Interaction of Culture Medium and Artificial Light Type on Pigmentation of Micro-Propagated Opuntia Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lighting System Characteristics
2.1.1. Artificial LED System
2.1.2. Laser Light Characteristics
2.2. Red Fruit Cactus Explant Preparation
Culture Media to Evaluate the Growth Effect Regulators with the LED and Laser Light Influence
2.3. Total Phenol and Flavonoid Determination
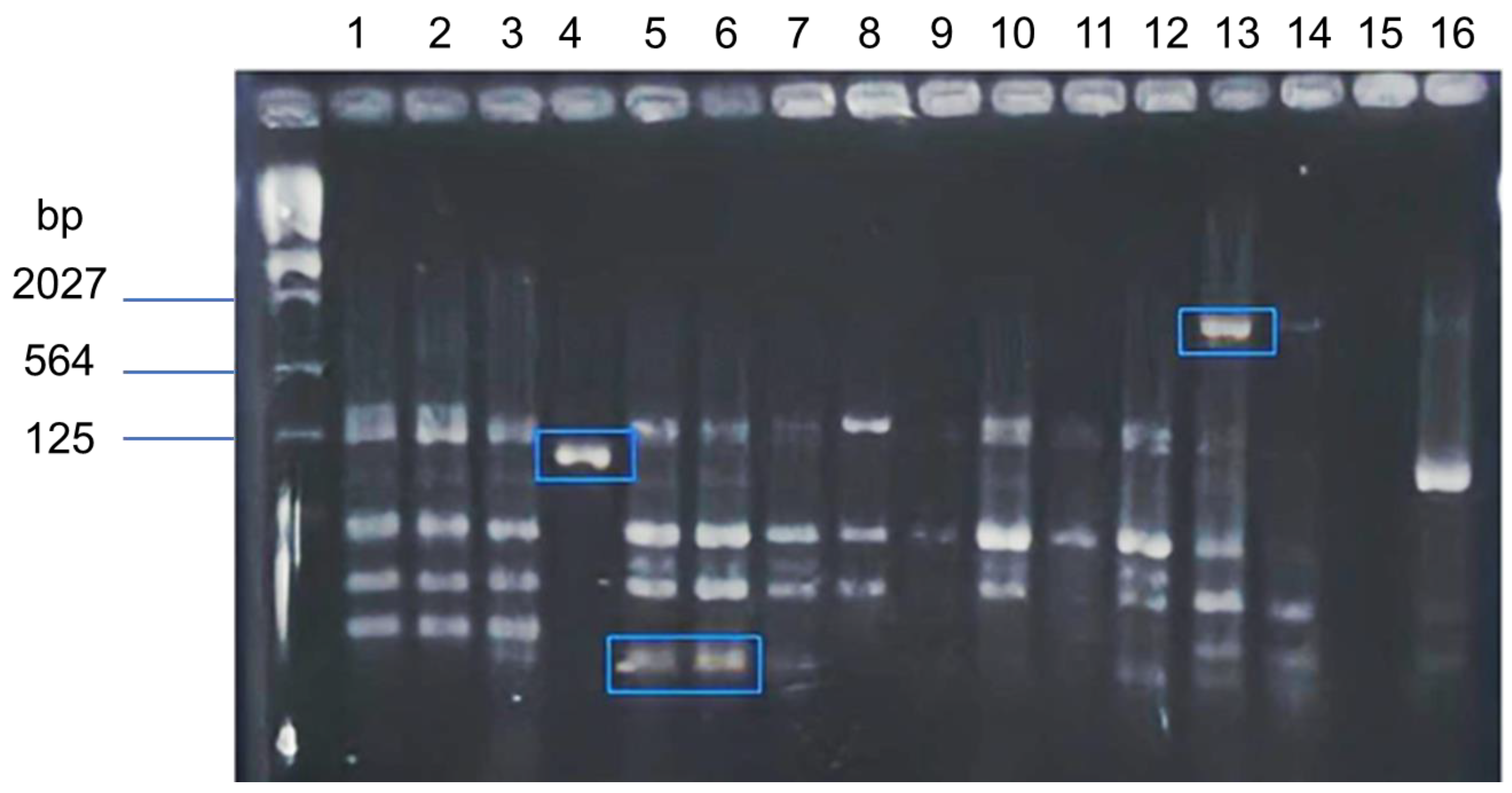
2.4. Genes Involved in Pigment Synthesis Identification
2.4.1. Genomic Deoxyribonucleic Acid (DNA) Extractions from Opuntia Spp.
2.4.2. DNA Fragment Amplification and Sequencing
2.5. Statistical Data Analyses
3. Results and Discussion
3.1. Prickly Pear Explant Development Effects of Components in the Culture Medium and Lighting Treatments
Phenolic and Flavonoid Content
3.2. Data Analysis
3.3. Genes Involved in Pigment Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amador-Rodríguez, K.Y.; Silos-Espino, H.; Valera-Montero, L.L.; Perales-Segovia, C.; Flores-Benítez, S.; Martínez-Bustos, F. Chemical and potentially functional compounds of selected prickly pears seeds (Opuntia spp.). Mitt. Klosterneubg. Rebe Wein Obs. Fruchteverwert. 2019, 69, 141–153. Available online: https://www.weinobst.at/dam/jcr:1e529db2-2805-41f5-96dc-052f52a22324/141-2019.pdf (accessed on 28 October 2023).
- Espinoza Sánchez, E.A.; Silos Espino, H.; Flores Benitez, S.; Valera Montero, L.L.; Rodríguez Salazar, E.; Gallegos Vázquez, C.; Guzmán Maldonado, H.S. Agrupamiento de genotipos de nopal (Opuntia spp.) de México por medio de la técnica de AFLPs y características del fruto. Phyton (B. Aires) 2014, 83, 299–306. [Google Scholar]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant Activities of Sicilian Prickly Pear (Opuntia ficus indica) Fruit Extracts and Reducing Properties of Its Betalains: Betanin and Indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Santoscoy, R.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic Composition, Antioxidant Capacity and in Vitro Cancer Cell Cytotoxicity of Nine Prickly Pear (Opuntia spp.) Juices. Plant Foods Hum. Nutr. 2009, 64, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ramos, M.; García-Mateos, M.; Corrales-García, J.; Ybarra-Moncada, C.; Castillo-González, A.M. Compuestos antioxidantes en variedades pigmentadas de tuna (Opuntia sp.). Rev. Fitotec. 2015, 38, 349–357. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bahulikar, R.A.; Kauthale, V.K.; Punde, K.K. Micropropagation purpose of spineless cactus (Opuntia ficus-indica Mill.) for fodder production. In X International Congress on Cactus Pear and Cochineal: Cactus—The New Green Revolution in Drylands; ISHS: Leuven, Belgium, 2022; Volume 1343, pp. 109–114. [Google Scholar] [CrossRef]
- Mabrouk, A.; Abbas, Y.; El Goumi, Y.; El Maaiden, E.; El Kharrassi, Y.; Antry-Tazi, S.E.; Fakiri, M. Optimization of prickly pear cacti (Opuntia spp.) micropropagation using an experimental design method. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e1577. [Google Scholar] [CrossRef]
- García-Saucedo, P.A.; Valdez-Morales, M.; Valverde, M.E.; Cruz-Hernandez, A.; Paredes-Lopez, O. Plant regeneration of three Opuntia genotypes used as human food. Plant Cell Tissue Organ Cult. 2005, 80, 215–219. [Google Scholar] [CrossRef]
- Gomes, F.L.A.F.; Heredia, F.F.; E Silva, P.B.; Facó, O.; de Paiva Campos, F.D.A. Somatic embryogenesis and plant regeneration in Opuntia ficus-indica (L.) Mill. (Cactaceae). Sci. Hort. 2006, 108, 15–21. [Google Scholar] [CrossRef]
- Bouzroud, S.; El Maaiden, E.; Sobeh, M.; Prasad Devkota, K.; Boukcim, H.; Kouisni, L.; El Kharrassi, Y. Micropropagation of Opuntia and Other Cacti Species Through Axillary Shoot Proliferation: A Comprehensive Review. Front. Plant Sci. 2022, 13, 926653. [Google Scholar] [CrossRef]
- Han, T.; Vaganov, V.; Cao, S.; Li, Q.; Ling, L.; Cheng, X.; Tu, M. Improving “Color Rendering” of LED lighting for the growth of lettuce. Sci. Rep. 2017, 7, 45944. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Kim, E.; Park, J.; Lee, J. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed]
- Bello-Bello, J.J.; Perez-Sato, J.A.; Cruz-Cruz, C.A.; Martínez-Estrada, E. Light-emitting diodes: Progress in plant micropropagation. IntechOpen 2017, 6, 93–103. [Google Scholar] [CrossRef]
- Ruangrak, E.; Khummueng, W. Effects of artificial light sources on accumulation of phytochemical contents in hydroponic lettuce. J. Hortic. Sci. Biotechnol. 2019, 94, 378–388. [Google Scholar] [CrossRef]
- Miler, M.; Kulus, D.; Woźny, A.; Rymarz, D.; Hajzer, M.; Wierzbowski, K.; Nelke, R.; Szeffs, L. Application of wide-spectrum light-emitting diodes in micropropagation of popular ornamental plant species: A study on plant quality and cost reduction. In Vitro Cell. Dev. Biol. Plant. 2018, 55, 99–108. [Google Scholar] [CrossRef]
- Lotfi, L.; Mars, M.; Werbrouck, S. Optimizing pear micropropagation and rooting with light emitting diodes and trans-cinnamic acid. J. Plant Growth Regul. 2019, 88, 173–180. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and anti-oxidants by means of the Folin-Ciocalteau reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA Isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987, 19, 11–15. Available online: https://webpages.charlotte.edu/~jweller2/pages/BINF8350f2011/BINF8350_Readings/Doyle_plantDNAextractCTAB_1987.pdf (accessed on 28 October 2023).
- Felker, P.; Stintzing, F.C.; Müssig, E.; Leitenberger, M.; Carle, R.; Vogt, T.; Bunch, R. Colour inheritance in cactus pear (Opuntia ficus-indica) fruits. Ann. Appl. Biol. 2008, 152, 307–318. [Google Scholar] [CrossRef]
- Muñoz Jáuregui, A.M.; Ramos-Escudero, D.F.; Alvarado-Ortiz Ureta, C.; Castañeda Castañeda, B. Evaluación de la capacidad antioxidante y contenido de compuestos fenólicos en recursos vegetales promisorios. Rev. Soc. Quím. Perú. 2007, 73, 142–149. [Google Scholar]
- Del Socorro Santos Díaz, M.; Barba de la Rosa, A.P.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia spp.: Characterization and Benefits in Chronic Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 8634249. [Google Scholar] [CrossRef] [PubMed]
- Norambuena, L.; Marchant, L.; Bennison, P.; Hirschberg, C.B.; Silva, H.; Orellana, A. Transport of UDP-galactose in plants: Identification and functional characterization of AtUTR1, and Arabidopsis thaliana UDP-galactose/UDP-glucose transporter 2002. J. Biol. Chem. 2002, 277, 32923–32929. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Tian, S.; Yang, L.; Zhang, Z.; Liu, Y. A systematic review of the uridine diphosphate-galactose/glucose-4-epimerase (UGE) in plants. J. Plant Growth Regul. 2021, 93, 267–278. [Google Scholar] [CrossRef]

| Cactus Explants | MS Components | Additional Components | ||
|---|---|---|---|---|
| I | II | III | ||
| A | 50% and activated carbon (1 g/L) | sucrose (at 10, 20, 30, 50, and 60 g/L), maguey honey (10 g/L) | BAP (1 mg/L) | white LED and red LED light |
| B | 50% (liquid state) with activated carbon (1 g/L) 50% (liquid state) without activated carbon (1 g/L) | sucrose (at 25, 30 y 50 g/L), maguey honey (at 15, 25, and 50 g/L) | BAP (1 mg/L) | white LED light |
| C | 50% and 100% with activated carbon (1 g/L) and 50% and 100% without activated carbon (1 g/L) | sucrose (50 g/L), maguey honey (25 g/L) | 2,4-D (1, 2 y; 3 mg/L) | white LED and red LED light |
| D | 50% and activated carbon (1 g/L) | sucrose (30, 50, and 75 g/L) | 2,4-D (1, 2, 3 y; 4 mg/L) | white LED and red LED light |
| E | 50% and AgNO3 (0, 1, 2, and 4 y; 8 mg/L) | sucrose (30 g/L). mannitol (3% and 4%) | IAA (1 g/L) and BAP (3 mL/L) | white LED and red LED light |
| F | 50% and activated carbon (1 g/L) | fructose (at 10, 15, 20, and 30 g/L) | 2,4-D (1, 2 y; 3 mg/L) and kinetin (0.5, 1, and 2 mg/L) | white LED and red LED light |
| G | 50% and AgNO3 (0,0.75, 1, and 2 mg/L) | fructose (2 g/L), prickly pear juice (0, 2, 4, and 6 mL/L), mannitol (3, 4, and 5 g/L) | 2,4-D (2 mg/L) and IAA (1 g/L) | white LED light |
| H | 50% and activated carbon (1 g/L) | sucrose (30 g/L), fructose (20 g/L), prickly pear juice (16 mL/L), and mannitol (0.375 mL/L) | BAP (3 mg/L), 2,4-D (2 y; 3 mg/L), IAA (0.5 mg/L), kinetin (1 mg/L), and IAA (1 mg/L) | white, red, and blue LED light and laser beam (during 30 s) |
| I | 50%, pH of 5.5, 5.7, and 6, citric acid (50 mg/L), and activated carbon (1 g/L) | sucrose (5, 10 y 20 g/L), prickly pear juice (10, 20, 30, and 40 mL/L) | 2,4-D (0.1, 0.5, 0.75 and 1 g/L) and kinetin (0.1, 0.2, 0.5, and 1 mg/L), putrescine (10 mg/L) | white LED light |
| J | 50% and AgNO3 (0, 2, and 4 y; 8 mg/L) and activated carbon (1 g/L) | sucrose (3 and 100 g/L), mannitol (30 and 40 g/L) | 2,4-D (3 mg/L) | white LED light |
| K | 50% and activated carbon (1, 2 g/L) | sucrose (30, 40, and 60 g/L), maguey honey (10 g/L) | BAP (1 mg/L) | white LED light |
| L | MS (0%, 10%, and 50%), pH 5.7, and activated carbon (1 g/L) | maguey honey (15, 20, 25, and 30 g/L) | none | white LED light |
| M | 50% and activated carbon (1 g/L) | dextrose (10 g/L), prickly pear juice (7.5 mL/L), and maguey honey (7.5 mL/L) | picloram (1 and 2 mg/L), 2,4-D (0.7 mg/L) | white LED light |
| Explant Cactus | Genotype | Morphological Response | Major Response | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Callus | Shoot | Roots | |||||||
| Type | Amount Per Treatment | (%) | (cm) | (%) | (cm) | (%) | (cm) | ||
| A | Tapón | 12 | 33.3 | 1.4 ± 0.2 | 41.6 | 1.7 ± 0.8 | 58.3 | 8.5 ± 6.6 | Red and white LED lights have the same effect on explant growth. |
| Copena VI | 8 | 25.0 | 1.2 ± 0.2 | 62.5 | 2.4 ± 1.4 | 100.0 | 7.6 ± 5.1 | ||
| B | Tapón | 12 | There was no influence on explant growth in callus, shoots, or roots. | The explants were necrotic after 15 days. | |||||
| Copena VI | 10 | The explant did not develop callus, shoots, or roots. | |||||||
| C | Tapón | 6 | 14.2 | 0.5 ± 0.3 | 100.0 | 1.7 ± 0.6 | 33.3 | 4.6 ± 2.7 | Light treatments had poor response in callus formation. |
| Copena VI | 7 | 14.2 | 0.7 ± 0.2 | 21.8 | 0.7 ± 0.4 | 7.3 ± 5.0 | |||
| D | Tapón | 3 | No callus formation | 66.0 | 0.6 ± 0.3 | 66.6 | 8.3 ± 0.5 | Root development. | |
| Copena VI | 3 | Absent | 33.3 | 0.2 ± 0.1 | 100.0 | 1.6 ± 1.1 | |||
| E | Tapón | 3 | 100.0 | 4.0 ± 3.0 | 66.3 | 0.8 ± 0.35 | No root formation. | Outstanding callus development. | |
| Copena VI | 4 | 25.0 | 0.3 ± 0.0 | 33.3 | 0.7 ± 0.0 | Absent | |||
| F | Tapón | 7 | 42.0 | 4.0 ± 3.0 | 71.0 | 0.6 ± 0.3 | No root formation. | White LED light affected shoot generation. | |
| Copena VI | 7 | Absent | 100.0 | 0.7 ± 0.0 | Absent | Red LED light effect on sprout development. | |||
| G | Tapón | 5 | Present | No effect in shoots and roots. | Embryogenesis generation in the genotype Tapón Aguanoso with small callus. | ||||
| Copena VI | 5 | Active | Not present in shoots and roots. | ||||||
| H | Tapón | 5 | Active | No effect shoots and roots. | The callus produced exhibited red pigmentation, and the laser light induced embryogenesis. | ||||
| Copena VI | 5 | Present | Not present in shoots and roots. | ||||||
| I | Tapón | 5 | Light | 80.0 | 2.12 ± 0.8 | 80.0 | 4.7 ± 1.8 | Very few but vigorous shoots were produced, and roots were formed. No red pigmentation. | |
| Copena VI | 4 | Light | 83.6 | 2.0 ± 1.06 | 83.0 | 5.6 ± 6.2 | |||
| J | Tapón | 3 | Light | 66.6 | 0.8 ± 0.28 | Absent | Few shoots, no roots, and a few whitish calluses. No red pigmentation. | ||
| Copena VI | 4 | Light | 66.6 | 0.4 ± 0.14 | No present. | ||||
| K | Tapón | 4 | No effect | 50.0 | 1.5 ± 0.70 | 50.0 | 17.8 ± 6.5 | Limited sprouting and thin roots. No red pigmentation. | |
| Copena VI | 4 | No present | 75.0 | 1.8 ± 0.76 | 75.0 | 13.4 ± 9.1 | |||
| L | Tapón | 2 | No present | 100.0 | Very small | 100.0 | 1.7 ± 0.0 | Very small shoots and roots. | |
| Copena VI | 2 | No effect | 100.0 | Too small | 100.0 | 2.7 ± 1.7 | |||
| M | Tapón | 12 | Occurrence | Not present | No effect | Callus with red pigmentation. | |||
| Copena VI | 10 | Presence | No effect | Absent | |||||
| Genotype | Simple Phenolic Acids in Prickly Pears (mg/100 g) | Total Phenolics mg GAE/100 g | Flavonoids mg EC/100 g | |||
|---|---|---|---|---|---|---|
| Gallic | Coumaric Acid | Ferulic | Chlorogenic | |||
| Tapón | 1887.2 ± 1.7 | 1121.0 ± 49.5 | 863.32 ± 111.8 | 380.22 ± 22.05 | 391.39 ± 2.1 | 67.06 ± 0.015 |
| Copena V1 | 2283.30 ± 6.2 | 2155.0 ± 35.0 | 2176 ± 27.9 | 314.14 ± 26.47 | 374 ± 5.9 | 68.5 ± 0.03 |
| Genotype | DNA Sequence in the 5′-3′ Enzyme for Each Cactus Genotype. |
|---|---|
| Atlixco | CTCACTCATCTCTTCACCTGTTCTTGGATGCAAACAGTCAGTACTAACACTAGAATGCCTGTTTGGATGTCCTGATTGAACAACATGCTCAAGTGAAGAAATTATCAATTAGGTTTGTTATCATCAAAACACTACATCCTAAAGAGGATGAATTCAACAAGGTGGCAGTGTCAAGGGCATTGAACGGCATTGGACTAGCCATTGTAATACCCGCCATTCAGTCCCTCGTGGCAGACTCAACTGAGGATCACAACCGTGGCACAGCCTTTGGGTGGCTACAGCTCACAGGAAACATGGGCTCAATCCTTGGCGGCCTTTGTTCTGTCTTGTTGGCTTCCAGGTCCTTCATGGGAGTCCCTGGTTGGAGGATAGCCTTCCATTTAGTTGGCCTAGTTAGTGTCATTGTTGGAGTTCTGGTTTGCCTCTTTGCCAATGATCCCCGCTATGCAGGAAGTGATCACAAGGCTAGAGAGGAGAAACGTCTGTCCTTTTGGTTAGAATTGAAAGGCGGTGTTGCAGGAAGCAAAATCAGTTCTCCGAATTCCATCATTTCAGATATTGTTGCCCAAGGTGTGTCTGGGTCTTTTCCGTGTCAGCATTGTCATTTAACATCATTGTGGGTGGGAACTCCGAA. |
| Cristalina | GTAGGTGTAGATGGCAAGTACTTCTTGGCTTTTCATTAATTCTGTTCTGCTATCAACTGCCTATGTTTACTCCATTTTAGCAACTTAGTGATGTGAACCAGATTCTTTGTTTGAACAAAGGCAAACTCTTCATCCAATATCAAAGTCCCCAAAATGGTTATTTACAAACTTATTAATAACTTAAGAAATTGGCCACCTTCTAGGAAGGAGTTCCCCTCTCCTCAACCTCTAAACAATACTGTTTCCAATACCTATATTTCCCGTTATGCAAGGTCAAGAACTTAGCTTTCTTTATTTGGAATCTAAGGGTTTTTCTGGGTGTTGCAAGTGAGGCCGCAGAAACTTTCTTTGATTTGACCGAATTTGGCAGGGTAAAATTTACTGCCTTTTTCGAAAGTTTTACTTCATGGTTTGCCAATCCTGGGTTAGAGAAATCAGGGGTAAATCCCTTAAAACCCAAAGCAACCCCCCTGGAATTGCCCCAAGGCGGTTTCGGGGGGGCGCCCCTGGCCCAAGTCCCAACCCGATTGGTCGGACCCCTCCCATAACGGTTTTCCTAGGGCGGGGGGTGTTTAAACCCGGGGGTTTGGGGAGGGGGGGGAATTTTAAGAACCAAACACCCTCGAGAGGGAAATTTTTTAAAAATGGGGGGTTCCAGGATATTTTTGTTTTTGGCGTTATAAAAAGAATGTTGATTAAAAAAAAGCAGTCCCAGGATG. |
| Tapón aguanoso | CGCAATCGCTTATTAGGGACATATACGCAGCTTGGTCCCGCTACCCAAACAAGAAAAGAAATAGATTTTTGGGGGTTTTTTTTTCACAAAATGGGCTTCGGTAAAAAGTTCGCCTTATTTTTGAAGAATTGGAACAAGTTTTGAAAGGACAAATCCTCAAGGAAACTGTCTTAAATTCAAAACAATTACGGCCTCGATTTAATTTCGGAATTGTCACTGATCCTATAATCCATACATAATGTCTAGTGTGGTGGAGACGGGTTTGTAACCAGTACCCTCTGGCTGGACCAAATACGAAAAAGGCCGATTCCCCTGGATTCCTCGAACAAAATCCTGGGGGAATACCGGCCTTGTGGAATCGAG. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silos Espino, H.; Escalera García, R.; Moncada González, D.; Valera-Montero, L.L.; Flores Benítez, S.; Ortiz Morales, M.; Guzmán Maldonado, H.S.; Escalante-Garcia, N.; Olvera-Gonzalez, E. Interaction of Culture Medium and Artificial Light Type on Pigmentation of Micro-Propagated Opuntia Plants. Horticulturae 2023, 9, 1348. https://doi.org/10.3390/horticulturae9121348
Silos Espino H, Escalera García R, Moncada González D, Valera-Montero LL, Flores Benítez S, Ortiz Morales M, Guzmán Maldonado HS, Escalante-Garcia N, Olvera-Gonzalez E. Interaction of Culture Medium and Artificial Light Type on Pigmentation of Micro-Propagated Opuntia Plants. Horticulturae. 2023; 9(12):1348. https://doi.org/10.3390/horticulturae9121348
Chicago/Turabian StyleSilos Espino, Hector, R. Escalera García, D. Moncada González, Luis L. Valera-Montero, S. Flores Benítez, M. Ortiz Morales, H. S. Guzmán Maldonado, Nivia Escalante-Garcia, and Ernesto Olvera-Gonzalez. 2023. "Interaction of Culture Medium and Artificial Light Type on Pigmentation of Micro-Propagated Opuntia Plants" Horticulturae 9, no. 12: 1348. https://doi.org/10.3390/horticulturae9121348
APA StyleSilos Espino, H., Escalera García, R., Moncada González, D., Valera-Montero, L. L., Flores Benítez, S., Ortiz Morales, M., Guzmán Maldonado, H. S., Escalante-Garcia, N., & Olvera-Gonzalez, E. (2023). Interaction of Culture Medium and Artificial Light Type on Pigmentation of Micro-Propagated Opuntia Plants. Horticulturae, 9(12), 1348. https://doi.org/10.3390/horticulturae9121348






