Trichoderma viride Isolate Tvd44 Enhances Potato Growth and Stimulates the Defense System against Potato Virus Y
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolation, Molecular Identification, and Culture Preparation of Trichoderma viride
2.2. Viral Source and Molecular Identification
2.3. Antiviral Activity Assay in the Greenhouse
2.4. Disease Assessment
2.5. Growth Parameter Evaluation
2.6. Estimation of Antioxidant Enzyme Activity
2.6.1. Leaf Sample Preparation
2.6.2. Peroxidase (POX) Activity
2.6.3. Polyphenol Oxidase (PPO) Activity
2.7. Protein Content
2.8. Chlorophyll Photosynthetic Pigment
2.9. Transcriptional Level of Defense-Related Genes
2.9.1. RNA Extraction and cDNA Synthesis
2.9.2. Quantitative PCR (qPCR) Assay
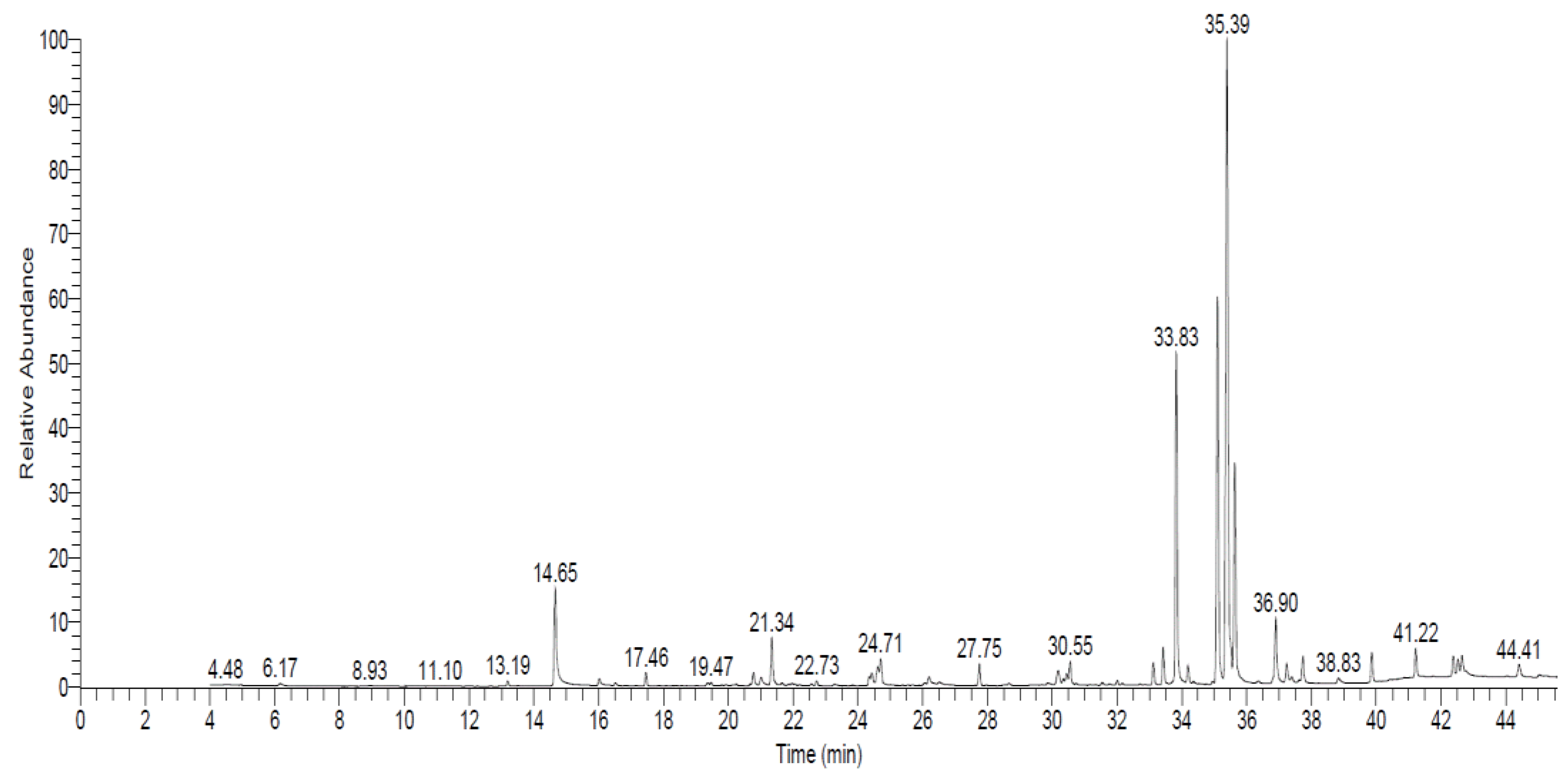
2.10. GC–MS Analysis
2.11. Statistical Analysis
3. Results
3.1. Fungal Isolation and Molecular Identification
3.2. Viral Source and Identification
3.3. Disease Assessment
3.4. Growth Parameter Evaluation
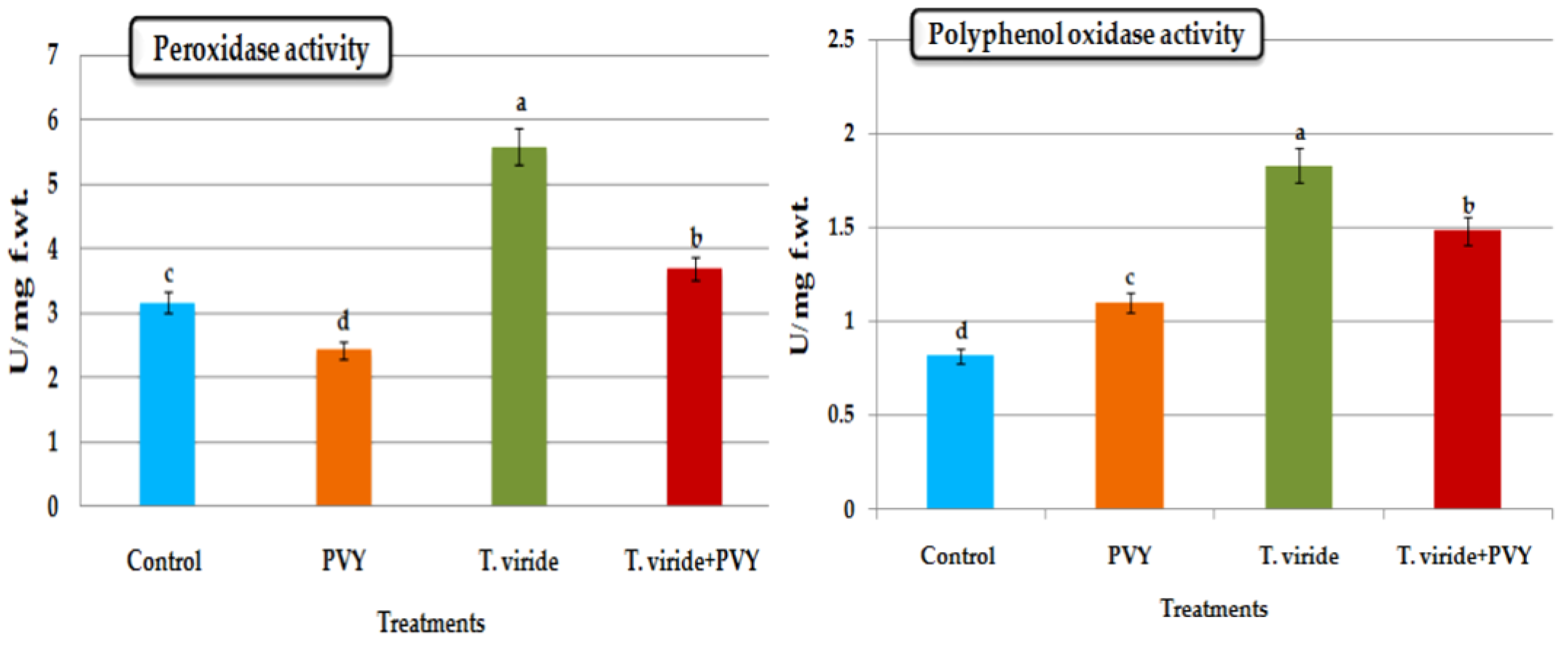
3.5. Estimation of Antioxidant Enzyme Activity
3.5.1. Peroxidase (POX) Activity
3.5.2. Polyphenol Oxidase (PPO) Activity
3.6. Protein Content
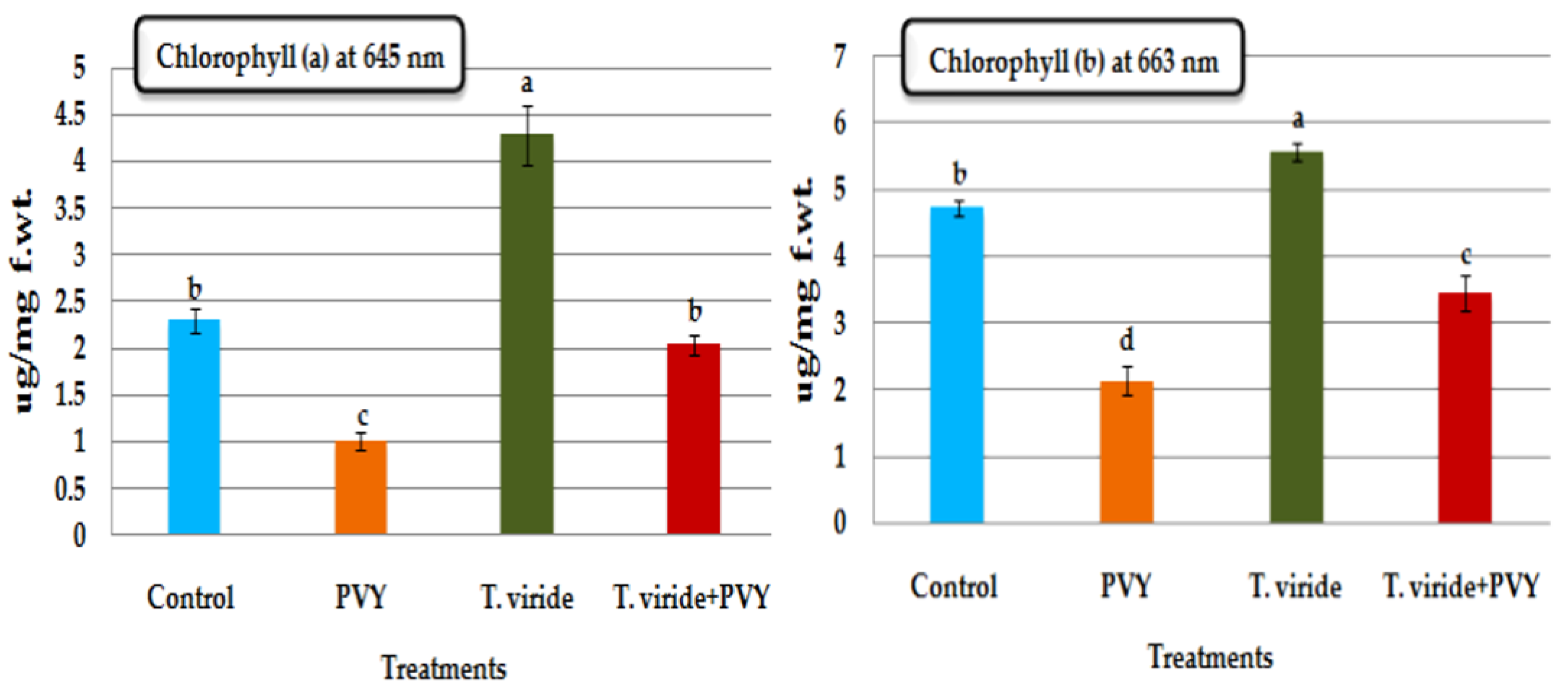
3.7. Chlorophyll
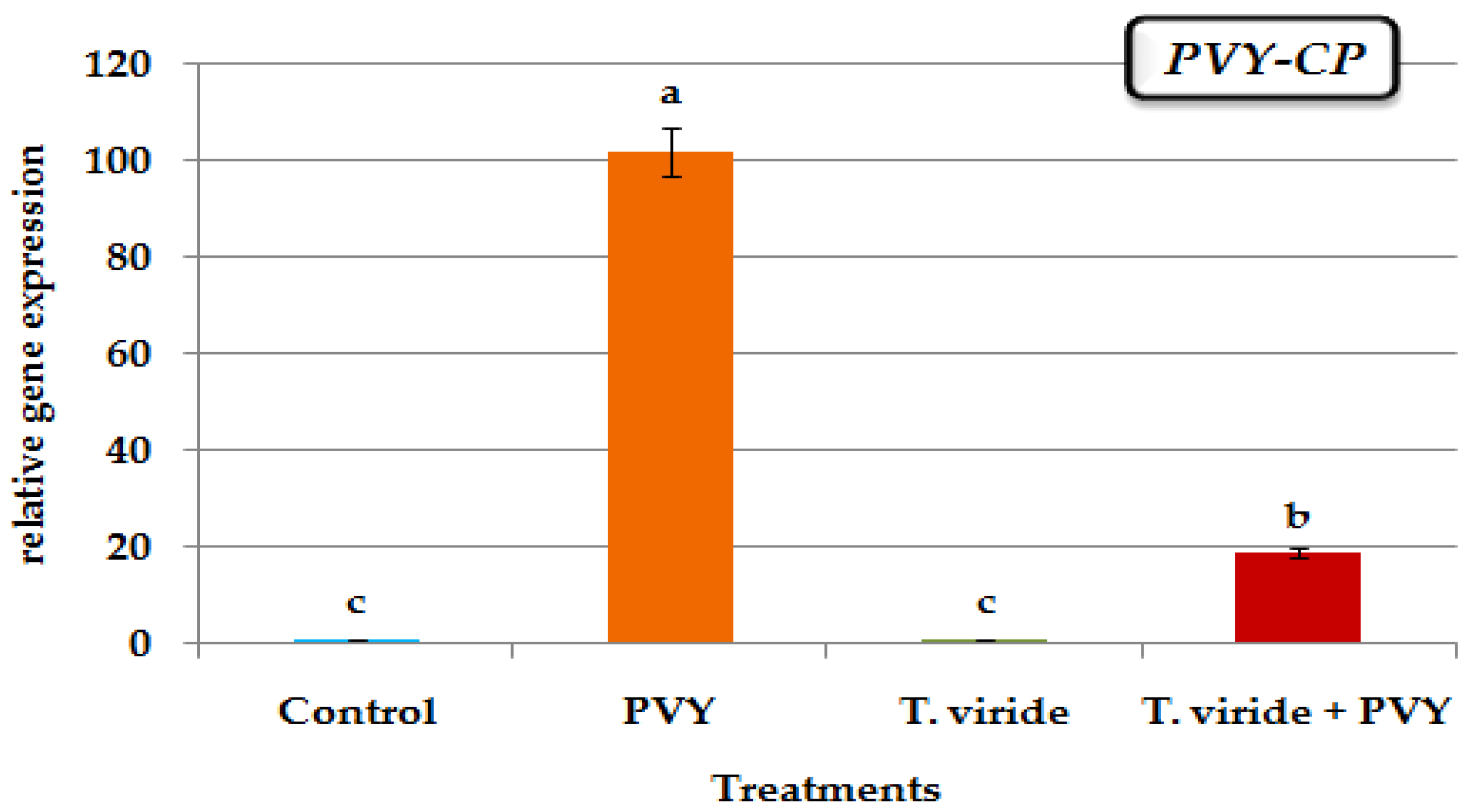
3.8. Effect of T. viride on PVY Accumulation Level
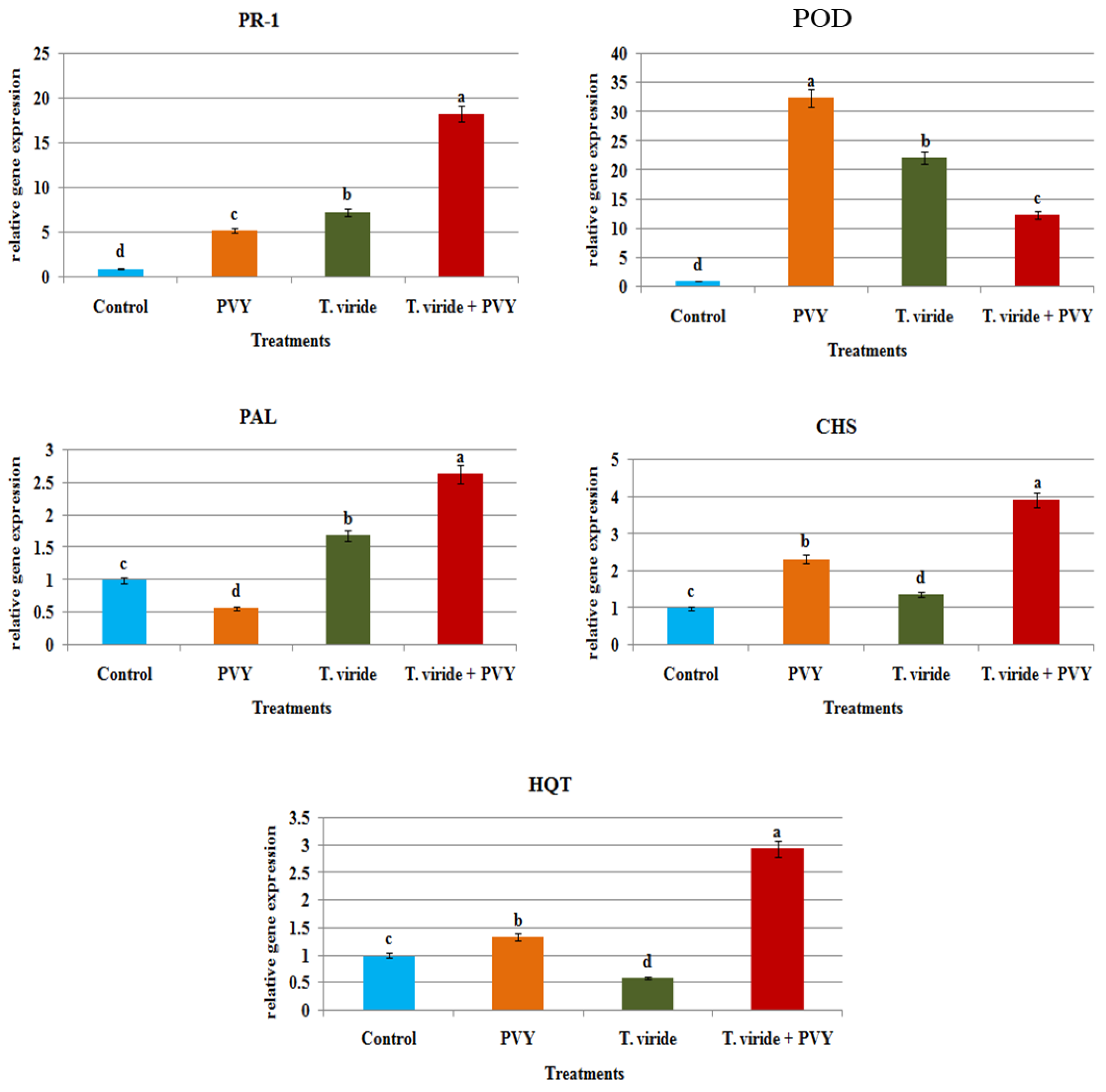
3.9. Defense-Related Transcriptional Levels
3.10. Identification of Bioactive Metabolites of Tvd44
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, J.S.; Adams, B.; Parsons, T.J.; French, R.; Lane, L.C.; Jensen, S.G. Molecular cloning, sequencing, and phylogenetic relationships of a new potyvirus: Sugarcane streak mosaic virus, and a reevaluation of the classification of the Potyviridae. Mol. Phylogenet. Evol. 1998, 10, 323–332. [Google Scholar] [CrossRef]
- Bhoi, T.K.; Samal, I.; Majhi, P.K.; Komal, J.; Mahanta, D.K.; Pradhan, A.K.; Saini, V.; Raj, M.N.; Ahmad, M.A.; Behera, P.P. Insight into aphid mediated Potato Virus Y transmission: A molecular to bioinformatics prospective. Front. Microbiol. 2022, 13, 1001454. [Google Scholar] [CrossRef]
- Valkonen, J.P.T.; Gebhardt, C.; Zimnoch-Guzowska, E.; Watanabe, K.N. Resistance to Potato virus Y in potato. In Potato Virus Y: Biodiversity, Pathogenicity, Epidemiology and Management; Springer: Berlin/Heidelberg, Germany, 2017; pp. 207–241. [Google Scholar]
- Elsharkawy, M.M.; Alotibi, F.O.; Al-Askar, A.A.; Adnan, M.; Kamran, M.; Abdelkhalek, A.; Behiry, S.I.; Saleem, M.H.; Ahmad, A.A.; Khedr, A.A. Systemic Resistance Induction of Potato and Tobacco Plants against Potato Virus Y by Klebsiella oxytoca. Life 2022, 12, 1521. [Google Scholar] [CrossRef]
- Aramburu, J.; Galipienso, L.; Matas, M. Characterization of potato virus Y isolates from tomato crops in northeast Spain. Eur. J. Plant Pathol. 2006, 115, 247–258. [Google Scholar] [CrossRef]
- Kopp, A.; Kondrák, M.; Bánfalvi, Z. Molecular mechanisms of resistance to potato virus X and Y in potato. Acta Phytopathol. Entomol. Hung. 2015, 50, 151–160. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Hafez, E. Plant Viral Diseases in Egypt and Their Control. In Cottage Industry of Biocontrol Agents and Their Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 403–421. [Google Scholar]
- Heflish, A.A.; Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Protective and Curative Effects of Trichoderma asperelloides Ta41 on Tomato Root Rot Caused by Rhizoctonia solani Rs33. Agronomy 2021, 11, 1162. [Google Scholar] [CrossRef]
- Metcalf, D.A.; Wilson, C.R. The process of antagonism of Sclerotium cepivorum in white rot affected onion roots by Trichoderma koningii. Plant Pathol. 2001, 50, 249–257. [Google Scholar] [CrossRef]
- Behiry, S.; Soliman, S.A.; Massoud, M.A.; Abdelbary, M.; Kordy, A.M.; Abdelkhalek, A.; Heflish, A. Trichoderma pubescens Elicit Induced Systemic Resistance in Tomato Challenged by Rhizoctonia solani. J. Fungi 2023, 9, 167. [Google Scholar] [CrossRef]
- Abeysinghe, S. Systemic resistance induced by Trichoderma harzianum RU01 against Uromyces appendiculatus on Phaseolus vulgaris. J. Natl. Sci. Found. Sri Lanka 2009, 37, 203–207. [Google Scholar] [CrossRef]
- Howell, C.R. Cotton seedling preemergence damping-off incited by Rhizopus oryzae and Pythium spp. and its biological control with Trichoderma spp. Phytopathology 2002, 92, 177–180. [Google Scholar] [CrossRef]
- Maheshwary, N.; Gangadhara Naik, B.; Amoghavarsha Chittaragi, M.; Naik, S.K.; Nandish, M. Compatibility of Trichoderma asperellum with fungicides. Pharma Innov. J 2020, 9, 136–140. [Google Scholar]
- Malmierca, M.G.; Barua, J.; McCormick, S.P.; Izquierdo-Bueno, I.; Cardoza, R.E.; Alexander, N.J.; Hermosa, R.; Collado, I.G.; Monte, E.; Gutiérrez, S. Novel aspinolide production by Trichoderma arundinaceum with a potential role in Botrytis cinerea antagonistic activity and plant defence priming. Environ. Microbiol. 2015, 17, 1103–1118. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Scala, F.; Ruocco, M.; Lorito, M. The molecular biology of the interactions between Trichoderma spp., phytopathogenic fungi, and plants. Phytopathology 2006, 96, 181–185. [Google Scholar] [CrossRef]
- Vitti, A.; Pellegrini, E.; Nali, C.; Lovelli, S.; Sofo, A.; Valerio, M.; Scopa, A.; Nuzzaci, M. Trichoderma harzianum T-22 induces systemic resistance in tomato infected by Cucumber mosaic virus. Front. Plant Sci. 2016, 7, 1520. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Al-Askar, A.A.; Arishi, A.A.; Behiry, S.I. Trichoderma hamatum Strain Th23 Promotes Tomato Growth and Induces Systemic Resistance against Tobacco mosaic Virus. J. Fungi 2022, 8, 228. [Google Scholar] [CrossRef]
- Deepa, N.; Sreenivasa, M.Y. Biocontrol strategies for effective management of phytopathogenic fungi associated with cereals. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 177–189. [Google Scholar]
- Calistru, C.; McLean, M.; Berjak, P. In vitro studies on the potential for biological control of Aspergillus flavus and Fusarium moniliforme by Trichoderma species. Mycopathologia 1997, 137, 115–124. [Google Scholar] [CrossRef]
- Castle, A.; Speranzini, D.; Rghei, N.; Alm, G.; Rinker, D.; Bissett, J. Morphological and molecular identification of Trichoderma isolates on North American mushroom farms. Appl. Environ. Microbiol. 1998, 64, 133–137. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Samuels, G.J.; Dodd, S.L.; Gams, W.; Castlebury, L.A.; Petrini, O. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 2002, 94, 146–170. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Aseel, D.G.; Rashad, Y.M.; Hammad, S.M. Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against Tomato mosaic Virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef]
- Aseel, D.G.; Abdelkhalek, A.; Alotibi, F.O.; Samy, M.A.; Al-Askar, A.A.; Arishi, A.A.; Hafez, E.E. Foliar Application of Nanoclay Promotes Potato (Solanum tuberosum L.) Growth and Induces Systemic Resistance against Potato Virus, Y. Viruses 2022, 14, 2151. [Google Scholar] [CrossRef]
- Mansour, A.; Al-Musa, A. Tomato yellow leaf curl virus: Host range and virus-vector relationships. Plant Pathol. 1992, 41, 122–125. [Google Scholar] [CrossRef]
- Imran, M.; Khan, M.A.; Azeem, M.; Ahmed, N.; Binyamin, R.; Riaz, A. Screening of tomato germplasm for the source of resistance and its management against ToMV. Pak. J. Phytopathol. 2012, 24, 53–57. [Google Scholar]
- de Souza, M.B.; Stamford, N.P.; Silva, E.V.; Berger, L.R.R.; e Silva, S.C.E.R.; Costa, A.F.; Ferraz, A.P.F. Defense response by inter-active bio-protector and chitosan to Sclerotium rolfsii Wilt disease on cowpea, Brazilian Oxisol. Afr. J. Agric. Res. 2018, 13, 1053–1062. [Google Scholar]
- Angelini, R.; Manes, F.; Federico, R. Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chick-pea stems. Planta 1990, 182, 89–96. [Google Scholar] [CrossRef]
- Kumar, K.B.; Khan, P.A. Peroxidase & polyphenol oxidase in excised ragi (Eleusine corocana cv PR 202) leaves during senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hung, R.; Lee, S.; Bennett, J.W. Arabidopsis thaliana as a model system for testing the effect of Trichoderma volatile organic compounds. Fungal Ecol. 2013, 6, 19–26. [Google Scholar] [CrossRef]
- Palta, J.P. Leaf chlorophyll content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Rashad, Y.M.; Aseel, D.G.; Hafez, E.E. Antifungal potential and defense gene induction in maize against Rhizoctonia root rot by seed extract of Ammi visnaga (L.) Lam. Phytopathol. Mediterr. 2018, 57, 73–88. [Google Scholar]
- Hafez, E.E.; Abdelkhalek, A.A.; Abd El-Wahab, A.S.E.-D.; Galal, F.H. Altered gene expression: Induction/suppression in leek elicited by Iris Yellow Spot Virus infection (IYSV) Egyptian isolate. Biotechnol. Biotechnol. Equip. 2013, 27, 4061–4068. [Google Scholar] [CrossRef]
- Rashad, Y.; Aseel, D.; Hammad, S.; Elkelish, A. Rhizophagus irregularis and Rhizoctonia solani differentially elicit systemic transcriptional expression of polyphenol biosynthetic pathways genes in sunflower. Biomolecules 2020, 10, 379. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Behiry, S.I.; Al-Askar, A.A. Bacillus velezensis PEA1 Inhibits Fusarium oxysporum Growth and Induces Systemic Resistance to Cucumber mosaic Virus. Agronomy 2020, 10, 1312. [Google Scholar] [CrossRef]
- Hamrouni, R.; Molinet, J.; Dupuy, N.; Taieb, N.; Carboue, Q.; Masmoudi, A.; Roussos, S. The effect of aeration for 6-pentyl-alpha-pyrone, conidia and lytic enzymes production by Trichoderma asperellum strains grown in solid-state fermentation. Waste Biomass Valorization 2020, 11, 5711–5720. [Google Scholar] [CrossRef]
- Salwan, R.; Rialch, N.; Sharma, V. Bioactive volatile metabolites of Trichoderma: An overview. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms: Discovery and Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 87–111. [Google Scholar]
- Stracquadanio, C.; Quiles, J.M.; Meca, G.; Cacciola, S.O. Antifungal activity of bioactive metabolites produced by Trichoderma asperellum and Trichoderma atroviride in liquid medium. J. Fungi 2020, 6, 263. [Google Scholar] [CrossRef]
- Gothai, S.; Vijayarathna, S.; Chen, Y.; Kanwar, J.R.; Wahab, H.A.; Sasidharan, S. In vitro-scientific evaluation on anti-Candida albicans activity, antioxidant properties, and phytochemical constituents with the identification of antifungal active fraction from traditional medicinal plant Couroupita guianensis Aubl. Flower. J. Complement. Med. Res. 2018, 8, 85. [Google Scholar] [CrossRef]
- Mariastutt, H.D.; Listiyowati, S.R.I.; Wahyudi, A.T.R.I. Antifungal activity of soybean rhizosphere actinomycetes producing bioactive compounds against Fusarium oxysporum. Biodiversitas J. Biol. Divers. 2018, 19, 2127–2133. [Google Scholar] [CrossRef]
- Abdullah, R.R. Insecticidal activity of secondary metabolites of locally isolated fungal strains against some cotton insect pests. J. Plant Prot. Pathol. 2019, 10, 647–653. [Google Scholar] [CrossRef]
- ChunYan, H.; Hong, P.; ZhenYu, Z.; Jing, S. Evaluation of antioxidant and antitumour activities of lemon essential oil. J. Med. Plant Res. 2010, 4, 1910–1915. [Google Scholar]
- Govindasamy, V.; Balasubramanian, R. Biological control of groundnut rust, Puccinia arachidis, by Trichoderma harzianum/Biologische Bekämpfung des Erdnuß-Rostes, Puccinia arachidis mit Trichoderma harzianum. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 1989, 96, 337–345. [Google Scholar]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E. Myths and dogmas of biocontrol changes in perceptions derived from research on Trichoderma harzinum T-22. Plant Dis. 2000, 84, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 2001, 235, 235–242. [Google Scholar] [CrossRef]
- Susiana, P.; Achmadi, P.; Retno, P.S.; Rina, S.K.; Kadarwati, B. The resistance of potatoes by application of Trichoderma viride antagonists fungus. E3S Web Conf. 2018, 73, 6014. [Google Scholar] [CrossRef]
- Jamil, A. Antifungal and plant growth promoting activity of Trichoderma spp. against Fusarium oxysporum f. sp. lycopersici colonizing tomato. J. Plant Prot. Res. 2021, 61, 243–253. [Google Scholar]
- Awad-Allah, E.F.A.; Shams, A.H.M.; Helaly, A.A.; Ragheb, E.I.M. Effective Applications of Trichoderma spp. as Biofertilizers and Biocontrol Agents Mitigate Tomato Fusarium Wilt Disease. Agriculture 2022, 12, 1950. [Google Scholar] [CrossRef]
- Aggarwal, R.; Gupta, S.; Singh, V.B.; Sharma, S. Microbial detoxification of pathotoxin produced by spot blotch pathogen Bipolaris sorokiniana infecting wheat. J. Plant Biochem. Biotechnol. 2011, 20, 66–73. [Google Scholar] [CrossRef]
- Harman, G.E.; Petzoldt, R.; Comis, A.; Chen, J. Interactions between Trichoderma harzianum strain T22 and maize inbred line Mo17 and effects of these interactions on diseases caused by Pythium ultimum and Colletotrichum graminicola. Phytopathology 2004, 94, 147–153. [Google Scholar] [CrossRef]
- Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Cherepanova, E.A.; Alekseev, V.Y.; Sarvarova, E.R.; Kasimova, A.R.; Maksimov, I. V By modulating the hormonal balance and ribonuclease activity of tomato plants Bacillus subtilis induces defense response against potato virus X and potato virus Y. Biomolecules 2022, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Beris, D.; Theologidis, I.; Skandalis, N.; Vassilakos, N. Bacillus amyloliquefaciens strain MBI600 induces salicylic acid dependent resistance in tomato plants against Tomato spotted wilt virus and Potato virus Y. Sci. Rep. 2018, 8, 10320. [Google Scholar] [CrossRef] [PubMed]
- El-Gendi, H.; Al-Askar, A.A.; Király, L.; Samy, M.A.; Moawad, H.; Abdelkhalek, A. Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae 2022, 8, 301. [Google Scholar] [CrossRef]
- Tamandegani, P.R.; Sharifnabi, B.; Massah, A.; Zahravi, M. Induced reprogramming of oxidative stress responses in cucumber by Trichoderma asperellum (Iran 3062C) enhances defense against Cucumber mosaic virus. Biol. Control 2021, 164, 104779. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Aseel, D.G.; Király, L.; Künstler, A.; Moawad, H.; Al-Askar, A.A. Induction of Systemic Resistance to Tobacco mosaic virus in Tomato through Foliar Application of Bacillus amyloliquefaciens Strain TBorg1 Culture Filtrate. Viruses 2022, 14, 1830. [Google Scholar] [CrossRef]
- Saravanan, T.; Bhaskaran, R.; Muthusamy, M. Pseudomonas fluorescens induced enzymological changes in banana roots (Cv. rasthali) against Fusarium wilt disease. Plant Pathol. J. 2004, 3, 72–80. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Abdel-Gawd, S.; Sleem, E. Induction of Systemic resistance and enhanced enzyme activity by Trichoderma sp. Shmosa Tri (FJ 937359) in Squash against Zucchini Yellow Mosaic Virus (ZYMV). In Proceedings of the Egypt. J. Bot. 3rd International Conference, Helwan, Egypt, 17–18 April 2013; pp. 539–558. [Google Scholar]
- Abdelkhalek, A.; Yassin, Y.; Abdel-Megeed, A.; Abd-Elsalam, K.A.; Moawad, H.; Behiry, S.I. Rhizobium leguminosarum bv. viciae-Mediated Silver Nanoparticles for Controlling Bean Yellow Mosaic Virus (BYMV) Infection in Faba Bean Plants. Plants 2023, 12, 45. [Google Scholar] [CrossRef]
- Petrova, D.; Chaneva, G.; Stoimenova, E.; Kapchina-Toteva, V. Effect of Cucumber mosaic virus on the contents of chlorophyll, proline, the degree of lipid peroxidation and phenotypic expression of pepper lines with different susceptibility to virus. Oxid. Commun. 2012, 35, 182–189. [Google Scholar]
- Hoegen, E.; Strömberg, A.; Pihlgren, U.; Kombrink, E. Primary structure and tissue-specific expression of the pathogenesis-related protein PR-1b in potato. Mol. Plant Pathol. 2002, 3, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Rohfritsch, O.; Fritig, B.; Legrand, M. Phenylalanine ammonia-lyase in tobacco (molecular cloning and gene expression during the hypersensitive reaction to Tobacco mosaic virus and the response to a fungal elicitor). Plant Physiol. 1994, 106, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Nawrath, C.; Métraux, J.-P. Salicylic acid induction–deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 1999, 11, 1393–1404. [Google Scholar]
- Chen, H.; Zhang, Z.; Teng, K.; Lai, J.; Zhang, Y.; Huang, Y.; Li, Y.; Liang, L.; Wang, Y.; Chu, C. Up-regulation of LSB1/GDU3 affects Geminivirus infection by activating the salicylic acid pathway. Plant J. 2010, 62, 12–23. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Dewdney, J.; Blee, K.A.; Stone, J.M.; Asai, T.; Plotnikov, J.; Denoux, C.; Hayes, T.; Gerrish, C.; Davies, D.R. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 2006, 47, 851–863. [Google Scholar] [CrossRef]
- Chamnongpol, S.; Willekens, H.; Moeder, W.; Langebartels, C.; Sandermann, H.; Van Montagu, M.; Inzé, D.; Van Camp, W. Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc. Natl. Acad. Sci. USA 1998, 95, 5818–5823. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Luo, Y.; Qin, S.; Xi, L.; Wan, B.; Du, L. Induction of systemic resistance against Tobacco mosaic virus by Ningnanmycin in tobacco. Pestic. Biochem. Physiol. 2014, 111, 14–18. [Google Scholar] [CrossRef]
- Xu, H.; Nie, J. Identification, characterization, and molecular detection of Alfalfa mosaic virus in potato. Phytopathology 2006, 96, 1237–1242. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Su, H.; Song, S.; Yan, X.; Fang, L.; Zeng, B.; Zhu, Y. Endogenous salicylic acid shows different correlation with baicalin and baicalein in the medicinal plant Scutellaria baicalensis Georgi subjected to stress and exogenous salicylic acid. PLoS ONE 2018, 13, e0192114. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, D.-D.; Dong, X.-W.; Zhao, P.-B.; Chen, L.-L.; Song, X.-Y.; Wang, X.-J.; Chen, X.-L.; Shi, M.; Zhang, Y.-Z. Antimicrobial peptaibols induce defense responses and systemic resistance in tobacco against Tobacco mosaic virus. FEMS Microbiol. Lett. 2010, 313, 120–126. [Google Scholar] [CrossRef] [PubMed]
- André, C.M.; Schafleitner, R.; Legay, S.; Lefèvre, I.; Aliaga, C.A.A.; Nomberto, G.; Hoffmann, L.; Hausman, J.-F.; Larondelle, Y.; Evers, D. Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress. Phytochemistry 2009, 70, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Marais, J.P.J.; Deavours, B.; Dixon, R.A.; Ferreira, D. The stereochemistry of flavonoids. In The Science of Flavonoids; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–46. [Google Scholar]
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Regente, M.; Jacobi, S.; Del Rio, M.; Pinedo, M.; de la Canal, L. Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pestic. Biochem. Physiol. 2017, 140, 30–35. [Google Scholar] [CrossRef]
- Bate, N.J.; Orr, J.; Ni, W.; Meromi, A.; Nadler-Hassar, T.; Doerner, P.W.; Dixon, R.A.; Lamb, C.J.; Elkind, Y. Quantitative relationship between phenylalanine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate-determining step in natural product synthesis. Proc. Natl. Acad. Sci. USA 1994, 91, 7608–7612. [Google Scholar] [CrossRef]
- Howles, P.A.; Sewalt, V.J.H.; Paiva, N.L.; Elkind, Y.; Bate, N.J.; Lamb, C.; Dixon, R.A. Overexpression of L-phenylalanine ammonia-lyase in transgenic tobacco plants reveals control points for flux into phenylpropanoid biosynthesis. Plant Physiol. 1996, 112, 1617–1624. [Google Scholar] [CrossRef]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746. [Google Scholar] [CrossRef]
- Leiss, K.A.; Maltese, F.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. Identification of chlorogenic acid as a resistance factor for thrips in chrysanthemum. Plant Physiol. 2009, 150, 1567–1575. [Google Scholar] [CrossRef]
- Tsao, R.; Marvin, C.H.; Broadbent, A.B.; Friesen, M.; Allen, W.R.; Mcgarvey, B.D. Evidence for an isobutylamide associated with host-plant resistance to western flower thrips, Frankliniella occidentalis, in chrysanthemum. J. Chem. Ecol. 2005, 31, 103–110. [Google Scholar] [CrossRef]
- Gupta, R.C. Carbamate pesticides. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 661–664. [Google Scholar]
- Degani, O.; Khatib, S.; Becher, P.; Gordani, A.; Harris, R. Trichoderma asperellum secreted 6-Pentyl-α-Pyrone to control Magnaporthiopsis maydis, the maize late wilt disease agent. Biology 2021, 10, 897. [Google Scholar] [CrossRef]




| Gene | Primer Sequence (5′–3′) | Functional Annotation |
|---|---|---|
| PR-1 | Forward: CCAAGACTATCTTGCGGTTC Reverse: GAACCTAAGCCACGATACCA | Pathogenesis related protein-1 |
| POD | Forward: TGGAGGTCCAACATGGCAAGTTCT Reverse: TGCCACATCTTGCCCTTCCAAATG | Peroxidase |
| PAL1 | Forward: ACGGGTTGCCATCTAATCTGACA Reverse: CGAGCAATAAGAAGCCATCGCAAT | Phenylalanine ammonia-lyase |
| CHS | Forward: CACCGTGGAGGAGTATCGTAAGGC Reverse: TGATCAACACAGTTGGAAGGCG | Chalcone synthase |
| HQT | Forward: CCCAATGGCTGGAAGATTAGCTA Reverse: CATGAATCACTTTCAGCCTCAACAA | Hydroxycinnamoyl Co A quinate hydroxycinnamoyl transferase |
| Beta-actin | Forward: ATGCCATTCTCCGTCTTGACTTG Reverse: GAGTTGTATGTAGTCTCGTGGATT | Housekeeping gene |
| ITS | Forward: TCCGTAGGTGAACCTGCGG Reverse: TCCTCCGCTTATTGATATGC | Internal transcribed spacer |
| PVY-CP | Forward: CAACTCCAGATGGAACAATTG Reverse: CCATTCATCACAGTTGGC | Potato virus Y coat protein |
| Treatment | Disease Incidence (%) * | Disease Incidence Grade | PDI (%) |
|---|---|---|---|
| C | 00.0 c | - | 00.0 c |
| T | 00.0 c | - | 00.0 c |
| V | 89.4 a | high | 83.6 a |
| T + V | 21.4 b | moderate | 11.2 b |
| Treatment * | Plant Height (cm) | Shoot Length (cm) | Root Length (cm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | No. of Leaves |
|---|---|---|---|---|---|---|
| C | 25.0 ± 2.65 b | 15.7 ± 2.08 a | 08.0 ± 2.00 b | 03.6 ± 0.61 b | 1.02 ± 0.24 bc | 29.7 ± 1.53 b |
| T | 37.7 ± 2.52 a | 18.0 ± 1.00 a | 19.7 ± 3.51 a | 5.27 ± 0.72 a | 1.39 ± 0.51 b | 57.7 ± 2.08 a |
| V | 15.7 ± 2.10 c | 10.0 ± 1.00 b | 05.7 ± 1.53 b | 1.50 ± 0.50 c | 0.53 ± 0.21 c | 11.7 ± 5.85 c |
| T + V | 34.3 ± 4.04 a | 16.3 ± 3.51 a | 18.0 ± 01.0 a | 4.53 ± 0.31 ab | 2.15 ± 0.15 a | 31.3 ± 2.08 b |
| * RT | RA% | Compound | Molecular Formula | Biological Activity | References |
|---|---|---|---|---|---|
| 14.65 | 4.29 | 6-Amyl-α-pyrone | C10H14O2 | Antifungal | [39,40,41] |
| 33.83 | 14.06 | trans-[(2,3-Diphenylcyclopropyl)methyl] phenyl sulfide | C22H20OS | Anticandidal and antioxidant | [42] |
| 35.11 | 16.90 | 1,1-Dicyano-2-methyl-4-(p-cyanophenyl)propene | C13H9N3 | Antifungal and insecticidal | [43] |
| 35.39 | 31.78 | Thiocarbamic acid, N,N-dimethyl, S-1,3-diphenyl-2-butenyl ester | C19H21NOS | Antimicrobial | [44] |
| 35.63 | 9.02 | 1-Propene, 3-(2-cyclopentenyl)-2-methyl-1,1-diphenyl | C21H22 | Antifungal | [42] |
| 36.90 | 2.95 | S-(1,3-diphenylbutyl) dimethyl thiocarbamate | C19H21NOS | Antioxidant and anticancer activity | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aseel, D.G.; Soliman, S.A.; Al-Askar, A.A.; Elkelish, A.; Elbeaino, T.; Abdelkhalek, A. Trichoderma viride Isolate Tvd44 Enhances Potato Growth and Stimulates the Defense System against Potato Virus Y. Horticulturae 2023, 9, 716. https://doi.org/10.3390/horticulturae9060716
Aseel DG, Soliman SA, Al-Askar AA, Elkelish A, Elbeaino T, Abdelkhalek A. Trichoderma viride Isolate Tvd44 Enhances Potato Growth and Stimulates the Defense System against Potato Virus Y. Horticulturae. 2023; 9(6):716. https://doi.org/10.3390/horticulturae9060716
Chicago/Turabian StyleAseel, Dalia G., Seham A. Soliman, Abdulaziz A. Al-Askar, Amr Elkelish, Toufic Elbeaino, and Ahmed Abdelkhalek. 2023. "Trichoderma viride Isolate Tvd44 Enhances Potato Growth and Stimulates the Defense System against Potato Virus Y" Horticulturae 9, no. 6: 716. https://doi.org/10.3390/horticulturae9060716
APA StyleAseel, D. G., Soliman, S. A., Al-Askar, A. A., Elkelish, A., Elbeaino, T., & Abdelkhalek, A. (2023). Trichoderma viride Isolate Tvd44 Enhances Potato Growth and Stimulates the Defense System against Potato Virus Y. Horticulturae, 9(6), 716. https://doi.org/10.3390/horticulturae9060716









