An Analysis of Volatile Compounds and Study of Release Regularity in the Flower of Amorphophallus titanum in Four Periods
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Method
2.2.1. Collection of Volatiles
2.2.2. Volatile Compounds Analysis
2.2.3. Qualitative and Quantitative Analysis of Volatiles
2.2.4. Analysis of Volatile Metabolome
3. Results
3.1. Analysis on the Composition and Content of Volatile Compounds of Titan Arum Flower Volatiles in Four Periods
3.2. PCA of Volatile Compounds of the Titan Arum Flower in Four Periods
3.3. Screening and Analysis of Important Volatile Compounds in the Titan Arum Flower
4. Discussion
| Published Year | Authors and Literature | Odor Collection Method | Main Conclusions |
|---|---|---|---|
| 1997 | Kite and Hetterscheid [2] | Traps packed with Tenax GR | Dimethyl disulfide (75%), dimethyl trisulfide (10%) |
| 1998 | Kite et al. [1] | Traps packed with Tenax TA | Dimethyl disulfide (10–90%), dimethyl trisulfide (1–10%) |
| 2010 | Shirasu et al. [10] | Solid phase microextraction (SPME) | Volatile compounds absorbed to SPME: most were dimethyl disulfide, dimethyl trisulfide and methyl thiolacetate, and the main odorant causing the smell during the flower-opening phase was identified as dimethyl trisulfide |
| 2012 | Fujioka et al. [15] | Collected odors into the sample bags | Dimethyl trisulfide (DMTS) is the main odor component of the flower detected by the human nose |
| 2017 | Raman et al. [20] | Modified headspace–solid phase microextraction (HS-SPME) | Compounds such as butyric acid, g-butyrolactone, isovaleric acid, phenol, and trimethyl pyrazine in the appendix part of the inflorescence could contribute to the carrion-like odor of the bloom, and some sweet-smelling compounds such as butyl acetate, 4-hydroxy-4-methyl-2-pentanone, 2-ethyl hexanol, linalool, benzylalcohol, 2-phenoxyethanol and methyl dihydrojasmonate could also be detected |
| 2017 | Kite and Hetterscheid. [16] | Traps packed with Tenax TA | Dimethyl disulfide (70%), dimethyl trisulfide (25%), dimethyl tetrasulfide (1%) and dimethyl pentasulfide (<1%) |
| 2023 | Kang et al. [48] | Thermal desorption tubes | Dimethyl disulfide was present in the highest abundance during the female flowering phase, followed by dimethyl trisulfide, methyl thiolacetate, methanethiol and 3-methylbutanal |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kite, G.C.; Hetterscheid, W.L.A.; Lewis, M.J.; Boyce, P.C.; Ollerton, J.; Cocklin, E.; Diaz, A.; Simmonds, M.S.J. Inflorescence odours andpollinators of Arum and Amorphophallus (Araceae). In Reproductive Biology; Royal Botanic Gardens Kew: Richmond, UK, 1998; pp. 295–315. [Google Scholar]
- Kite, G.C.; Hetterschieid, W.L.A. Inflorescence odours of Amorphophalus and Pseudodracontium (Araceae). Phytochemistry 1997, 46, 71–75. [Google Scholar] [CrossRef]
- Barthlott, W.; Szarzynski, J.; Vlek, P.; Lobin, W.; Korotkova, N. A torch in the rain forest: Thermogenesis of the Titan arum (Amorphophallus titanum). Plant Biol. 2009, 11, 499–505. [Google Scholar] [CrossRef]
- Claudel, C.; Lev-Yadun, S. Odor polymorphism in deceptive Amorphophallus species—A review. Plant Signal. Behav. 2021, 16, 1991712. [Google Scholar] [CrossRef] [PubMed]
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Peñuelas, J. Floral volatile organic compounds: Between attraction and deterrence of visitors under global change. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 56–67. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; StåHl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Arimura, G.; Ozawa, R.; Kugimiya, S.; Takabayashi, J.; Bohlmann, J. Herbivore-Induced Defense Response in a Model Legume. Two-Spotted Spider Mites Induce Emission of (E)-β-Ocimene and Transcript Accumulation of (E)-β-Ocimene Synthase in Lotus japonicus. Plant Physiol. 2004, 135, 1976–1983. [Google Scholar] [CrossRef]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef]
- McCallum, E.J.; Cunningham, J.P.; Lücker, J.; Zalucki, M.P.; De Voss, J.J.; Botella, J.R. Increased plant volatile production affects oviposition, but not larval development, in the moth Helicoverpa armigera. J. Exp. Biol. 2011, 214, 3672–3677. [Google Scholar] [CrossRef]
- Shirasu, M.; Fujioka, K.; Kakishima, S.; Nagai, S.; Tomizawa, Y.; Tsukaya, H.; Murata, J.; Manome, Y.; Touhara, K. Chemical Identity of a Rotting Animal-Like Odor Emitted from the Inflorescence of the Titan Arum (Amorphophallus titanum). Biosci. Biotechnol. Biochem. 2010, 74, 2550–2554. [Google Scholar] [CrossRef]
- Jain, A.K. Data clustering: 50 years beyond K-means. Pattern Recognit. Lett. 2010, 31, 651–666. [Google Scholar] [CrossRef]
- Keyzers, R.A.; Boss, P.K. Changes in the Volatile Compound Production of Fermentations Made from Musts with Increasing Grape Content. J. Agric. Food Chem. 2010, 58, 1153–1164. [Google Scholar] [CrossRef]
- Gemert, L.J.V. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter and Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Claudel, C.; Buerki, S.; Chatrou, L.W.; Antonelli, A.; Alvarez, N.; Hetterscheid, W. Large-scale phylogenetic analysis of Amorphophallus (Araceae) derived from nuclear and plastid sequences reveals new subgeneric delineation. Bot. J. Linn. Soc. 2017, 184, 32–45. [Google Scholar] [CrossRef]
- Fujioka, K.; Shirasu, M.; Manome, Y.; Ito, N.; Kakishima, S.; Minami, T.; Tominaga, T.; Shimozono, F.; Iwamoto, T.; Ikeda, K.; et al. Objective Display and Discrimination of Floral Odors from Amorphophallus titanum, Bloomed on Different Dates and at Different Locations, Using an Electronic Nose. Sensors 2012, 12, 2152–2161. [Google Scholar] [CrossRef]
- Kite, G.C.; Hetterscheid, W.L.A. Phylogenetic trends in the evolution of inflorescence odours in Amorphophallus. Phytochemistry 2017, 142, 126–142. [Google Scholar] [CrossRef]
- Dobson, H. Floral volatiles in insect biology. Insect-Plant Interact. 1994, 5, 47–81. [Google Scholar]
- Junker, R.R.; Heidinger, I.; Blüthgen, N. Floral Scent Terpenoids Deter the Facultative Florivore Metrioptera bicolor (Ensifera, Tettigoniidae, Decticinae). J. Orthoptera Res. 2010, 19, 69–74. [Google Scholar] [CrossRef]
- Heiduk, A.; Brake, I.; Tolasch, T.; Frank, J.; Jürgens, A.; Meve, U.; Dötterl, S. Scent chemistry and pollinator attraction in the deceptive trap flowers of Ceropegia dolichophylla. S. Afr. J. Bot. 2010, 76, 762–769. [Google Scholar] [CrossRef]
- Raman, V.; Tabanca, N.; Demİrcİ, B.; Khan, I.A. Studies on the floral anatomy and scent chemistry of titan arum (Amorphophallus titanum, Araceae). Turk. J. Bot. 2017, 41, 63–74. [Google Scholar] [CrossRef]
- Borg-Karlson, A.K.; Englund, F.O.; Unelius, C.R. Dimethyl oligosulphides, major volatiles released from Sauromatum guttatum and Phallus impudicus. Phytochemistry 1994, 35, 321–323. [Google Scholar] [CrossRef]
- Hall, M.J.R. Trapping the flies that cause myiasis: Their responses to host-stimuli. Ann. Trop. Med. Parasitol. 1995, 89, 333–357. [Google Scholar] [CrossRef]
- Nilssen, A.C.; Tømmerås, B.Å.; Schmid, R.; Evensen, S.B. Dimethyl trisulphide is a strong attractant for some calliphorids and a muscid but not for the reindeer oestrids Hypoderma tarandi and Cephenemyia trompe. Entomol. Exp. Et Appl. 1996, 79, 211–218. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollsten, L. Floral scent in bat-pollinated plants: A case of convergent evolution. Bot. J. Linn. Soc. 1995, 119, 45–57. [Google Scholar] [CrossRef]
- Punekar, S.A.; Kumaran, K.P.N. Pollen morphology and pollination ecology of Amorphophallus species from North Western Ghats and Konkan region of India. Flora—Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 326–336. [Google Scholar] [CrossRef]
- Gibernau, M.; Gibernau, M. Pollinators and visitors of aroid inflorescences: An addendum. Aroideana. J. Int. Aroid Soc. 2011, 34, 70–83. [Google Scholar]
- Chai, S.K.; Wong, S.Y. Five pollination guilds of aroids (Araceae) at Mulu National Park (Sarawak, Malaysian Borneo). Webbia 2019, 74, 353–371. [Google Scholar] [CrossRef]
- Claudel, C. The many elusive pollinators in the genus Amorphophallus. Arthropod-Plant Interact. 2021, 15, 833–844. [Google Scholar] [CrossRef]
- Percival, M.S. Review of The Principles of Pollination Ecology, by K. Faegri & L. van der Pijl. J. Ecol. 1967, 55, 589. [Google Scholar]
- Johnson, S.D.; Schiestl, F.P. Floral Mimicry; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Dötterl, S.; David, A.; Boland, W.; Silberbauer-Gottsberger, I.; Gottsberger, G. Evidence for Behavioral Attractiveness of Methoxylated Aromatics in a Dynastid Scarab Beetle-Pollinated Araceae. J. Chem. Ecol. 2012, 38, 1539–1543. [Google Scholar] [CrossRef]
- Tóth, M.; Szarukán, I.; Nagy, A.; Furlan, L.; Benvegnu, I.; Magda, R.C.; Ábri, T.; Kéki, T.; Körösi, S.; Pogonyi, A.; et al. European corn borer (Ostrinia nubilalis Hbn., Lepidoptera: Crambidae): Comparing the performance of a new bisexual lure with that of synthetic sex pheromone in five countries. Pest Manag. Sci. 2017, 73, 2504–2508. [Google Scholar] [CrossRef]
- Lohonyai, Z.; Vuts, J.; Fail, J.; Tóth, M.; Imrei, Z. Field response of two cetoniin chafers (Coleoptera, scarabaeidae) to floral compounds in ternary and binary combinations. Acta Phytopathol. Et Entomol. Hung. 2018, 53, 259–269. [Google Scholar] [CrossRef]
- Martin, N.; Neelz, V.; Spinnler, H. Suprathreshold intensity and odour quality of sulphides and thioesters. Food Qual. Prefer. 2004, 15, 247–257. [Google Scholar] [CrossRef]
- Vazquez-Landaverde, P.A.; Torres, J.A.; Qian, M.C. Quantification of trace volatile sulfur compounds in milk by solid-phase microextraction and gas chromatography-pulsed flame photometric detection. J. Dairy Sci. 2006, 89, 2919–2927. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Wang, Z.Y. Volatile Compounds in Fresh and Processed Shiitake Mushrooms (Lentinus edodes Sing.). Food Sci. Technol. Res. 2000, 6, 166–170. [Google Scholar] [CrossRef]
- Lindsay, R.C.; Rippe, J.K. Enzymic Generation of Methanethiol to Assist in the Flavor Development of Cheddar Cheese and Other Foods. In Biogeneration of Aromas; American Chemical Society: Washington, DC, USA, 1986; pp. 286–308. [Google Scholar]
- Derbali, E.; Makhlouf, J.; Vezina, L.P. Biosynthesis of sulfur volatile compounds in broccoli seedlings stored under anaerobic conditions. Postharvest Biol. Technol. 1998, 13, 191–204. [Google Scholar] [CrossRef]
- Marks, H.S.; Hilson, J.A.; Leichtweis, H.C.; Stoewsand, G.S. S-Methylcysteine sulfoxide in Brassica vegetables and formation of methyl methanethiosulfinate from Brussels sprouts. J. Agric. Food Chem. 1992, 40, 2098–2101. [Google Scholar] [CrossRef]
- Nakagawa, K.; Umeda, T.; Higuchi, O.; Tsuzuki, T.; Suzuki, T.; Miyazawa, T. Evaporative Light-Scattering Analysis of Sulforaphane in Broccoli Samples: Quality of Broccoli Products Regarding Sulforaphane Contents. J. Agric. Food Chem. 2006, 54, 2479–2483. [Google Scholar] [CrossRef]
- Atsuko, I.; Ryoko, K.; Yoshikazu, H.; Toshihide, N.; Hiroshi, I.; Nami, G.Y. Screening and Identification of Precursor Compounds of Dimethyl Trisulfide (DMTS) in Japanese Sake. J. Agric. Food Chem. 2009, 57, 189–195. [Google Scholar]
- Makimoto, J.; Wakabayashi, K.; Inoue, T.; Ikeda, Y.; Kanda, R.; Isogai, A.; Fujii, T.; Nakae, T. Mutagenesis, breeding, and characterization of sake yeast strains with low production of dimethyl trisulfide precursor. J. Biosci. Bioeng. 2020, 130, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.D.M.; McKernan, G.; Bryce, J.H. A source of dimethyl disulfide and dimethyl trisulfide in grain spirit produced with a Coffey still. J. Am. Soc. Brew. Chem. 1998, 56, 99–103. [Google Scholar] [CrossRef]
- Curioni, P.M.G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Schmidt, A.; Rennenberg, H.; Wilson, L.G.; Filner, P. Formation of methanethiol from methionine by leaf tissue. Phytochemistry 1985, 24, 1181–1185. [Google Scholar] [CrossRef]
- Chin, H.; Lindsay, R.C. Mechanisms of formation of volatile sulfur compounds following the action of cysteine sulfoxide lyases. J. Agric. Food Chem. 1994, 42, 1529–1536. [Google Scholar] [CrossRef]
- Boelens, M.; De Valois, P.J.; Wobben, H.J.; Arne, V.D.G. Volatile flavor compounds from onion. J. Agric. Food Chem. 1971, 19, 984–991. [Google Scholar] [CrossRef]
- Kang, L.L.; Kaur, J.; Winkeler, K.; Kubiak, D.; Hill, J.E. How the volatile organic compounds emitted by corpse plant change through flowering. Sci. Rep. 2023, 13, 372. [Google Scholar] [CrossRef]
- Skubatz, H.; Nelson, T.A.; Dong, A.M.; Bendich, M. Infrared thermography of Arum lily inflorescences. Planta 1990, 182, 432–436. [Google Scholar] [CrossRef]
- Lamprecht, I.; Seymour, R.S. Thermologic investigations of three species of Amorphophallus. J. Therm. Anal. Calorim. 2010, 102, 127–136. [Google Scholar] [CrossRef]
- Stensmyr, M.C.; Urru, I.; Collu, I.; Celander, M.; Hansson, B.S.; Angioy, A.M. Pollination: Rotting smell of dead-horse arum florets. Nature 2002, 420, 625–626. [Google Scholar] [CrossRef]
- Angioy, A.M.; Stensmyr, M.C.; Urru, I.; Puliafito, M.; Collu, I.; Hansson, B.S. Function of the heater: The dead horse arum revisited. Proc. Biol. Sci. 2004, 271, S13–S15. [Google Scholar] [CrossRef]

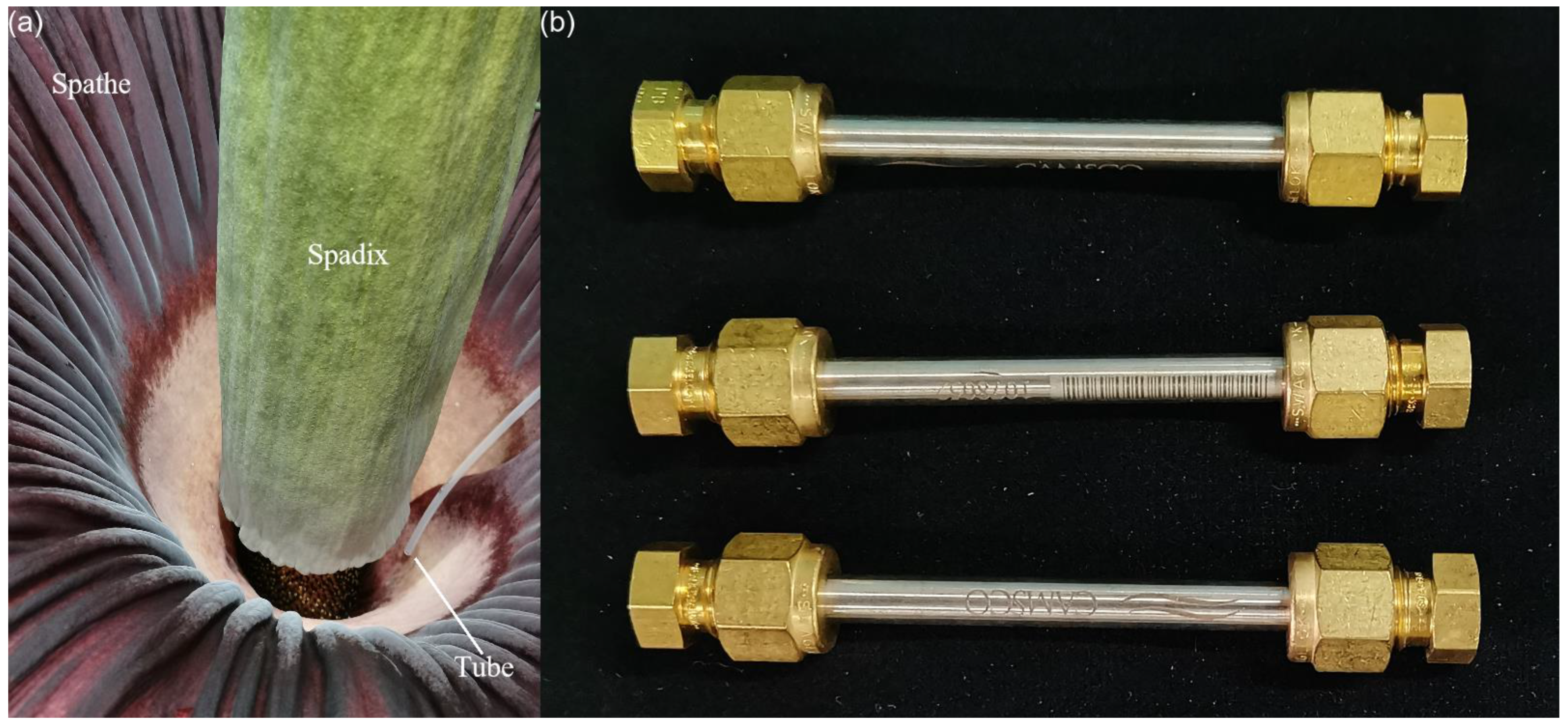

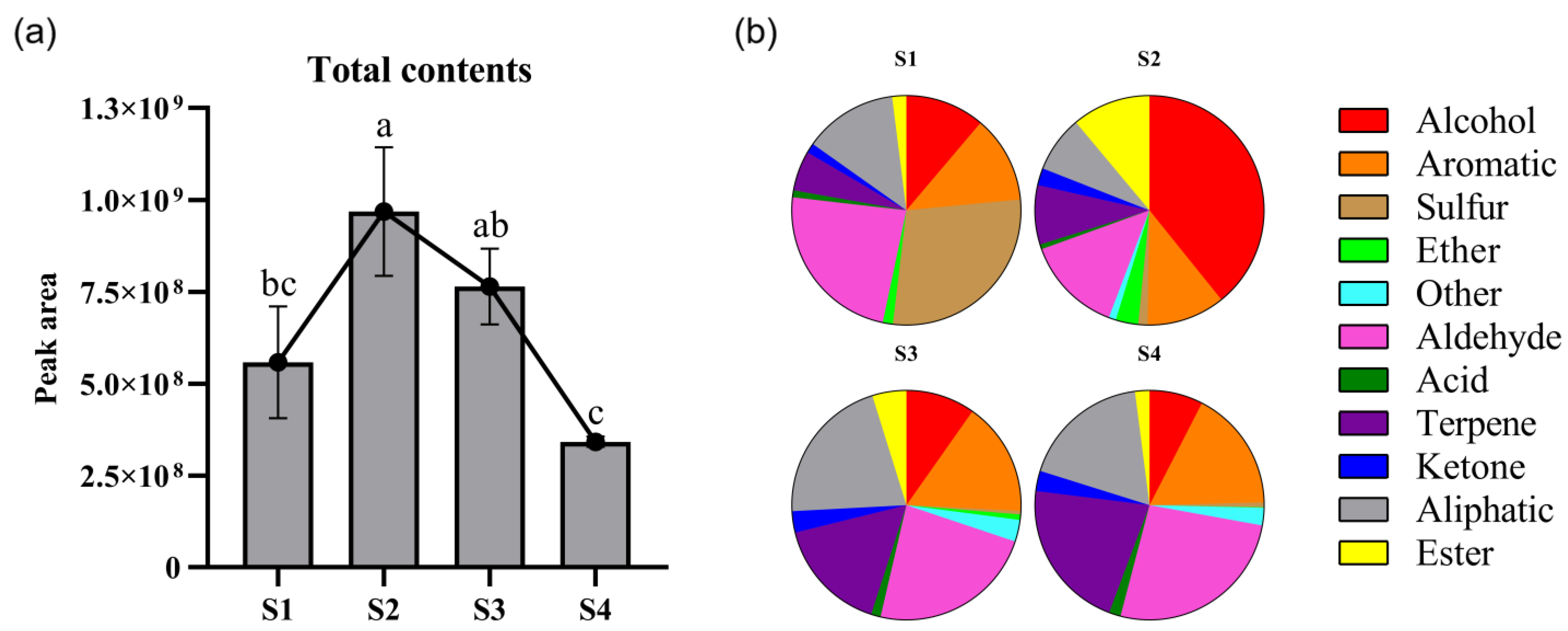
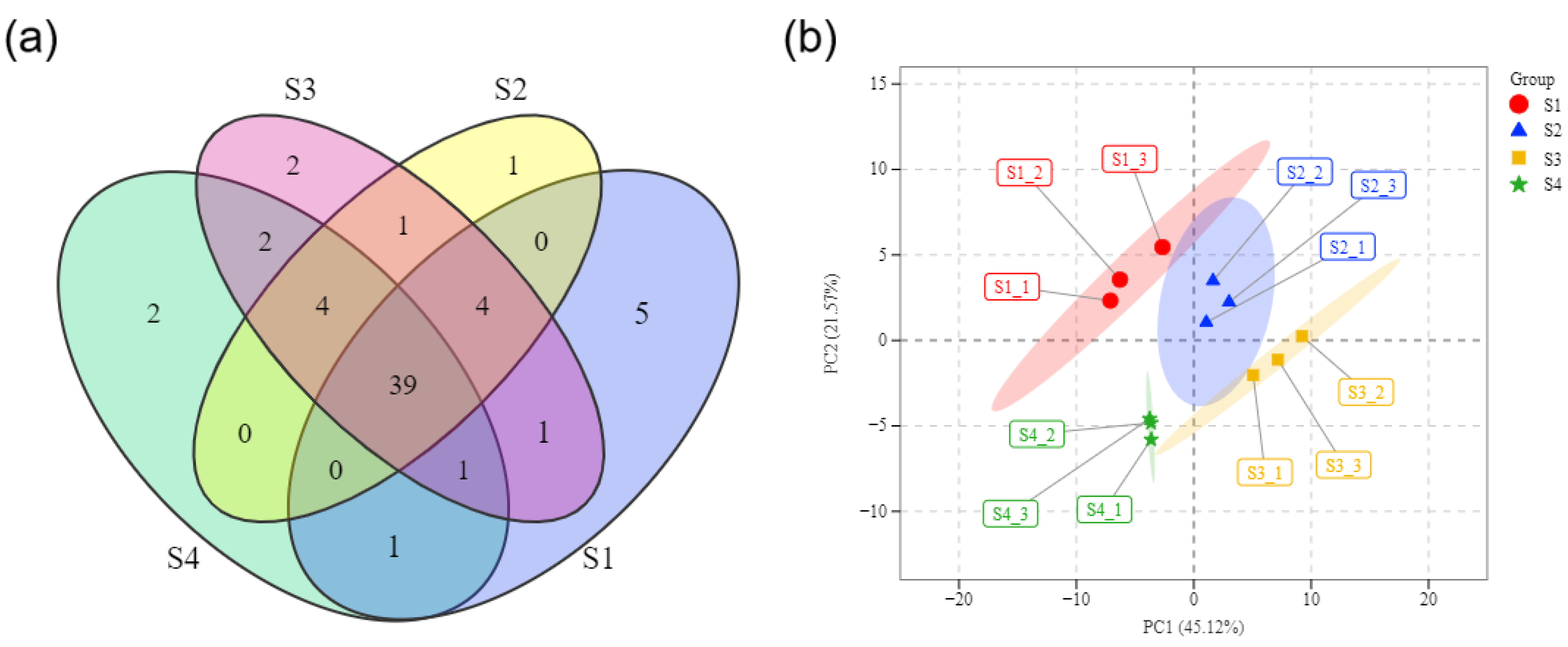
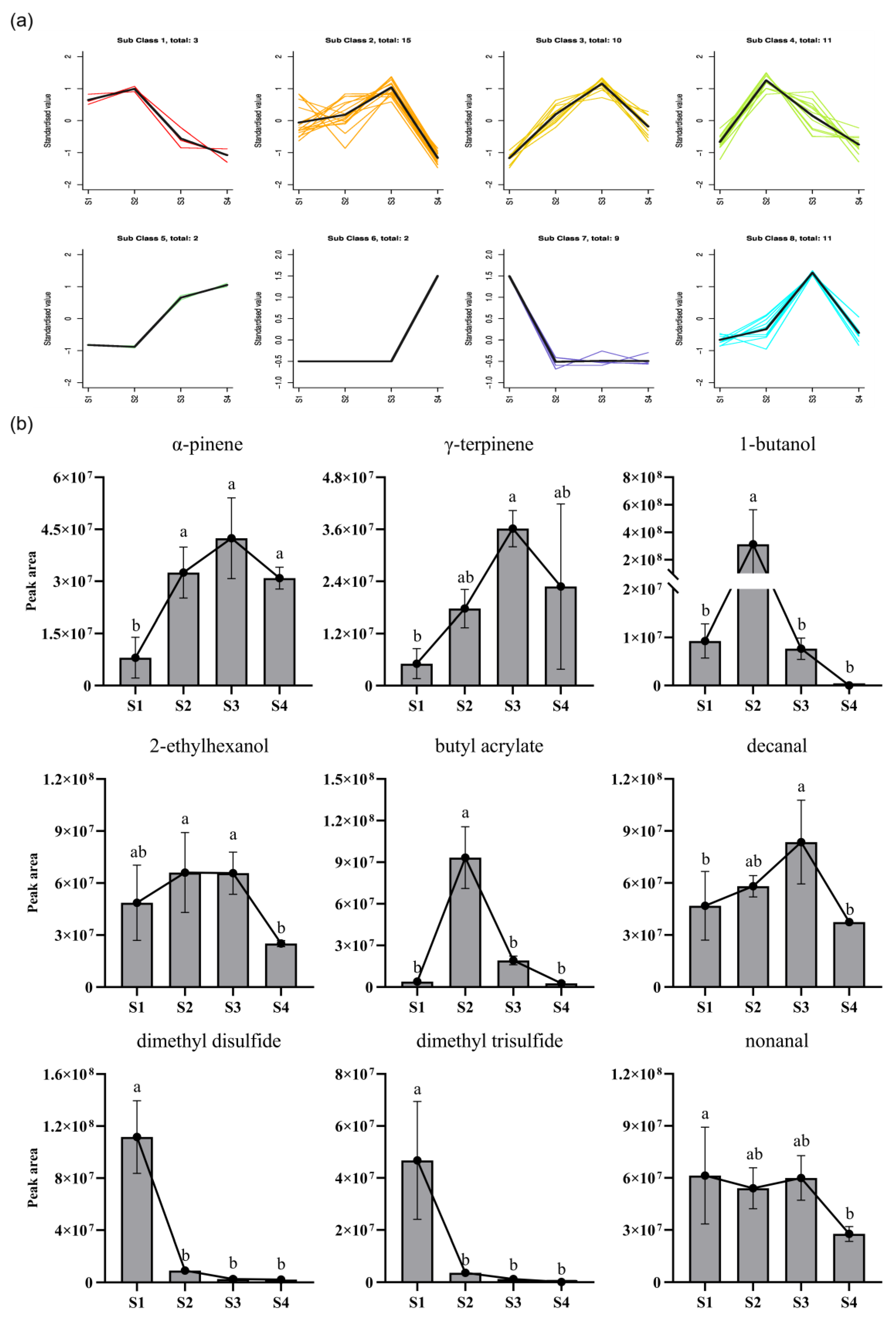

| No. | Mea/Lite RI 1 | Volatile Component | CAS | S1 | S2 | S3 | S4 | D/R Odor Threshold (mg/m3) 2 |
|---|---|---|---|---|---|---|---|---|
| Acid (2) | ||||||||
| M008 | 576/610 | acetic acid | 64-19-7 | 0.98 | 0.68 | 1.34 | 1.09 | 0.001–10/0.05–25 |
| M053 | 1272/1273 | nonanoic acid | 112-05-0 | - | - | - | 0.57 | 0.02–0.12/0.0016–0.0032 |
| Alcohol (3) | ||||||||
| M002 | 463/427 | ethanol | 64-17-5 | 0.83 | 0.04 | 0.16 | 0.21 | 0.17–1350/8.7–9230 |
| M009 | 662/659 | 1-butanol | 71-36-3 | 1.66 | 32.33 | 1.00 | - | 0.01–162/0.35–285 |
| M039 | 995/1030 | 2-ethylhexanol | 104-76-7 | 8.72 | 6.83 | 8.60 | 7.35 | 0.4–0.8/0.74 |
| Aldehyde (8) | ||||||||
| M001 | 408/404 | acetaldehyde | 75-07-0 | 0.34 | 0.46 | 0.70 | 0.88 | 0.0027–1/0.027–10 |
| M011 | 643/652 | 3-methylbutanal | 590-86-3 | 0.62 | 0.33 | 0.49 | 0.74 | 0.00035–0.0016/- |
| M015 | 806/800 | hexanal | 66-25-1 | 1.25 | 0.69 | 1.44 | 3.42 | 0.0011–0.33/0.02–0.16 |
| M025 | 905/901 | heptanal | 111-71-7 | - | - | - | 1.06 | 0.00085–0.26/0.009–0.15 |
| M036 | 1005/1003 | octanal | 124-13-0 | 1.91 | 0.66 | 1.38 | 1.05 | 0.000052–0.17/0.005–0.01 |
| M043 | 1104/1104 | nonanal | 124-19-6 | 10.99 | 5.58 | 7.86 | 8.13 | 0.002–0.23/0.0096–0.06 |
| M050 | 1204/1206 | decanal | 112-31-2 | 8.39 | 6.00 | 10.94 | 10.98 | 0.0026–0.063/0.03–0.094 |
| M057 | 1402/1409 | dodecanal | 112-54-9 | - | - | 0.70 | - | 0.033/0.014–0.033 |
| Aliphatic hydrocarbon (10) | ||||||||
| M004 | 498/464 | 1,4-pentadiene | 591-93-5 | 0.93 | - | 3.21 | 3.12 | -/- |
| M016 | 788/821 | 2,4-dimethyl-heptane | 2213-23-2 | 0.28 | - | - | - | -/- |
| M024 | 916/900 | nonane | 111-84-2 | - | - | 0.96 | - | 12/108 |
| M032 | 981/991 | 2,2,4,6,6-pentamethylheptane | 13475-82-6 | 1.00 | - | - | - | -/- |
| M035 | 1015/1000 | decane | 124-18-5 | 0.08 | 0.08 | 0.16 | - | 3.6–160/- |
| M049 | 1214/1200 | dodecane | 112-40-3 | 0.76 | 0.69 | 0.75 | 0.63 | 0.77–50/- |
| M055 | 1294/1322 | heptamethylnonane | 4390-04-9 | 5.11 | 3.07 | 6.13 | 5.53 | -/- |
| M056 | 1413/1400 | tetradecane | 629-59-4 | 2.72 | 2.13 | 5.91 | 4.98 | 5/- |
| M061 | 1512/1500 | pentadecane | 629-62-9 | 1.40 | 1.16 | 2.22 | 2.04 | -/- |
| M062 | 1612/1600 | hexadecane | 544-76-3 | 0.92 | 0.82 | 1.68 | 1.84 | 0.5/- |
| Aromatic compound (13) | ||||||||
| M013 | 794/763 | toluene | 108-88-3 | 1.46 | 2.08 | 2.02 | 3.83 | 0.12–590/3.5–260 |
| M017 | 893/855 | ethylbenzene | 100-41-4 | 0.95 | 0.62 | 0.55 | 0.76 | 0.026–78.3/- |
| M018 | 907/866 | m-xylene | 108-38-3 | 3.20 | 1.90 | 0.78 | 1.67 | 0.052–86/1.1–2.4 |
| M021 | 907/887 | o-xylene | 95-47-6 | 1.18 | 0.77 | 0.57 | 1.05 | 0.77–23.6/1–3.1 |
| M029 | 982/962 | benzaldehyde | 100-52-7 | 0.86 | 0.96 | 1.53 | 0.97 | <0.01–3400/0.33–4.1 |
| M033 | 1020/990 | 1,2,4-trimethylbenzene | 95-63-6 | 0.11 | 0.24 | 0.35 | 0.35 | 0.14–0.7/0.3–1.1 |
| M038 | 1042/1022 | o-cymene | 527-84-4 | 0.59 | 0.68 | 2.04 | 1.28 | -/0.004–0.005 |
| M041 | 1029/1065 | acetophenone | 98-86-2 | 0.70 | 0.50 | 0.95 | 0.66 | 0.01–1.5/2.9 |
| M046 | 1231/1182 | naphthalene | 91-20-3 | 0.75 | 0.99 | 1.28 | 1.18 | 0.007–0.45/0.05–5.34 |
| M051 | 1212/1225 | 2-phenoxyethanol | 122-99-6 | 0.29 | 0.22 | 0.81 | 1.02 | -/- |
| M054 | 1345/1307 | 1-methylnaphthalene | 90-12-0 | 0.37 | 0.32 | 0.47 | - | -/- |
| M059 | 1458/1436 | 1,4-dimethylnaphthalene | 571-58-4 | - | 0.32 | 0.83 | 0.59 | -/- |
| M063 | 1711/1692 | 3,4-diethylbiphenyl | 61141-66-0 | 1.82 | 1.48 | 3.85 | 3.72 | -/- |
| Ester (7) | ||||||||
| M007 | 586/612 | ethyl acetate | 141-78-6 | - | 0.22 | 0.17 | - | 0.88–623/3.6–270 |
| M014 | 761/767 | diethyl carbonate | 105-58-8 | 0.78 | - | - | 0.18 | -/- |
| M023 | 874/861 | butyl acrylate | 141-32-2 | 0.71 | 9.64 | 2.51 | 0.80 | 0.0015–0.01/0.014 |
| M027 | 884/908 | butyl propionate | 590-01-2 | 0.13 | 0.78 | 0.40 | 0.19 | 0.19/- |
| M034 | 984/995 | butyl butanoate | 109-21-7 | 0.11 | 0.20 | 0.18 | 0.15 | 0.028/- |
| M044 | 1088/1059 | dimethyl 2-methylbutanedioate | 1604-11-1 | - | - | 1.14 | 0.23 | -/- |
| M047 | 1281/1192 | methyl salicylate | 119-36-8 | 0.27 | 0.25 | 0.42 | 0.50 | 0.002–119/0.0035–22.3 |
| Ether (3) | ||||||||
| M010 | 657/661 | 1-methoxy-2-propanol | 107-98-2 | 0.43 | - | - | - | 30.908–121/- |
| M019 | 892/858 | dibutyl ether | 142-96-1 | 0.46 | 3.17 | 0.76 | 0.07 | 0.4–8/1.3 |
| M026 | 936/906 | 2-butoxyethanol | 111-76-2 | 0.54 | - | - | - | 0.21–1.9/1.7 |
| Ketone (6) | ||||||||
| M003 | 455/486 | acetone | 67-64-1 | - | 0.90 | - | - | 1–27,900/4.1–1900 |
| M005 | 545/576 | methyl vinyl ketone | 78-94-4 | 0.58 | 0.68 | 0.89 | - | (0.5) 3 |
| M006 | 555/598 | 2-butanone | 78-93-3 | - | - | 0.41 | 1.27 | 0.21–250/16–163 |
| M020 | 853/887 | 3-heptanone | 106-35-4 | 0.43 | - | - | - | -/- |
| M022 | 891/894 | cyclohexanone | 108-94-1 | 0.36 | 0.39 | 0.45 | 0.46 | 0.48–880/0.48 |
| M060 | 1420/1453 | geranyl acetone | 3796-70-1 | - | 0.44 | 1.25 | 1.12 | -/- |
| Other (1) | ||||||||
| M052 | 1208/1229 | benzothiazole | 95-16-9 | - | 1.02 | 3.07 | 2.46 | -/- |
| Sulfur compound (2) | ||||||||
| M012 | 722/746 | dimethyl disulfide | 624-92-0 | 20.00 | 0.94 | 0.34 | 0.63 | 0.0011–0.078/0.011–0.029 |
| M030 | 972/970 | dimethyl trisulfide | 3658-80-8 | 8.38 | 0.38 | 0.16 | 0.04 | -/0.0073 |
| Terpene (8) | ||||||||
| M028 | 948/937 | α-pinene | 80-56-8 | 1.44 | 3.36 | 5.55 | 9.06 | 0.1–105/25–29 |
| M031 | 993/1028 | p-mentha-1(7),3-diene | 99-84-3 | 0.23 | 0.69 | 0.96 | 1.79 | -/- |
| M037 | 1031/1060 | γ-terpinene | 99-85-4 | 0.91 | 1.83 | 4.73 | 6.69 | 2.5–55/1.4–1.6 |
| M040 | 1059/1032 | eucalyptol | 470-82-6 | 0.66 | 0.80 | 1.01 | 0.53 | 0.003–2/0.00062–0.05 |
| M042 | 1064/1088 | terpinolene | 586-62-9 | 0.46 | - | 0.35 | - | -/- |
| M045 | 1164/1169 | menthol | 1490-04-6 | 1.47 | 0.93 | 1.84 | 1.68 | -/- |
| M048 | 1143/1189 | α-terpineol | 98-55-5 | - | 0.30 | 0.79 | 0.72 | 0.01–0.86/0.0125–110 |
| M058 | 1398/1405 | longifolene | 475-20-7 | 0.46 | 0.44 | 0.92 | 0.76 | -/- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zhang, P.; Liu, D.; Feng, Y.; Chi, M.; Guo, Z.; Wang, X.; Zhong, J.; Sun, M. An Analysis of Volatile Compounds and Study of Release Regularity in the Flower of Amorphophallus titanum in Four Periods. Horticulturae 2023, 9, 487. https://doi.org/10.3390/horticulturae9040487
Liu D, Zhang P, Liu D, Feng Y, Chi M, Guo Z, Wang X, Zhong J, Sun M. An Analysis of Volatile Compounds and Study of Release Regularity in the Flower of Amorphophallus titanum in Four Periods. Horticulturae. 2023; 9(4):487. https://doi.org/10.3390/horticulturae9040487
Chicago/Turabian StyleLiu, Dongyan, Peng Zhang, Donghuan Liu, Yuan Feng, Miao Chi, Ziyu Guo, Xi Wang, Jian Zhong, and Ming Sun. 2023. "An Analysis of Volatile Compounds and Study of Release Regularity in the Flower of Amorphophallus titanum in Four Periods" Horticulturae 9, no. 4: 487. https://doi.org/10.3390/horticulturae9040487
APA StyleLiu, D., Zhang, P., Liu, D., Feng, Y., Chi, M., Guo, Z., Wang, X., Zhong, J., & Sun, M. (2023). An Analysis of Volatile Compounds and Study of Release Regularity in the Flower of Amorphophallus titanum in Four Periods. Horticulturae, 9(4), 487. https://doi.org/10.3390/horticulturae9040487






