Abstract
Strawberry (Fragraria x ananassa Duch) cv. Tochiotome is nutrient rich. However, it has a short shelf life, especially at room temperature. Coating is one of the methods used to prolong fruit shelf life. Coating materials, such as nano chitosan (NC) and Aloe vera (AV) gel, are edible and safe to consume. The objective was to analyze the interaction between AV gel and NC concentrations and determine the optimal concentration to improve the quality of strawberries at room temperature. This experiment used a factorial randomized block design with treatments of NC (0, 0.5, and 1%) and AV gel (0, 25, and 50%). The results showed an interaction between AV gel and NC concentrations for all variables. The optimum concentrations to improve the fruit quality of strawberries cv. Tochiotome were 25% AV gel with 1% NC, which increased the value by more than twofold compared with the control (no AV gel and NC) until four days after storage. The fruit quality characteristics were fruit hardness (6.57 N), weight loss (1.23%), titratable acidity (0.74 mL), total phenolic (1.79 µg GAE/FW), total flavonoid (29.85 mg QE/g), vitamin C concentration (43.83 mg/100 g), and shelf life (4.66 days). More than 1% NC may enhance quality. In conclusion, AV and NC are a potential treatment for improving the postharvest quality of strawberry.
1. Introduction
Strawberries, which are native to subtropical climates, are currently grown almost everywhere in the world, from subtropical to tropical countries. Indonesia is a tropical country, with coordinates of latitude 6° N–11° S and longitude 95° W–141° E; a temperature range between 25.1 and 35.4 °C; and a relative humidity range between 76 and 88%. Therefore, strawberries can be cultivated in Indonesia, particularly at higher elevations. Strawberry is a nutrient-rich fruit as a source of bioactive compounds because of its high levels of vitamin C, folate, and phenolic constituents [1]. Strawberry fruit also contains various bioactive compounds for improving human health and preventing illness [2]. Basu et al. [3] reported that consuming strawberries reduces the risk of type 2 diabetes by improving the antioxidant status of blood plasma. Recently, many strawberry cultivars have been cultivated in Indonesia, but some did not produce high-quality fruit, for example having a sour fruit taste. Introducing strawberries from other regions with existing high quality, such as Japan, with its sweet taste of strawberries, is an alternative to overcome that problem. Our preliminary study on three Japanese strawberry cultivars cultivated on medium land (730 m above sea level) showed that strawberry cv. Tochiotome produced larger and heavier fruits than the other two [4]. In addition, a previous study reported that Tochiotome was one of the major cultivars used in the warm region of Japan [5]. Moreover, it produced the most fruit [6].
The problem of introduced horticulture crop in the lowland area is the high temperature that affects plant growth and development [7,8,9]. In strawberry, high temperature causes the loss of fruit quality, leading to an extremely short shelf life at room temperature because of the pathogens that can quickly attack the fruit. Strawberry fruit infected with pathogens has a shorter shelf life [10]. A previous study by Rahman et al. [11] revealed that fully mature strawberry fruit’s shelf life was less than two days at ambient temperature (25 °C). Similarly, Balitjestro [12] also stated that the shelf life of strawberry fruit in Indonesia is around one day, except if the fruit is harvested when still immature. Coating the strawberry fruit is a postharvest alternative solution to this problem [13]. To be safe for humans, materials used for coating should be both edible and biodegradable. Usually, it is called a natural edible coating [14]. Yousuf et al. [15] stated that natural edible coating could extend fruit shelf life and maintain fruit quality during storage. Coatings are based on three materials: carbohydrates, proteins, and lipids. Chitosan and AV are carbohydrate-based coatings [16].
Chitosan is derived from fungus and crustacean families such as shrimp and crabs. Chitosan has a better gas barrier than other polymers due to its polysaccharide-based coating, which enables self-sustaining defense [17,18]. Recently, nanoparticle technology was applied to chitosan coating. Therefore, it is possible to use nano chitosan (NC) for fruit surface coating. Nanoparticle chitosan can function as a natural fungicide to inhibit microbial growth on fruit surfaces [10]. NC can be antibacterial and antifungal. Consequently, it is more appropriate for use as a food preservative [19]. Strawberry fruit can be well preserved for up to 15 days if 2% NC is applied and the fruit is stored at 0 °C and 90% relative humidity [20]. Strawberries have a fruit shelf life of only nine days at a temperature of 6 °C and a relative humidity of 50% [21].
Aloe vera (AV) is a perennial succulent commonly used for healing due to its pharmacological and therapeutic properties [22,23]. Balcik and Koral [24] reported that AV had degradable and antibacterial properties. As a result, it offers the potential for an edible coating to extend fruit shelf life and preserve strawberry quality. AV, similar to NC, belongs to the polysaccharide group [25]. The concentration of 20% AV preserved the quality of strawberry fruit for up to 15 days and prevented weight loss by 5% [26]. The storage temperature also impacts the fruit quality, with 0 °C being the ideal temperature for maintaining strawberry fruit quality during storage or transportation [27].
AV gel and NC are suitable materials for edible coatings due to prolonging fruit shelf life, preserving fruit quality, and being safe for human consumption. However, few studies on the optimal concentration of AV gel and NC are applied to the storage of strawberries at room temperature. This study aimed to investigate the influence of the interaction between AV gel and NC concentrations and the optimal concentration on the postharvest quality and shelf life of cv. Tochiotome strawberries maintained at room temperature.
2. Results
2.1. Chitosan in Combination with AV Significantly Increased Fruit Firmness during Storage at Room Temperature
The study showed that chitosan-based and AV-based edible coating significantly improved strawberry fruit firmness during storage, mainly when combined (Figure 1a,b). After two days of storage (DAS), the uncoated fruits (c0a0) firmness value was 3.92 N, while fruits coated with edible coating had higher fruit firmness values of: 6.67 (c0a1), 5.10 (c0a2), 5.69 (c1a0), 6.47 (c1a1), 6.37 (c1a2), 6.28 (c2a0), 7.75 (c2a1), and 6.86 N (c2a2). Interestingly, fruits coated with 1% chitosan and 25% AV (c2a1) had the best fruit firmness value at 2 DAS. Observations at 4 DAS revealed a more interesting finding. Uncoated fruits had a similar firmness value to c0a2 (50% AV), but uncoated fruits had a significantly lower firmness value than c0a1 (25% AV). Interestingly, a similar occurrence was observed with chitosan-based coatings at 4 DAS. The addition of 50% AV decreased fruit firmness, while 25% AV gel increased fruit firmness (Figure 1b).
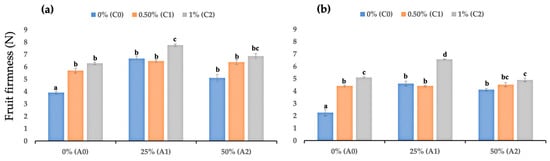
Figure 1.
(a) Fruit firmness values of coated and uncoated fruits at 2 DAS; (b) fruit firmness values of coated and uncoated fruits at 4 DAS. Data represent mean ± standard error (SE) of three replicates with ten fruits per replicate. Bars with same letter are not significantly different according to the Tukey-HSD test at 5%. p value for the test was 0.05 < p-value < 0.025.
2.2. Chitosan in Combination with AV Prevented Fruit Weight Loss during Storage at Room Temperature
In this study, we observed the effect of edible coating on strawberry fruit weight loss during the 2 and 4 DAS. At 2 DAS, uncoated fruits had the highest fruit weight loss amongst all the treatments, with a weight loss of 3.43%, followed by fruits coated with only 25% AV with a weight loss of 2.86%, and fruits coated with only 50% AV with a weight loss of 2.19% (Figure 2a). Fruits coated with chitosan-based edible coating showed significantly lower fruit weight loss at 2 DAS than uncoated fruits and fruits with AV-based coating. At 2 DAS, adding AV into the edible chitosan coating did not significantly affect fruit weight loss. Interestingly, at 4 DAS, adding AV into the edible chitosan coating significantly changed fruit weight loss (Figure 2b). Fruits coated with chitosan and AV had a lower weight loss percentage than those coated with only chitosan.
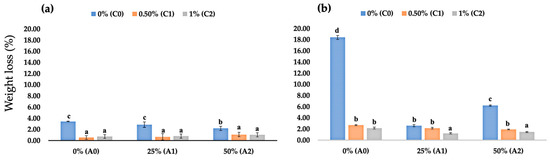
Figure 2.
(a) Fruit weight loss values of coated and uncoated fruits at 2 DAS; (b) fruit weight loss values of coated and uncoated fruits at 4 DAS. Data represent mean ± standard error (SE) of three replicates with ten fruits per replicate. Bars with same letter are not significantly different according to the Tukey-HSD test at 5%. p value for the test was 0.05 < p-value < 0.025.
2.3. Chitosan in Combination with AV Prevented TSS from Increasing during Storage at Room Temperature
Based on our observations at 2 DAS, uncoated strawberry fruits had the highest fruit TSS, followed by fruits coated only with 50% AV gel and 25% AV gel with average values of 9.77 °brix, 8.60 °brix, and 8.30 °brix, respectively. Interestingly, fruits coated with chitosan or a mixture of chitosan and AV had lower TSS values than uncoated fruits and fruits coated with only AV. Interestingly, the TSS values of fruits coated with chitosan or a mixture between chitosan and AV were similar at 2 DAS. However, the TSS values differed significantly at 4 DAS. Fruits coated with the mixture of chitosan and AV had lower TSS values than those with only chitosan or AV (Figure 3b). At 4 DAS, fruits coated with 1% chitosan combined with 25% AV had the lowest TSS values amongst the edible coatings fruits, with an average TSS value of 8.37.
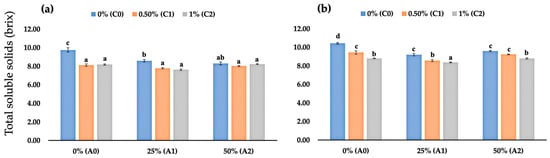
Figure 3.
(a) Fruit TSS values of coated and uncoated fruits at 2 DAS; (b) fruit TSS values of coated and uncoated fruits at 4 DAS. Data represent mean ± standard error (SE) of three replicates with ten fruits per replicate. Bars with the same letters are not significantly different according to the Tukey-HSD test at 5%. p value for the test was 0.05 < p-value < 0.025.
2.4. Chitosan in Combination with AV Maintained Fruit Titratable Acidity during Storage at Room Temperature
Our observations at 2 DAS revealed that uncoated fruits had the lowest titratable acidity (TA), i.e., 0.75 mL. In contrast, fruits coated with 1% chitosan had the highest TA values among others, with an average value of 0.88 mL (1% chitosan), 0.95 mL (1% chitosan + 25% AV), and 0.90 mL (1% chitosan + 50% AV) (Figure 4a). At 4 DAS, uncoated fruits had the lowest TA among stored fruits, i.e., 0.35 mL. Interestingly, fruits coated with only 1% chitosan had lower TA values than those coated with a combination of 1% chitosan and 25% or 50% AV (Figure 4b).
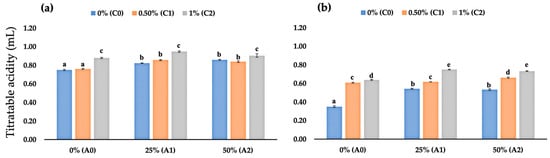
Figure 4.
(a) Fruit TA values of coated and uncoated fruits at 2 DAS; (b) fruit TA values of coated and uncoated fruits at 4 DAS. Data represent mean ± standard error (SE) of three replicates with ten fruits per replicate. Bars with same letter are not significantly different according to the Tukey-HSD test at 5%. p value for the test was 0.05 < p-value < 0.025.
2.5. Chitosan in Combination with AV Preserved Fruit Phenolics and Flavonoid Contents during Storage at Room Temperature
Our observation revealed that applying chitosan, AV, or a mixture of both as edible coating did not significantly affect fruit total phenolics at 2 DAS (Figure 5a). However, at 4 DAS, significant changes were observed. Fruits coated with edible coating had higher phenolic content than uncoated fruits, except for fruits coated with 50% AV and a mixture of 0.5% chitosan combined with 50% AV (Figure 5b). Fruits coated with 1% chitosan and fruits coated with a mix of 1% chitosan combined with 25% AV had the highest total phenolic content amongst the stored fruits with an average total phenolic content of 1.67 and 1.80 μg GAE/FW gel, which was 116.8% and 133.7% higher than uncoated fruits, respectively.
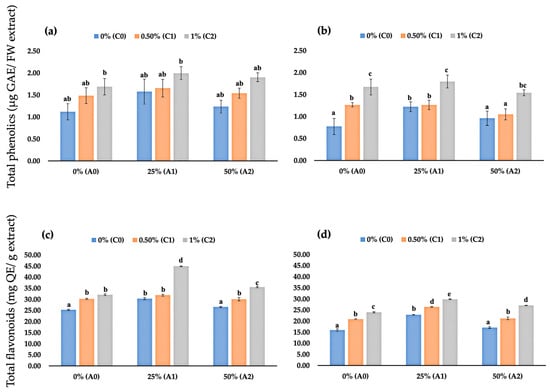
Figure 5.
(a) Fruit total phenolics content of coated and uncoated fruits at 2 DAS; (b) fruit total phenolics content of coated and uncoated fruits at 4 DAS; (c) fruit total flavonoid content of coated and uncoated fruits at 2 DAS; (d) fruit total flavonoid content of coated and uncoated fruits at 4 DAS. Data represent mean ± standard error (SE) of three replicates with ten fruits per replicate. Bars with same letter are not significantly different according to the Tukey-HSD test at 5%. p value for the test was 0.05 < p-value < 0.025.
In this study, we also observed the total flavonoid content of stored fruits. Interestingly, the total flavonoid content of stored fruits differed significantly at 2 DAS. Uncoated fruits and fruits coated with 50% AV had the lowest total flavonoid content, with an average value of 25.31 and 26.58 mg QE/g gel, respectively (Figure 5c). While fruits coated with 1% chitosan combined with 25% AV had the highest total flavonoid content among the stored fruits, with an average value of 44.93 mg QE/g gel. A similar result was observed at 4 DAS; uncoated fruits and fruits coated with 50% AV had the lowest total flavonoid content with an average value of 15.66 and 17.13 mg QE/g gel, respectively. While fruits coated with 1% chitosan combined with 25% AV had the highest total flavonoid content among the stored fruits, with an average value of 29.86 mg QE/g gel (Figure 5d).
2.6. Chitosan in Combination with AV Preserved Fruit Vitamin C Content during Storage at Room Temperature
Our observation revealed that applying chitosan, AV, or a mixture of both as edible coating significantly affected fruit vitamin C content at 2 DAS and 4 DAS (Figure 6a,b). At 2 DAS, uncoated fruits had the lowest vitamin C content among all stored fruits, with an average value of 47.54 mg/100 g. In contrast, fruits coated with a mixture of 1% chitosan and 25% AV had the highest vitamin C content among all stored fruits, with an average value of 60.22 mg/100 g (Figure 6a). Interestingly, at 4 DAS, mixture-based edible coating performed better than chitosan or AV edible coating (Figure 6b). Fruits coated with chitosan and AV had a higher vitamin C content than uncoated fruits and fruit coated with only chitosan or AV.
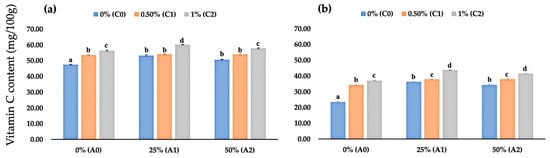
Figure 6.
(a) Fruit vitamin C content of coated and uncoated fruits at 2 DAS; (b) fruit vitamin C content of coated and uncoated fruits at 4 DAS. Data represent mean ± standard error (SE) of three replicates with ten fruits per replicate. Bars with same letter are not significantly different according to the Tukey-HSD test at 5%. p value for the test was 0.05 < p-value < 0.025.
2.7. Chitosan in Combination with AV Prolonged Strawberry Shelf-Life at Room Temperature
In this study, the application of the edible coating, made specifically by combining chitosan with AV, significantly affected strawberry fruit shelf life during storage at room temperature (Figure 7). At 2 DAS, uncoated fruits had the shortest shelf life among all stored fruits, with an average value of 2.3 days, while fruits coated with a mixture of 1% chitosan and 25% or 50% AV had the most extended shelf life among all stored fruits, with an average shelf life of 4.7 and 4.3 days, respectively (Figure 7).
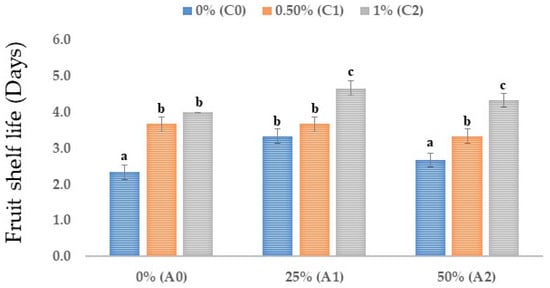
Figure 7.
Fruit shelf life during storage at room temperature. Data represent mean ± standard error (SE) of three replicates with ten fruits per replicate. Bars with same letter are not significantly different according to the Tukey-HSD test at 5%. p value for the test was 0.05 < p-value < 0.025.
3. Discussion
Strawberry is a horticultural crop with a short shelf life. Several methods, such as good postharvest handling, were developed to reduce strawberry postharvest loss [28,29,30]. Several studies had shown that the shelf life of strawberries could be extended by several methods, such as cooling, atmosphere modification, and coating [31]. This study reported that chitosan, AV, and its combination effectively reduced postharvest loss and improved strawberry fruit shelf life.
Firmness is an important physical parameter used to assess the quality of fruits during ripeness, storage, and distribution [32]. Applying chitosan and AV significantly affected strawberry fruit’s firmness and weight loss. Coated fruits had higher fruit firmness and less weight loss than uncoated fruits, especially at 4 DAS. Water loss strongly affected fruit firmness and weight loss values during room temperature storage [31]. Both edible coatings acted as a gas barrier and reduced fruit respiration, reducing fruit water loss [33,34]. Interestingly, this study found that adding 25% AV significantly increased the chitosan-based edible coating’s effectiveness in preventing water loss, thus increasing its effectiveness in maintaining fruit weight and firmness during storage. Previous studies indicated that adding AV gel enhanced edible coatings’ hydrophobic properties, further limiting coated fruit respiration [25,35]. Thus, we believed that adding 25% AV in this research increased the gas barrier properties of chitosan-based edible coating by reducing its permeability to water.
During storage, the fruit’s TSS gradually increased. The increasing TSS is caused by the breakdown of carbohydrates into simple sugars used in respiration [36]. Applying chitosan, AV, and a mixture of both prevented the increase of fruit TSS value during storage with varying degrees of effectiveness. The most effective edible coating for maintaining fruit TSS during storage was made by combining 1% chitosan with 25% AV. This edible coating was the most effective for preventing fruit water loss during storage, thus reducing fruit respiration and maintaining fruit TSS values during storage. Several studies reported similar results for the effect of chitosan and AV, such as in banana, papaya, guava, sweet cherry, and mango [37,38,39,40].
The TA is mainly used to estimate the organic acid content [41,42]. In this study, uncoated fruits had the lowest TA content. It was assumed that the chitosan coating modified the internal atmosphere of the fruit by reducing the O2 uptake and keeping CO2 at a high level [43], affecting respiration activities among all stored fruits. Hence, its titratable acidity was the lowest. In contrast, fruits with an edible coating, especially an edible coating made by combining 1% chitosan and 25% AV, had the highest titratable acidity values (Figure 4a,b). The thin layer of edible coating on the surface of the fruit reduced the fruit’s capability to fix O2, thereby reducing respiration [36]. The reduction in fruit titratable acidity during storage was mainly caused by using organic acids for respiration [44]. Therefore, fruits with higher respiration had lower titratable acidity than those with lower respiration activities.
Oxidation is one of the major causes of vitamin C, flavonoid, and phenolics reduction during fruit storage [45,46]. Therefore, to preserve these contents, it is paramount to limit the exposure of stored fruits to O2 [45]. Edible coatings made using chitosan and AV formed a thin layer on the fruit’s surface, limiting O2 contact with stored fruits, hence limiting oxidation [36,47]. Interestingly, an edible coating made by combining chitosan with AV performed better than chitosan-only or AV-only edible coatings (Figure 6a,b). This result indicated a strong synergistic relationship between both substances when applied as an edible coating.
The application of chitosan, AV, and a mixture of both as edible coating managed to prolong strawberry fruit shelf life at room temperature by more than double the uncoated value. This was likely caused by the gas barrier and hydrophobic properties of both substances [36,47] and AV’s antifungal and antibacterial properties [48]. Our study revealed that adding AV at the appropriate concentration significantly improved chitosan-based edible coating effectiveness in preserving strawberry fruit during storage at room temperature. However, it is also important to note that adding AV at high concentrations had an adverse effect on the chitosan-based edible coating. The addition of 50% AV concentration made the edible coating too thick and too wet, thus actually increasing the amount of pathogen growth within the strawberry fruit surface.
4. Materials and Methods
4.1. Fruit Preparation
The plant material used in this experiment was strawberry cv. Tochiotome introduced from Japan and cultivated in the Faculty of Agriculture’s screen plastic house at Universitas Padjadjaran, Indonesia. The coordinates of the location are 6°44′–7°83′ South Latitude and 107°21′–108°21′ East Longitude, at an altitude of 760 m above sea level. The average temperature in the growing location was around 29 °C. The fruits were harvested at similar maturities, at full maturity stage or more than 75% red color [11]. According to BBCH, the harvested strawberries are stage 85 (the first fruits have cultivar-specific color). The harvested fruits were selected and the fruits with mechanical or biological damage were rejected. The selected fruits were then placed in a cooler box during transportation to the laboratory.
4.2. Edible Coating Preparations and Applications
For AV preparation, AV was washed using 0.03% chlorine, and then the leaf skin was separated from the gel using a knife [47]. The gel was ground using a blender until smooth and heated at 75 °C. For 100 mL of the gel, 1 g of glycerin, 0.5 g of carboxymethyl cellulose (CMC), and 5 g of ascorbic acid was added and blended into the gel. Distilled water was added to the gel for the appropriate treatment concentration (25 and 50%/v:v). NC coating solution was prepared by mixing 5 g of ascorbic acid and 100 mL of distilled water by a magnetic stirrer for 10 min and the solution was then mixed with 0.5 and 1 g of NC to make the concentration of 0.5% and 1%, respectively. Before treatment, AV and NC solution were added to an emulsifier (polysorbate 80) 0.1 mL/100 mL of solution, whereas the fruit was dipped in a sodium hypochlorite solution 0.05% for 2–3 s and dried with a fan dryer [49]. The fruits were treated with a combination of AV (0, 25, and 50%) and NC from shrimp shell (0, 0.5, and 1%). The experiment consisted of three biological replicates, and each replicate consisted of ten fruits. For AV and NC treatment, the fruits were dipped in AV solution and then dipped again in NC solution for 2–3 s and dried using an air dryer. The control fruits were just dipped in distillated water. The treated fruits were packed into the perforated polystyrene box and stored for postharvest analysis at room temperature 24 °C and 80% humidity.
4.3. Total Soluble Solids and Titratable Acidity Analysis
TSS was measured according to the method described by [50,51] using a PAL-J refractometer (Atago, Tokyo, Japan). TA was analyzed using the titration method from [50] with some modifications. In brief, a 25 mL sample of strawberry juice was placed in an Erlenmeyer flask, followed by 250 mL of distilled water and three drops of bromothymol blue. The sample was titrated with 0.1 N NaOH until it reached 8.1.
4.4. Vitamin C Analysis
Vitamin C was measured using the UV–Vis spectrophotometric method [52]. Then, 2 g of sample was mixed with 70% ethanol and then was filtered using filter paper in an Erlenmeyer flask until 50 mL. Subsequently, 2 mL filtered sample was taken into the cuvette and tested by spectrophotometry using a wavelength of 267 nm. Data were obtained by calculating using the standard curve of ascorbic acid.
4.5. Polyphenol Analysis
Polyphenol contenr was calculated using the Folin–Ciocalteu method by [51] with some modifications. First, 0.2 g of dried strawberry fruit was put into a microtube and mixed with 1 mL of methanol. The samples were centrifuged at 6000× g rpm for 5 min and 50 μL of supernatant was added to the test tube. Then, 2.5 mL of 10% Folin–Ciocalteu solution and 2 mL of 7.5% sodium carbonate were added to the test tube and then incubated for 15 min at 45 °C. After incubation, the samples were measured using an Orion AquaMate 8000 UV–vis Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at 765 nm.
4.6. Total Flavonoid Analysis
The total flavonoid content was calculated using the AlCl3 method with quercetin as the standard calibration curve, following a previous study by [51] with some modifications. Briefly, 1 g of dry sample was dissolved in 25 mL of ethanol and then homogenized and centrifuged at 6000× g rpm for 5 min. Then, 2 mL of supernatant was put into a test tube and 2 mL of 2% AlCl3 and 5 mL of 5% acetic acid. The samples were incubated for 30 min, and the total flavonoid was measured using an Orion AquaMate 8000 UV–vis spectrophotometer (Thermo Scientific, Waltham, MA, USA) at 415 nm.
4.7. Fruit Firmness and Fruit Weight Loss
Fruit firmness was measured according to the method described by [51] using a hand penetrometer of Universal Hardness Tester (Nippon Optical Works, Tokyo, Japan). Then, the fruit weight loss was measured using the formula by [53], as follows:
weight loss (%) = (wo − wt)/wo × 100%.
wo = fruit weight at day 0 (g),
wt = fruit weight at day t after storage (g).
4.8. Evaluation of Fruit Shelf Life
In evaluating shelf life, the fruits were harvested at similar fruit maturation, 50–75% red stage, where its fruits are acceptable to consumers in the market. The harvested time was initiated as 0 days after storage (DAS). According to [11], the shelf life was determined by counting the days from the beginning of storage until the fruit quality was lost, indicated by the fruit’s appearance as being unmarketable. Observations were made from the beginning of storage up to 50% of the marketable fruit samples. Marketable fruits possessed several basic criteria, i.e., fungus-free, not rotten, and not mushy.
4.9. Statistical Analysis
Fruit quality variables, such as fruit firmness, weight loss, TSS, titratable acidity, total phenolics, total flavonoids, vitamin C content, and fruit shelf life, were statistically analyzed by using one-way ANOVA and then continued by Tukey-HSD test to determine which differences in concentrations were a significant between the control and coated samples. All data analysis was done in the statistical Analysis ToolPak of Microsoft Excel. The level of significance used was (p < 0.05).
5. Conclusions
The present study concluded that the optimum concentrations to improve the fruit quality of strawberries cv. Tochiotome were 25% AV gel and 1% NC, which increased the value of fruit quality more than twofold compared with the control (no AV gel and NC) until four days after storage. The fruit quality characteristics consisted of fruit hardness (6.57 N), weight loss (1.23%), titratable acidity (0.74 mL), total phenolic (1.79 µg GAE/FW), total flavonoid (29.85 mg QE/g), vitamin C concentration (43.83 mg/100 g), and shelf life (4.66 days). Increasing the concentration of NC from 0.5% to 1% enhanced the shelf life and fruit quality components of strawberry cv. Tochiotome. Future research is required to determine whether increasing the concentration by more than 1% results in a more favorable effect.
Author Contributions
Conceptualization, S.M., J.S.H. and M.A.; methodology, S.M. and F.F.; validation, S.M., J.S.H., K.N., B.P.N.R. and M.A.; formal analysis, F.F., K.N. and B.P.N.R.; investigation, F.F., B.P.N.R. and K.N.; data curation, S.M. and F.F.; writing—original draft preparation, F.F., K.N. and B.P.N.R.; writing—review and editing, S.M., J.S.H. and M.A.; visualization, F.F., S.M. and B.P.N.R.; supervision, S.M., J.S.H. and M.A.; funding acquisition, S.M. and J.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universitas Padjadjaran under the scheme of Unpad Doctoral Dissertation Research 2019–2021. The APC was funded by Universitas Padjadjaran.
Data Availability Statement
Not applicable.
Acknowledgments
We also thank all of the members of our laboratory for helpful discussions throughout the work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Van Buren, L.; Wagner, E.; Wiseman, S.; Put, F.V.D.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruits and vegetables reflects their phenolic and vitamin C composition. Free. Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-López, A.; Giampieri, F.; Battino, M. Promising Health Benefits of the Strawberry: A Focus on Clinical Studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef]
- Basu, A.; Morris, S.; Nguyen, A.; Betts, N.M.; Fu, D.; Lyons, T.J. Effects of Dietary Strawberry Supplementation on Antioxidant Biomarkers in Obese Adults with Above Optimal Serum Lipids. J. Nutr. Metab. 2016, 2016, 3910630. [Google Scholar] [CrossRef] [PubMed]
- Farida, F. Adaptability Study of Strawberry Introduced From Japan in Indonesian Tropical Climate. Doctoral Thesis, Doctoral Program of Agricultural Sciences, Universitas Padjadjaran, Sumedang, Indonesia, 10 June 2023. [Google Scholar]
- Kawagishi, K.; Kawaguchi, A.; Kakizaki, Y.; Goto, M. Year-round strawberry production system in Hokkaido, the Northernmost region of Japan. In Proceedings of the VII International Strawberry Symposium, Beijing, China, 18–22 February 2012; Volume 1049, pp. 813–818. [Google Scholar]
- Yoshida, Y.; Koyama, N.; Tamura, H. Color and anthocyanin composition of strawberry fruit: Changes during fruit development and differences among cultivars, with special reference to the occurrence of pelargonidin 3- malonylglucoside. J. Jpn. Soc. Hortic. Sci. 2002, 71, 355–361. [Google Scholar] [CrossRef]
- Suradinata, Y.R.; Hamdani, J.S.; Mubarok, S. Response of potato cultivars ‘Atlantic’ and ‘Medians’ to the modified micro-climate at medium altitude. Res. Crops 2019, 20, 542–548. [Google Scholar]
- Nuraini, A.; Nugroho, P.S.; Sutari, W.; Mubarok, S.; Hamdani, J.S. Effects of cytokinin and paclobutrazol application time on growth and yield of G2 potato (Solanum tuberosum L.) Medians cultivar at medium altitude in Indonesia. Agric. Nat. Resour. 2021, 55, 171–176. [Google Scholar]
- Hamdani, J.S.; Nuraini, A.; Mubarok, S. The use of paclobutrazol and shading net on growth and yield of potato ‘medians’ tuber of G2 in medium land of Indonesia. J. Agron. 2018, 17, 62–67. [Google Scholar] [CrossRef]
- Saqib, S.; Zaman, W.; Ayaz, A.; Habib, S.; Bahadur, S.; Hussain, S.; Muhammad, S.; Ullah, F. Postharvest disease inhibition in fruit by synthesis and characterization of chitosan iron oxide nanoparticles. Biocatal. Agric. Biotechnol. 2020, 28, 101729. [Google Scholar] [CrossRef]
- Rahman, M.M.; Moniruzzaman, M.; Ahmad, M.R.; Sarker, B.C.; Khurshid Alam, M. Maturity Stages Affect the Postharvest Quality and Shelf-Life of Fruits of Strawberry Genotypes Growing in Subtropical Regions. J. Saudi Soc. Agric. Sci. 2016, 15, 28–37. [Google Scholar] [CrossRef]
- Balitjestro Peningkatan Kualitas Buah Segar Stroberi Melalui Pengangan Panen dan Pascapanen. Available online: http://jestro.is-best.net/peningkatan-kualitas-buah-segar-stroberi-melalui-penanganan-panen-dan-pascapanen/?i=1 (accessed on 15 April 2023).
- Hosseinifarahi, M.; Jamshidi, E.; Amiri, S.; Kamyab, F.; Radi, M. Quality, phenolic content, antioxidant activity, and the degradation kinetic of some quality parameters in strawberry fruit coated with salicylic acid and Aloe vera gel. J. Food Process. Preserv. 2020, 44, e14647. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Xia, J.; Li, Y.; Zhang, L.; Feng, H.; Zhang, X. Flexible sensing enabled agri-food cold chain quality control: A review of mechanism analysis, emerging applications, and system integration. Trends Food Sci. Technol. 2023, 133, 189–204. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent Developments in Shelf-Life Extension of Fresh-Cut Fruits and Vegetables by Application of Different Edible Coatings: A Review. LWT 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible Coatings and Antimicrobial Nanoemulsions for Enhancing Shelf Life and Reducing Foodborne Pathogens of Fruits and Vegetables: A Review. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M. Nanoedible Films for Food Packaging: A Review. J. Mater. Sci. 2019, 54, 12290–12318. [Google Scholar] [CrossRef]
- Lu, J.; Fan, X.; Hu, J.; Li, J.; Rong, J.; Wang, W.; Chen, Y.; Liu, W.; Chen, J.; Chen, Y. Construction and Function of Robust and Moist Bilayer Chitosan-based Hydrogel Wound Dressing. Mater. Des. 2023, 226, 111604. [Google Scholar] [CrossRef]
- Nugraheni, P.S.; Soeriyadi, A.H.; Sediawan, W.B.; Ustadi; Budhijanto, W. Influence of Salt Addition and Freezing-Thawing on Particle Size and Zeta Potential of Nano-Chitosan. IOP Conf. Ser. Earth Env. Sci. 2019, 278, 012052. [Google Scholar] [CrossRef]
- Tezotto-Uliana, J.V.; Fargoni, G.P.; Geerdink, G.M.; Kluge, R.A. Chitosan Applications Pre- or Postharvest Prolong Raspberry Shelf-Life Quality. Postharvest Biol. Technol. 2014, 91, 72–77. [Google Scholar] [CrossRef]
- Nasrin, T.A.A.; Rahman, M.A.; Hossain, M.A.; Islam, M.N.; Arfin, M.S. Postharvest Quality Response of Strawberries with AV Coating during Refrigerated Storage. J. Hortic. Sci. Biotechnol. 2017, 92, 598–605. [Google Scholar] [CrossRef]
- Choi, S.; Chung, M.-H. A Review on the Relationship between AV Components and Their Biologic Effects. Semin. Integr. Med. 2003, 1, 53–62. [Google Scholar] [CrossRef]
- Jadhav, A.N.; Rathod, S.R.; Kolte, A.P.; Bawankar, P.V. Effect of AV as a Local Drug Delivery Agent in the Management of Periodontal Diseases: A Systematic Review and Meta-Analysis. J. Indian Soc. Periodontol. 2021, 25, 372–378. [Google Scholar] [CrossRef]
- Balcik Misir, G.; Koral, S. Effects of Edible Coatings Based on Ultrasound-Treated Fish Proteins Hydrolysate in Quality Attributes of Chilled Bonito Fillets. J. Aquat. Food Prod. Technol. 2019, 28, 999–1012. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest Sweet Cherry Quality and Safety Maintenance by AV Treatment: A New Edible Coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Sanchez, L.T.; Garcia, O.R.; Gutierrez, R.; Luna, J.C.; Villa, C.C. Increasing Shelf Life of Strawberries (Fragaria ssp.) by Using a Banana Starch-chitosan-AV Gel Composite Edible Coating. Int. J. Food Sci. Technol. 2020, 55, 92–98. [Google Scholar] [CrossRef]
- Mitcham, B. Quality Assurance for Strawberries: A Case Study. Perish. Handl. Newsl. Issue 1996, 85, 6–9. [Google Scholar]
- Mubarok, S.; Suminar, E.; Abidat, A.H.; Setyawati, C.A.; Setiawan, E.; Buswar, A.S. Overview of Melatonin’s Impact on Postharvest Physiology and Quality of Fruits. Horticulturae 2023, 9, 586. [Google Scholar] [CrossRef]
- Mubarok, S.; Qonit, M.A.H.; Rahmat, B.P.N.; Budiarto, R.; Suminar, E.; Nuraini, A. An overview of ethylene insensitive tomato mutants: Advantages and disadvantages for postharvest fruit shelf-life and future perspective. Front. Plant Sci. 2023, 14, 1079052. [Google Scholar] [CrossRef]
- Mubarok, S.; Dahlania, S.; Suwali, N. Dataset on the change of postharvest quality of Physalis peruviana L. as an effect of ethylene inhibitor. Data Brief 2019, 24, 103849. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Rega, P.; Migliozzi, T.; Capuano, L.R.; Scortichini, M.; Petriccione, M. Effect of cold storage and shelf life on physiological and quality traits of early ripening pear cultivars. Sci. Hortic. 2013, 162, 341–350. [Google Scholar] [CrossRef]
- Qamar, J.; Ejaz, S.; Anjum, M.A.; Nawaz, A.; Hussain, S.; Ali, S.; Saleem, S. Effect of Aloe vera Gel, Chitosan and Sodium Alginate Based Edible Coatings on Postharvest Quality of Refrigerated Strawberry Fruits of cv. Chandler. J. Hortic. Sci. Technol. 2018, 1, 8–16. [Google Scholar] [CrossRef]
- Nguyen, V.T.B.; Nguyen, D.H.H.; Nguyen, H.V.H. Combination Effects of Calcium Chloride and Nano-Chitosan on the Postharvest Quality of Strawberry (Fragaria × Ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Garcia, O.R.; Villa, C.C. The Influence of AV Gel Incorporation on the Physicochemical and Mechanical Properties of Banana Starch-Chitosan Edible Films. J. Sci. Food Agric. 2018, 98, 4042–4049. [Google Scholar] [CrossRef]
- Shah, S.; Hashmi, M.S. Chitosan–AV Gel Coating Delays Postharvest Decay of Mango Fruit. Hortic. Environ. Biotechnol. 2020, 61, 279–289. [Google Scholar] [CrossRef]
- Petriccione, M.; de Sanctis, F.; Pasquariello, M.S.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Mencarelli, F. The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during postharvest life. Food Bioprocess Technol. 2015, 8, 394–408. [Google Scholar] [CrossRef]
- Kittur, F.S.; Saroja, N.; Tharanathan, H.R.N. Polysaccharide-based composite coating formulations for shelf-life extension of fresh banana and mango. Eur. Food Res. Technol. 2001, 213, 306–311. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar]
- Hadiwijaya, Y.; Putri, I.E.; Mubarok, S.; Hamdani, J.S. Rapid and non-destructive prediction of total soluble solids of guava fruits at various storage periods using handheld near-infrared instrument. IOP Conf. Ser. Earth Environ. Sci. 2020, 458, 012022. [Google Scholar]
- Mubarok, S.; Rahman, I.M.; Kamaluddin, N.N.; Solihin, E. Impact of 1-Methylcyclopropene combined with chitosan on postharvest quality of tropical banana ‘Lady Finger’. Int. J. Food Prop. 2022, 25, 1171–1185. [Google Scholar] [CrossRef]
- Volpe, S.; Cavella, S.; Torrieri, E. Biopolymer Coatings as Alternative to Modified Atmosphere Packaging for Shelf Life Extension of Minimally Processed Apples. Coatings 2019, 9, 569. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate Coatings Preserve Fruit Quality and Bioactive Compounds during Storage of Sweet Cherry Fruit. Food Bioproc Technol. 2012, 5, 2990–2997. [Google Scholar] [CrossRef]
- Thuraidah, A.; Dairobi, A. Pengaruh Kalsium Klorida (CaCl2) dan Lama Penyimpanan Terhadap Kadar Vitamin C Anggur (Vitis vinifera). Med. Lab. Technol. J. 2015, 1, 61–71. [Google Scholar] [CrossRef]
- Del Martínez-González, M.C.; Bautista-Baños, S.; Correa-Pacheco, Z.N.; Corona-Rangel, M.L.; Ventura-Aguilar, R.I.; Del Río-García, J.C.; Ramos-García, M.D.L. Effect of Nanostructured Chitosan/Propolis Coatings on the Quality and Antioxidant Capacity of Strawberries During Storage. Coatings 2020, 10, 90. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Koushesh Saba, M.; Emamifar, A. AV and Ascorbic Acid Coatings Maintain Postharvest Quality and Reduce Microbial Load of Strawberry Fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Nasution, R.P.; Trisnowati, S.; Putra, E.T.S. Pengaruh Lama Penyinaran Ultraviolet-c Dan Cara Pengemasan Terhadap Mutu Buah Stroberi (Fragaria × Ananassa Duchesne) Selama Penyimpanan. Vegetalika 2013, 2, 87–99. [Google Scholar]
- Nicolau-Lapeña, I.; Abadias, M.; Bobo, G.; Aguiló-Aguayo, I.; Lafarga, T.; Viñas, I. Strawberry sanitization by peracetic acid washing and its effect on fruit quality. Food Microbiol. 2019, 83, 159–166. [Google Scholar] [CrossRef]
- Mubarok, S.; Okabe, Y.; Fukuda, N.; Ariizumi, T.; Ezura, H. Potential Use of a Weak Ethylene Receptor Mutant, Sletr1-2, as Breeding Material to Extend Fruit Shelf Life of Tomato. J. Agric. Food Chem. 2015, 63, 7995–8007. [Google Scholar] [CrossRef] [PubMed]
- Mubarok, S.; Ezura, H.; Qonit, M.A.H.; Prayudha, E.; Anas; Suwali, N.; Kusumiyati; Kurnia, D. Alteration of Nutritional and Antioxidant Level of Ethylene Receptor Tomato Mutants, Sletr1-1 and Sletr1-2. Sci. Hortic. 2019, 256, 108546. [Google Scholar] [CrossRef]
- Karinda, M.; Fatimawali, F.; Citraningtyas, G. Perbandingan Hasil Penetapan Kadar Vitamin C Mangga Dodol Dengan Menggunakan Metode Spektrofotometri UV-Vis Dan Iodometri. Pharmacon 2013, 2, 86–89. [Google Scholar]
- AOAC. AOAC International Official Methods of Analysis of AOAC International, 19th ed.; AOAC: Gaithersburg, MD, USA, 2012. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).