Quality Evaluation of Mustard Microgreens Grown on Peat and Jute Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Culture Conditions and Growing Substrates
2.1.1. Growing Substrates
2.1.2. Growing Conditions
2.2. Determination of Yield
2.3. Sensory Test
2.4. Determination of Leaf Texture/Toughness
2.5. Determination of Total Phenolic Compounds
2.6. Determination of Chlorophylls and Total Carotenoids
2.7. Statistics
3. Results
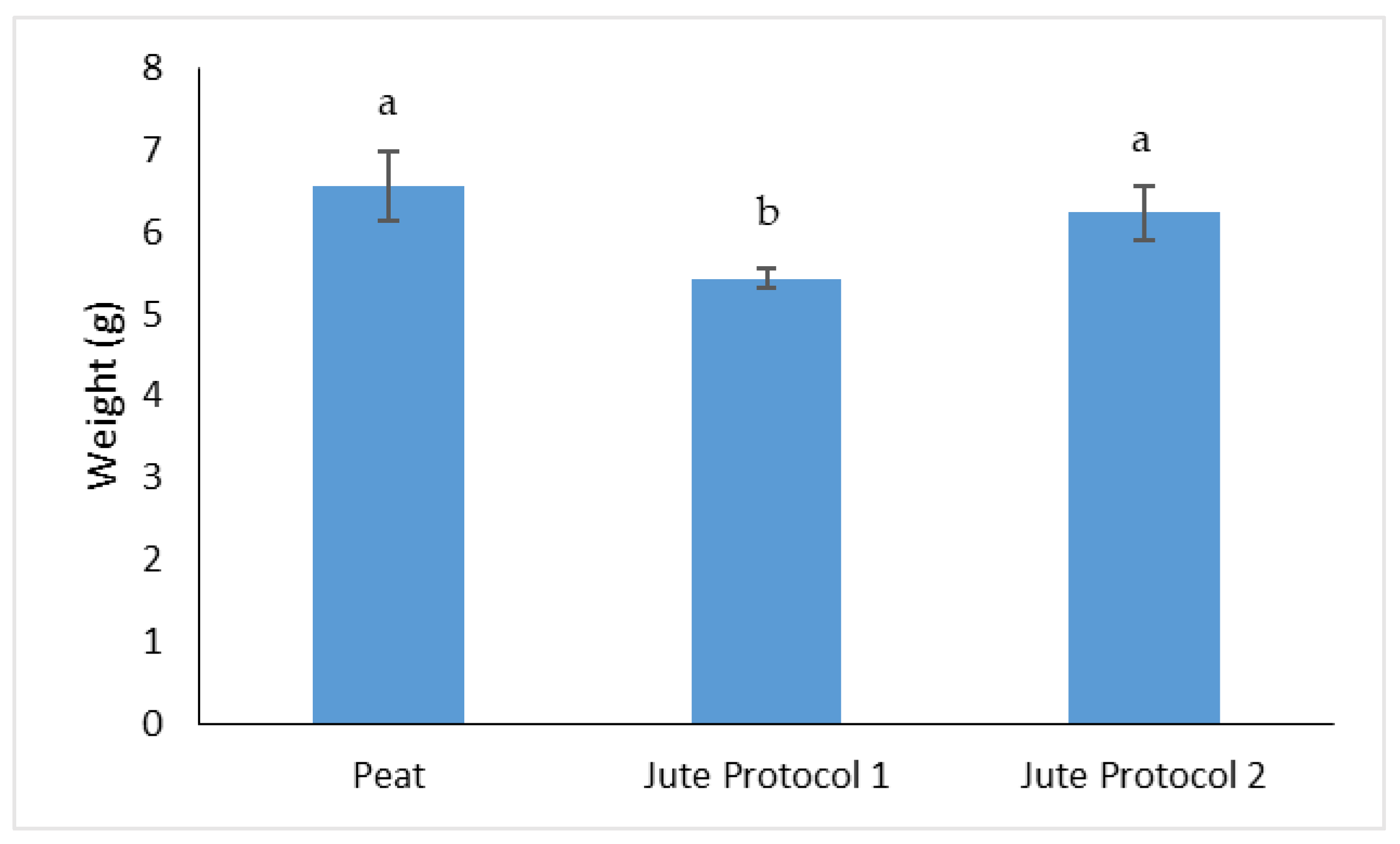
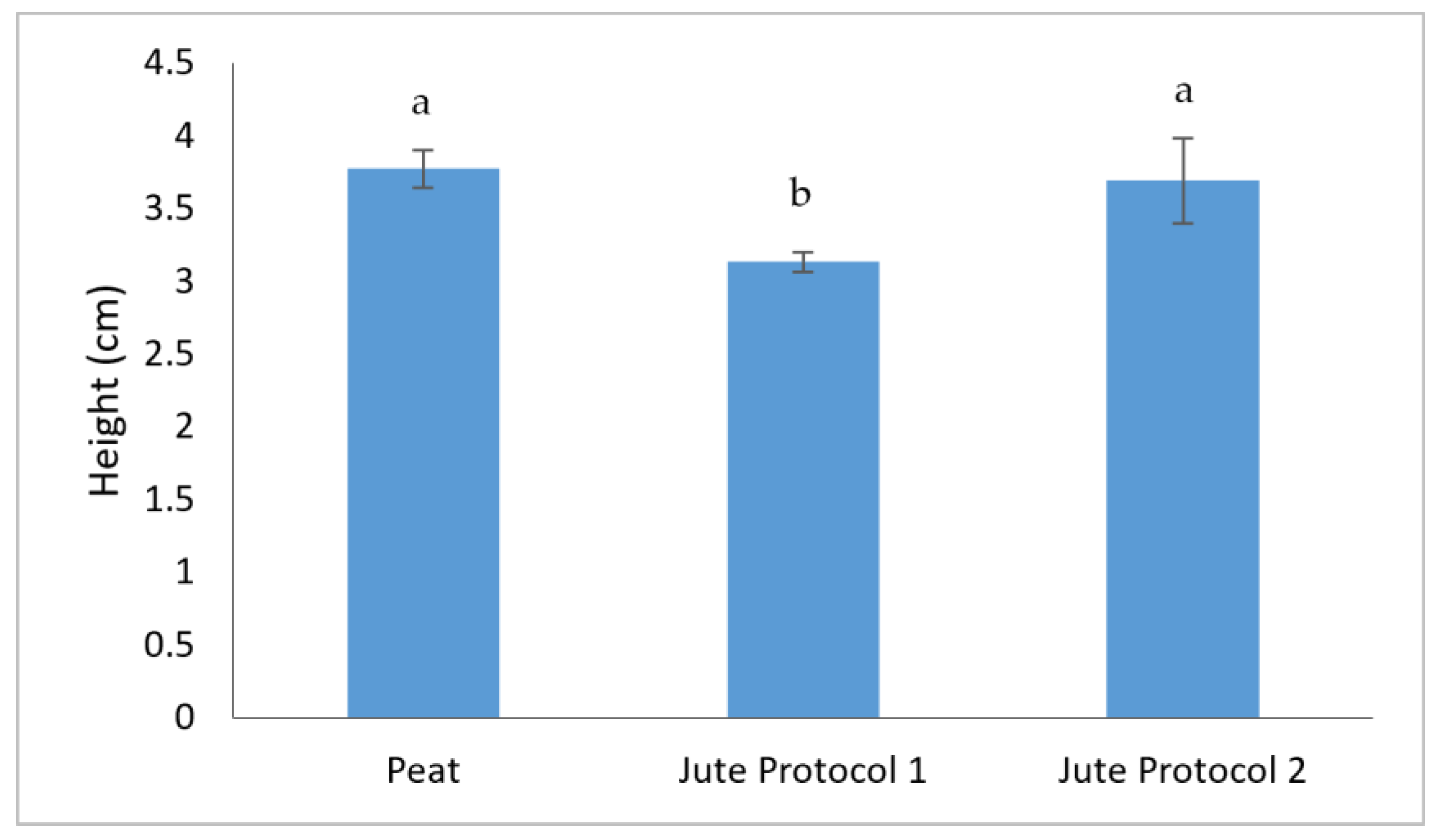
3.1. Determination of Yield
3.2. Sensory Test
3.3. Determination of Leaf Texture/Toughness
3.4. Determination of Total Phenolic Compounds
3.5. Determination of Chlorophylls and Total Carotenoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verlinden, S. Microgreens: Definitions, Product Types, and Production Practices, 1st ed.; John Wiley & Sons Inc.: Charleston, WV, USA, 2020; Chapter 3; ISBN 978-11-1962-540-7. [Google Scholar] [CrossRef]
- Teng, J.; Liao, P.; Wang, M. The Role of Emerging Micro-Scale Vegetables in Human Diet and Health Benefits—An Updated Review Based on Microgreens. Food Funct. 2021, 12, 1914–1932. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.W. Sprouts and Microgreens—Novel Food Sources for Healthy Diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.; Noguera, P.; Bur, S. National Inventory of Organic Wastes for Use as Growing Media for Ornamental Potted Plant Production: Case Study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Negri, M.; Santoro, P.; Ferrante, A. Quality Evaluation of Indoor-Grown Microgreens Cultivated on Three Different Substrates. Horticulturae 2021, 7, 96. [Google Scholar] [CrossRef]
- Othman, A.J.; Vodorezova, E.S.; Mardini, M.; Hanana, M.B. Dataset for the Content of Bioactive Components and Phytonutrients of (Ocimum basilicum and Brassica rapa) Microgreens. Data Brief 2022, 40, 107737. [Google Scholar] [CrossRef]
- Di Gioia, F.; de Bellis, P.; Mininni, C.; Santamaria, P.; Serio, F. Physicochemical, Agronomical and Microbiological Evaluation of Alternative Growing Media for the Production of Rapini (Brassica rapa L.) Microgreens. J. Sci. Food Agric. 2017, 97, 1212–1219. [Google Scholar] [CrossRef]
- Holland Bio Products. Available online: https://www.hollandbioproducts.com/hollandbiomat (accessed on 16 October 2022).
- Wieczorek, M.N.; Dunkel, A.; Szwengiel, A.; Czaczyk, K.; Drożdżyńska, A.; Zawirska-Wojtasiak, R.; Jeleń, H.H. The Relation between Phytochemical Composition and Sensory Traits of Selected Brassica Vegetables. LWT 2022, 156, 113028. [Google Scholar] [CrossRef]
- Xiao, Z.; Codling, E.E.; Luo, Y.; Nou, X.; Lester, G.E.; Wang, Q. Microgreens of Brassicaceae: Mineral Composition and Content of 30 Varieties. J. Food Compos. Anal. 2016, 49, 87–93. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, F. Phytochemistry and Biological Activity of Mustard (Brassica juncea): A Review. CyTA—J. Food 2020, 18, 704–718. [Google Scholar] [CrossRef]
- Kim, M.S.; Duizer, L.M.; Grygorczyk, A. Application of a Texture Analyzer Friction Rig to Evaluate Complex Texture Attributes in Apples. Postharvest Biol. Technol. 2022, 186, 111820. [Google Scholar] [CrossRef]
- Pistón, F.; Pèrez, A.G.; Sanz, C.; Refoyo, A. Strawberry Postharvest Shelf Life is Related to Total Acid Content and Fruit Firmness. Acta Hortic. 2021, 1309, 869–872. [Google Scholar] [CrossRef]
- Rosa, A.; Pinna, I.; Piras, A.; Porcedda, S.; Masalaa, C. Flavoring of sea salt with Mediterranean aromatic plants affects salty taste perception. J. Sci. Food Agric. 2022, 102, 6005–6013. [Google Scholar] [CrossRef]
- Volf, M.; Volfová, T.; Seifert, C.L.; Ludwig, A.; Engelmann, R.A.; Jorge, L.R.; Richter, R.; Schedl, A.; Weinhold, A.; Wirth, C.; et al. A Mosaic of Induced and Non-Induced Branches Promotes Variation in Leaf Traits, Predation and Insect Herbivore Assemblages in Canopy Trees. Ecol. Lett. 2022, 25, 729–739. [Google Scholar] [CrossRef]
- Takeuchi, M.; Zalucki, M.P. Feeding Behaviour in Australian Gregarious Lophyrotoma Sawflies (Hymenoptera: Pergidae). Aust. Entomol. 2022, 61, 494–504. [Google Scholar] [CrossRef]
- Wang, H.G.; Wouk, J.; Anderson, R.; Marquis, R.J. Strong Influence of Leaf Tie Formation and Corresponding Weak Effect of Leaf Quality on Herbivory in Eight Species of Quercus. Ecol. Entomol. 2022, 47, 69–80. [Google Scholar] [CrossRef]
- Salgado-Luarte, C.; González-Teuber, M.; Madriaza, K.; Gianoli, E. Trade-off between Plant Resistance and Tolerance to Herbivory: Mechanical Defenses Outweigh Chemical Defenses. Ecology 2022, 103, 3860. [Google Scholar] [CrossRef]
- Fang, Y.; Jiang, Z.; Li, L.; Li, J.; He, J.; Liu, S.; Wu, Y.; Cui, L.; Huang, X. Response of Tropical Seagrass Palatability Based on Nutritional Quality, Chemical Deterrents and Physical Defence to Ammonium Stress and Its Subsequent Effect on Herbivory. Mar. Environ. Res. 2022, 182, 105785. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; De Bellis, L.; Miceli, A. Nutraceutical Properties of Mulberries Grown in Southern Italy (Apulia). Antioxidants 2019, 8, 223. [Google Scholar] [CrossRef]
- Negro, C.; Sabella, E.; Nicolì, F.; Pierro, R.; Materazzi, A.; Panattoni, A.; Aprile, A.; Nutricati, E.; Vergine, M.; Miceli, A.; et al. Biochemical Changes in Leaves of Vitis vinifera cv. Sangiovese Infected by Bois Noir Phytoplasma. Pathogens 2020, 9, 269. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and Program to Determine Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. In Advances in Photosynthesis Research, Proceedings of the VIth International Congress on Photosynthesis, Brussels, Belgium, 1–6 August 1983; Sybesma, C., Ed.; Springer: Dordrecht, The Netherlands, 1984; Volume 2, pp. 9–12. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; de Pascale, S.; Rouphael, Y. Phenolic Constitution, Phytochemical and Macronutrient Content in Three Species of Microgreens as Modulated by Natural Fiber and Synthetic Substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef]
- Xiao, Z.; Rausch, S.R.; Luo, Y.; Sun, J.; Yu, L.; Wang, Q.; Chen, P.; Yu, L.; Stommel, J.R. Microgreens of Brassicaceae: Genetic Diversity of Phytochemical Concentrations and Antioxidant Capacity. LWT 2019, 101, 731–737. [Google Scholar] [CrossRef]
- De la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and Correlation of Sensory Attributes and Chemical Compositions of Emerging Fresh Produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue Light Dosage Affects Carotenoids and Tocopherols in Microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; de Pascale, S.; Rouphael, Y. Functional Quality in Novel Food Sources: Genotypic Variation in the Nutritive and Phytochemical Composition of Thirteen Microgreens Species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]



| Substrate | N |
|---|---|
| Peat | 0.78 ± 0.08 b |
| Jute protocol 2 | 0.65 ± 0.06 a |
| Substrate | mg/g FW | mg/g DW |
|---|---|---|
| Peat | 1.27 ± 0.42 a | 24.39 ± 8.17 a |
| Jute protocol 2 | 1.54 ± 0.38 a | 29.68 ± 7.23 a |
| Substrate | Chlorophyll a | Chlorophyll b | Total Carotenoids | |||
|---|---|---|---|---|---|---|
| (µg/g FW) | (µg/g DW) | (µg/g FW) | (µg/g DW) | (µg/g FW) | (µg/g DW) | |
| Peat | 332 ± 20 a | 6400 ± 370 a | 166 ± 22 a | 3192 ± 382 a | 56 ± 3 b | 1086 ± 60 b |
| Jute protocol 2 | 360 ± 23 a | 6938 ± 410 a | 177 ± 25 a | 3413 ± 445 a | 71 ± 5 a | 1369 ± 83 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min Allah, S.; Dimita, R.; Negro, C.; Luvisi, A.; Gadaleta, A.; Mininni, C.; De Bellis, L. Quality Evaluation of Mustard Microgreens Grown on Peat and Jute Substrate. Horticulturae 2023, 9, 598. https://doi.org/10.3390/horticulturae9050598
Min Allah S, Dimita R, Negro C, Luvisi A, Gadaleta A, Mininni C, De Bellis L. Quality Evaluation of Mustard Microgreens Grown on Peat and Jute Substrate. Horticulturae. 2023; 9(5):598. https://doi.org/10.3390/horticulturae9050598
Chicago/Turabian StyleMin Allah, Samar, Rosanna Dimita, Carmine Negro, Andrea Luvisi, Alessio Gadaleta, Carlo Mininni, and Luigi De Bellis. 2023. "Quality Evaluation of Mustard Microgreens Grown on Peat and Jute Substrate" Horticulturae 9, no. 5: 598. https://doi.org/10.3390/horticulturae9050598
APA StyleMin Allah, S., Dimita, R., Negro, C., Luvisi, A., Gadaleta, A., Mininni, C., & De Bellis, L. (2023). Quality Evaluation of Mustard Microgreens Grown on Peat and Jute Substrate. Horticulturae, 9(5), 598. https://doi.org/10.3390/horticulturae9050598









