Abstract
Saffron (Crocus sativus) is the most expensive spice in the world and a valuable medicinal plant. In this study, the flowering, vegetative growth performance, and daughter corm formation of potted saffron were investigated in six growing media: loamy soil, silty soil, sandy soil, peatmoss, peatmoss + sandy soil, and peatmoss + foam. The highest values of the stigma fresh weight, the root weight, and the number and diameter of daughter corms were observed in plants grown in soil with a light texture, i.e., sandy soil or peatmoss + foam, although smaller daughter corms were produced by sandy soil-grown plants. Compared with loamy soil (heavy soil), the peatmoss + foam growing medium increased the leaf pigment levels and the number of leaves and daughter corms, and it produced the highest number of daughter corms with diameters of ≥2 cm. Compared with plants in other media, saffron plants grown in loamy soil had higher catalase and peroxidase activities but lower polyphenol oxidase activity. Overall, saffron could be cultivated and produced in growing media with various pH values (4.00–9.25), although a mixture of peatmoss and foam was the optimal growing medium for potted saffron production. These findings are beneficial for selecting the optimal growing media/soil for saffron cultivation in farmlands.
1. Introduction
Saffron (Crocus sativus L.) is a highly priced spice infertile perennial plant belonging to the family Iridaceae []. The dormancy of saffron can extend from May to late summer (i.e., June, July, and August), and the plant flowers in autumn []. Saffron is classified as a monocotyledonous herbaceous triploid plant and is cultivated for its stigma (called threads), with 90–93% of global production occurring in Iran [,,,]. The stigmas are collected and dried, after which they are used mainly as coloring and seasoning agents in foods but also in many industries (e.g., in dyes, textiles, drugs, culinary adjuncts, and flavorings) and for their pharmacological properties, including anticancer, antioxidant, antitumor, and antimutagenic activities [,]. The production of 0.5 g of dry stigma reportedly requires 75–100 saffron flowers, which provide about 225–300 stigma threads. Thus, saffron is considered the most expensive spice in the world, with 1 kg costing EUR 1500–2200 [,]. Saffron is cultivated in only a few places worldwide, which are ordered in terms of their saffron cultivation area as follows (in hectares): Iran (108,000), Afghanistan (7557), India (3674), Greece (1000), Morocco (850), Spain (150), Italy (70), and France (37) []. The bioactive ingredients in saffron spice that provide its phytochemical, pharmaceutical, or therapeutic attributes include crocins, crocetin, picrocrocin, and safranal, which also provide the color, bitterness, and aromatic properties of the spice [,,,,]. Saffron has also been reported to ameliorate complications related to diabetes mellitus [,].
Growing substrates affect plant growth and development through their physical and chemical properties [,]. Such substrates must have low physical strength and high porosity [], the latter of which is essential for ensuring that air, water, and nutrients reach the roots. However, a growing substrate’s most important quality is how it adsorbs, retains, and releases water []. A high water retention capacity leads to insufficient aeration of the roots, which can be suffocated []. Chemical properties, including pH, cation exchange capacity, electrical conductivity (EC), and nutrient availability, also affect plant growth []. A growing substrate with a high cation exchange capacity is more favorable, as essential nutrients can be contained in the substrate (adsorbed to the particles) []. In a substrate mixture, peatmoss provides good aeration and a moderate-to-high water retention capacity []. Therefore, because of its chemical and physical properties [,], peatmoss is the substrate component used most in greenhouse mixes to balance water and aeration in the substrate.
The saffron cultivation is limited in farmland located at high altitudes under cold climate conditions [,,]. The high demand and low supply have made saffron the most expensive spice in the world [,]. Introducing saffron cultivation to different regions with a moderate climate could possibly increase the saffron supply. Successful saffron cultivation and growth depend on many factors, including climatic factors (mainly temperature and humidity), cultural practices (e.g., the control of weeds, pests, and diseases; irrigation; and sowing date), and soil properties (e.g., pH, salinity, texture, and soil organic matter) [,,,]. These factors affect the quantity and quality of the saffron yield as well as its growth. The optimum soil pH is considered 6.8–7.8, the EC should be <2 dS m−1 [,,], and the ideal seasonal rainfall is 600 mm []. The preferred soil for saffron cultivation is well-irrigated, loose, friable, low-density, and well-drained clay soil []. Additionally, the soil properties could influence the vegetative growth and saffron yield, thus impacting the economic production of saffron. Therefore, the present study aimed to investigate the flowering yield, vegetative growth performance, and daughter corm yield responses of saffron to varied growing media, i.e., peatmoss, silty, sandy, and loamy soils.
2. Materials and Methods
2.1. Plant Materials
Saffron corms (10−11 cm in circumference; 3.2–3.5 cm in diameter) were obtained from Bloembollenbedrijf J. C. Koot (Vennewatersweg, The Netherlands). All corms were peeled, and uninjured and uninfected corms were selected. The corms were sterilized via dipping in a 0.5 g L−1 fungicide solution (Aromil-Plus 50 WP; Mobedco-Vet, Amman, Jordan) containing 15% (w/w) metalaxyl and 35% (w/w) copper oxychloride to prevent fungal infestation. The corms were dried prior to planting.
2.2. Growing Media Treatments and Analyses
The saffron corms were planted in plastic pots (12 cm in diameter; 1 corm per pot) filled with six different growing media—M1 (loamy soil), M2 (silty soil), M3 (sandy soil), M4 (peatmoss), M5 (peatmoss + sandy soil), and M6 (peatmoss + foam)—and placed in the open field conditions. The growing substrates are described in more detail in Table 1.

Table 1.
Information about the growing media used to grow saffron plants.
The analyses of the growing media were performed before starting the experiment and at the end of the experiment or at the harvest (Table 2 and Table 3). The pH values of all media were measured in a 1:1 suspension using a calibrated pH meter 3510 (Jenway, Staffordshire, UK), whereas the EC was measured in a 1:5 ratio (soil:deionized water) using an EC Meter (MI 170, Italy). Available nitrogen (NH4+) was determined using the micro Kjeldahl method [], and available phosphorus was determined according to the method of Olsen and Sommers []. Calcium and magnesium were measured according to the method described by Jackson []. The concentrations of copper (Cu+2), iron (Fe+2), zinc (Zn+), and manganese (Mn+) were quantified using an atomic absorption spectrophotometer []. Sodium (Na+) and potassium (K+) were extracted according to the methods described by Page et al. [], and their concentrations were determined using a flame photometer PFP7 (Jenway, Staffordshire, UK).

Table 2.
pH and salinity (EC) values (before cultivation and at the end of the experiment) of the growing media used to grow saffron plants.

Table 3.
Content of macro- and micronutrients in the growing media used to grow saffron plants.
2.3. Cultural Practices
Saffron corms were transplanted on 20 November 2020, and the plants were grown for 18 weeks until daughter corms were harvested. All corms were cultured at a depth of 10 cm. The pots were irrigated (depending on the climate) every 10 days from November to March, every 6 days during April, and every 3 days during May, with 100 mL per pot every time using cans. Fertilizers (0.25 g L−1 of complete fertilizer (NPK: 19:19:19), 0.2 g L−1 CaNO3 (N = 15.5% and CaO = 26.3%) (Yara company, Cairo, Egypt), 0.15 g L−1 MgSO4 (Mg = 16%, S = 12.5), 0.1 g L−1 chelated microelements (Fe = 4%, Zn = 4%, Mn = 3%, Cu = 0.1%, B = 1.5, and Mo = 0.5%) (Queisna Agriculture Development, Menofia, Egypt)) were dissolved in an irrigation tank and added directly to the plots during irrigation once in December, January, and February, respectively, and twice from March to May. Changes in air temperature, humidity, and rainfall were recorded (Figure 1; https://www.worldweatheronline.com/kafr-el-zayat-weather-history/al-gharbiyah/eg.aspx; accessed on 9 June 2021). Weeding was performed by hand during the growing period. Crust breaking operations were applied using a trowel after both full flower harvests. No disease or insect infestation was detected; thus, no control chemicals were applied.
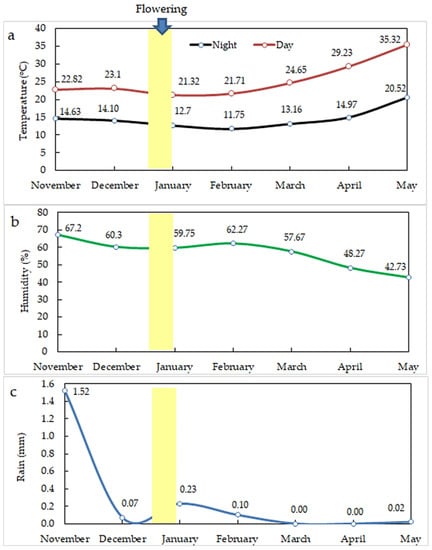
Figure 1.
Changes in average day and night temperature (a), humidity (b), and rainfall (c) from November 2020 through May 2021.
2.4. Flowering, Vegetative Growth, and Daughter Corm Formation Parameters
The root length, the number of roots, and the root fresh weight were determined 1 week after corm planting in different growing media (Figure 2b). Flowering parameters (i.e., the number of flowers per plant and stigma fresh and dry weight) were recorded 4 weeks after corm transplanting, and the flowering period lasted 8 days (Figure 2c). The stigma dry weight was determined after drying the stigmas for 1 h at 40 °C [,]. Vegetative growth parameters (i.e., leaf length, the number of leaves, plant fresh and dry weight, and root fresh and dry weight) were measured after 9 weeks of corm sprouting (Figure 2d,e). The dry weights of plants and roots were determined after oven drying for 48 h at 65 °C. After leaf senescence, the plants were lifted, the mother corm parts were removed, and the replacement corms were separated. Corm formation parameters (i.e., the number of corms per plant, corm diameter, and corm fresh weight) were recorded 18 weeks after corm planting.

Figure 2.
General overview of the different stages of saffron growth in plants grown in different growing media. Photographs represent (a) the corms used in the experiment, (b) root initiation after 7 days, (c) flowering after 30 days, and (d,e) vegetative growth after 63 days of culture in loamy soil (M1) and peatmoss + foam (M6), respectively.
2.5. Measurement of Leaf Pigments
The contents of chlorophyll a (Chl a), chlorophyll b (Chl b), and total carotenoids in fully expanded young leaves were determined using spectrophotometric analyses. Chlorophyll was extracted from leaf tissue by adding 0.5 g of fresh leaves to 5 mL of N,N-dimethyl formamide for 48 h in the dark at 4 °C. The absorbance was measured at 470, 647, and 663 nm for Chl a, Chl b, and carotenoids, respectively, using a spectrophotometer (Double Beam UV/Visible Spectrophotometer Libra S80PC, UK). Chlorophyll and carotenoid concentrations were calculated from the spectrophotometric data using the formulae of Moran and Porath [].
2.6. Measurement of Antioxidant Enzymes
Based on the evaluation of rooting and the vegetative growth of saffron plants during the experiment, only four growing media, namely, M1 (loamy soil), M3 (sandy soil), M4 (peatmoss), and M6 (peatmoss + foam), were used to investigate saffron plants growing under stress conditions. To measure the activity of antioxidant enzymes, 0.5 g of saffron leaves were homogenized in liquid nitrogen with 1.5 mL of the respective extraction buffer using a prechilled mortar and pestle. The homogenate was filtered through four layers of cheesecloth and centrifuged at 22,000× g and 4 °C for 20 min. The supernatant was recentrifuged at 22,000× g and 4 °C for 20 min. Catalase (CAT; EC 1.11.1.6) activity was measured by determining the consumption of H2O2 at 240 nm []. Peroxidase (POD; EC 1.11.1.7) activity was determined according to the procedure proposed by Hammerschmidt et al. []. Polyphenol oxidase (PPO; EC 1.10.3.1) activity was determined according to the method described by Malik and Singh [].
2.7. Statistical Analyses
The experiment was set up in a completely randomized design. Each treatment had four replicates, and each replicate was represented by five pots, with each pot containing one plant, rendering 20 plants per treatment. The means were compared using Tukey’s range test via SAS (version 6.12; SAS Institute, Cary, NC, USA).
3. Results
3.1. Root Initiation and Saffron Corm Growth in Different Growing Media
The type of growing media significantly influenced the number, length, and fresh and dry weight of roots after 1 and 9 weeks of planting (Figure 3). The M3 (sandy soil), M4 (peatmoss), and M5 (peatmoss + sandy soil) media provided favorable root initiation after 1 week (Figure 3). After 9 weeks, the M3 medium contained plants with the highest number of roots (≥140), followed by the M4 medium, whereas the M1 medium (loamy soil) contained plants with the fewest roots. The longest roots (21 cm) were recorded in the M4 medium, followed by the M5 medium, whereas the shortest roots (4 cm) were recorded in the M1 medium. Interestingly, after 9 weeks, the fresh weight of roots per corm increased gradually from the M1 medium to the M6 medium, with the highest value recorded in the M6 medium, followed by the M5 medium (peatmoss + foam; peatmoss + sand). Similarly, the root dry weight recorded the highest values in those two peatmoss mixtures (Figure 3c). Nine weeks after planting, a well-developed root system was observed in all growing media, except for M1, which had a low level of rooting (Figure 4).
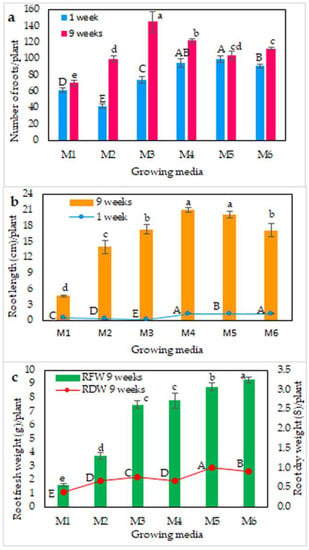
Figure 3.
Growth of saffron roots in different growing media. (a) Number of roots after 1 and 9 weeks of planting, (b) root length after 1 and 9 weeks of planting, and (c) root fresh and dry weights after 9 weeks of planting. M1: loamy soil; M2: silty soil; M3: sandy soil; M4: peatmoss; M5: peatmoss + sand; M6: peatmoss + foam. Different letters within a set of values denote significant differences at p ≤ 0.05, according to Tukey’s test.

Figure 4.
Growth of saffron roots after 1 and 9 weeks of planting in different growing media. Photographs represent rooting after (a) 1 and (b) 9 weeks, respectively. M1: loamy soil; M2: silty soil; M3: sandy soil; M4: peatmoss; M5: peatmoss + sand; M6: peatmoss + foam.
3.2. Flowering, Vegetative Growth, and Leaf Pigments of Saffron Grown in Different Growing Media
The type of growing media significantly influenced the stigma fresh and dry weight but did not influence the number of flowers per plant. In M4 (peatmoss), M5 (peatmoss + sandy soil), and M6 (peatmoss + foam), saffron plants had the highest stigma fresh and dry weight, whereas loamy soil contained plants with the lowest stigma fresh and dry weight (Table 4).

Table 4.
Effects of growing media on the flowering of saffron 4 weeks after planting.
Vegetative growth in terms of the leaf length, the shoot fresh and dry weight, and the number of leaves per plant was significantly influenced by the growing media (Table 5). The highest vegetative growth parameter values were recorded in plants grown in peatmoss alone, with sandy soil, or with foam. The lowest vegetative growth parameter values were observed in plants grown in loamy and silty soils.

Table 5.
Effect of growing media on the vegetative growth of saffron 9 weeks after planting.
The M6 medium with peatmoss and foam produced the highest values of Chl a (3.5 mg g−1 fresh weight), Chl b (0.8 mg g−1 fresh weight), and carotenoids (0.33 mg g−1 fresh weight) in saffron plants, whereas loamy soil produced the lowest leaf pigment values in saffron (Figure 5).
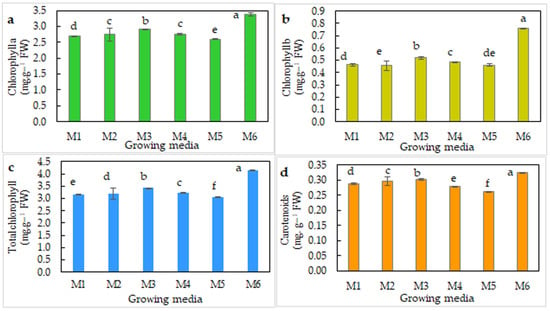
Figure 5.
Content of saffron leaf pigments (a) chlorophyll a, (b) chlorophyll b, (c) total chlorophyll, and (d) carotenoids 9 weeks after planting in different growing media. M1: loamy soil; M2: silty soil; M3: sandy soil; M4: peatmoss; M5: peatmoss + sand; M6: peatmoss + foam. Different letters within a set of values denote significant differences at p ≤ 0.05, according to Tukey’s test.
3.3. Antioxidant Enzyme Activity in Saffron Leaves from Plants Grown in Different Growing Media
The type of growing media significantly influenced the antioxidant enzyme activity of saffron plants (Figure 6). The highest CAT activity was detected in plants grown in loamy soil, whereas the lowest CAT activity was detected in plants grown in sandy soil (Figure 6a). Saffron plants grown in peatmoss + foam exhibited lower POD activity but higher PPO activity than saffron grown in any other growing media (Figure 6b,c).
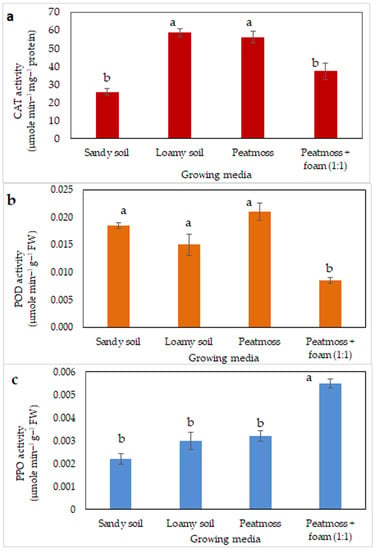
Figure 6.
Antioxidant enzyme activity levels in saffron leaves from saffron plants grown in different growing substrates 9 weeks after planting. Photographs (a–c) represent the values of catalase (CAT), peroxidase (POD), and polyphenol oxidase (PPO), respectively. Different letters within a set of values denote significant differences at p ≤ 0.05, according to Tukey’s test.
3.4. Daughter Corm Production of Saffron Grown in Different Growing Media
The diameter and fresh weight of daughter corms, the total number of corms per plant, and the number of cormlets with diameters of ≥2.0 cm were significantly influenced by different growing media (Table 6). The highest number of daughter corms was recorded in saffron plants grown in sandy soil, but these corms were small (Figure 7a). The M6 growing medium with peatmoss and foam contained saffron plants with the highest corm diameter, fresh weight, and number of corms with diameters of ≥2.0 cm, i.e., those that can flower in the next season (Figure 7b). The lowest level of daughter corm production was recorded in saffron grown in loamy soil.

Table 6.
Effects of growing media on the corm production of saffron 18 weeks after planting.
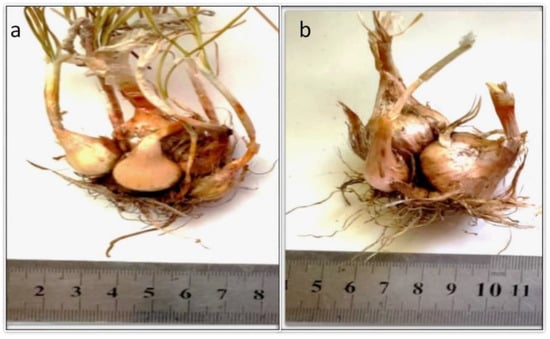
Figure 7.
Saffron daughter corm formation after 18 weeks of culture in (a) sandy soil (M3) and (b) peatmoss + foam (M6).
4. Discussion
The soils used as growing media in this study differed in terms of their pH and salinity (EC) values. Sandy soil had the highest pH value (9.25), whereas the peatmoss + foam medium had the lowest pH (4.00). The pH of sandy soil media was reduced from 9.25 to 7.10, whereas the opposite trend was observed for the peatmoss + foam medium, in which the pH was increased from 4.00 to 5.70 (Table 2). The cultivation of saffron plants led to a decrease in the salinity of most growing substrates after harvesting (e.g., sandy soil from 0.143 to 0.11 dS m−1), whereas the peatmoss media showed an increase in EC values after harvesting (e.g., peatmoss + sand medium from 0.242 to 0.560 dS m−1). The highest growth parameter values were recorded in plants grown in peatmoss alone and its mixtures with sand or foam, especially peatmoss + foam, followed by peatmoss + sandy soil. Conversely, plants grown in heavy loamy soil showed stunted growth. These results indicate that the growth and production of saffron depend mainly on soil texture and soil pH, with the best growth and production observed in soil with a lighter texture and a near-neutral soil pH (i.e., around pH 7). These results are consistent with the previous findings [,]. Shajari et al. [] found that, compared with a heavy soil texture, a light soil texture increased the saffron flower yield by 15%. Saffron can tolerate a wide range of pedologic and forcing conditions []. The growing substrate/soil pH levels and salinity were reduced as a result of saffron cultivation, indicating that the saffron crop could probably act as a soil conditioner (Table 2).
The current study supports the notion that soil texture is the soil character that controls the growth and production of key saffron attributes (e.g., stigmas and cormlets). Soil with a light texture is favorable for saffron production because it provides sufficient soil aeration that enables strong root growth. When the soil has a heavy texture, e.g., loamy soil, root growth is limited (Figure 4; Table 3 and Table 4), as demonstrated by the low-quality parameter values (i.e., intensive rooting, root length, production of desirable cormlets, diameter of cormlets, and dry weight of stigma) of saffron grown in loamy soil. Indeed, compared with plants grown in the peatmoss + foam medium, the desirable production of cormlets and their diameter decreased by 56.6% and 21.5%, respectively, in plants grown in loamy soil. Other soil characters that influence the success of saffron production include the soil pH, a low bulk density, the soil salinity, a well-developed friable structure, the organic matter content, the well-drained soil, and the soil fertility [].
During the cultivation of saffron, it is not usually necessary to add high levels of fertilizer and water []; thus, it is possible to cultivate saffron under arid/semi-arid conditions and in new reclaimed soils. Under the conditions of low soil organic matter (0.4%), low rainfall (206 mm), neutral soil pH (7.34), low soil salinity (1.18 dS m−1), and light soil texture (55% sand), integrated nutrient management (including chemical, organic, and biological fertilizers) can improve some soil properties (e.g., soil cation exchange capacity, soil bulk density, and nutrient content) and saffron biomass production []. In addition, saffron production can be managed under conditions in which the soil (5.64 dS m−1) and irrigation water (2.9–5.8 dS m−1) are saline by selecting the proper planting date [].
The present results suggest that a peatmoss and foam medium is suitable for saffron production because many saffron yield parameters were high in this soil substrate, with the increases relative to loamy soil recorded as follows: number of leaves per plant (21.4%), diameter of cormlets (21.5%), desirable size cormlets (56.7%), fresh stigmata weight per plant (19.6%), and photosynthetic pigment content (37.5%). Additionally, the activities of CAT and POD were higher in saffron grown in loamy soil, indicating that these plants suffered from oxidative stress in this soil substrate. Thus, mixing peatmoss with a lighter soil, such as a sandy soil or foam, gives a superior reference growing medium for the production of saffron. Given that new reclaimed or desert soils have a sandy texture, the present results suggest that saffron could be cultivated in such soils, even if the soil acidity is high (up to pH 9.25). Moreover, this study indicates that loamy soil, which has a heavy texture, a high bulk density, a badly developed friable structure, and potentially poor drainage and may be polluted [], is not suitable for saffron production. Overall, forming a mixture of light soil and peatmoss is considered acceptable for producing saffron, although an economic evaluation of this agroprocess is still required.
5. Conclusions
The type of growing media used to grow saffron influenced flowering, vegetative growth, and daughter corm formation according to the physical and chemical properties of the various soil substrates. Heavy soil, such as loamy soil, negatively affected saffron growth, whereas a mixture of peatmoss and foam or sand was the optimal growing media for potted saffron production. The high activities of CAT and POD observed in saffron grown in loamy soil indicate that these plants suffered from oxidative stress in this soil substrate. Overall, saffron could be cultivated and produced in growing media with various pH values (4.00–9.25), but a mixture of peatmoss and foam was the growing medium in which potted saffron was optimal.
Author Contributions
Conceptualization, M.E.E.-M. and Y.H.D.; methodology, M.E.E.-M. and Y.H.D.; formal analysis, M.E.E.-M. and Y.H.D.; investigation and data curation, M.E.E.-M., Y.H.D. and M.K.S.; validation, H.E.-R. and M.K.S.; visualization, H.E.-R. and M.K.S.; writing—original draft preparation, M.E.E.-M., Y.H.D. and H.E.-R.; writing—review and editing, Y.H.D., M.E.E.-M., H.E.-R. and M.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Researchers Supporting Project number (RSP2023R375), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are presented within the article.
Acknowledgments
The authors acknowledge the Researchers Supporting Project number (RSP-2023R375), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nemati, Z.; Harpke, D.; Gemicioglu, A.; Kerndorff, H.; Blattner, F.R. Saffron (Crocus sativus) is an autotriploid that evolved in Attica (Greece) from wild Crocus cartwrightianus. Mol. Phylogenet. Evol. 2019, 136, 14–20. [Google Scholar] [CrossRef]
- Banhangi, F.M.; Moghaddam, P.R.; Asadi, G.A.; Khorramde, S. Do corm seeding rate and planting depth influence growth indicators of saffron (Crocus sativus L.)? Ind. Crops Prod. 2021, 174, 114145. [Google Scholar] [CrossRef]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the king of spices: An overview. Sci. Hortic. 2020, 272, 109560. [Google Scholar] [CrossRef]
- Kothari, D.; Thakur, M.; Joshi, R.; Kumar, A.; Kumar, R. Agro-Climatic Suitability Evaluation for Saffron Production in Areas of Western Himalaya. Front. Plant Sci. 2021, 12, 657819. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Thakur, R.; Kumar, R. Saffron (Crocus sativus L.): Gold of the spices—A comprehensive review. Hortic. Environ. Biotechnol. 2021, 62, 661–677. [Google Scholar] [CrossRef]
- Moradi Rikabad, M.; Pourakbar, L.; Siavash Moghaddam, S.; PopovicDjordjevic, J. Agrobiological, chemical and antioxidant properties of saffron (Crocus sativus L.) exposed to TiO2 nanoparticles and ultraviolet-B stress. Ind. Crops Prod. 2019, 137, 137–143. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Desenko, V.; Ivanauskas, L.; Georgiyants, V. Standard operating procedure of Ukrainian saffron cultivation according with good agricultural and collection practices to assure quality and traceability. Ind. Crops Prod. 2020, 151, 112376. [Google Scholar] [CrossRef]
- Giorgi, A.; Pentimalli, D.; Giupponi, L.; Panseri, S. Quality traits of saffron (Crocus sativus L.) produced in the Italian Alps. Open Agric. 2017, 2, 52–57. [Google Scholar] [CrossRef]
- Musazadeh, V.; Zarezadeha, M.; Faghfouri, A.H.; Keramati, M.; Ghoreishi, Z.; Farnam, A. Saffron, as an adjunct therapy, contributes to relieve depression symptoms: An umbrella meta-analysis. Pharmacol. Res. 2022, 175, 105963. [Google Scholar] [CrossRef] [PubMed]
- Azgomi, R.N.D.; Karimia, A.; Zarshenasa, M.M.; Jazani, A.M. The mechanisms of saffron (Crocus sativus’) on the inflammatory pathways of diabetes mellitus: A systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102365. [Google Scholar] [CrossRef]
- Jazani, M.A.; Karimi, A.; Nasimi, R.; Azgomi, D. The potential role of saffron (Crocus Sativus L.) and its components in oxidative stress in diabetes mellitus: A systematic review. Clin. Nutr. ESPEN 2022, 48, 148–157. [Google Scholar] [CrossRef]
- Saleh, H.A.R.; YI El-Nashar, M.F. Serag-El-Din, and Y.H. Dewir. Plant growth, yield and bioactive compounds of two culinary herbs as affected by substrate type. Sci. Hortic. 2019, 243, 464–471. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Alsadon, A.; Ibrahim, A.; El-Mahrouk, M. Effects of growing substrate, mode of nutrient supply, and saffron corm size on flowering, growth, photosynthetic competence, and cormlet formation in hydroponics. HortTechnology 2022, 32, 234–240. [Google Scholar] [CrossRef]
- Hajiaghaei Kamrani, M. The effects of physicochemical properties of hydroponic substrates on the uptake of macronutrients in potato shoot, root and minitubers. J. Plant Nutr. 2021, 44, 2476–2485. [Google Scholar] [CrossRef]
- Verdonck, O.; Demeyer, P. The influence of the particle sizes on physical properties of growing media. Acta Hortic. 2004, 644, 99–101. [Google Scholar] [CrossRef]
- Rahman, M.; Quamruzzaman, M.; Begum, P.; Sani MN, H.; Chawdhery, M.R.; Ahmed, S.; Ali, M. Physical and chemical properties of different substrate mixtures and their effects on growth and yield of lettuce. Bangladesh Hortic. 2017, 2, 39–46. [Google Scholar]
- Silber, A. Chemical characteristics of soilless media. In Soilless Culture: Theory and Practice Chapter 4; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; pp. 113–148. [Google Scholar] [CrossRef]
- Graber, E.R.; Meller Harel, Y.; Kolton, M.; Cytryn, E.; Silber, A.; Rav David, D.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Bigelow, C.; Bowman, D.; Cassel, D. Physical properties of three sand size classes amended with inorganic materials or sphagnum peat moss for putting green rootzones. Crop Sci. 2004, 44, 900–907. [Google Scholar] [CrossRef]
- Prasad, M.; Maher, M.J. Physical and chemical properties of fractionated peat. Acta Hort. 1993, 342, 257–264. [Google Scholar] [CrossRef]
- Sahin, U.; Anapali, O.; Ercisli, S. Physico-chemical and physical properties of some substrates used in horticulture. Die Gart. 2002, 67, 55–60. [Google Scholar]
- Douglas, M.H.; BM Smallfield, A.R. Wallace, and J.A. McGimpsey. Saffron (Crocus sativus L.): The effect of mother corm size on progeny multiplication, flower and stigma production. Sci. Hortic. 2002, 166, 50–58. [Google Scholar] [CrossRef]
- Khilare, V.; Tiknaik, A.; Prakash, B.; Ughade, B.; Korhale, G.; Nalage, D.; Ahmed, N.; Khedkar, C.; Khedkar, G. Multiple tests on saffron find new adulterant materials and reveal that IST grade saffron is rare in the market. Food Chem. 2019, 272, 635–642. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, V.; Devi, K.; Sharma, M.; Singh, M.K.; Ahuja, P.S. State of art of saffron (Crocus sativus L.) agronomy: A comprehensive review. Food Rev. Int. 2008, 25, 44–85. [Google Scholar] [CrossRef]
- Aytekin, A.; Acikgoz, O.A. Hormone and microorganism treatments in the cultivation of saffron (Crocus sativus L.) plants. Molecules 2008, 13, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Winterhalter, P.; Straubinger, M. Saffron-renewed interest in an ancient spice. Food Rev. Int. 2000, 16, 39–59. [Google Scholar] [CrossRef]
- Rahimi, H.; Shokrpour, M.; Tabrizi Raeini, L.; Esfandiari, E. A study on the effects of environmental factors on vegetative characteristics and corm yield of saffron (Crocus sativus). Iran J. Hortic. Sci. 2017, 48, 45–52. [Google Scholar]
- Fallahi, H.R.; Mahmoodi, S. Influence of organic and chemical fertilisation on growth and flowering of saffron under two irrigation regimes. Saffron Agron. Technol. 2018, 6, 147–166. [Google Scholar] [CrossRef]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Scrano, L.; Cicco, N.; Candido, V. The influence of soil physical and chemical properties on saffron (Crocus sativus L.) growth, yield and quality. Agronomy 2020, 10, 1154. [Google Scholar] [CrossRef]
- Esmaeilian, Y.; Amiri, M.B.; Neamatollahi, E. High density planting and manure affect flower yield, corm characteristics, and volatile compounds of saffron (Crocus sativus L.). Ind. Crops Prod. 2022, 176, 114363. [Google Scholar] [CrossRef]
- Zarghani, F.; Karimi, A.; Khorasani, R.; Lakzian, A. To evaluate the effect of soil physical and chemical characteristics on the growth characteristics of saffron (Crocus sativus L.) corms in Tornbate Heydariyeh area. J. Agroecol. 2016, 8, 120–133. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Alsadon, A. Effects of nutrient solution electrical conductivity on the leaf gas exchange, biochemical stress markers, growth, stigma yield, and daughter corm yield of saffron in a plant factory. Horticulturae 2022, 8, 673. [Google Scholar] [CrossRef]
- Fallahi, H.R.; Mahmoodi, S. Impact of water availability and fertilisation management on saffron (Crocus sativus L.) biomass allocation. J. Horti. Postharvest Res. 2018, 1, 131–146. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India, Private Limited: New Delhi, India, 1973. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeny, D.R. Methods of Soil Analysis, Part ii, 2nd ed.; Agronomy Monograph ASA and SSSA: Madison, WI, USA, 1982. [Google Scholar]
- Maggio, A.; Raimondi, G.; Martino, A.; De Pascale, S. Soilless cultivation of saffron in Mediterranean environment. Acta Hortic. 2006, 718, 515–522. [Google Scholar] [CrossRef]
- Moran, R.; Porath, D. Chlorophyll determination in intact tissues using N,N-Dimethyl formamide. Plant Physiol. 1982, 69, 1370–1381. [Google Scholar] [CrossRef]
- Aebi, H. Catalases. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1984; Volume 2, pp. 673–684. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M.B. Plant Emymology and Histoenzymology; Kalyani Publishers: Delhi, India, 1980. [Google Scholar]
- Shajari, M.A.; Moghaddam, P.R.; Ghorbani, R.; Koocheki, A. The possibility of improving saffron (Crocus sativus L.) flower and corm yield through the irrigation and soil texture managements. Sci. Hortic. 2020, 271, 109485. [Google Scholar] [CrossRef]
- García-Rodríguez, M.V.; Moratalla-López, N.; López-Córcoles, H.; Alonso, G.L. Saffron quality obtained under different forcing conditions, considering various vegetative stages of corms. Sci. Hortic. 2021, 277, 109811. [Google Scholar] [CrossRef]
- Shahandeh, H. Soil conditions for sustainable saffron production. In Saffron Science, Technology and Health; Woodhead Publishing Series in Food Science, Technology and Nutritionpp; Koocheki, A., Khajeh-Hosseini, M., Eds.; Woodhead Publishing: Chennai, India, 2020; pp. 59–66. [Google Scholar] [CrossRef]
- Ghanbari, J.; Khajoei-Nejad, G. Integrated nutrient management to improve some soil characteristics and biomass production of saffron. Ind. Crops Prod. 2021, 166, 113447. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Hashemi, S.E.; Del Borghi, A.; Spasiano, D.; Rad, M.; Race, M. Feasibility study of saffron cultivation using a semi-saline water by managing planting date, a new statement. Environ. Res. 2022, 203, 111853. [Google Scholar] [CrossRef]
- Elbehiry, F.; Elbasiouny, H.; El-Ramady, H.; Brevik, E.C. Mobility, distribution, and potential risk assessment of selected trace elements in soils of the Nile Delta, Egypt. Env. Monit Assess 2019, 191, 713. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).