrossRef]
- Project 18-029C. Evaluation of Citrus Rootstocks Response to HLB in Large-Scale Existing Field Trials. 2022. Available online: https://slideplayer.com/slide/17630075/ (accessed on 7 October 2022).
- Grosser, B.J.; Gmitter, F.; Bowman, K. New Rootstocks in the Citrus Breeding Pipeline. Available online: https://citrusindustry.net/2020/07/15/new-rootstocks-in-the-citrus-breeding-pipeline/ (accessed on 7 February 2023).
- Grosser, J.W.; Ollitrault, P.; Olivares-Fuster, O. Invited review: Somatic hybridization in citrus: An effective tool to facilitate variety improvement. Vitr. Cell. Dev. Biol. Plant 2000, 36, 434–449. [Google Scholar] [CrossRef]
- Grosser, J.W. Citrus Rootstock Named “UFR-4”. Publication Number: US 2015/0195974 P1. United States Plant Patent Aplication Publication; Florida Foundation Seed Producers, Inc.: Marianna, FL, USA, 9 July 2015; pp. 1–6. [Google Scholar]
- Grosser, J.W.; Omar, A.A.; Gmitter, J.A.; Syvertsen, J.P. Salinity tolerance of ‘Valencia’ orange trees on allotetraploid rootstocks. In Proceedings of the Florida State Horticultural Society, Delray Beach, FL, USA, 3–5 June 2012; Volume 125, pp. 50–55. [Google Scholar]
- Grosser, J.W.; Chandler, J.L.; Ling, P.; Barthe, G.A. New somatic hybrid rootstock candidates for tree-size control and high juice quality. In Proceedings of the Florida State Horticultural Society, St. Petersburg, FL, USA, 5–7 June 2011; Volume 124, pp. 131–135. [Google Scholar]
- Arjona-López, J.M.; Capote, N.; López-Herrera, C.J. Improved real-time PCR protocol for the accurate detection and quantification of Rosellinia necatrix in avocado orchards. Plant Soil 2019, 443, 605–612. [Google Scholar] [CrossRef]
- Campbell, C.L.; Madden, L. V Temporal analysis of epidemics I: Descriptions and comparisons of disease progress curve. In Introduction to Plant Disease Epidemiology; Campbell, C.L., Madden, L.V., Eds.; Wiley: New York, NY, USA, 1990; pp. 161–202. [Google Scholar]
- Simko, I.; Piepho, H.-P. The area under the disease progress stairs: Calculation, advantage, and application. Phytopathology 2012, 102, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.M. Distortion of fungal hyphæ in the presence of certain inhibitors. Nature 1947, 159, 850. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; ISBN 3-900051-07-0. Available online: http://www.r-project.org/ (accessed on 29 June 2022).
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics: With Special Reference to the Biological Sciences; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1960. [Google Scholar]
- de Mendiburu, F. Statistical Procedures for Agricultural Research; Package “Agricolae”, Version 1.4-4; Comprehensive R Archive Network, Institute for Statistics and Mathematics: Vienna, Austria, 2013. [Google Scholar]
- Wickham, H. Data Analysis. In ggplot2. Use R! Springer: Cham, Switzerland, 2016; pp. 189–201. [Google Scholar]
- Kunwar, S.; Grosser, J.; Gmitter, F.G.; Castle, W.S.; Albrecht, U. Field performance of ‘Hamlin’ orange trees grown on various rootstocks in Huanglongbing-endemic conditions. HortScience 2021, 56, 244–253. [Google Scholar] [CrossRef]
- Tallón Vila, C.I. Biotechnology Applied to the Genetic Improvement of Citrus Rootstocks. In Development of a Protocol for Micropropagation and Adventitious Regeneration for Use in Generating Salt Toleran Mutant Lines; Universidad de Murcia: Murcia, Spain, 2015. [Google Scholar]
- McCarty, C.D.; Bitter, W.P.; Cole, D.A. Comparisons between Troyer and Carrizo citrange. Citrograph 1974, 59, 294–310. [Google Scholar]
- López-Herrera, C.J.; Pérez-Jiménez, R.M.; Barceló-Muñoz, A.; Zea-Bonilla, T. Evaluación de patrones de aguacate por su tolerancia a la podredumbre blanca. Rev. Chapingo Ser. Hortic. 1999, 5, 267–270. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).