Net Photosynthesis and Biomass Production in Stevia, Eggplant, and Cowpea Can Be Improved by Fertilization with Cyanobacteria (Limnospira maxima)
Abstract
:1. Introduction
2. Materials and Methods
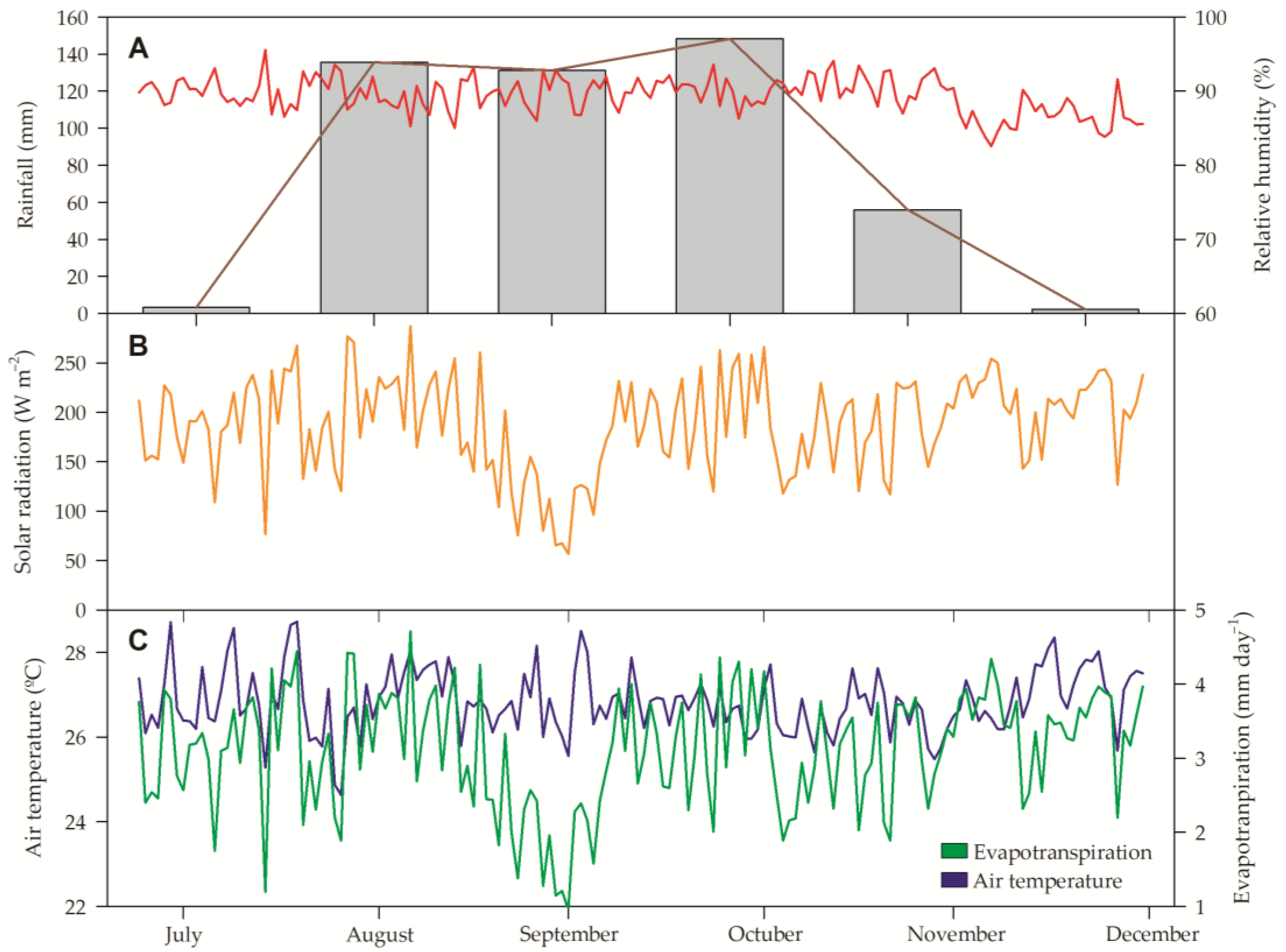
2.1. Experimental Site and Plant Material
2.2. Cultivation and Preparation of Limnospira maxima Extract
2.3. Experimental Design and Treatments
2.4. Gas Exchange and Chlorophyll a Fluorescence Parameters
2.5. Phenotypic Characteristics
2.6. Plasticity Index
2.7. Statistical Analysis
3. Results
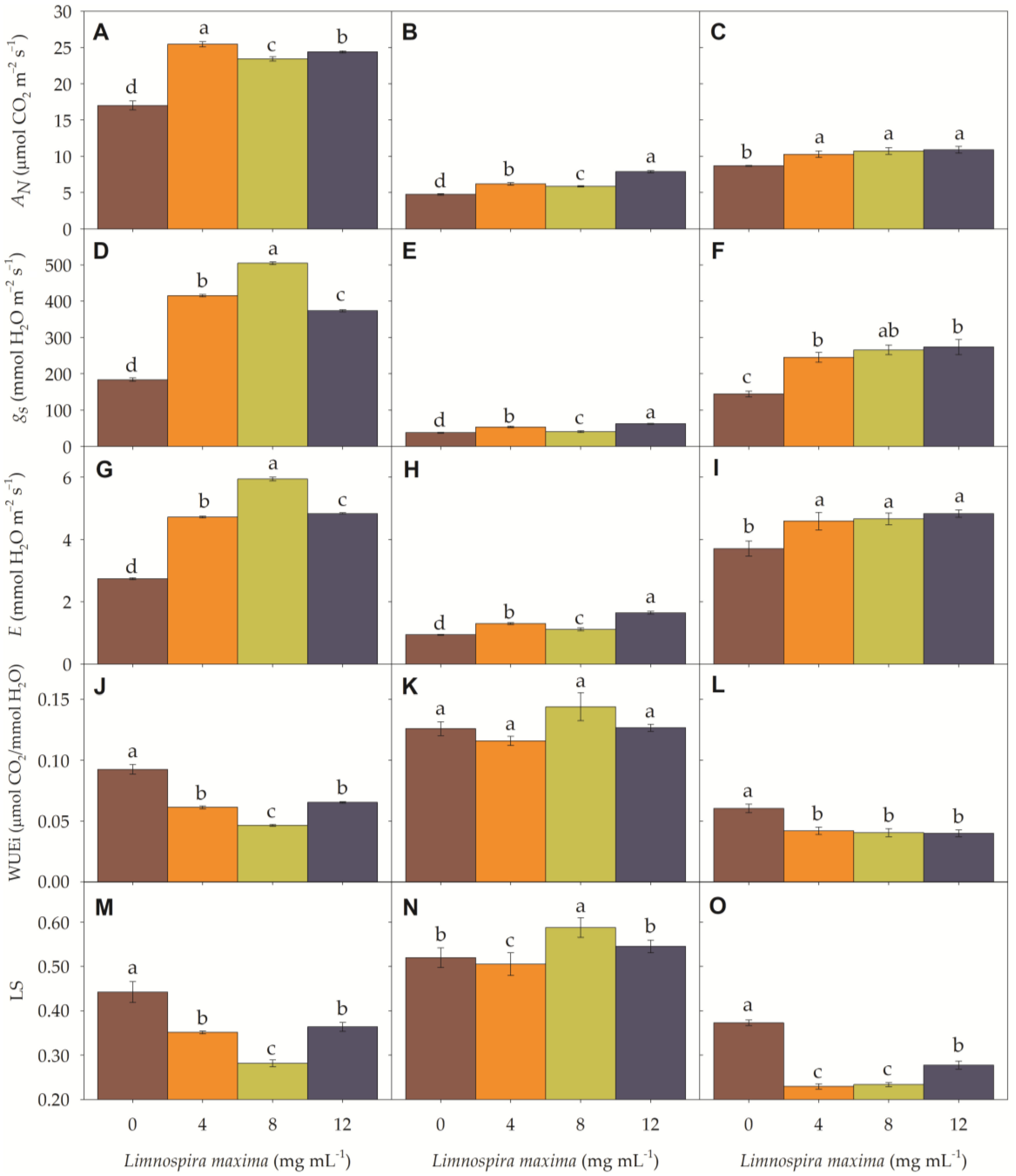
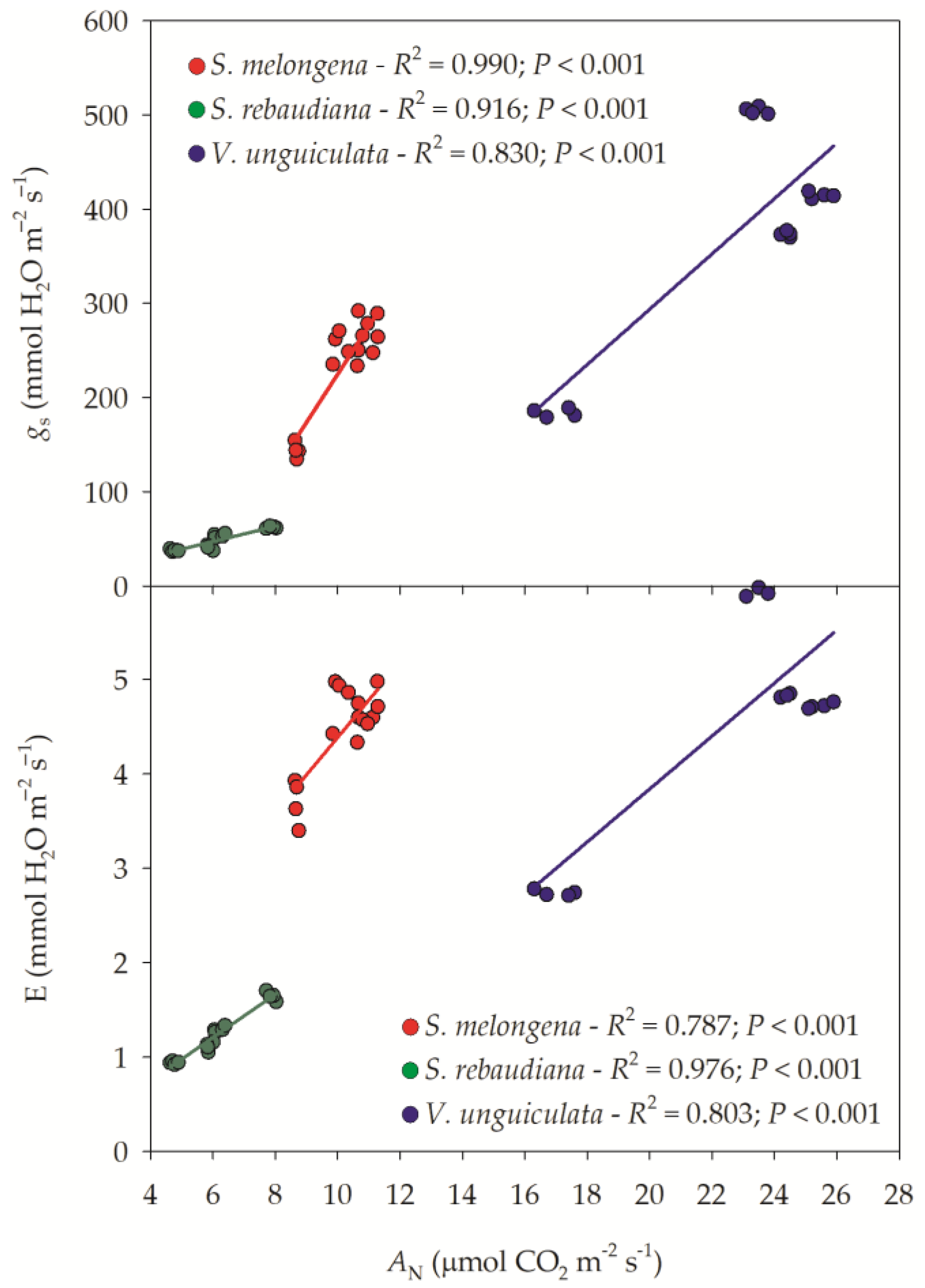
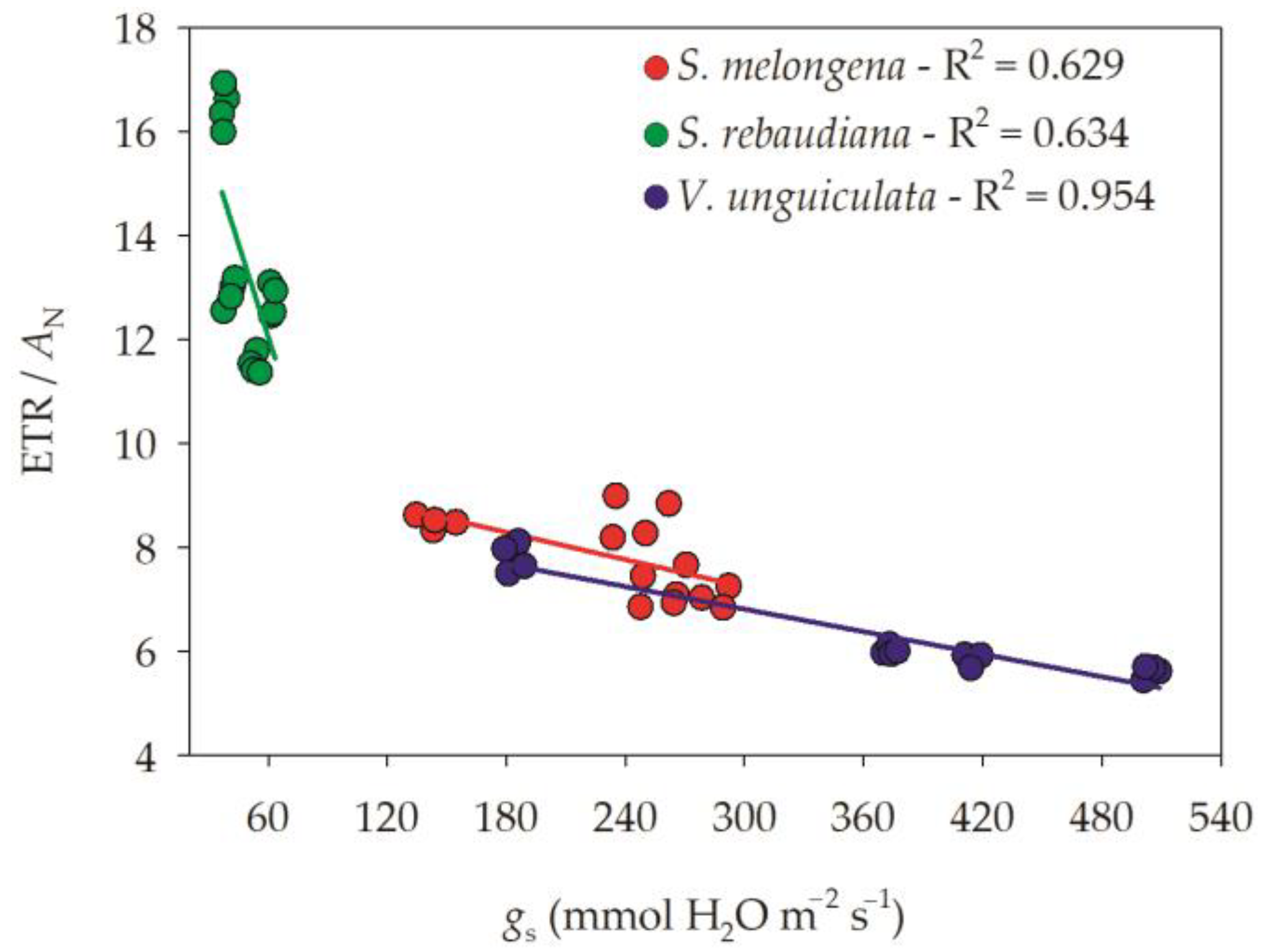
3.1. Gas Exchange Parameters
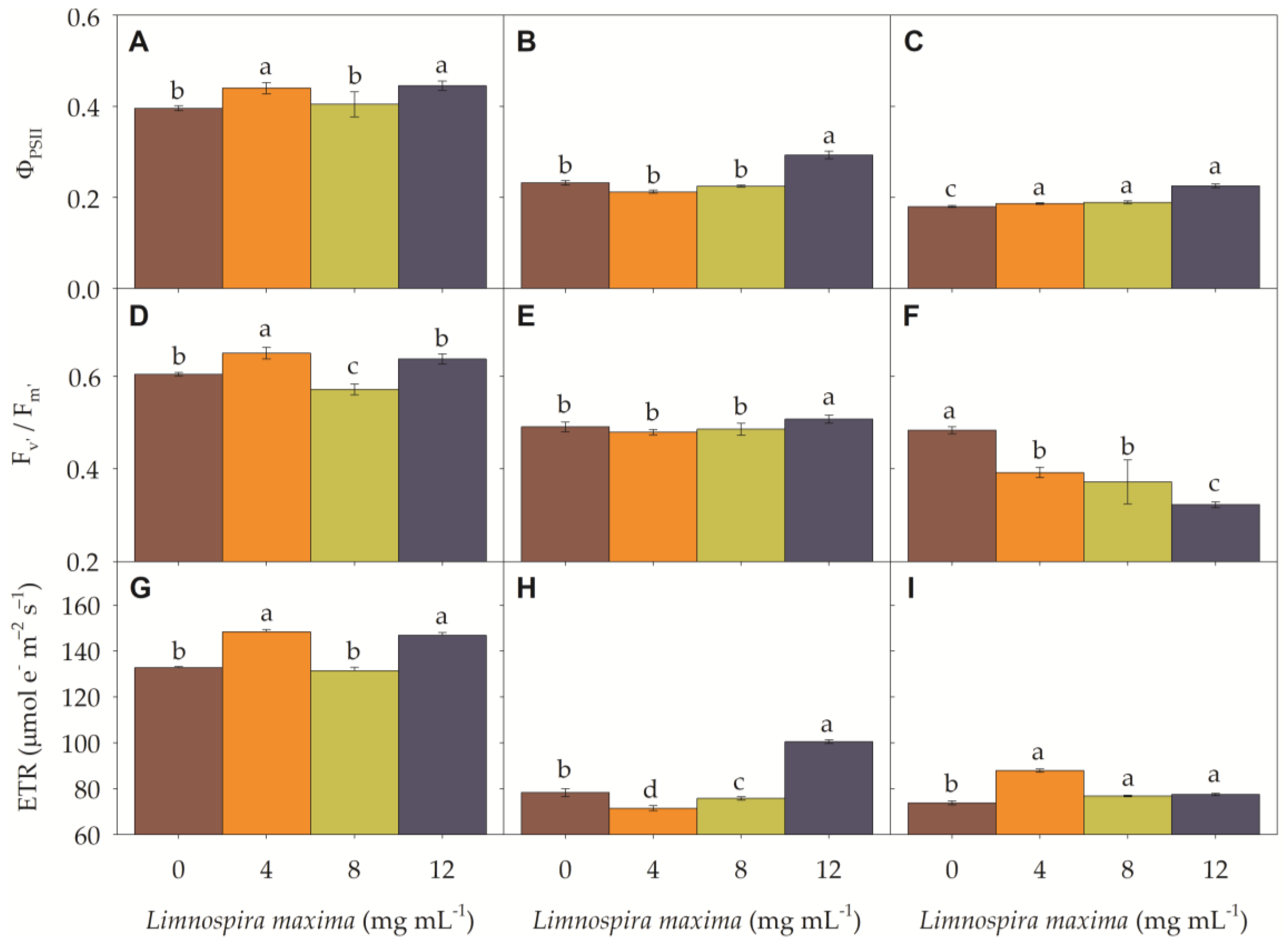
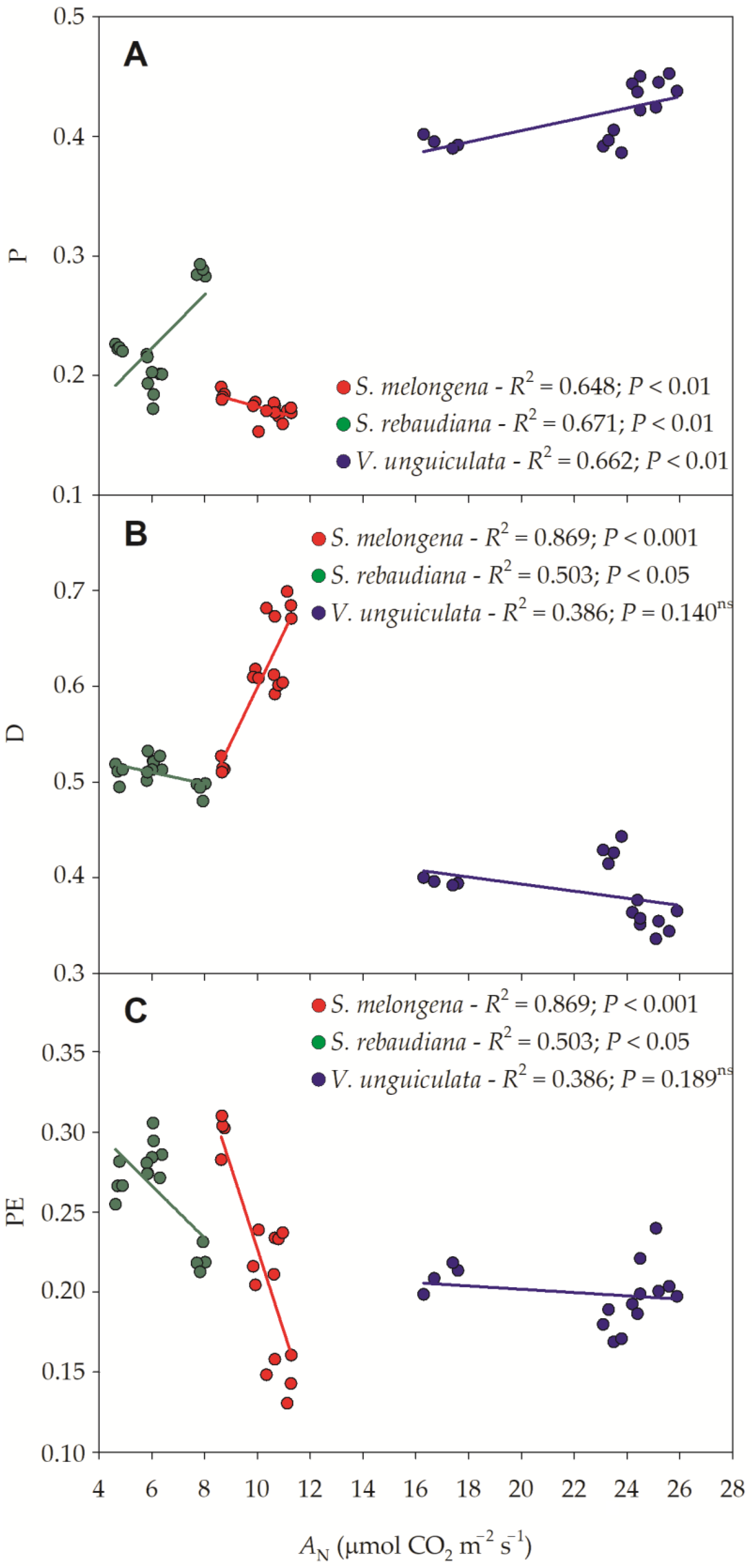
3.2. Chlorophyll a Fluorescence
3.3. Photosynthetic Efficiency
3.4. Phenotypic Characteristics
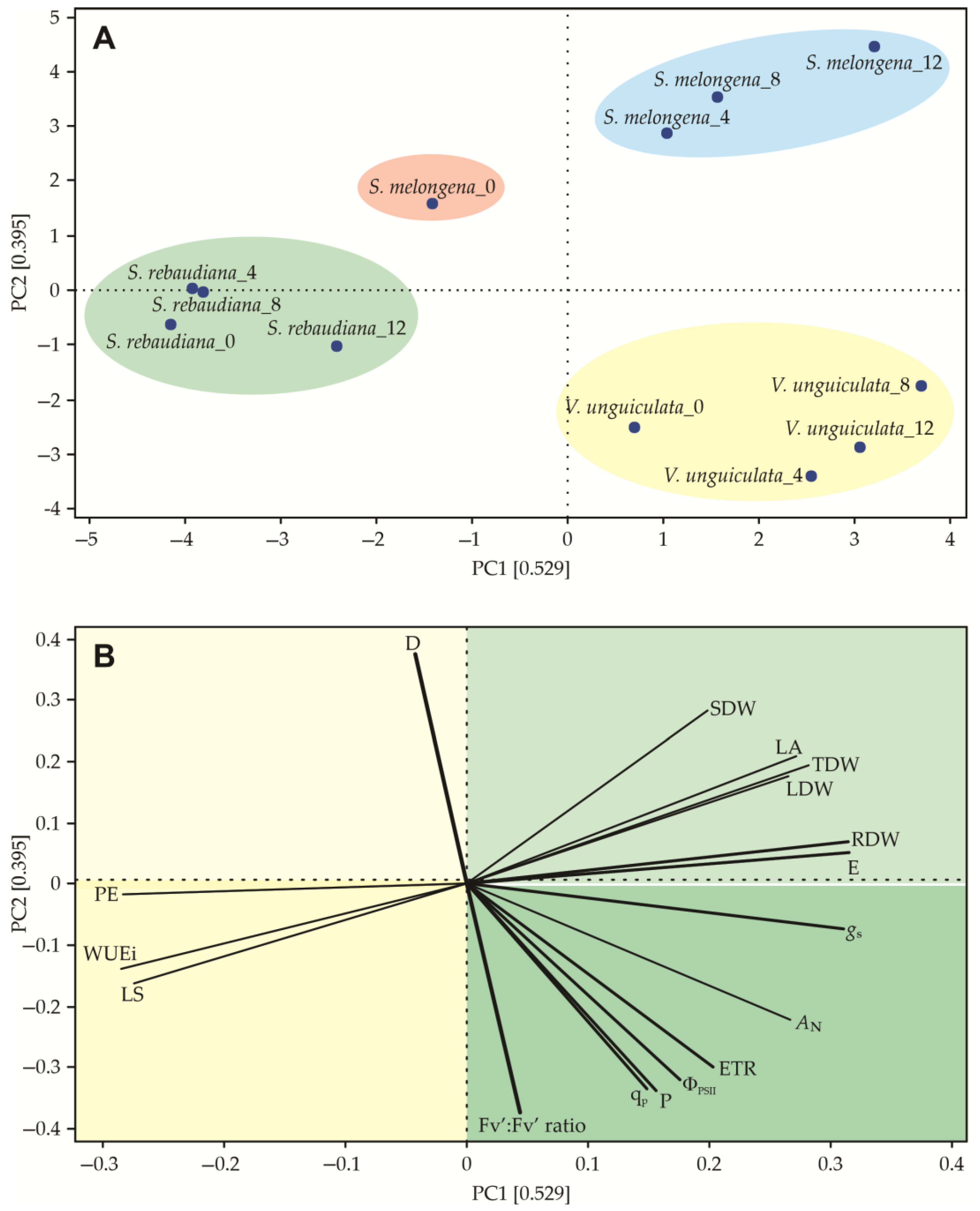
3.5. Principal Component Analysis (PCA)
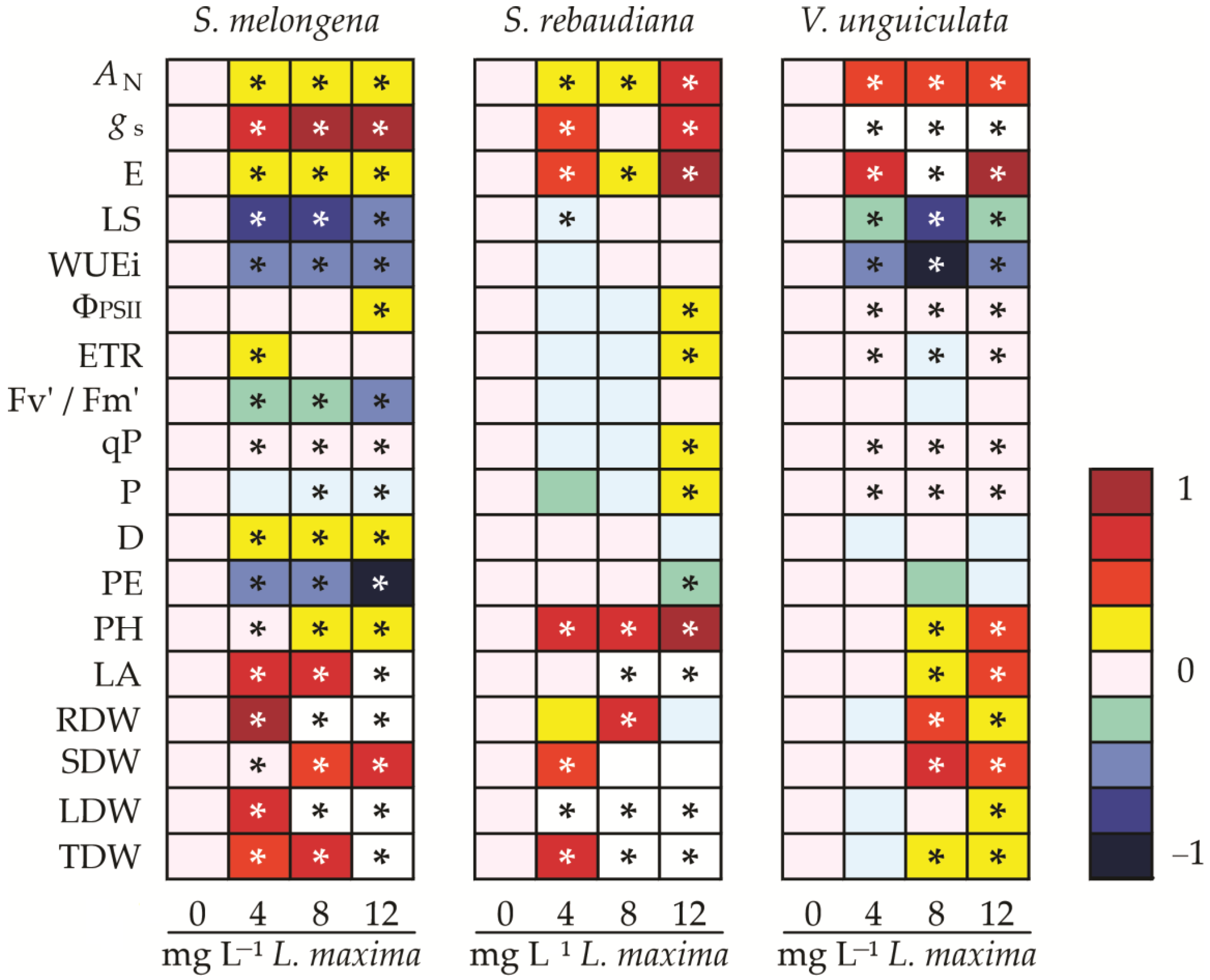
3.6. Heatmap
3.7. Plasticity Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gialeli, M.; Troumbis, A.Y.; Giaginis, C.; Papadopoulou, S.K.; Antoniadis, I.; Vasios, G.K. The Global Growth of ‘Sustainable Diet’ during Recent Decades, a Bibliometric Analysis. Sustainability 2023, 15, 11957. [Google Scholar] [CrossRef]
- Gumbi, N.; Gumbi, L.; Twinomurinzi, H. Towards Sustainable Digital Agriculture for Smallholder Farmers: A Systematic Literature Review. Sustainability 2023, 15, 12530. [Google Scholar] [CrossRef]
- Wu, L.; Du, R.; Liu, Q. A Study on Smallholder Farmers’ Willingness to Sustain Cooperation with New Farmers—Analysis Based on the Perspective of Smallholder Farmers’ Satisfaction. Sustainability 2023, 15, 11363. [Google Scholar] [CrossRef]
- Bibi, F.; Rahman, A. An Overview of Climate Change Impacts on Agriculture and Their Mitigation Strategies. Agriculture 2023, 13, 1508. [Google Scholar] [CrossRef]
- Mahajan, M.; Anuradha; Pal, P.K. Attaining Higher Biomass and Steviol Glycosides Yields of Stevia rebaudiana through Adjustment of Plant Population and Nitrogen Rate. Ind. Crops Prod. 2021, 165, 113426. [Google Scholar] [CrossRef]
- Nandi, P. VMR Stevia Market Size, Share, Trends, Scope, Opportunities and Forecast. Verified Market Research 2022. Available online: https://www.marketresearchfuture.com/reports/stevia-market-1747?utm_term=&utm_campaign=&utm_ source =adwords&utm_medium=ppc&hsa_acc=2893753364&hsa_cam=19912237177&hsa_grp=148712481999&hsa_ad=659589502539&has _src=g&hsa_tgt=dsa-2080758265640&hsa_kw=&hsa_mt=&hsa_net=adwords&hsa_ver=3&gad_source=1&gclid=Cj0KCQiA67 CrBhC1ARIsACKAa8RAJH-QbB9wkM8ddKKPfkcOf655469cNxDeV6CptoSfwr6JK4CK0ocaAiaFEALw_wcB (accessed on 3 December 2023).
- Santo, É.D.E.; Ishii, M.; Pinto, U.M.; Matsudo, M.C.; Carvalho, J.C.M.D. Obtaining Bioproducts from the Studies of Signals and Interactions between Microalgae and Bacteria. Microorganisms 2022, 10, 2029. [Google Scholar] [CrossRef]
- Olorunwa, O.J.; Adhikari, B.; Shi, A.; Barickman, T.C. Screening of Cowpea (Vigna unguiculata (L.) Walp.) Genotypes for Waterlogging Tolerance Using Morpho-Physiological Traits at Early Growth Stage. Plant Sci. 2022, 315, 111136. [Google Scholar] [CrossRef]
- Chen, W.; Modi, D.; Picot, A. Soil and Phytomicrobiome for Plant Disease Suppression and Management under Climate Change: A Review. Plants 2023, 12, 2736. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of Liquid Seaweed Extracts on Growth of Tomato Seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Orona-Navar, A.; Aguilar-Hernández, I.; Nigam, K.D.P.; Cerdán-Pasarán, A.; Ornelas-Soto, N. Alternative Sources of Natural Pigments for Dye-Sensitized Solar Cells: Algae, Cyanobacteria, Bacteria, Archaea and Fungi. J. Biotechnol. 2021, 332, 29–53. [Google Scholar] [CrossRef]
- Bhowmik, D.; Dubey, J.; Mehra, S. Evaluating Potential of Spirulina as Innoculant for Pulses. Acad. J. Plant Sci. 2010, 3, 161–164. [Google Scholar]
- Ekeuku, S.O.; Chong, P.N.; Chan, H.K.; Mohamed, N.; Froemming, G.R.A.; Okechukwu, P.N. Spirulina Supplementation Improves Bone Structural Strength and Stiffness in Streptozocin-Induced Diabetic Rats. J. Tradit. Complement. Med. 2022, 12, 225–234. [Google Scholar] [CrossRef]
- Markou, G.; Chentir, I.; Tzovenis, I. Microalgae and Cyanobacteria as Food: Legislative and Safety Aspects. In Cultured Microalgae for the Food Industry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 249–264. ISBN 978-0-12-821080-2. [Google Scholar]
- Liang, Y.; Yang, C.; Sainju, U.M.; Zhang, N.; Zhao, F.; Wang, W.; Wang, J. Differential Responses of Soil Microbial N-Cycling Functional Genes to 35 Yr Applications of Chemical Fertilizer and Organic Manure in Wheat Field Soil on Loess Plateau. Agronomy 2023, 13, 1516. [Google Scholar] [CrossRef]
- Mógor, Á.F.; De Oliveira Amatussi, J.; Mógor, G.; Bocchetti De Lara, G. Bioactivity of Cyanobacterial Biomass Related to Amino Acids Induces Growth and Metabolic Changes on Seedlings and Yield Gains of Organic Red Beet. Am. J. Plant Sci. 2018, 9, 966–978. [Google Scholar] [CrossRef]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Efficacy of Microalgal Extracts as Biostimulants through Seed Treatment and Foliar Spray for Tomato Cultivation. Ind. Crops Prod. 2020, 151, 112453. [Google Scholar] [CrossRef]
- Mógor, Á.F.; Ördög, V.; Lima, G.P.P.; Molnár, Z.; Mógor, G. Biostimulant Properties of Cyanobacterial Hydrolysate Related to Polyamines. J. Appl. Phycol. 2018, 30, 453–460. [Google Scholar] [CrossRef]
- Toribio, A.J.; Suárez-Estrella, F.; Jurado, M.M.; López, M.J.; López-González, J.A.; Moreno, J. Prospection of Cyanobacteria Producing Bioactive Substances and Their Application as Potential Phytostimulating Agents. Biotechnol. Rep. 2020, 26, e00449. [Google Scholar] [CrossRef]
- Mala, R.; Ruby Celsia, A.S.; Mahalakshmi, R.; Rajeswari, S. Agronomic Biofortification of Amaranthus dubius with Macro Nutrients and Vitamin A. IOP Conf. Ser. Mater. Sci. Eng. 2017, 225, 012214. [Google Scholar] [CrossRef]
- Geries, L.S.M.; Elsadany, A.Y. Maximizing Growth and Productivity of Onion (Allium cepa L.) by Spirulina Platensis Extract and Nitrogen-Fixing Endophyte Pseudomonas Stutzeri. Arch. Microbiol. 2021, 203, 169–181. [Google Scholar] [CrossRef]
- Righini, H.; Francioso, O.; Martel Quintana, A.; Roberti, R. Cyanobacteria: A Natural Source for Controlling Agricultural Plant Diseases Caused by Fungi and Oomycetes and Improving Plant Growth. Horticulturae 2022, 8, 58. [Google Scholar] [CrossRef]
- Żymańczyk-Duda, E.; Samson, S.O.; Brzezińska-Rodak, M.; Klimek-Ochab, M. Versatile Applications of Cyanobacteria in Biotechnology. Microorganisms 2022, 10, 2318. [Google Scholar] [CrossRef]
- Muratova, A.; Gorelova, S.; Golubev, S.; Kamaldinova, D.; Gins, M. Rhizosphere Microbiomes of Amaranthus spp. Grown in Soils with Anthropogenic Polyelemental Anomalies. Agronomy 2023, 13, 759. [Google Scholar] [CrossRef]
- Bloom, A.J. Mineral Nutrition; Plant Physiology and Development; Taiz, L., Zeiger, E., Møller, I.M., Murphy, A., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 107–130. [Google Scholar]
- Pineda-Rodriguez, Y.Y.; Pompelli, M.F.; Jarma-Orozco, A.; Rodríguez, N.V.; Rodriguez-Paez, L.A. A New and Profitable Protocol to DNA Extraction in Limnospira maxima. Methods Protoc. 2023, 6, 62. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Barata-Luís, R.; Vitorino, H.S.; Gonçalves, E.R.; Rolim, E.V.; Santos, M.G.; Almeida-Cortez, J.S.; Ferreira, V.M.; Lemos, E.E.; Endres, L. Photosynthesis, Photoprotection and Antioxidant Activity of Purging Nut under Drought Deficit and Recovery. Biomass Bioenergy 2010, 34, 1207–1215. [Google Scholar] [CrossRef]
- Valladares, F.; Balaguer, L.; Martinez-Ferri, E.; Perez-Corona, E.; Manrique, E. Plasticity, Instability and Canalization: Is the Phenotypic Variation in Seedlings of Sclerophyll Oaks Consistent with the Environmental Unpredictability of Mediterranean Ecosystems? New Phytol. 2002, 156, 457–467. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Espitia-Romero, C.A.; De Diós Jaraba-Navas, J.; Rodriguez-Paez, L.A.; Jarma-Orozco, A. Stevia rebaudiana under a CO2 Enrichment Atmosphere: Can CO2 Enrichment Overcome Stomatic, Mesophilic and Biochemical Barriers That Limit Photosynthesis? Sustainability 2022, 14, 14269. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Cultivation of Microalgae and Cyanobacteria: Effect of Operating Conditions on Growth and Biomass Composition. Molecules 2020, 25, 2834. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Wolosiuk, R.A. Photosynthesis: The Carbon Reactions, 5th ed.; Plant Physiology; Taiz, L., Zeiger, E., Eds.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2010; pp. 247–280. [Google Scholar]
- Altaf, M.A.; Shu, H.; Hao, Y.; Zhou, Y.; Mumtaz, M.A.; Wang, Z. Vanadium Toxicity Induced Changes in Growth, Antioxidant Profiling, and Vanadium Uptake in Pepper (Capsicum annum L.) Seedlings. Horticulturae 2021, 8, 28. [Google Scholar] [CrossRef]
- Jing, F.; Shi, S.; Guan, J.; Lu, B.; Wu, B.; Wang, W.; Ma, R.; Nan, P. Analysis of Phenotypic and Physiological Characteristics of Plant Height Difference in Alfalfa. Agronomy 2023, 13, 1744. [Google Scholar] [CrossRef]
- Snider, J.L.; Chastain, D.R.; Meeks, C.D.; Collins, G.D.; Sorensen, R.B.; Byrd, S.A.; Perry, C.D. Predawn Respiration Rates during Flowering Are Highly Predictive of Yield Response in Gossypium hirsutum When Yield Variability Is Water-Induced. J. Plant Physiol. 2015, 183, 114–120. [Google Scholar] [CrossRef]
- Bustan, A.; Goldschmidt, E.E. Estimating the Cost of Flowering in a Grapefruit Tree. Plant Cell Environ. 1998, 21, 217–224. [Google Scholar] [CrossRef]
- Lee, A.K.; Suh, J.K.; Roh, M.S. Flowering and Changes in Respiration in Asiatic Hybrid Lilies as Influenced by Bulb Vernalization. Sci. Hortic. 2010, 123, 366–371. [Google Scholar] [CrossRef]
- Urban, L.; Lu, P.; Thibaud, R. Inhibitory Effect of Flowering and Early Fruit Growth on Leaf Photosynthesis in Mango. Tree Physiol. 2004, 24, 387–399. [Google Scholar] [CrossRef]
- Urban, L.; Jegouzo, L.; Damour, G.; Vandame, M.; Francois, C. Interpreting the Decrease in Leaf Photosynthesis during Flowering in Mango. Tree Physiol. 2008, 28, 1025–1036. [Google Scholar] [CrossRef]
- Edmeades, G.O.; Daynard, T.B. The Relationship between final Rield and Photosynthesis at Flowering in Individual Maize Plants. Can. J. Plant Sci. 1979, 59, 585–601. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Szufa, S.; Grzesik, M.; Piotrowski, K.; Janas, R. The Promotive Effect of Cyanobacteria and Chlorella Sp. Foliar Biofertilization on Growth and Metabolic Activities of Willow (Salix viminalis L.) Plants as Feedstock Production, Solid Biofuel and Biochar as C Carrier for Fertilizers via Torrefaction Process. Energies 2021, 14, 5262. [Google Scholar] [CrossRef]
- Bais, J.; Kandel, H.; DeSutter, T.; Deckard, E.; Keene, C. Soybean Response to N Fertilization Compared with Co-Inoculation of Bradyrhizobium japonicum and Azospirillum brasilense. Agronomy 2023, 13, 2022. [Google Scholar] [CrossRef]
- Kollmen, J.; Strieth, D. The Beneficial Effects of Cyanobacterial Co-Culture on Plant Growth. Life 2022, 12, 223. [Google Scholar] [CrossRef]
- Falcioni, R.; Antunes, W.C.; Demattê, J.A.M.; Nanni, M.R. Biophysical, Biochemical, and Photochemical Analyses Using Reflectance Hyperspectroscopy and Chlorophyll a Fluorescence Kinetics in Variegated Leaves. Biology 2023, 12, 704. [Google Scholar] [CrossRef]
- Jiang, N.; Yang, Z.; Zhang, H.; Xu, J.; Li, C. Effect of Low Temperature on Photosynthetic Physiological Activity of Different Photoperiod Types of Strawberry Seedlings and Stress Diagnosis. Agronomy 2023, 13, 1321. [Google Scholar] [CrossRef]
- Wang, T.; Li, L.; Qin, Y.; Lu, B.; Xu, D.; Zhuang, W.; Shu, X.; Zhang, F.; Wang, N.; Wang, Z. Effects of Seasonal Changes on Chlorophyll Fluorescence and Physiological Characteristics in the Two Taxus Species. Plants 2023, 12, 2636. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, C.; Bai, Y.; Zhang, N.; Yu, Q.; Zhao, F.; Lu, X. Far-Red Chlorophyll Fluorescence Radiance Tracks Photosynthetic Carbon Assimilation Efficiency of Dark Reactions. Appl. Sci. 2021, 11, 10821. [Google Scholar] [CrossRef]
- Gashash, E.A.; Osman, N.A.; Alsahli, A.A.; Hewait, H.M.; Ashmawi, A.E.; Alshallash, K.S.; El-Taher, A.M.; Azab, E.S.; Abd El-Raouf, H.S.; Ibrahim, M.F.M. Effects of Plant-Growth-Promoting Rhizobacteria (PGPR) and Cyanobacteria on Botanical Characteristics of Tomato (Solanum lycopersicon L.) Plants. Plants 2022, 11, 2732. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Attia, M.S.; Salem, M.S.; Refaay, D.A.; Alhoqail, W.A.; Senousy, H.H. Cyanobacteria-Mediated Immune Responses in Pepper Plants against Fusarium Wilt. Plants 2022, 11, 2049. [Google Scholar] [CrossRef]
- Santos, É.; Massa, A.; Azevedo, J.; Martins, D.; Reimão, M.; Vasconcelos, V.; Campos, A.; Freitas, M. Cyanobacterial Biomass Used as Biofertilizer in Lettuce Plants: Effects on Growth and Cyanotoxin Accumulation. Biol. Life Sci. Forum 2022, 14, 35. [Google Scholar] [CrossRef]
- Bello, A.S.; Saadaoui, I.; Ahmed, T.; Hamdi, H.; Cherif, M.; Dalgamouni, T.; Al Ghazal, G.; Ben-Hamadou, R. Enhancement in Bell Pepper (Capsicum annuum L.) Plants with Application of Roholtiella sp. (Nostocales) under Soilless Cultivation. Agronomy 2021, 11, 1624. [Google Scholar] [CrossRef]
- Shariatmadari, Z.; Riahi, H.; Seyed Hashtroudi, M.; Ghassempour, A.; Aghashariatmadary, Z. Plant Growth Promoting Cyanobacteria and Their Distribution in Terrestrial Habitats of Iran. Soil Sci. Plant Nutr. 2013, 59, 535–547. [Google Scholar] [CrossRef]
- Kholssi, R.; Marks, E.A.N.; Miñón, J.; Maté, A.P.; Sacristán, G.; Montero, O.; Debdoubi, A.; Rad, C. A Consortium of Cyanobacteria and Plant Growth Promoting Rhizobacteria for Wheat Growth Improvement in a Hydroponic System. S. Afr. J. Bot. 2021, 142, 247–258. [Google Scholar] [CrossRef]
- Prasanna, R.; Kanchan, A.; Ramakrishnan, B.; Ranjan, K.; Venkatachalam, S.; Hossain, F.; Shivay, Y.S.; Krishnan, P.; Nain, L. Cyanobacteria-Based Bioinoculants Influence Growth and Yields by Modulating the Microbial Communities Favourably in the Rhizospheres of Maize Hybrids. Eur. J. Soil Biol. 2016, 75, 15–23. [Google Scholar] [CrossRef]
- Prasanna, R.; Ramakrishnan, B.; Simranjit, K.; Ranjan, K.; Kanchan, A.; Hossain, F.; Nain, L. Cyanobacterial and Rhizobial Inoculation Modulates the Plant Physiological Attributes and Nodule Microbial Communities of Chickpea. Arch. Microbiol. 2017, 199, 1311–1323. [Google Scholar] [CrossRef]
- Mutale-joan, C.; Redouane, B.; Najib, E.; Yassine, K.; Lyamlouli, K.; Laila, S.; Zeroual, Y.; Hicham, E.A. Screening of Microalgae Liquid Extracts for Their Bio Stimulant Properties on Plant Growth, Nutrient Uptake and Metabolite Profile of Solanum lycopersicum L. Sci. Rep. 2020, 10, 2820. [Google Scholar] [CrossRef]
- Le Moigne, T.; Crozet, P.; Lemaire, S.D.; Henri, J. High-Resolution Crystal Structure of Chloroplastic Ribose-5-Phosphate Isomerase from Chlamydomonas reinhardtii—An Enzyme Involved in the Photosynthetic Calvin-Benson Cycle. Int. J. Mol. Sci. 2020, 21, 7787. [Google Scholar] [CrossRef]
- Kim, H.-J.; Ku, K.-M.; Choi, S.; Cardarelli, M. Vegetal-Derived Biostimulant Enhances Adventitious Rooting in Cuttings of Basil, Tomato, and Chrysanthemum via Brassinosteroid-Mediated Processes. Agronomy 2019, 9, 74. [Google Scholar] [CrossRef]
- Espinosa-Antón, A.A.; Hernández-Herrera, R.M.; González, M.G. Extractos bioactivos de algas marinas como bioestimulantes del crecimiento y la protección de las plantas. Biotecnol. Veg. 2020, 20, 257–282. [Google Scholar]
- Schiattone, M.I.; Boari, F.; Cantore, V.; Castronuovo, D.; Denora, M.; Di Venere, D.; Perniola, M.; Renna, M.; Sergio, L.; Candido, V. Effects of Nitrogen, Azoxystrobin and a Biostimulant Based on Brown Algae and Yeast on Wild Rocket Features at Harvest and During Storage. Agronomy 2021, 11, 2326. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. Growth and Nutraceutical Properties Are Enhanced by Biostimulants in a Long-Term Period: Chemical and Metabolomic Approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef]
- Baroni, A.M. Caracterização do Sistema Fotossintético em Folhas de Plantas Jovens de Caesalpinia echinata Lam. (pau-brasil). Ph.D. Thesis, Instituto de Botânica, São Paulo, Brazil, 2005. [Google Scholar]
- Mengarda, L.H.G.; Milanez, C.R.D.; Silva, D.M.; Aguilar, M.A.G.; Cuzzuol, G.R.F. Morphological and Physiological Adjustments of Brazilwood (Caesalpinia echinata Lam.) to Direct Solar Radiation. Braz. J. Plant Physiol. 2012, 24, 161–172. [Google Scholar] [CrossRef]
- Balah, M.A.; Balah, A.M. Growth and Ecological Characteristics of Physalis angulata Invasive Weed Species in Several Invaded Communities. Biologia 2022, 77, 325–338. [Google Scholar] [CrossRef]
- Pájaro-Esquivia, Y.S.; Domínguez-Haydar, Y.; Tinoco-Ojanguren, C. Intraspecific Variation in Morpho-Functional Traits and Plastic Response to Water and Light in Seedlings of Aspidosperma polyneuron (Apocynaceae). Flora 2021, 282, 151903. [Google Scholar] [CrossRef]
- Mhlanga, P.F.; Shaik, S. Phenotypic Plasticity in Cucurbita maxima and C. moschata Seedlings as a Measure of Drought Stress Tolerance under Pot Screening. S. Afr. J. Bot. 2023, 159, 290–301. [Google Scholar] [CrossRef]
- Adu, M.O. Causal Shoot and Root System Traits to Variability and Plasticity in Juvenile Cassava (Manihot esculenta Crantz) Plants in Response to Reduced Soil Moisture. Physiol. Mol. Biol. Plants 2020, 26, 1799–1814. [Google Scholar] [CrossRef]
- Goswami, D.; Parmar, S.; Vaghela, H.; Dhandhukia, P.; Thakker, J.N. Describing Paenibacillus Mucilaginosus Strain N3 as an Efficient Plant Growth Promoting Rhizobacteria (PGPR). Cogent Food Agric. 2015, 1, 1000714. [Google Scholar] [CrossRef]
- Alshallash, K.S.; Mohamed, M.F.; Dahab, A.A.; Abd El-Salam, H.S.; El-Serafy, R.S. Biostimulation of Plectranthus amboinicus (Lour.) Spreng. with Different Yeast Strains: Morphological Performance, Productivity, Phenotypic Plasticity, and Antioxidant Activity. Horticulturae 2022, 8, 887. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, Soil and Plants: A Critical Review of Microalgae as Renewable Resources for Agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
| pH | O.C | S | P | Ca | Mg | K | Na | Cu | Fe | Mn | B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1:1 | % | mg kg−1 | cmol (+) kg−1 | mg kg−1 | |||||||
| 6.34 | 0.75 | 16.4 | 36.0 | 2.91 | 1.36 | 0.46 | 0.20 | 0.69 | 12.9 | 14.8 | 0.32 |
| N | P | K | Mg | Ca | Na | Protein | Carbohydrates | Ash |
|---|---|---|---|---|---|---|---|---|
| mg g−1 DW | g g−1 DW | |||||||
| 0.11 | 0.41 | 0.35 | 0.12 | 0.11 | 0.40 | 0.02 | 0.017 | 0.002 |
| Species | Limnospira maxima (mg mL−1) | Root Dry Weight (g) | Stem Dry Weight (g) | Leaf Dry Weight (g) | Total Dry Weight (g) | Canopy/Root Ratio |
|---|---|---|---|---|---|---|
| V. unguiculata | 0 | 2.27 ± 0.02 c | 1.64 ± 0.02 d | 2.82 ± 0.02 b | 6.72 ± 0.03 c | 1.97 ± 0.03 b |
| 4 | 2.22 ± 0.02 d | 1.84 ± 0.02 c | 2.61 ± 0.02 c | 6.68 ± 0.04 c | 2.00 ± 0.04 b | |
| 8 | 3.13 ± 0.03 a | 2.64 ± 0.02 a | 2.82 ± 0.03 b | 8.60 ± 0.06 a | 1.74 ± 0.02 c | |
| 12 | 2.63 ± 0.01 b | 2.44 ± 0.02 b | 3.24 ± 0.03 a | 8.31 ± 0.03 b | 2.15 ± 0.02 a | |
| S. rebaudiana | 0 | 0.49 ± 0.05 c | 0.60 ± 0.08 d | 0.59 ± 0.03 d | 1.67 ± 0.10 d | 2.45 ± 0.21 d |
| 4 | 0.60 ± 0.07 b | 0.87 ± 0.05 c | 1.30 ± 0.06 c | 2.77 ± 0.13 c | 3.67 ± 0.47 c | |
| 8 | 0.74 ± 0.04 a | 1.91 ± 0.07 b | 1.58 ± 0.03 b | 4.23 ± 0.04 b | 4.71 ± 0.39 b | |
| 12 | 0.46 ± 0.10 c | 2.36 ± 0.10 a | 1.71 ± 0.07 a | 4.54 ± 0.02 a | 9.26 ± 2.43 a | |
| S. melongena | 0 | 1.20 ± 0.006 d | 3.23 ± 0.06 d | 1.78 ± 0.05 d | 6.21 ± 0.11 d | 4.17 ± 0.10 a |
| 4 | 2.29 ± 0.003 c | 3.70 ± 0.08 c | 2.86 ± 0.11 c | 8.85 ± 0.13 c | 2.86 ± 0.05 c | |
| 8 | 2.45 ± 0.004 b | 4.30 ± 0.08 b | 3.81 ± 0.09 b | 10.57 ± 0.15 b | 3.31 ± 0.06 b | |
| 12 | 3.30 ± 0.006 a | 4.90 ± 0.05 a | 5.91 ± 0.09 a | 14.11 ± 0.13 a | 3.28 ± 0.04 b |
| Photosynthetic Features | S. melongena | S. rebaudiana | V. unguiculata | Mean Value |
|---|---|---|---|---|
| AN | 0.235 | 0.423 | 0.371 | 0.343 |
| gs | 0.539 | 0.423 | 0.648 | 0.537 |
| E | 0.318 | 0.459 | 0.550 | 0.442 |
| LS | 0.404 | 0.228 | 0.408 | 0.347 |
| WUEi | 0.434 | 0.306 | 0.531 | 0.424 |
| Mean value | 0.386 ± 0.116 a | 0.368 ± 0.097 a | 0.501 ± 0.113 a | 0.418 ± 0.080 A |
| Chlorophyll a fluorescence features | ||||
| ΦPSII | 0.225 | 0.306 | 0.154 | 0.228 |
| ETR | 0.176 | 0.309 | 0.133 | 0.206 |
| Fv′/Fm′ | 0.385 | 0.101 | 0.161 | 0.216 |
| qP | 0.095 | 0.337 | 0.071 | 0.168 |
| P | 0.197 | 0.412 | 0.146 | 0.252 |
| D | 0.270 | 0.098 | 0.242 | 0.203 |
| PE | 0.579 | 0.305 | 0.296 | 0.393 |
| Mean value | 0.275 ± 0.161 a | 0.267 ± 0.120 a | 0.172 ± 0.074 a | 0.238 ± 0.073 A |
| Morphological features | ||||
| PH | 0.200 | 0.548 | 0.355 | 0.368 |
| LA | 0.574 | 0.596 | 0.317 | 0.495 |
| RDW | 0.639 | 0.581 | 0.302 | 0.507 |
| SDW | 0.362 | 0.799 | 0.395 | 0.519 |
| LDW | 0.713 | 0.701 | 0.207 | 0.540 |
| TDW | 0.572 | 0.650 | 0.234 | 0.485 |
| Mean value | 0.510 ± 0.192 a | 0.646 ± 0.093 a | 0.301 ± 0.071 b | 0.486 ± 0.061 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariza-González, A.R.; Jarma-Orozco, A.; Jaraba-Navas, J.d.D.; Pico-González, A.I.; Herazo-Cárdenas, D.S.; Vegliante Arrieta, D.; Vallejo-Isaza, A.; Pineda-Rodriguez, Y.Y.; Rodriguez-Paez, L.A.; Pompelli, M.F. Net Photosynthesis and Biomass Production in Stevia, Eggplant, and Cowpea Can Be Improved by Fertilization with Cyanobacteria (Limnospira maxima). Horticulturae 2023, 9, 1309. https://doi.org/10.3390/horticulturae9121309
Ariza-González AR, Jarma-Orozco A, Jaraba-Navas JdD, Pico-González AI, Herazo-Cárdenas DS, Vegliante Arrieta D, Vallejo-Isaza A, Pineda-Rodriguez YY, Rodriguez-Paez LA, Pompelli MF. Net Photosynthesis and Biomass Production in Stevia, Eggplant, and Cowpea Can Be Improved by Fertilization with Cyanobacteria (Limnospira maxima). Horticulturae. 2023; 9(12):1309. https://doi.org/10.3390/horticulturae9121309
Chicago/Turabian StyleAriza-González, Anthony Ricardo, Alfredo Jarma-Orozco, Juan de Dios Jaraba-Navas, Ana Isabel Pico-González, Diana Sofia Herazo-Cárdenas, Daniela Vegliante Arrieta, Adriana Vallejo-Isaza, Yirlis Yadeth Pineda-Rodriguez, Luis Alfonso Rodriguez-Paez, and Marcelo F. Pompelli. 2023. "Net Photosynthesis and Biomass Production in Stevia, Eggplant, and Cowpea Can Be Improved by Fertilization with Cyanobacteria (Limnospira maxima)" Horticulturae 9, no. 12: 1309. https://doi.org/10.3390/horticulturae9121309
APA StyleAriza-González, A. R., Jarma-Orozco, A., Jaraba-Navas, J. d. D., Pico-González, A. I., Herazo-Cárdenas, D. S., Vegliante Arrieta, D., Vallejo-Isaza, A., Pineda-Rodriguez, Y. Y., Rodriguez-Paez, L. A., & Pompelli, M. F. (2023). Net Photosynthesis and Biomass Production in Stevia, Eggplant, and Cowpea Can Be Improved by Fertilization with Cyanobacteria (Limnospira maxima). Horticulturae, 9(12), 1309. https://doi.org/10.3390/horticulturae9121309









