Production of Quinoa Leafy Greens in High Tunnel for Season Extension in Missouri
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and High Tunnel Structure
2.2. Plant Materials, Experimental Design and Methods
2.3. Data Collection and Plant Harvest
2.4. Nutritional Analysis
2.5. Statistical Analysis
3. Results and Discussion
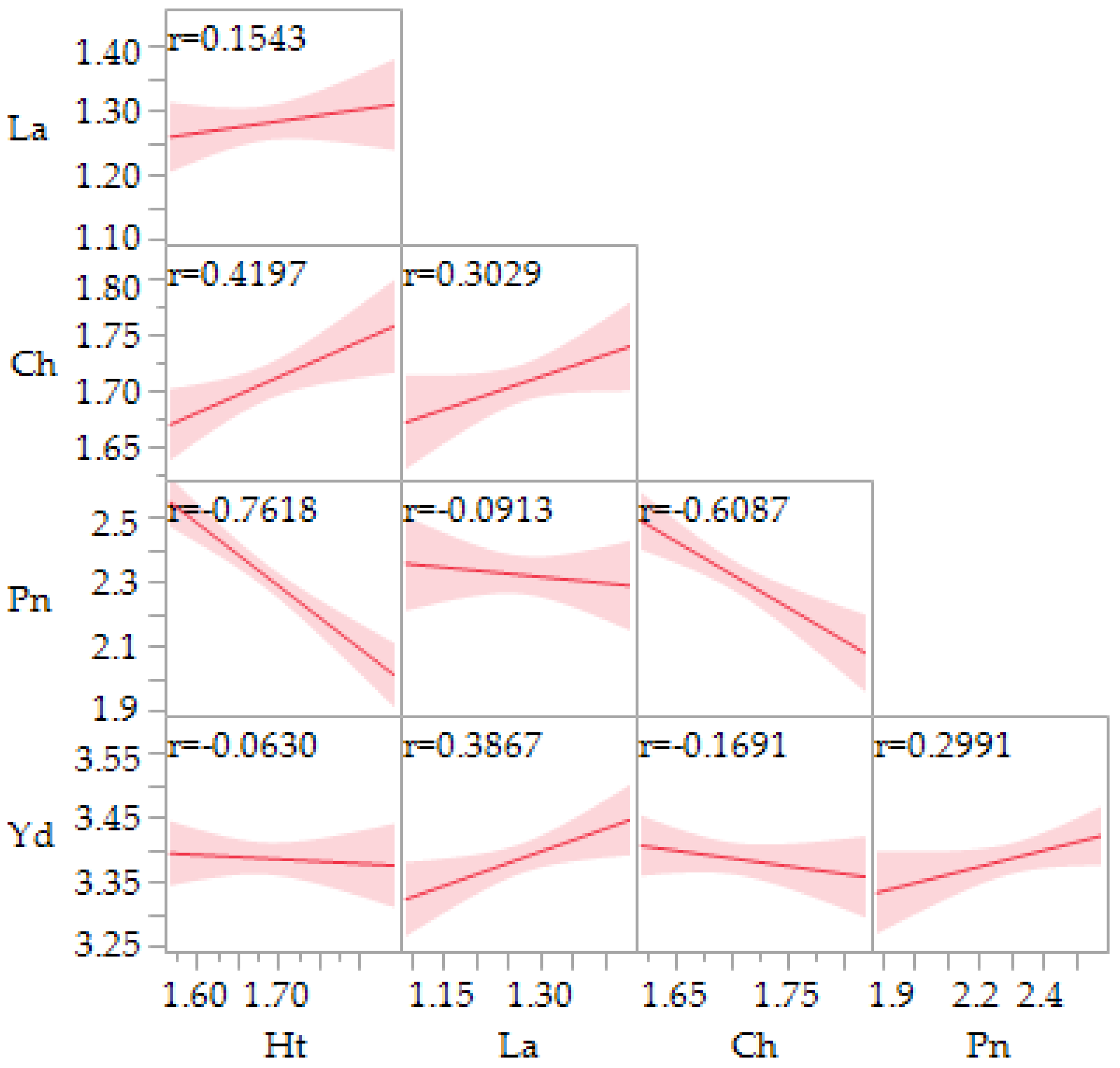
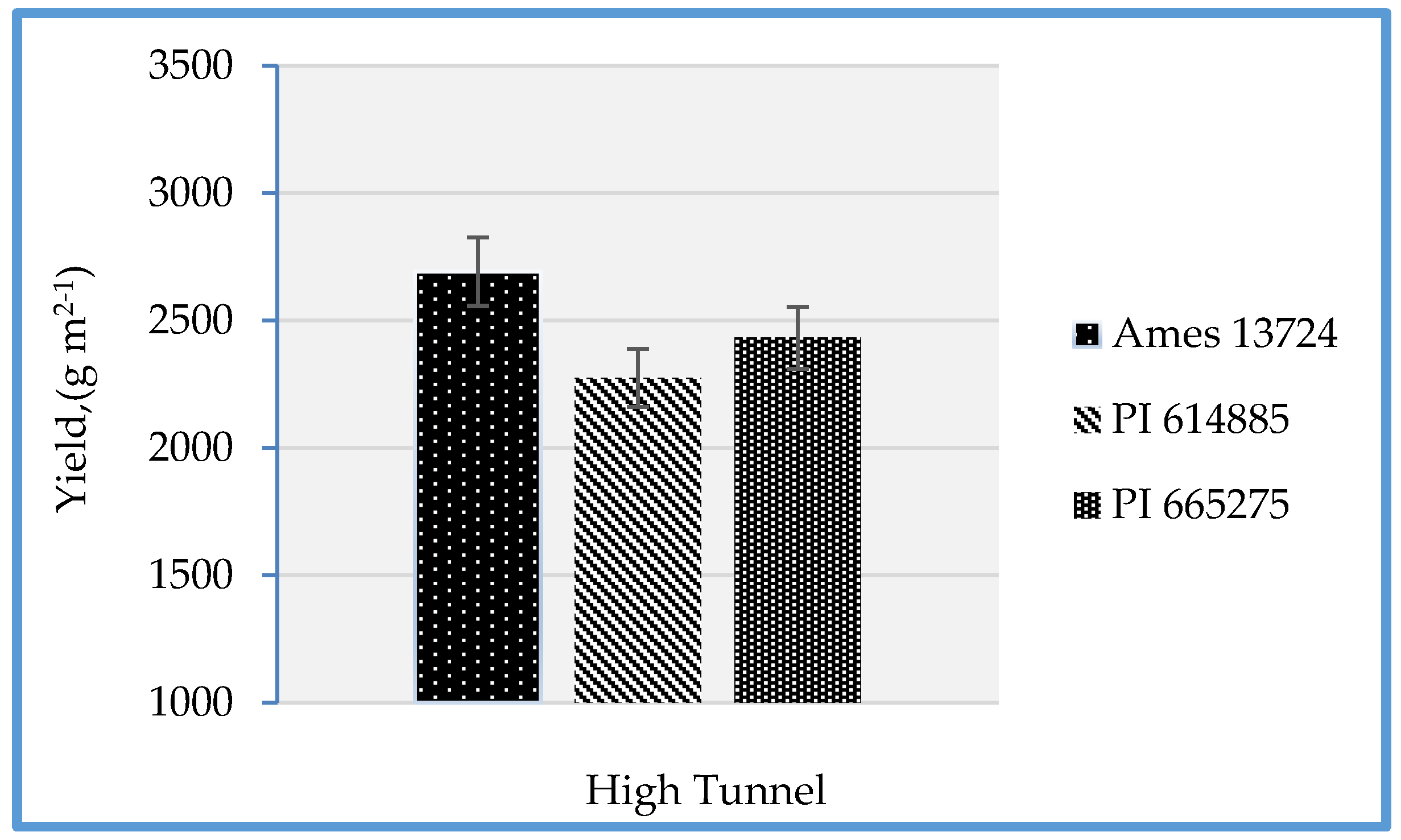
3.1. Plant Height, Chlorophyll Content, Leaf Area, Plant Number, and Yield
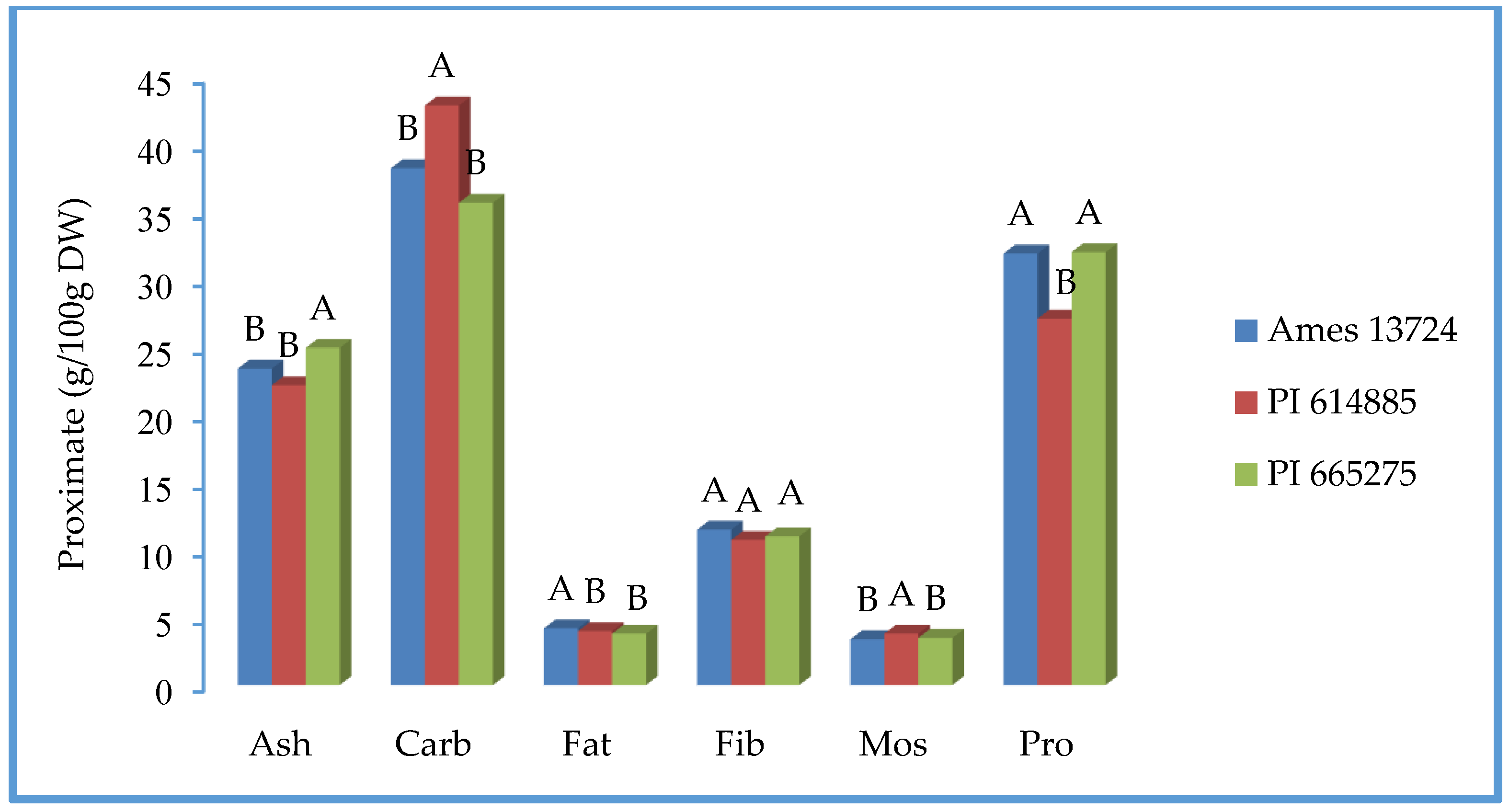
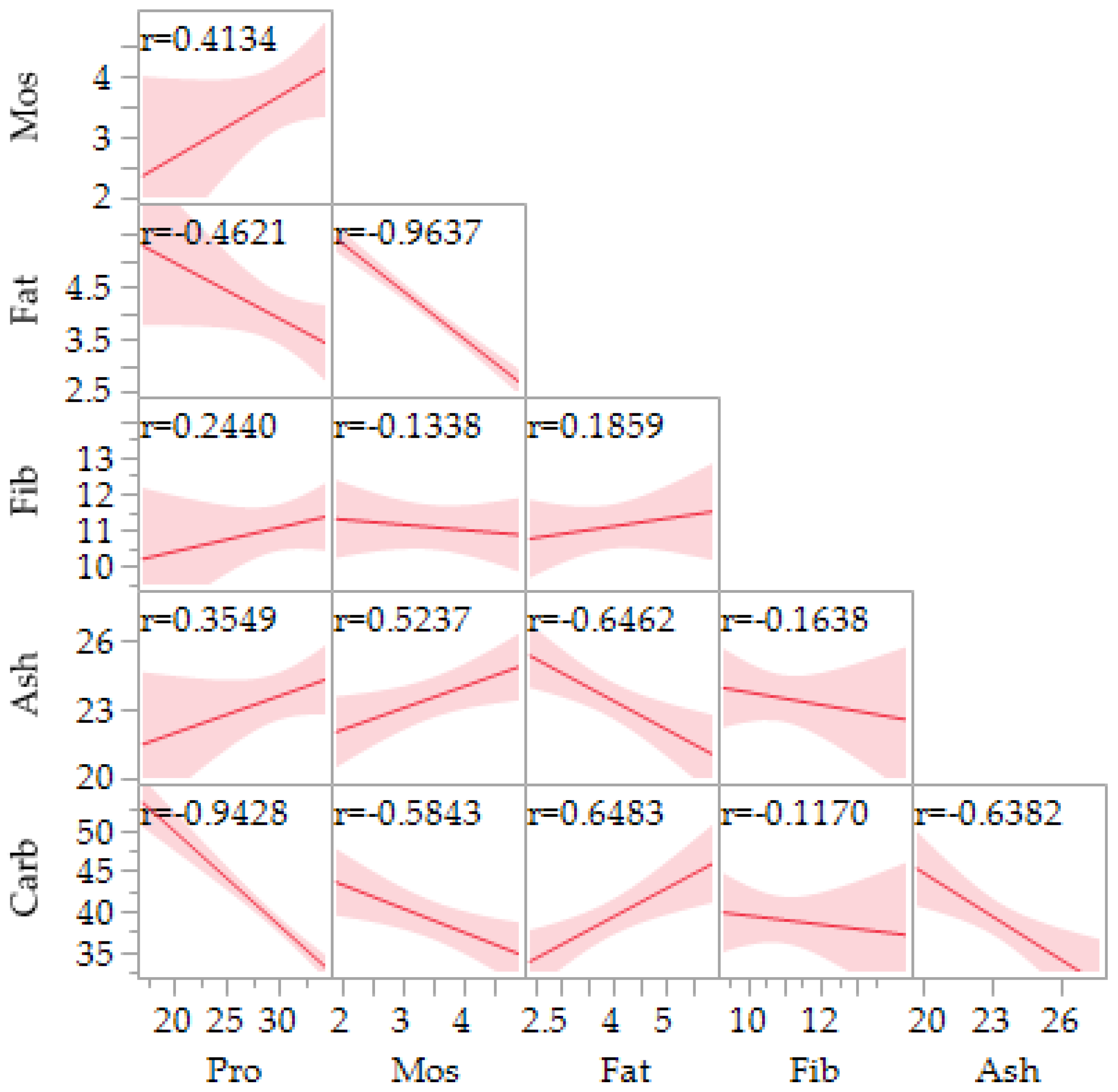
3.2. Nutritional Composition-Proximate Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Carey, E.E.; Jett, L.; Lamont, W.J., Jr.; Nennich, T.T.; Orzolek, M.D.; Williams, K.A. Horticultural Crop Production in High Tunnels in the United States: A Snapshot. HortTechnology 2009, 19, 37–43. [Google Scholar] [CrossRef]
- Lamont, W. Overview of the use of high tunnels worldwide. HortTechnology 2009, 19, 25–29. [Google Scholar] [CrossRef]
- Orzolek, M.D. Increasing economic return to high tunnel with specialty crops. Acta Hort. 2013, 987, 83–88. [Google Scholar] [CrossRef]
- NSAC: National Sustainable Agriculture Coalition. High Tunnels Create Opportunities for New Farmers. NSAC’s Blog. 8 July 2016. Available online: https://sustainableagriculture.net/blog/high-tunnels-create-opportunities/ (accessed on 20 August 2022).
- Bruce, A.B.; Farmer, J.R.; Maynard, E.T.; Valliant, J.C.D. Assessing the impact of the EQIP High Tunnel Initiative. J. Agril. Food Syst. Comm. Dev. 2017, 7, 159–180. [Google Scholar] [CrossRef]
- Bruce, A.B.; Maynard, E.T.; Farmer, J.R. Farmers’ perspectives on challenges and opportunities associated with using high tunnels for specialty crops. HortTechnology 2019, 29, 290–299. [Google Scholar] [CrossRef]
- O’Connell, S.; Rivard, C.; Peet, M.M.; Harlow, C.; Louws, F. High tunnel and field production of organic heirloom tomatoes: Yield, fruit quality, disease, and microclimate. HortScience 2012, 47, 1283–1290. [Google Scholar] [CrossRef]
- CDC: Centers for Disease Control and Prevention. Strategies to Prevent Obesity and Other Chronic Diseases: The CDC Guide to Strategies to Increase the Consumption of Fruits and Vegetable; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2011.
- U.S. Department of Health and Human Services (HHS); U.S. Department of Agriculture (USDA). 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services (HHS); U.S. Department of Agriculture (USDA): Denver, CO, USA, 2015.
- Barkat, N.; Singh, J.; Jayaprakash, G.K.; Patil, B.S. Effect of harvest time on the levels of phytochemicals, free radical-scavenging activity, alpha-amylase inhibition and bile acid binding capacity of spinach (Spinacia oleracea). J. Food Sci. Agron. 2018, 98, 3468–3477. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Nutrients, minerals, pigments and phytochemicals, and radical scavenging activity in Amaranthus blitum leafy vegetables. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, D.; Chen, L.Y.; Sultanbawa, Y. A comprehensive review of beneficial dietary phytochemicals in common traditional Southern African leafy vegetables. Food Sci. Nutr. 2018, 6, 714–727. [Google Scholar] [CrossRef]
- Borrelli, K.; Koenig, R.T.; Jaeckel, B.M.; Miles, C.A. Yield of Leafy Greens in High Tunnel Winter Production in the Northwest United States. Hortscience 2013, 48, 183–188. [Google Scholar] [CrossRef]
- NASS-USDA: National Agricultural Statistics Service—US Department of Agriculture. Census of Agriculture; Horticultural Specialties; 2014. Available online: http://www.agcensus.usda.gov/Publications (accessed on 6 July 2022).
- Reeder, B.; Foshee, W.; Blythe, E.; Kessler, R.; Kemble, J.; Vinson, E.; Dozier, W.; Wells, L. High Tunnel Production of Tomatoes for Season Extension in Southeast Alabama. Horticulturae 2020, 6, 94. [Google Scholar] [CrossRef]
- Rogers, M.A.; Wszelaki, A.L. Influence of High Tunnel Production and Planting Date on Yield, Growth, and Early Blight Development on Organically Grown Heirloom and Hybrid Tomato. Horttechnology 2012, 22, 452–462. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, M.M.; Bi, G.H.; Evans, B.; Harkess, R. Planting Date Effect on Yield of Tomato, Eggplant, Pepper, Zinnia, and Snapdragon in High Tunnel in Mississippi. J. Crop Improvement 2014, 28, 27–37. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Deepak, O. Chenopodium quinoa: An Indian perspective. Ind. Crops Prod. 2006, 23, 73–87. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Swieca, M.; Sulkowski, M.; Dziki, D.; Baraniak, B.; Czyz, J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts- In vitro study. Food Chem. Toxicol. 2013, 57, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Jancurová, M.; Minarovičová, L.; Dandár, A. Quinoa-A Review. Czech J. Food Sci. 2009, 27, 71–79. [Google Scholar] [CrossRef]
- Vazquez-Luna, A.; Cortes, V.P.; Carmona, F.F.; Diaz-Sobac, R. Quinoa leaf as a nutritional alternative. Ciencia Invest. Agraria. 2019, 46, 137–143. [Google Scholar] [CrossRef]
- Adamczewska-Sowińska, K.; Sowiński, J.; Jama-Rodzeńska, A. The effect of sowing date and harvest time on leafy greens of quinoa (Chenopodium quinoa willd.) yield and selected nutritional parameters. Agriculture 2021, 11, 405. [Google Scholar] [CrossRef]
- El-Samad, E.; Hussin, S.; El-Naggar, A.; El-Bordeny, N.; Eisa, S. The potential use of quinoa as a new non-traditional leafy vegetable crop. Biosci. Res. 2018, 15, 3387–3403. [Google Scholar]
- Pathan, S.; Eivazi, F.; Valliyodan, B.; Paul, K.; Ndunguru, G.; Clark, K. Nutritional composition of the green leaves of quinoa (Chenopodium quinoa Willd.). J. Food Res. 2019, 8, 55–65. [Google Scholar] [CrossRef]
- Le, L.; Gong, X.; An, Q.; Xiang, D.; Zou, L.; Peng, L.; Wu, X.; Tan, W.; Nie, Z.; Wu, Q.; et al. Quinoa sprouts as potential vegetable source: Nutrient composition and functional contents of different quinoa sprout varieties. Food Chem. 2021, 357, 129752. [Google Scholar] [CrossRef]
- Saini, S.; Saini, K. Chenopodium album Linn: An outlook on weed cum nutritional vegetable along with medicinal properties. Emergent Life Sci. Res. 2020, 6, 28–33. [Google Scholar] [CrossRef]
- Wan, Y.; Zhou, M.; Le, L.; Gong, X.; Jiang, L.; Huang, J.; Cao, X.; Shi, Z.; Tan, M.; Cao, Y.; et al. Evaluation of morphology, nutrients, phytochemistry and pigments suggests the optimum harvest date for high-quality quinoa leafy vegetable. Scientia Hort. 2022, 304, 111240. [Google Scholar] [CrossRef]
- Yadav, R.K.; Tomar, B.S.; Pachauri, N.; Jain, V. Studies of nutritional properties and antioxidant potential in green leafy vegetables. J. Sci. Food Agril. 2018, 2, 7–13. [Google Scholar]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef] [PubMed]
- AOAC: Association of Official Analytical Chemist. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- JMP. Version 2; The SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- El-Naggar, A.; Hussin, S.; Abd El-Samad, E.; Eisa, S. Quinoa as a new leafy vegetable crop in Egypt. Arab Univ. J. Agril. Sci. 2018, 26, 745–753. [Google Scholar] [CrossRef]
- Pathan, S.; Siddiqui, R. Nutritional composition and bioactive components in quinoa greens—A Review. Nutrients. 2022, 14, 558. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Akbarpour, V.; Dashti, F.; Shojaeian, A. Effect of different levels of nitrogen fertilizer on yield, nitrate accumulation and several quantitative attributes of five Iranian spinach accessions. Amer. Eurasian J. Agric. Environ. Sci. 2010, 8, 468–473. [Google Scholar]
- Mbwambo, O.; Abukutsa-Onyango, M.O.; Dinssa, F.F.; Ojiewo, C. Performances of elite amaranth genotypes in grain and leaf yields in Northern Tanzanian. J. Hort. 2015, 7, 16–23. [Google Scholar] [CrossRef]
- Koudela, M.; Petříková, K. Nutrients content and yield in selected cultivars of leaf lettuce (Lactuca sativa L. var. crispa). Hort. Sci. 2008, 35, 99–106. [Google Scholar] [CrossRef]
- Samaha, F.F.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, T.; Williams, M.; Gracely, E.J.; Stern, L.; et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N. Engl. J. Med. 2003, 348, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Onuminya, T.O.; Shodiya, O.E.; Olubiyi, O.O. Comparative proximate and phytochemical analyses of leafy vegetables of Lagos state. Niger. J. Pure Appl. Sci. 2017, 30, 3097–3103. [Google Scholar] [CrossRef]

| Year | Seeding Date | Harvesting Date | Duration Days | Inside Temp °C | Outside Temp °C | ||||
|---|---|---|---|---|---|---|---|---|---|
| min | max | av | min | max | av | ||||
| 2020 | 30 March | 11 May | 42 | na 1 | na | na | −4.00 | 29.8 | 12.37 |
| 2021 | 24 March | 5 May | 42 | −0.17 | 33.72 | 17.82 | −3.70 | 28.9 | 13.1 |
| 2022 | 30 March | 11 May | 42 | 0.1 | 35.82 | 18.41 | −3.80 | 32.6 | 13.31 |
| Parameter | Source | DF | SS | F Ratio | Prob > F |
|---|---|---|---|---|---|
| Yd | Var | 2 | 1866502 | 8.1 | 0.0031 ** |
| Year | 2 | 170761 | 0.7 | 0.4902 | |
| Var × Year | 4 | 1091271 | 2.4 | 0.091 | |
| La | Var | 2 | 21 | 4.5 | 0.0259 * |
| Year | 2 | 261 | 56.5 | <0.0001 *** | |
| Var × Year | 4 | 16 | 1.8 | 0.1817 | |
| Ch | Var | 2 | 102 | 7.8 | 0.0037 ** |
| Year | 2 | 283 | 21.5 | <0.0001 *** | |
| Var × Year | 4 | 70 | 2.7 | 0.067 | |
| Pn | Var | 2 | 5434 | 1.5 | 0.252 |
| Year | 2 | 42945 | 11.8 | 0.0005 *** | |
| Var × Year | 4 | 54199 | 7.4 | 0.001 *** | |
| Ht | Var | 2 | 94 | 4.8 | 0.2881 |
| Year | 2 | 688 | 35.1 | <0.0001 *** | |
| Var × Year | 4 | 155 | 4.0 | 0.1790 |
| Lines 1 | Yield (g m2−1) | Leaf Area (cm2) | Leaf Chlorophyll | Plant Number per Unit Area | Height (cm) |
|---|---|---|---|---|---|
| Ames 13724 | 2913.2 a | 20.1 a | 50.7 a | 270.3 a | 48.0 a |
| PI 665275 | 2407.9 b | 18.7 ab | 48.1 b | 251.1 a | 46.2 ab |
| PI 614885 | 2315.8 b | 17.9 b | 45.9 b | 236.6 a | 43.4 b |
| Parameter | Source | DF | SS | F Ratio | Prob > F |
|---|---|---|---|---|---|
| Mos | Variety | 2 | 0.6 | 14.0 | 0.0007 *** |
| Year | 1 | 20.1 | 988.2 | <0.0001 *** | |
| Variety × Year | 2 | 0.1 | 3.6 | 0.0596 | |
| Pro | Variety | 2 | 88 | 11.6 | 0.0019 ** |
| Year | 1 | 70 | 18.5 | 0.0012 ** | |
| Variety × Year | 2 | 123 | 16.3 | 0.0005 *** | |
| Fat | Variety | 2 | 0.5 | 4.3 | 0.039 * |
| Year | 1 | 17.5 | 309.0 | <0.0001 *** | |
| Variety × Year | 2 | 0.3 | 2.6 | 0.1182 | |
| Fib | Variety | 2 | 1.8 | 0.6 | 0.5596 |
| Year | 1 | 0.2 | 0.1 | 0.7347 | |
| Variety × Year | 2 | 4.5 | 1.5 | 0.2621 | |
| Ash | Variety | 2 | 23 | 10.2 | 0.0026 ** |
| Year | 1 | 27 | 23.4 | 0.0004 *** | |
| Variety × Year | 2 | 5 | 2.3 | 0.1383 | |
| Carb | Variety | 2 | 160 | 17.1 | 0.0003 *** |
| Year | 1 | 241 | 51.6 | <0.0001 *** | |
| Year × Variety | 2 | 79 | 8.5 | 0.005 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathan, S.; Ndunguru, G.; Islam, M.R.; Jhumur, S.T.; Ayele, A.G. Production of Quinoa Leafy Greens in High Tunnel for Season Extension in Missouri. Horticulturae 2023, 9, 209. https://doi.org/10.3390/horticulturae9020209
Pathan S, Ndunguru G, Islam MR, Jhumur ST, Ayele AG. Production of Quinoa Leafy Greens in High Tunnel for Season Extension in Missouri. Horticulturae. 2023; 9(2):209. https://doi.org/10.3390/horticulturae9020209
Chicago/Turabian StylePathan, Safiullah, Grato Ndunguru, Md R. Islam, Sadia T. Jhumur, and Addissu G. Ayele. 2023. "Production of Quinoa Leafy Greens in High Tunnel for Season Extension in Missouri" Horticulturae 9, no. 2: 209. https://doi.org/10.3390/horticulturae9020209
APA StylePathan, S., Ndunguru, G., Islam, M. R., Jhumur, S. T., & Ayele, A. G. (2023). Production of Quinoa Leafy Greens in High Tunnel for Season Extension in Missouri. Horticulturae, 9(2), 209. https://doi.org/10.3390/horticulturae9020209





