Field Performance of ‘Valencia’ Sweet Orange Trees Grafted onto Pummelo Interstocks and Swingle Citrumelo Rootstocks under Huanglongbing (HLB) Endemic Conditions
Abstract
1. Introduction
2. Materials and Methods
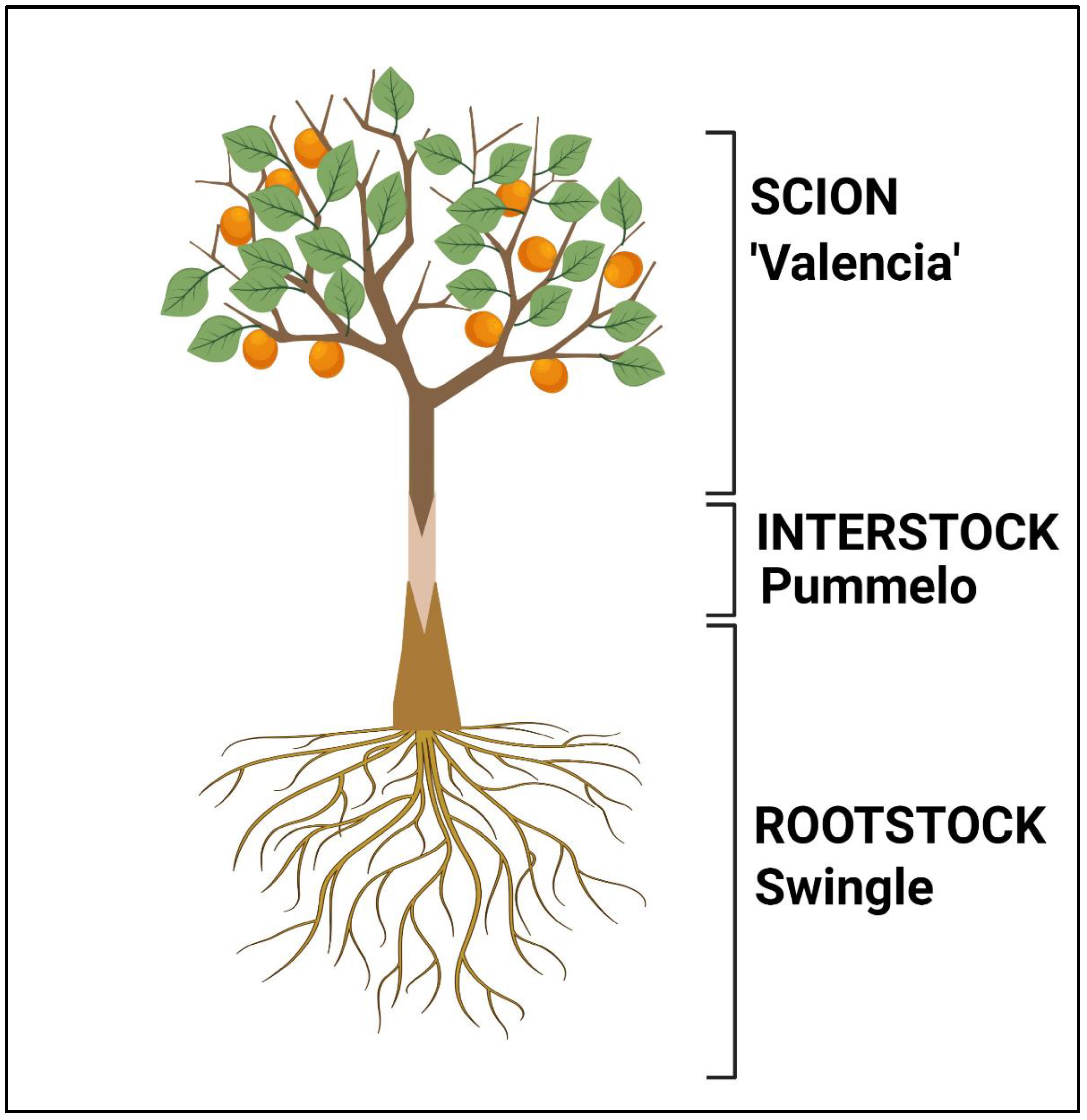
2.1. Plant Materials
2.2. CaLas Assessment in ‘Valencia’ Leaves
2.3. Physiological and Biochemical Variables
2.3.1. Foliar Photosynthetic Pigments and Starch Quantification
2.3.2. Total Phenolic Compounds and DPPH Radical Scavenging Activity
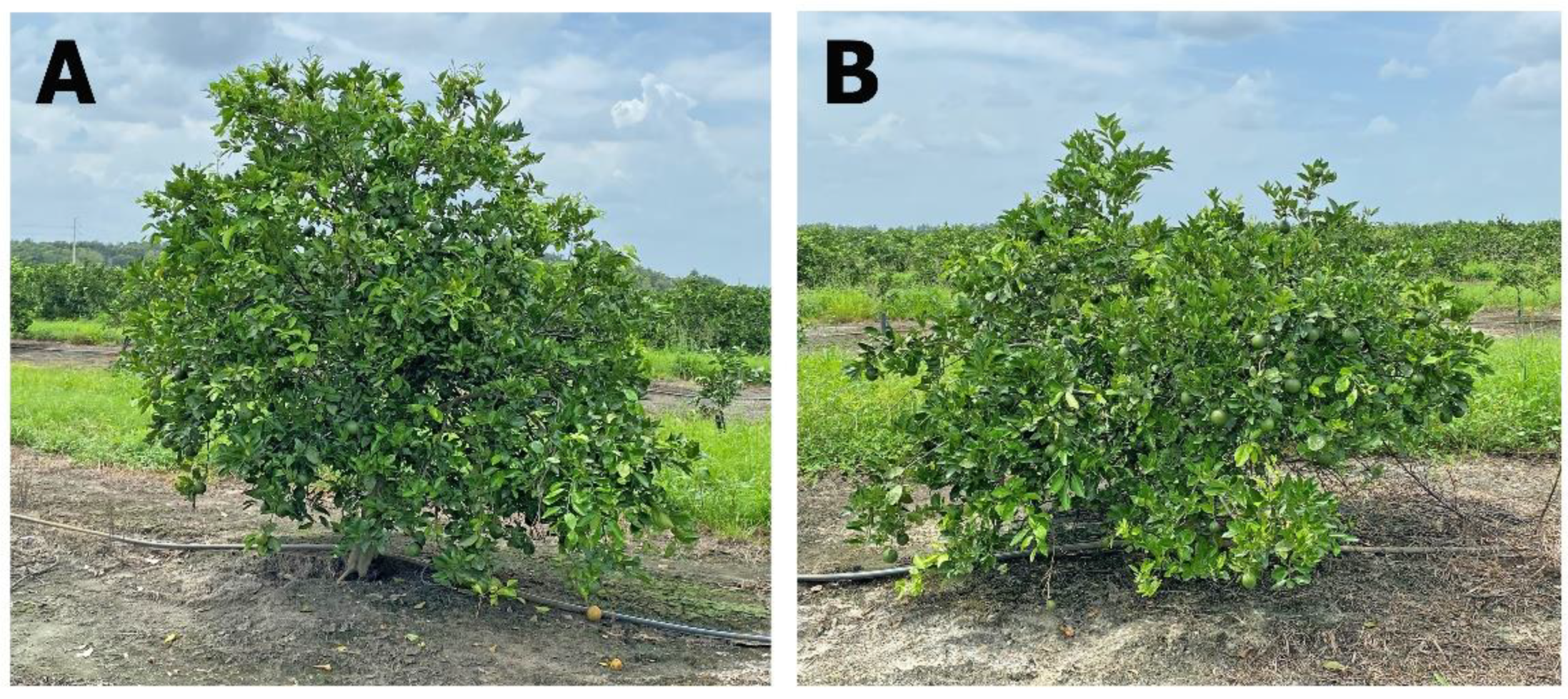
2.3.3. Tree Canopy
2.4. Gene Expression Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. CaLas Assessment in ‘Valencia’ Leaves
3.2. Physiological and Biochemical Variables
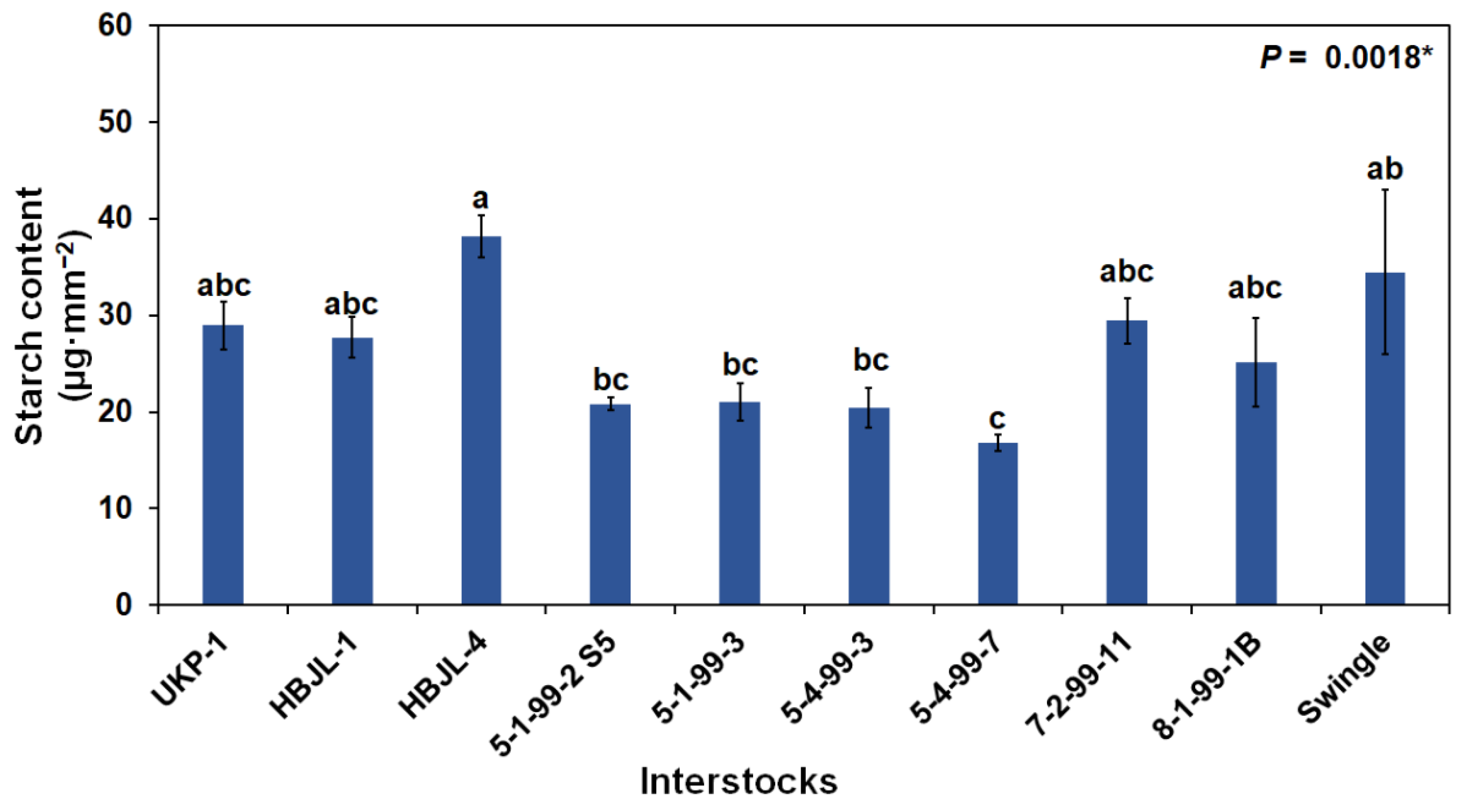
3.2.1. Foliar Photosynthetic Pigments and Starch Content
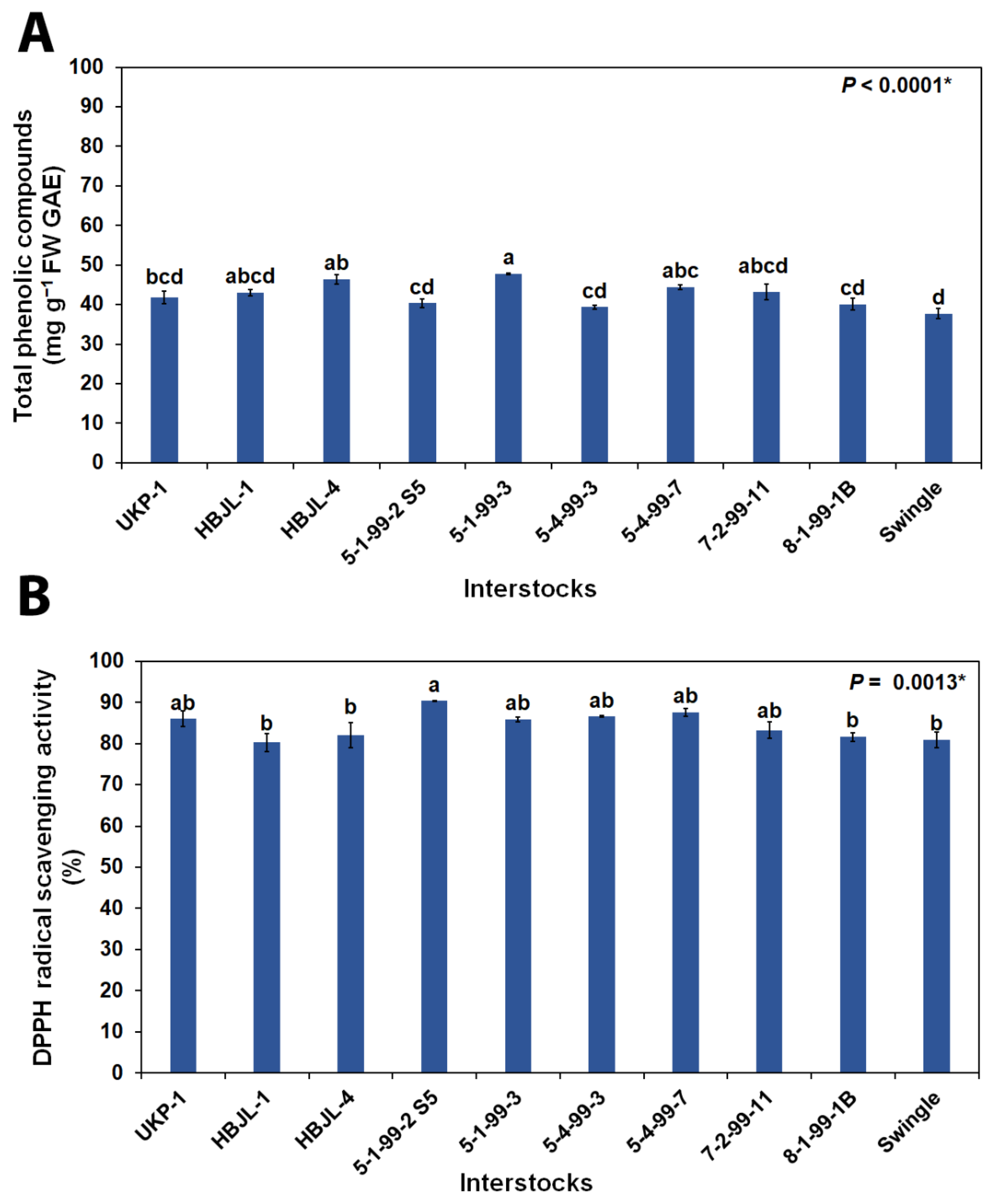
3.2.2. Total Phenolic Compounds and DPPH Radical Scavenging Activity
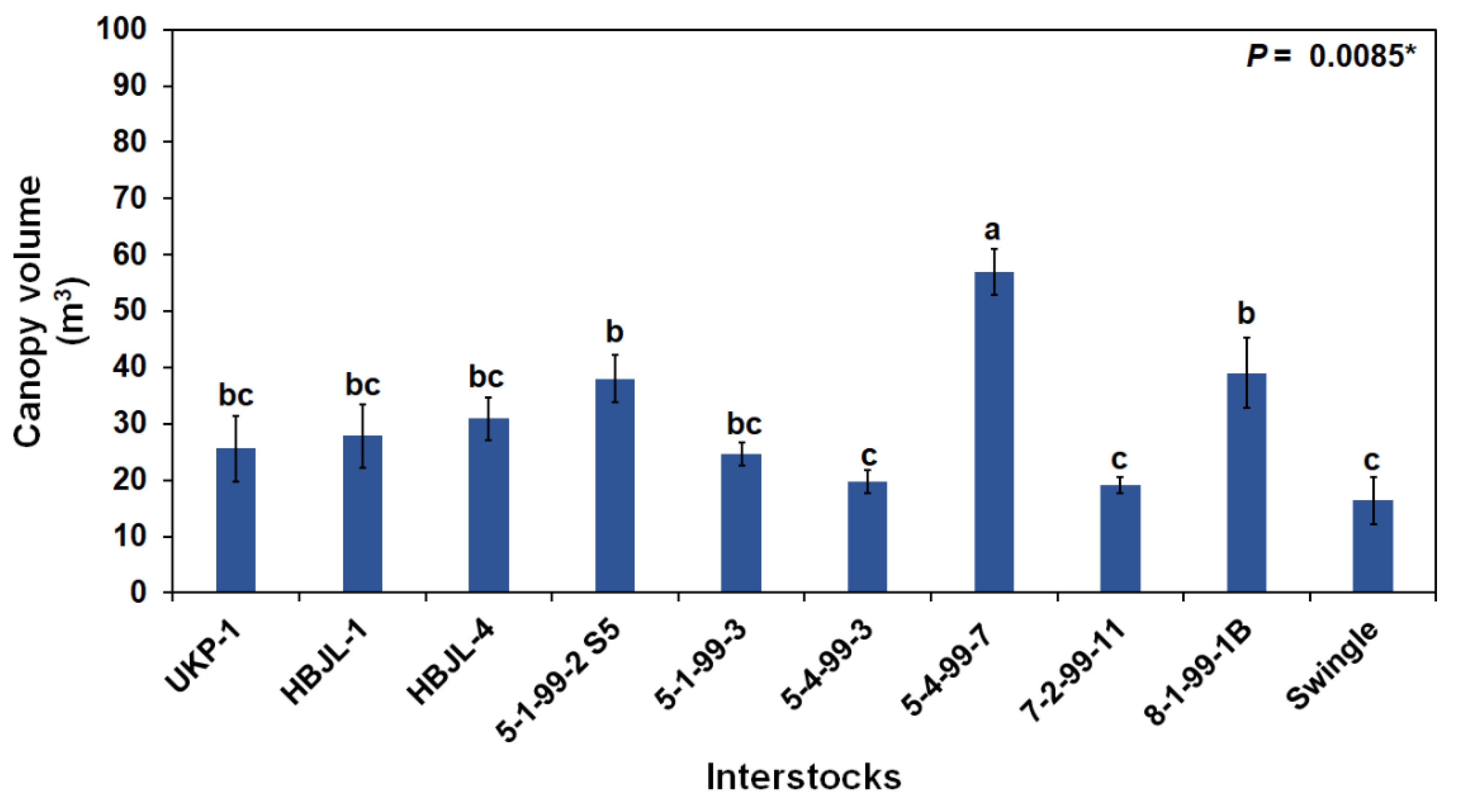
3.3. Tree Canopy
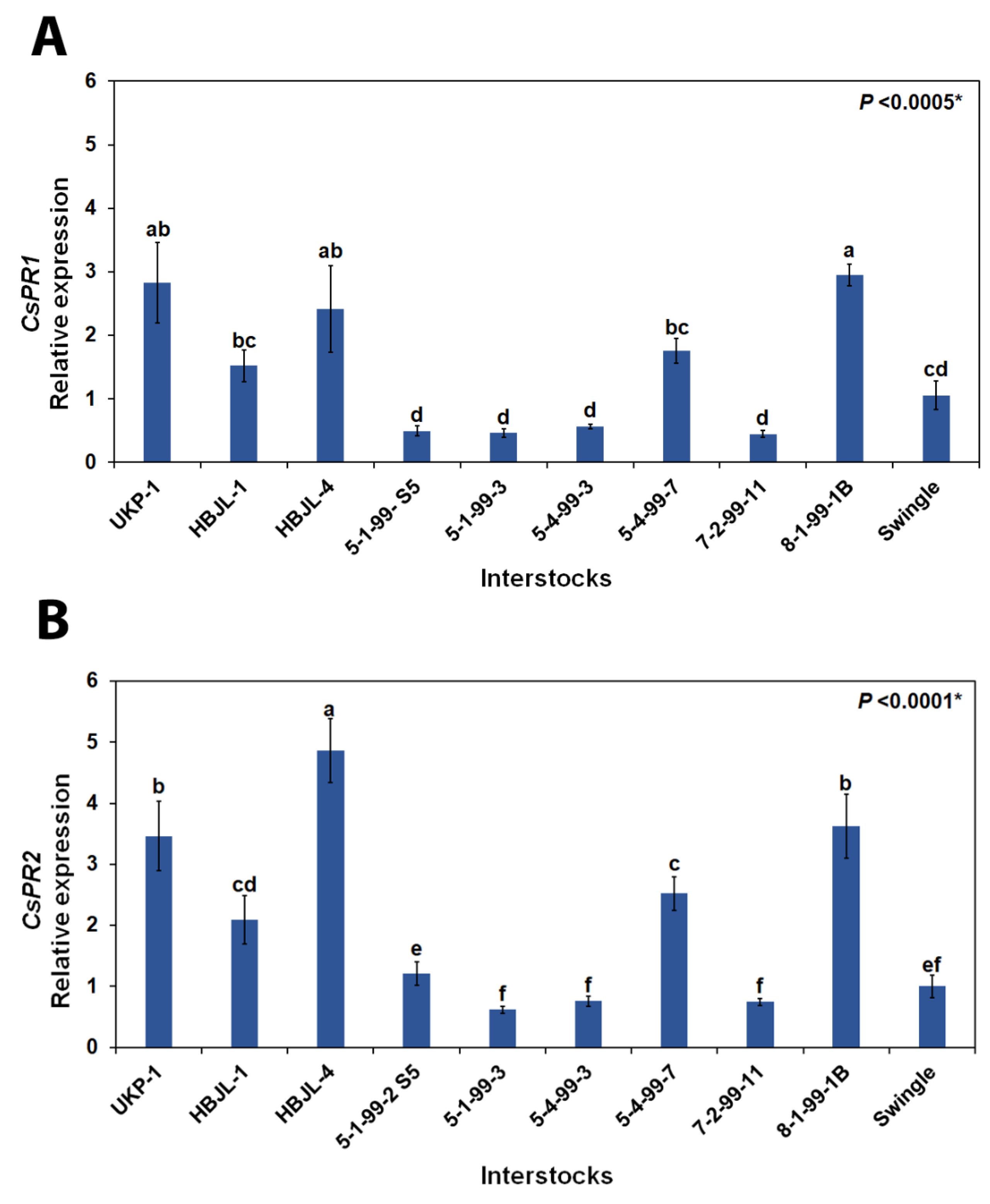
3.4. Gene Expression of Pathogenesis-Related (PR1 and PR2) Genes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zapata, S.D.; Peguero, F.; Sétamou, M.; Alabi, O.J. Economic Implications of Citrus Greening Disease Management Strategies. J. Agric. Resour. Econ. 2022, 47, 300–323. [Google Scholar]
- Killiny, N. Made for each other: Vector–pathogen interfaces in the huanglongbing pathosystem. Phytopathology 2022, 112, 26–43. [Google Scholar] [CrossRef]
- Trivedi, P.; Duan, Y.; Wang, N. Huanglongbing, a systemic disease, restructures the bacterial community associated with citrus roots. Appl. Environ. Microbiol. 2010, 76, 3427–3436. [Google Scholar] [CrossRef]
- Killiny, N.; Jones, S.E.; Nehela, Y.; Hijaz, F.; Dutt, M.; Gmitter, F.G.; Grosser, J.W. All roads lead to Rome: Towards understanding different avenues of tolerance to huanglongbing in citrus cultivars. Plant Physiol. Biochem. 2018, 129, 1–10. [Google Scholar] [CrossRef]
- Peng, Z.; Bredeson, J.V.; Wu, G.A.; Shu, S.; Rawat, N.; Du, D.; Parajuli, S.; Yu, Q.; You, Q.; Rokhsar, D.S. A chromosome-scale reference genome of trifoliate orange (Poncirus trifoliata) provides insights into disease resistance, cold tolerance and genome evolution in Citrus. Plant J. 2020, 104, 1215–1232. [Google Scholar] [CrossRef]
- Weber, K.; Mahmoud, L.M.; Stanton, D.; Welker, S.; Qiu, W.; Grosser, J.W.; Levy, A.; Dutt, M. Insights Into the Mechanism of Huanglongbing (HLB) Tolerance in the Australian Finger Lime (Citrus australasica). Front. Plant Sci. 2022, 13, 1019295. [Google Scholar] [CrossRef] [PubMed]
- Dutt, M.; Mahmoud, L.M.; Chamusco, K.; Stanton, D.; Chase, C.D.; Nielsen, E.; Quirico, M.; Yu, Q.; Gmitter, F.G., Jr.; Grosser, J.W. Utilization of somatic fusion techniques for the development of HLB tolerant breeding resources employing the Australian finger lime (Citrus australasica). PLoS ONE 2021, 16, e0255842. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Soares, J.; Pang, Z.; Huang, Y.; Sun, Z.; Wang, N.; Grosser, J.; Dutt, M. Potential mechanisms of AtNPR1 mediated resistance against Huanglongbing (HLB) in citrus. Int. J. Mol. Sci. 2020, 21, 2009. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.; Weber, K.C.; Qiu, W.; Mahmoud, L.M.; Grosser, J.W.; Dutt, M. Overexpression of the salicylic acid binding protein 2 (SABP2) from tobacco enhances tolerance against Huanglongbing in transgenic citrus. Plant Cell Rep. 2022, 41, 2305–2320. [Google Scholar] [CrossRef]
- Killiny, N.; Hijaz, F.; Gonzalez-Blanco, P.; Jones, S.E.; Pierre, M.O.; Vincent, C.I. Effect of adjuvants on oxytetracycline uptake upon foliar application in citrus. Antibiotics 2020, 9, 677. [Google Scholar] [CrossRef]
- Killiny, N.; Hijaz, F.; Al-Rimawi, F.; Nehela, Y.; Batuman, O. Translocation of oxytetracycline in citrus plants after root drench and stem delivery. Proc. Fla. State Hort. Soc. 2019, 132, 68–71. [Google Scholar]
- Huang, C.-Y.; Araujo, K.; Sánchez, J.N.; Kund, G.; Trumble, J.; Roper, C.; Godfrey, K.E.; Jin, H. A stable antimicrobial peptide with dual functions of treating and preventing citrus Huanglongbing. Proc. Natl. Acad. Sci. USA 2021, 118, e2019628118. [Google Scholar] [CrossRef] [PubMed]
- Kwakye, S.; Kadyampakeni, D.M.; Morgan, K.; Vashisth, T.; Wright, A. Effects of Iron Rates on Growth and Development of Young Huanglongbing-affected Citrus Trees in Florida. HortScience 2022, 57, 1092–1098. [Google Scholar] [CrossRef]
- Yu, X.; Killiny, N. RNA interference-mediated control of Asian citrus psyllid, the vector of the huanglongbing bacterial pathogen. Trop. Plant Pathol. 2020, 45, 298–305. [Google Scholar] [CrossRef]
- Vashisth, T.; Livingston, T. Efficacy of in-field thermotherapy in comparison and combination of defoliation for mitigating Huanglongbing in sweet orange. HortScience 2020, 55, 251–257. [Google Scholar] [CrossRef]
- Albrecht, U.; McCollum, G.; Bowman, K.D. Influence of rootstock variety on Huanglongbing disease development in field-grown sweet orange (Citrus sinensis [L.] Osbeck) trees. Sci. Hortic. 2012, 138, 210–220. [Google Scholar] [CrossRef]
- Girardi, E.A.; Mourão Filho, F.d.A.A. Production of interstocked ‘Pera’sweet orange nursey trees on ‘Volkamer’ lemon and ‘Swingle’citrumelo rootstocks. Sci. Agric. 2006, 63, 5–10. [Google Scholar] [CrossRef]
- Aboutalebi, A.; Hasanzadeh, H. Salinity and citrus rootstocks and interstocks. Int. J. Plant Anim. Environ. Sci. 2014, 4, 654–672. [Google Scholar]
- Gil-Izquierdo, A.; Riquelme, M.T.; Porras, I.; Ferreres, F. Effect of the rootstock and interstock grafted in lemon tree (Citrus limon (L.) Burm.) on the flavonoid content of lemon juice. J. Agric. Food Chem. 2004, 52, 324–331. [Google Scholar] [CrossRef]
- Di Vaio, C.; Cirillo, C.; Buccheri, M.; Limongelli, F. Effect of interstock (M. 9 and M. 27) on vegetative growth and yield of apple trees (cv “Annurca”). Sci. Hortic. 2009, 119, 270–274. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Ren, X.; Bao, L.; Zhang, L.; Zhang, L.; Han, M.; Zhan, D. Root growth, yield and fruit quality of ‘Red Fuji’apple trees in relation to planting depth of dwarfing interstock on the Loess Plateau. Eur. J. Hortic. Sci. 2015, 80, 109–116. [Google Scholar] [CrossRef]
- Popara, G.; Magazin, N.; Keserović, Z.; Milić, B.; Milović, M.; Kalajdžić, J.; Manojlović, M. Rootstock and interstock effects on plum cv.‘Čačanska Lepotica’young tree performance and fruit quality traits. Erwerbs Obstbau 2020, 62, 421–428. [Google Scholar] [CrossRef]
- Rozpara, E.; Grzyb, Z. Frutana®—A new Interstock for sweet cherry trees. In I International Symposium on Rootstocks for Deciduous Fruit Tree Species 658; International Society for Horticultural Science: Leuven, Belgium, 2002; pp. 247–250. [Google Scholar]
- Wang, Z.; Yin, Y.; Hu, H.; Yuan, Q.; Peng, G.; Xia, Y. Development and application of molecular-based diagnosis for ‘Candidatus Liberibacter asiaticus’, the causal pathogen of citrus huanglongbing. Plant Pathol. 2006, 55, 630–638. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents; Portland Press Ltd.: London, UK, 1983. [Google Scholar]
- Rosales, R.; Burns, J.K. Phytohormone changes and carbohydrate status in sweet orange fruit from Huanglongbing-infected trees. J. Plant Growth Regul. 2011, 30, 312–321. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Vashisth, T.; Grosser, J. Comparison of controlled release fertilizer (CRF) for newly planted sweet orange trees under Huanglongbing prevalent conditions. J. Hortic 2018, 5, 7. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Vincent, C.; Guha, A.; Killiny, N.; Diepenbrock, L. Understory environment promotes photosynthetic efficiency and mitigates severity and function of an introduced, vectored pathosystem: A study of a feral citrus population in central Florida. Trees 2021, 35, 1711–1725. [Google Scholar] [CrossRef]
- Achor, D.; Etxeberria, E.; Wang, N.; Folimonova, S.; Chung, K.; Albrigo, L. Sequence of anatomical symptom observations in citrus affected with huanglongbing disease. Plant Pathol. J. 2010, 9, 56–64. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Huyck, P.J.; Vincent, C.I.; Gmitter, F.G., Jr.; Grosser, J.W.; Dutt, M. Physiological Responses and Gene Expression Patterns in Open-Pollinated Seedlings of a Pummelo-Mandarin Hybrid Rootstock Exposed to Salt Stress and Huanglongbing. Plants 2021, 10, 1439. [Google Scholar] [CrossRef]
- Ma, W.; Pang, Z.; Huang, X.; Xu, J.; Pandey, S.S.; Li, J.; Achor, D.S.; Vasconcelos, F.N.; Hendrich, C.; Huang, Y.; et al. Citrus Huanglongbing is a pathogen-triggered immune disease that can be mitigated with antioxidants and gibberellin. Nat. Commun. 2022, 13, 529. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N.; Valim, M.F.; Jones, S.E.; Hijaz, F. Effect of different rootstocks on the leaf metabolite profile of ‘Sugar Belle’mandarin hybrid. Plant Signal. Behav. 2018, 13, e1445934. [Google Scholar] [CrossRef]
- Plyuta, V.; Zaitseva, J.; Lobakova, E.; Zagoskina, N.; Kuznetsov, A.; Khmel, I. Effect of plant phenolic compounds on biofilm formation by Pseudomonas aeruginosa. Apmis 2013, 121, 1073–1081. [Google Scholar] [CrossRef]
- Wallis, C.; Rashed, A.; Chen, J.; Paetzold, L.; Workneh, F.; Rush, C. Effects of potato-psyllid-vectored ‘Candidatus Liberibacter solanacearum’infection on potato leaf and stem physiology. Phytopathology 2015, 105, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, L.M.; Dutt, M.; Vincent, C.I.; Grosser, J.W. Salinity-induced physiological responses of three putative salt tolerant citrus rootstocks. Horticulturae 2020, 6, 90. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Stanton, D.; Amin, B.H.; Grosser, J.W.; Dutt, M. Overexpression of the Arabidopsis NPR1 gene confers enhanced salt tolerance by regulating antioxidant and starch accumulation in citrus. Plant Cell Tissue Organ Cult. PCTOC 2022, 150, 695–707. [Google Scholar] [CrossRef]
- Lockard, R.G. Effects of Rootstocks and Length and Type of Interstock on Growth of Apple trees in Sand Culture1. J. Am. Soc. Hortic. Sci. 1974, 99, 321–325. [Google Scholar] [CrossRef]
- Tukey, H.; Brase, K. The dwarfing effect of an intermediate stem-piece of Malling IX apple. Proc. Am. Soc. Hortic. Sci. 1943, 42, 357–364. [Google Scholar]
- Carlson, R.F. The effects and relationships of intermediate stem sections on growth and behaviour of apple cultivars. Proc. Am. Soc. Hort. Sci. 1965, 87, 21–28. [Google Scholar]
- Dutt, M.; Mahmoud, L.M.; Nehela, Y.; Grosser, J.W.; Killiny, N. The Citrus sinensis TILLER ANGLE CONTROL 1 (CsTAC1) gene regulates tree architecture in sweet oranges by modulating the endogenous hormone content. Plant Sci. 2022, 323, 111401. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Ahmad, R. Influence of endogenous plant hormones on physiological and growth attributes of Kinnow mandarin grafted on nine rootstocks. J. Plant Growth Regul. 2022, 41, 1254–1264. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Li, J.; Liu, M.-M.; Yao, Q.; Chen, J.-Z. Transcriptome profiling to understand the effect of citrus rootstocks on the growth of ‘Shatangju’mandarin. PLoS ONE 2017, 12, e0169897. [Google Scholar]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef]
- van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
| Description (Gene Symbol) | Forward and Reverse Primer Sequences (5′ to 3′) |
|---|---|
| CQUL-F | TGGAGGTGTAAAAGTTGCCAAA |
| CQUL-R | CCAACGAAAAGATCAGATATTCCTCTA |
| CQUL-Probe | FAM-ATCGTCTCGTCAAGATTGCTATCCGTGATACTAG-IBFQ |
| Description (Gene Symbol) | Forward and Reverse Primer Sequences (5′ to 3′) |
|---|---|
| CsPR1 | AACTCGCCTCAAGACTACCT |
| CTGCAACTGTGTCGTTCCATA | |
| CsPR2 | ACTTCGCTCAGTACCTTGTTC |
| GGCAGTGGAAACCTTGATTTG | |
| β-actin | GCTGCCTGATGGCCAGATC |
| AGTTGTAGGTAGTCTCATGAA |
| Interstocks | Ct Values of CaLas |
|---|---|
| UKP-1 | 27.06 ± 0.34 ab1 |
| HBJL-1 | 27.09 ± 0.07 ab |
| HBJL-4 | 25.88 ± 0.96 b |
| 7-2-99-11 | 26.87 ± 0.95 ab |
| 5-4-99-3 | 26.10 ± 0.20 ab |
| 5-4-99-7 S2 | 26.15 ± 0.61 b |
| 5-1-99-2 | 27.14 ± 0.12 ab |
| 5-1-99-3 | 27.82 ± 0.46 a |
| 8-1-99-1B | 25.91 ± 0.57 b |
| Swingle | 27.06 ± 0.49 ab |
| p value | NS |
| Selection | Chlorophyll a mg−1 g FW | Chlorophyll b mg−1 g FW | Total Chlorophyll mg−1 g FW |
|---|---|---|---|
| UKP-1 | 3.68 a,1 | 2.76 ab | 6.44 c |
| HBJL-1 | 4.47 a | 3.22 a | 7.70 a |
| HBJL-4 | 4.07 a | 2.57 b | 6.65 bc |
| 5-1-99-2 S5 | 4.19 a | 3.04 ab | 7.23 abc |
| 5-1-99-3 | 4.09 a | 3.03 ab | 7.13 abc |
| 5-4-99-3 | 4.61 a | 2.89 ab | 7.50 ab |
| 5-4-99-7 | 3.90 a | 2.80 ab | 6.74 bc |
| 7-2-99-11 | 4.44 a | 3.07 ab | 7.51 ab |
| 8-1-99-1B | 4.50 a | 3.28 a | 7.78 a |
| Swingle | 4.61 a | 2.84 ab | 6.71 bc |
| p value | NS | 0.0057 * | 0.0313 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutt, M.; Mahmoud, L.M.; Grosser, J.W. Field Performance of ‘Valencia’ Sweet Orange Trees Grafted onto Pummelo Interstocks and Swingle Citrumelo Rootstocks under Huanglongbing (HLB) Endemic Conditions. Horticulturae 2023, 9, 719. https://doi.org/10.3390/horticulturae9060719
Dutt M, Mahmoud LM, Grosser JW. Field Performance of ‘Valencia’ Sweet Orange Trees Grafted onto Pummelo Interstocks and Swingle Citrumelo Rootstocks under Huanglongbing (HLB) Endemic Conditions. Horticulturae. 2023; 9(6):719. https://doi.org/10.3390/horticulturae9060719
Chicago/Turabian StyleDutt, Manjul, Lamiaa M. Mahmoud, and Jude W. Grosser. 2023. "Field Performance of ‘Valencia’ Sweet Orange Trees Grafted onto Pummelo Interstocks and Swingle Citrumelo Rootstocks under Huanglongbing (HLB) Endemic Conditions" Horticulturae 9, no. 6: 719. https://doi.org/10.3390/horticulturae9060719
APA StyleDutt, M., Mahmoud, L. M., & Grosser, J. W. (2023). Field Performance of ‘Valencia’ Sweet Orange Trees Grafted onto Pummelo Interstocks and Swingle Citrumelo Rootstocks under Huanglongbing (HLB) Endemic Conditions. Horticulturae, 9(6), 719. https://doi.org/10.3390/horticulturae9060719







