Abstract
The aim of this study was to find out how different postharvest temperatures and MeJA treatments affected the quality of table grapes, their antioxidant properties, and the amount of hydrogen peroxide and malondialdehyde they contained. For the investigation, postharvest Shine Muscat table grapes were treated with low and high temperatures and MeJA at concentrations of 10 and 100 μmol/L. The results indicated that treating grape berries with MeJA at concentrations of 10 and 100 μmol/L effectively reduced weight loss and mitigated the increase in soluble solid content while also mitigating the decrease in berry firmness and titratable acidity. Consequently, this treatment preserved the sensory and nutritional qualities of the berries and extended their shelf life. Meanwhile, the application of MeJA at a concentration of 10 μmol/L demonstrated superior effectiveness compared to the 100 μmol/L concentration and resulted in a significant enhancement of antioxidant activities by increasing levels of superoxide dismutase, catalase, ascorbate peroxidase, and polyphenol oxidase. Furthermore, the levels of hydrogen peroxide and malondialdehyde in the samples increased for all treatments throughout the storage period. Nevertheless, the levels of hydrogen peroxide and malondialdehyde generation following MeJA treatment remained much lower compared to samples treated at room temperature and low temperature. Therefore, the postharvest application of MeJA at a concentration of 10 μmol/L could play a critical role as a stimulator of fruit quality as well as enhance physicochemical parameters and antioxidant activities for extending the shelf life of grapes during storage.
1. Introduction
Table grape cultivars are among the most widely consumed non-climacteric fruits globally. China has been the world’s leading producer of table grapes since 2011, with over 582,728 hectares planted and an annual yield of 11,269,900 t in 2021 [1]. Over the past ten years, table grape agriculture in China has spread from the western and northern regions to the southwestern and southern provinces of Sichuan, Jiangsu, Guangxi, and Yunnan [2]. Table grapes have a low physiological activity rate, and traits like appearance, color, texture, flavor, and aroma determine their quality. The “veraison” stage marks the onset of ripening, during which the grapes undergo changes such as sugar accumulation, berry softening, anthocyanin synthesis, organic acid metabolism, and the accumulation of flavour compounds [3].
Soluble solid content (SSC) and sugar/acid ratios are key indicators of table grape quality, with specific minimum requirements set for each cultivar [4]. The synthesis of hundreds of different volatile compounds during ripening determines the flavour of table grapes, which is a complex and important aspect of their quality [5]. Table grapes are highly perishable after harvest and sensitive to water loss due to rachis and pedicel desiccation, resulting in browning, weight loss, and fruit softening. Additionally, the necrotrophic fungus Botrytis cinerea is primarily responsible for fungal degradation, which results in significant losses [6]. This fungus grows quickly and can spread through berries even at low temperatures (LT) around 0 °C. As a result, table grape preservation is difficult and depends on a variety of characteristics, with temperature and relative humidity being especially important.
Temperature is one of the most important factors affecting fruit storage. Fruit can have its shelf life extended after harvest by being stored at the right temperature, which also reduces quality deterioration and microbiological infection [7,8]. A growing body of research suggests that low-temperature storage plays a protective role in fruits by regulating antioxidant activity, thereby reducing the accumulation of reactive oxygen species (ROS). This mechanism helps to minimize nutrient consumption and preserve fruit quality [9,10,11]. According to Zhao et al. [12], preserving sweet cherry and nectarine at near-ice temperatures considerably increased the activities of superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX). Storage at 4 °C also kept the antioxidant enzyme activities in peach fruit active for a considerable amount of time [13]. The findings of the study demonstrated that low-temperature storage can effectively maintain high antioxidant enzyme activity and delay senescence in fruits. This preservation of antioxidant activity contributes to the preservation of stored fruit quality and extends the overall storage period.
Methyl jasmonate (MeJA) is a plant hormone that acts as a signal molecule involved in the growth and development of plants. It also plays a crucial role in the plant’s response to various abiotic and biotic stresses [14,15,16]. Several previous studies and investigations show that the exogenous application of MeJA has a positive impact on the quality and shelf life of various harvested fruits, such as wine grape and bell pepper, which could be attributed to improved oxidation resistance. Moreover, MeJA applications have been shown to extend shelf life and be beneficial in mitigating symptoms of chilling injury in sweet orange and pomegranate [17,18]. It has been proposed that using MeJA lowers the activity of enzymes that hydrolyse glycosidic connections between cell wall components to promote cell wall softening in fruits, enhancing firmness and resistance to mechanical damage while indirectly lowering microbial attack [19].
MeJA has recently been used to improve the chilling resistance of fruits and vegetables such as pepper and pineapple [20,21]. It is possible that a stronger antioxidant defence system and a lower malondialdehyde (MDA) level are linked to MeJA’s ability to protect fruit quality from chilling damage.
Treatment with MeJA raised the levels of ascorbic acid and carotenoids in cherry tomato fruits that had already been picked. It also caused bioactive metabolites to build up [22]. During storage, a particular quantity of exogenous MeJA treatment preserved the fruit quality of blood orange and retained greater total phenolic and anthocyanin content than in the control [23]. Additionally, postharvest MeJA treatment can preserve and even enhance the nutritional and medicinal quality of medlar, wine grape, mandarin fruit, and Centella asiatica [24,25,26,27].
It has been demonstrated in several studies that the application of MeJA can improve the innate disease resistance of plants against pathogen infection and ecological stresses such as cold [28]. Moreover, MeJA has been shown to have insecticidal properties in agricultural crops by enhancing the activities of chitinase and β-1,3 glucanases in plant leaves [29]. Furthermore, exogenous MeJA has been found to enhance chilling tolerance in horticultural crops by improving the expression of heat shock proteins (HSPs) and C-repeat binding factor (CBF) [30]. These findings suggest that treatment with MeJA could be a valuable approach to reducing postharvest diseases and enhancing fruit quality, ultimately resulting in an extended shelf life for horticultural produce. Therefore, the aim of our study is to investigate how temperature regulation and MeJA affect the antioxidant activities of the Shine Muscat grape from a physiochemical perspective during a period of storage.
2. Materials and Methods
2.1. Fruit Samples and Treatments
The investigation was conducted using white table grapes, namely the Shine Muscat varietal. The Shine Muscat grapes were manually collected from the experimental vineyard of Nanjing Agricultural University, situated in the Jiangsu Academy of Agricultural Sciences in China. The grape bunches were promptly transported to the laboratory in cardboard boxes within a span of one hour. Prior to the experiment, the bunches were organized to guarantee that they possessed identical dimensions and hues and were devoid of any imperfections or indications of illness. The experiment took place at the laboratory of the pomology department, situated at Nanjing Agricultural University in Jiangsu Province, China. The bunches were divided into four groups, as indicated in Table 1. There were 15 boxes in each group, and each group had three boxes that served as replications. Subsequently, the bunches were placed on filter paper and packed in plastic boxes weighing 500 g each prior to storage.

Table 1.
Experimental Conditions for Storage of Shine Muscat Grape Bunches for a Duration of 30 Days.
Analyses were conducted on days 0, 10, 20, and 30 following the treatments. For the RT treatment, no measurements were taken on the 30th day except for estimating the weight loss at the end of storage because it had reached the unacceptable limit for consumption and marketing. Following the physiological evaluation, five berries were taken as samples and tested three times for subsequent analyses. The samples were immediately frozen in liquid nitrogen and stored at −80 °C.
2.2. Measurement of Physicochemical Properties
The fruit’s quality was assessed by measuring the weight loss ratio, firmness, soluble solid content (SSC), and titratable acidity (TA) using the methodology outlined in the AOAC [31] guidelines. Five berries were utilised in order to ascertain the weight reduction of the samples, and their initial weight was measured prior to storage. The weights of the remaining samples were measured at each sampling stage. Weight loss was determined by subtracting the weight at each sampling stage from the initial weight of each sample, dividing the difference by the initial weight, and then multiplying the quotient by 100.
A digital penetrometer (GY-4 digital fruit penetrometer, China) was used to measure the penetration force required for a 6 mm diameter probe to enter the berry at a rate of 5 mm/s to a depth of 5 mm. The experiment involved using five berries, which were tested three times, and the measurements were recorded in Newtons (N).
Five berries from each bunch were mixed and ground to make a homogeneous sample that was the same for SSC testing. The measurement was made in triplicate at 20 °C using a digital refractometer (3T, Atago Co., Ltd., Tokyo, Japan). A few drops of juice were put on the prism of the refractometer, and the results were expressed as a percentage.
Five mL of juice was taken and titrated with 0.1 N sodium hydroxide (NaOH) to a phenolphthalein endpoint using a colour indicator (clear to pink) to measure the percentage of titratable acidity. Total acidity was represented as a percentage of tartaric acid in the results.
2.3. Measurement of Antioxidant Enzyme Activities
The evaluation of antioxidant enzyme activity was conducted using the method described by Modesti et al. [25]. The frozen berry tissue powder was dissolved in an extraction buffer (2:5 w/v) containing 100 mM potassium phosphate buffer (pH 7.8), 100 mM sodium EDTA (pH 7), 1.25 mM polyethylene glycol, and 2 mM dithiothreitol to obtain the total soluble proteins. After being thoroughly mixed in the mortar with a 5% solution of polyvinylpolypyrrolidone, the sample was then transferred to a 2 mL Eppendorf tube. The samples were subjected to a 30 min 14,000× g centrifugation at 4 °C. The supernatant was used to conduct enzyme activity experiments.
A mix of 1.5 mL of 100 μL crude enzyme extract, 50 mM of potassium phosphate buffer (pH 7.8), 0.1 mM sodium EDTA, 13 mM of methionine, 75 μM NBT, and 2 μM riboflavin was made to measure SOD activity. Riboflavin was added to start the reaction, and the absorbance at 560 nm was measured after 15 min of incubation at room temperature with constant illumination. The amount of enzyme that, under the abovementioned assay conditions, inhibits the rate of NBT degradation by 50% is considered one SOD unit. The activity of SOD was determined as U g/FW using a UV spectrophotometer (Shimadzu UV-1800, Tokyo, Japan) with proper calibration.
To evaluate the activity of CAT, a reaction mixture was made by mixing 1.5 mL of a crude enzyme extract with 100 L, 20 mM of H2O2, and 50 mM of a pH 7 potassium phosphate buffer. The procedure began with the introduction of H2O2, and as a result of its decomposition, the absorbance at 240 nm at 25° C for 1 min decreased. CAT activity was measured as μmol H2O2/g FW.
APX activity was determined using a reaction mixture with a final volume of 1.5 mL. The mixture consisted of 20 μL of pure enzyme extract, 100 mM of potassium phosphate buffer (pH 7), 0.25 mM of ascorbic acid, 0.70 mM of H2O2, and 0.66 mM of sodium EDTA (pH 7). The process was initiated by introducing H2O2, and the ascorbic acid oxidation was evaluated by measuring the reduction at 290 nm. The activity of APX was expressed as μmol H2O2/g FW.
Polyphenol oxidase (PPO) activity was determined by incubating 1.5 mL of a final volume containing 500 mM of catechol in 100 mM of sodium phosphate buffer, pH 6.4, with 20 μL of a crude enzyme extract and observing the rise in absorbance at 398 nm. The molar difference in catechol-specific activity was expressed as μmol/g FW.
2.4. Measurement of H2O2 and Malondialdehyde (MDA) Content
To assay the hydrogen peroxide (H2O2) and MDA content, we followed the method according to Wang et al. [32]. In brief, 4 mL of 0.1% cold trichloroacetic acid was used to homogenize 0.5 g of the sample. After 20 min in an ice bath, the homogenate was centrifuged at 12,000× g and 4 °C for 20 min. The supernatants produced were collected for analysis.
For H2O2 content measurement, the reaction system was prepared by collecting 0.5 mL of supernatant, 1 mL of 1 M potassium iodide, and 0.5 mL of 10 mM potassium phosphate buffer (pH 7.0). The reaction system was incubated at 25 °C for one hour in the dark before determining the absorbance at 390 nm. H2O2 was used as a standard, and the H2O2 content was expressed as μmol H2O2/g FW.
To measure the MDA content, 0.4 g of the sample was homogenized in 8 mL of 10% cold trichloroacetic acid and centrifuged at 10,000× g and 4 °C for 20 min. The resulting 2 mL of supernatant was mixed with 2 mL of 20% trichloroacetic acid containing 0.5% thiobarbituric acid and incubated at 95 °C for 30 min. After quick cooling on ice, the reaction mixture was centrifuged at 10,000× g for 10 min at 4 °C. The absorbance of the system was measured at 450, 532, and 600 nm. The MDA content was measured using the equation below and expressed on a (μmol/g FW) basis:
MDA content = 6.452 × (OD532 − OD600) − 0.559 × OD450
2.5. Statistical Analysis
The data were analysed using one-way analysis of variance (ANOVA) with CoStat software, as described by Snedecor and Cochran [33]. The results were evaluated as the mean ± standard error (SE). Significance analysis was performed using Duncan’s multiple range tests, with a p value < 0.05 considered significant.
3. Results
3.1. Effect of RT, LT, and MeJA on Weight Loss, Firmness, TSS, and TA
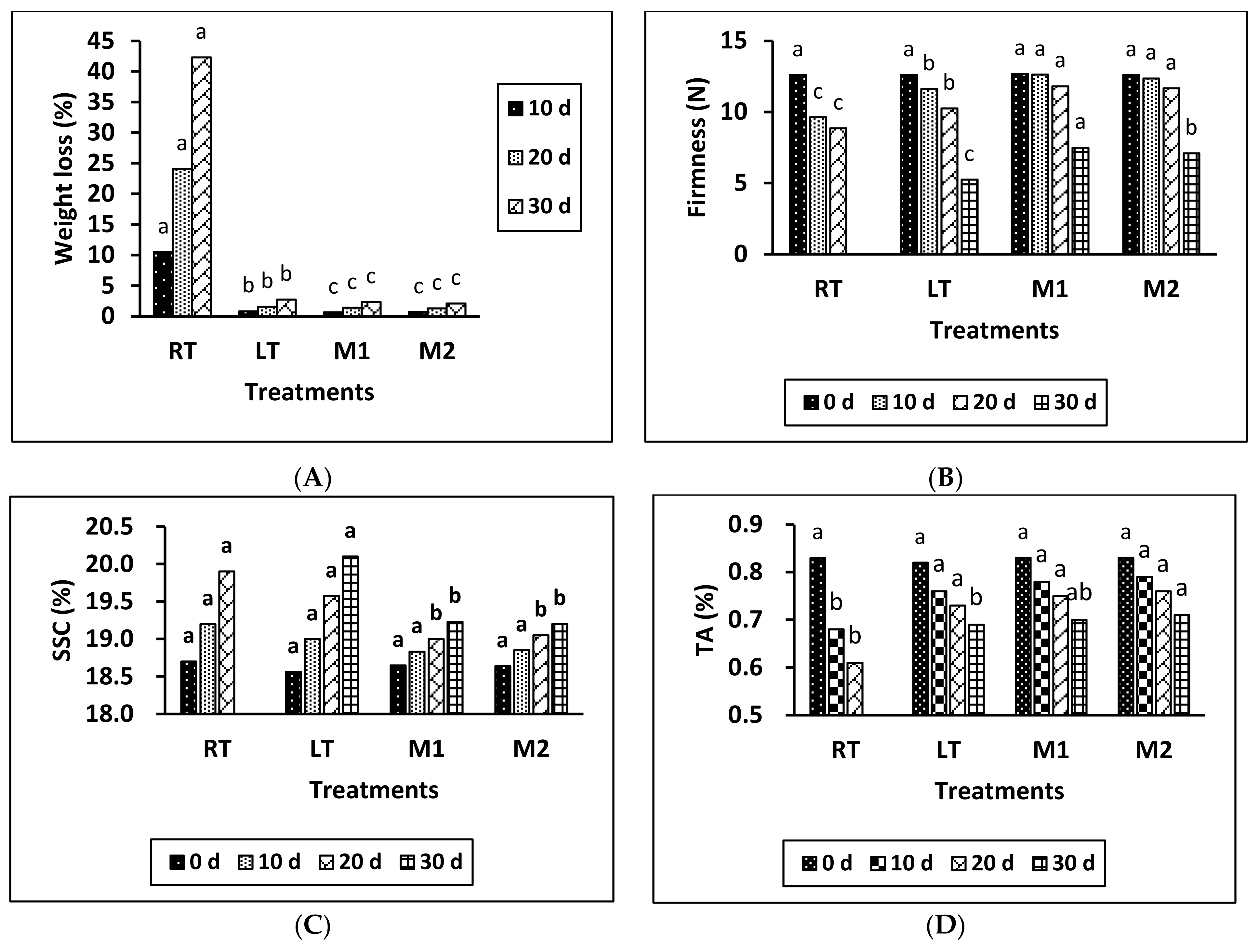
According to the results presented in Figure 1A,B, it is evident that there were significant differences between the treatments in terms of weight loss and berry firmness. In terms of weight loss, the application of MeJA (M1 and M2) resulted in significantly lower weight loss compared to both the RT and LT treatments across all storage intervals. Notably, there were no significant differences between the M1 and M2 treatments in terms of weight loss at any of the storage intervals. Moreover, the highest weight loss percentages were recorded for the RT group and the LT group during all storage intervals.
Figure 1.
Effects of temperature and MeJA on weight loss (A), firmness (B), SSC (C), and TA content (D) in grapes during the storage period. The data presented are expressed as the mean ± (SE) of triplicate assays. Significant differences between the RT, LT, and MeJA samples within the same period were determined using Duncan’s test (p < 0.05), indicated by different letters.
Considering berry firmness, the MeJA-treated samples exhibited significantly greater berry firmness compared to the untreated RT and LT samples. Additionally, berry firmness was consistently lower at the 10- and 20-day storage intervals for the RT treatment compared to the MeJA-treated samples.
In summary, the application of MeJA had a significant impact on reducing weight loss and maintaining berry firmness compared to both RT and LT treatments across the storage periods. Furthermore, there were no significant differences in weight loss or firmness between the M1 and M2 treatments at any of the storage intervals, except for the 30-day interval, where there was a significant difference in firmness between them.
According to the data provided in Figure 1C,D, the results indicate that the MeJA treatment effectively reduced the increase in SSC in comparison to samples stored at RT and LT, particularly over the period of 20 to 30 days of storage. Nevertheless, no notable disparities were detected among the various treatments over the 0- and 10-day storage periods. Furthermore, there was no discernible disparity observed between the M1 and M2 treatments after 20 and 30 days of storage. There was no discernible disparity between the RT and LT treatments during the storage period.
Furthermore, the TA percentage in all samples gradually decreased during storage. There were no significant differences between M1, M2, and LT treatments at 0, 10, and 20 days of storage, except for the 30-day storage interval, where a significant difference was observed between the M2 and LT treatments. The RT treatment exhibited a much lower TA percentage compared to the other treatments. Therefore, the main result is that the MeJA treatment greatly slowed the rise in SSC, especially after 30 days of storage. It also had different effects on TA compared to the other treatments.
3.2. Effect of RT, LT, and MeJA on SOD, CAT, APX, and PPO Activity
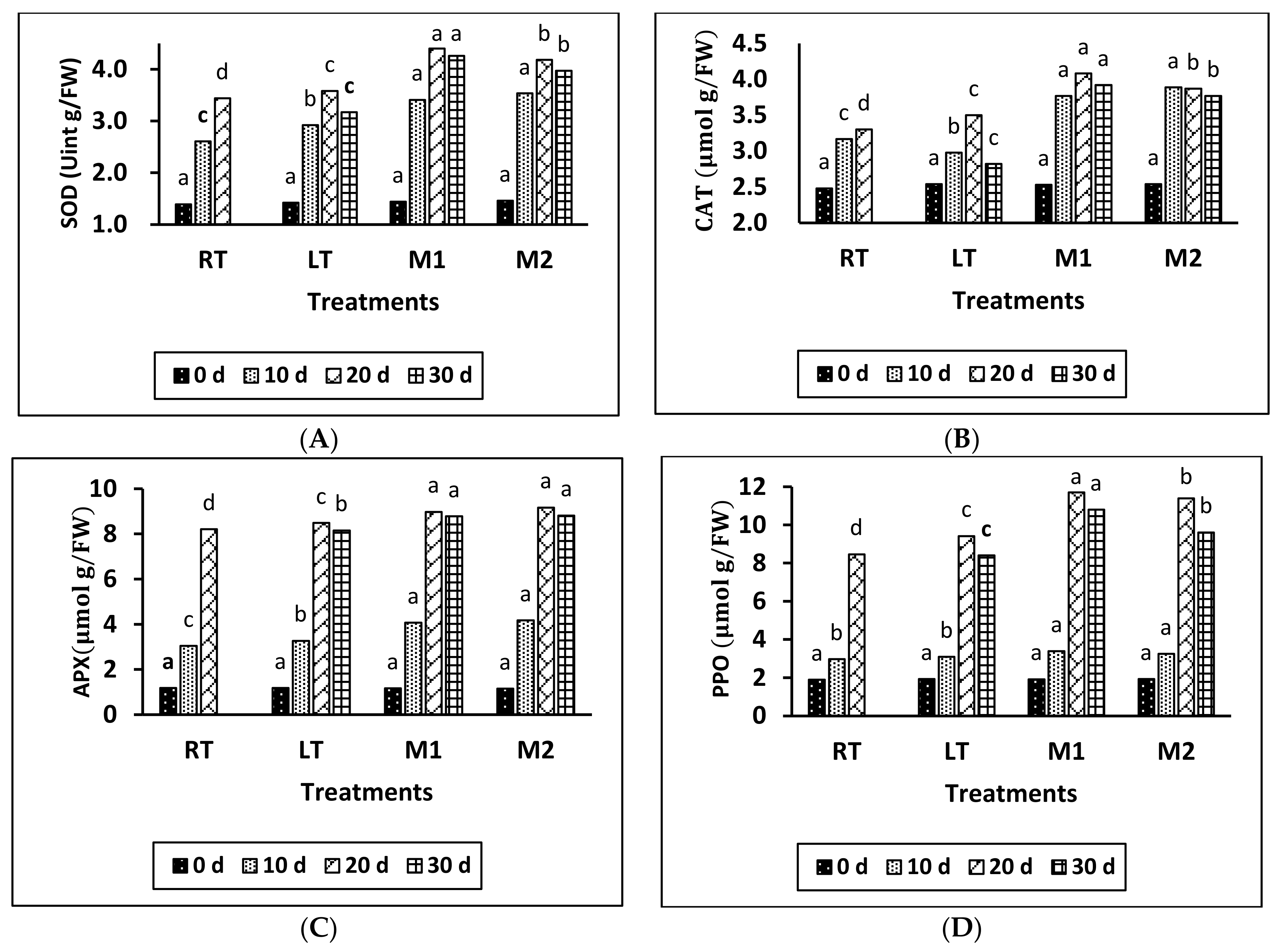
According to the data shown in Figure 2A–C, the levels of SOD, CAT, and APX activity initially increased in all treatment groups and gradually declined toward the end of the storage period. The highest activity was observed on day 20 in the M1, M2, LT, and RT treatments. The application of MeJA resulted in higher values of SOD, CAT, and APX compared to all other treatments. Additionally, a significant difference was observed between LT and RT, with RT exhibiting the lowest values of SOD, CAT, and APX compared to the other treatments. Moreover, a significant difference in the content of SOD, CAT, and PPO was found between M1 and M2 at 20 and 30 days of storage. However, there was no clear effect observed in APX between the M1 and M2 treatments during all storage periods.
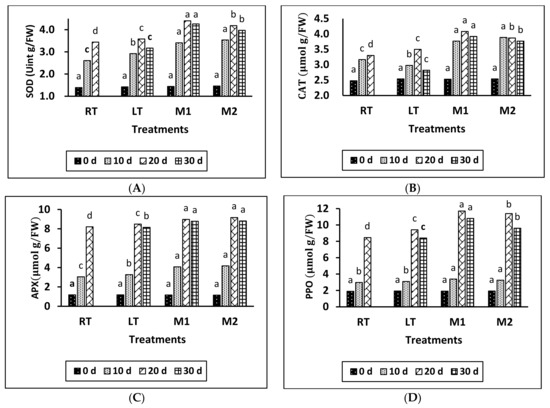
Figure 2.
Effect of different temperatures and MeJA on SOD (A), CAT (B), APX (C), and PPO activity (D) in grapes during storage period. The data presented are expressed as the mean ± (SE) of triplicate assays. Significant differences between the RT, LT, and MeJA samples within the same period were determined using Duncan’s test (p < 0.05), indicated by different letters.
Based on the results provided, it can be concluded that the application of MeJA significantly increased the activity of SOD, CAT, and APX in all of the treatments. Furthermore, notable differences were observed in these enzyme activities between LT and RT, with the LT treatment exhibiting significantly higher values of SOD, CAT, and APX than the RT treatment at 10 and 20 days of storage. To sum up, adding MeJA increased the activity of SOD, CAT, and APX in all of the treatments. There were notable differences in these enzyme activities between LT and RT, as well as between M1 and M2 at different storage times. However, there was no clear effect on APX between M1 and M2 treatments during all storage periods.
According to the findings in Figure 2D, PPO content showed a gradual rise until it reached its peak on day 20 of storage, then experienced a slight decline near the end of the storage period. On day 20, there was a notable disparity between the LT and RT treatments. Furthermore, a significant difference was observed between the MeJA treatments and the LT treatment. There was a notable disparity observed in the MeJA treatments (M1 and M2), specifically during the 20- and 30-day storage periods. The M1 treatment exhibited the highest values, followed by the M2, LT, and RT treatments.
3.3. Effect of RT, LT, and MeJA on H2O2 and MDA Content
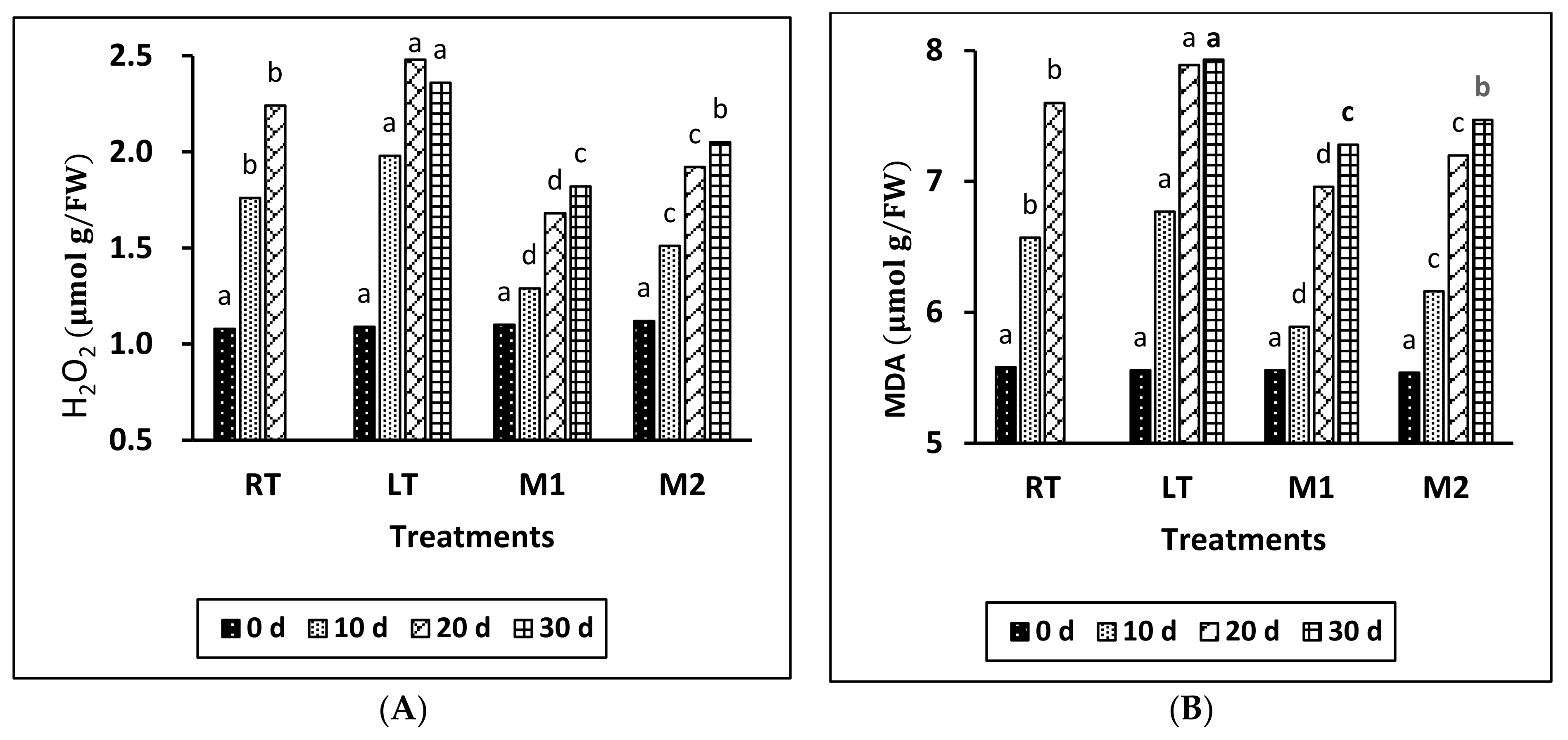
The findings of Figure 3A demonstrate that the concentration of H2O2 in all treatments indicated a progressive rise, culminating at the conclusion of the storage duration. The LT samples exhibited the highest concentration of H2O2, indicating a substantial statistical disparity when compared to the RT samples and those treated with MeJA during storage. In addition, M1 had the lowest H2O2 level compared to the other treatments during the storage period.
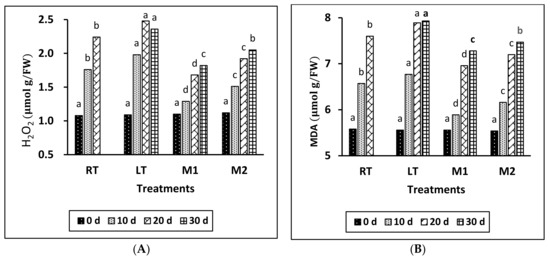
Figure 3.
Effect of different temperatures and MeJA treatment on H2O2 (A) and MDA content (B) in grapes during storage period. The data presented are expressed as the mean ± (SE) of triplicate assays. Significant differences between the RT, LT, and MeJA samples within the same period were determined using Duncan’s test (p < 0.05), indicated by different letters.
The analysis of the results presented in Figure 3B indicates a progressive increase in the content of MDA in all treated samples throughout the storage period. Notably, the MeJA treatments effectively preserved a lower content of MDA compared with the other treatments across the entire duration. Specifically, the M1 treatment demonstrated the lowest MDA content during the storage period, followed by the M2, RT, and LT treatments. A significant difference was observed between the LT and RT groups, as well as between the M1 and M2 treatments, during the storage period.
4. Discussion
4.1. Effect of RT, LT, and MeJA on Weight Loss, Firmness, TSS, and TA
The weight loss of fruits is a critical parameter used to evaluate their quality and marketability [34]. As shown in Figure 1A, weight loss percentage increased in all treatments during the storage period. This increase in weight loss was due to increased respiratory intake and transpiration water loss [35]. Weight loss increased with storage temperature. Less weight was lost under LT settings compared to RT conditions at the conclusion of the storage period. These findings align with previous studies that have shown a significant reduction in weight loss percentage in fruits such as blueberry, mango, and winter jujube when stored under LT conditions compared to the control group stored under RT conditions. These results reveal that MeJA treatment could enhance and preserve the quality of grapes. The findings agreed with [25,36,37]. They indicated that MeJA could inhibit weight loss in mangosteen, tomato, dragon fruit, papaya, table grape, and wine grape during storage periods. The decrease in weight loss resulting from MeJA treatment is likely attributable to inhibition of the fruit’s respiration process, which leads to a reduction in oxygen consumption and subsequent weight loss [38]. Furthermore, it improves the fruit’s capacity to retain moisture and decreases the rate of evaporation, resulting in less weight loss during storage [39]. MeJA also impacts fruit components, specifically organic acids and flavonoids, resulting in enhanced fruit quality and delayed deterioration [40].
Table grape firmness is an important quality parameter for producers, since severe softening might result in postharvest decay or consumer rejection [3]. Based on the results of this research, it was observed that both MeJA treatment and LT storage contributed to delaying the loss of firmness in grapes. This delay in firmness loss helped in maintaining the crisp flavour and freshness of the fruit. This effect may be attributed to the ability of LT to prevent postharvest physiological metabolism, as shown in Figure 1B. These findings agreed with [34,41,42]. They indicated that storage at LT prevented firmness reduction in blueberry, winter jujube, and apricot, delaying fruit ripening and senescence. Additionally, the reduction in fresh weight loss percentage can be due to MeJA treatment reducing the respiration rate during storage, presumably due to the maintenance of firmness and fruit quality [43].
MeJA treatment has been shown to increase firmness in fruits, as reported in previous studies [44]. This increase in firmness can be due to cell wall integrity stimulation-related enzymes, such as chitin synthase and phenylalanine ammonia-lyase (PAL).
Previous studies have indicated that the application of MeJA has varying effects on the expression of PAL. Furthermore, the PAL expression pattern differs between the pulp and core tissues. However, it is uncertain if PAL directly contributes to lignin formation in kiwifruit [44]. In peach fruit, MeJA was found to enhance phospholipid remodelling, which promotes the integrity of the cell wall and reduces electrolyte leakage. This may be due to the effect of MeJA on the related enzymes of the cell wall [45]. Moreover, MeJA has been shown to delay the degradation of enzymes, such as cellulose and pectin methylesterase in mandarin fruit [26].
SSC and TA are key quality parameters that determine the sugar, acid content, and flavour of fruits. In grapes, these parameters play a critical role in determining the unique flavour of the grape [46]. As shown in Figure 1C, SSC in all the treatment samples increased gradually during the storage period. The increase in SSC can be attributed to the elevated proportion of water loss from the grape berry along with the ongoing conversion of starch into sugar during the ripening process, leading to a greater concentration of SSC [34]. The results of this research agree with previous findings that MeJA-treated blueberry fruits resulted in lower SSC values compared to the untreated, particularly during the 14- and 21-day storage periods [47].
In contrast, the content of TA in all samples reduced slightly and continuously during storage, as shown in Figure 1D. This can be due to the organic acid consumption in the respiration process, with a minor decrease shown in treated samples [48]. At the end of storage, the SSC was lower and the TA content was higher in MeJA samples than in RT and LT samples, indicating that MeJA delayed the increase in SSC and reduced the decrease in TA content to maintain a higher storage quality.
The TA content observed during storage could be attributed to the berries’ metabolic activity during storage, as reported by Caleb et al. [49]. The results of this research are consistent with the results of El-Beltagi et al. [50], who indicated a decrease in TA content in pomegranate with increasing storage intervals under cold storage conditions, while MeJA treatment helped preserve the TA content compared to the control. Furthermore, it has been shown that blueberry fruits treated with MeJA exhibited a higher TA content compared to the control during storage, as reported by Huang et al. [47].
Briefly, the application of MeJA substantially suppressed the reduction of titratable acidity caused by storage-related damage while decreasing the sugars at the end of storage. The decrease in sugars is a result of the production and accumulation of phenolic compounds, which are driven by MeJA, as well as of the carbohydrates that play a crucial role as a material and energy source in the metabolism of phenolic compounds [51]. In this study, grape fruits preserved high quality criteria after 30 days of storage, although the SSC percentage was reduced.
4.2. Effect of RT, LT, and MeJA on SOD, CAT, APX, and PPO Activity
Antioxidant enzymes, including SOD, CAT, and APX, are essential components of the antioxidant system and play a main role in alleviating and eliminating ROS. These antioxidant enzymes also play a significant role during the fruit development stages, especially the ripening stage. According to Mittler [52], the antioxidants (CAT and APX) convert H2O2 to O2 and H2O, while SOD catalyses the dissociation process of O2− into H2O2 and O2. Previous studies have shown that SOD, CAT, and APX activities can alleviate chilling injuries and extend the shelf life of postharvest fruits by maintaining membrane integrity [53]. MeJA treatment improves the quality retention of Agaricus bisporus by inhibiting the activities of PPO, increasing the activities of antioxidant enzymes such as CAT and SOD, and reducing the expression levels of genes that encode PPO during storage [54].
Based on the findings of this study, it was found that MeJA had a significant impact on the content of SOD, CAT, APX, and PPO enzymes (as shown in Figure 2). This increase in enzyme activity was linked to better fruit quality parameters compared to the RT and LT treatments [36,44]. As a result, the amount of ROS in the fruits after harvest decreased [55]. Similar outcomes were observed in wine grapes treated with MeJA [25]. The results indicate that one mechanism by which MeJA-treated grapes preserve their quality is through their increased SOD, CAT, and APX enzyme content. According to [55], MeJA treatments that preserved higher SOD activity may have lowered O2− by converting it to H2O2. For example, the H2O2 content of MeJA-treated grapes increased slightly during storage, which may have been due to an increase in CAT enzyme content. Higher CAT enzyme content has been demonstrated to be the cause of conversion of H2O2 to water and O2 [56]. Treatment with 10 µM/L MeJA was more effective than treatment with 100 µM/L MeJA in increasing the levels of antioxidant enzymes during storage. The results of this study agreed with previous studies that demonstrated that a low concentration of MeJA can effectively suppress the biosynthesis of ethylene, a crucial hormone that plays a role in the ageing process of fruits and vegetables [57].
The postharvest treatment of grape fruits with MeJA has been observed to result in an increase in PPO enzyme activity during storage. This increase in activity is likely due to the enhanced activity of enzyme precursors and the regeneration of enzymes over time [58,59]. However, as enzyme levels drop toward the end of the storage period, there is a potential for the accumulation of reactive oxygen species (ROS), leading to damage to DNA and RNA, promotion of membrane peroxidation, and early senescence [60]. These effects can ultimately result in the loss of texture and quality, as well as impact the taste and nutritional value of the grape fruit. The data presented suggest that MeJA effectively modulates the stress response pathways, leading to the induction of PPO activity [61].
It is important to consider these findings when managing postharvest treatments for grape fruits in order to minimize the negative effects of PPO activity and ROS accumulation during storage. Strategies for controlling PPO activity and ROS accumulation, such as optimizing storage conditions and employing antioxidant treatments, may be beneficial in preserving the texture, quality, taste, and nutritional value of grape fruits during postharvest storage.
4.3. Effect of RT, LT, and MeJA on H2O2 and MDA Content
H2O2, a reactive oxygen species, can lead to oxidative stress, membrane peroxidation, and cell death in plants [62]. Based on the data provided in Figure 3A, the study indicates that the production of H2O2 and MDA content increased during storage, but following treatment with MeJA, the levels remained significantly lower compared to grapes treated with RT and LT. Furthermore, the activity of CAT and APX enzymes appeared to be linked to stress levels, as their increased activity might have contributed to a reduction in H2O2 accumulation in the tissue. This finding aligns with previous research on MeJA-treated wine grapes [25].
It is clear that the study suggests a potential role for MeJA in mitigating oxidative stress and H2O2 accumulation in grapes during storage. This aligns with previous research on other plant species [55]. The precise mechanism behind MeJA’s impact on H2O2 and MDA content, as well as its effect on CAT and APX enzymes, would likely benefit from further investigation and clarification.
MDA, a by-product of lipid peroxidation, is utilized as a marker to assess membrane damage induced by oxidative stress in plants [63]. In the present study, it was observed that samples treated with MeJA exhibited lower levels of MDA compared to other treatments, possibly due to an increase in enzymatic antioxidants. The reduction in MDA formation attributed to MeJA treatment suggests it may inhibit peroxidation reactions [64]. Conversely, the research highlighted a significant increase in MDA content in grapes treated with LT during storage, indicating that LT may accelerate the breakdown of membrane lipids. This effect could potentially impact cell compartmentalization and lead to a loss of cell integrity [65].
These findings suggest that MeJA treatment may play a role in reducing MDA content, potentially through the modulation of peroxidation reactions and the enhancement of enzymatic antioxidants. Additionally, the contrasting impact of LT on MDA content emphasizes the significance of environmental factors in influencing membrane lipid breakdown and cellular integrity during storage.
5. Conclusions and Prospects
Notably, this study presents compelling evidence for the efficacy of MeJA treatment, particularly at a concentration of 10 μmol/L, in preserving the quality attributes of grapes. This includes the reduction of weight loss, enhancement of firmness, and maintenance of titratable acidity (TA), alongside the mitigation of the increase in soluble solid content (SSC). Additionally, the application of MeJA led to elevated levels of crucial antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and polyphenol oxidase (PPO). Furthermore, it effectively reduced the increase in H2O2 and MDA content during cold storage. Hence, the application of MeJA as a stimulator of berry quality emerges as a promising strategy to enhance the physical, chemical, and physiological characteristics of grapes, ultimately improving berry quality.
According to this study, the application of MeJA as a chemical molecule in the winemaking process holds potential for decreasing the sugar levels in grapes, particularly in the delayed stages of storage. However, additional investigation is necessary to fully understand its effectiveness. It is vital to meticulously consider the suitable dosage in order to attain the desired outcome without jeopardising the excellence of the wine. Moreover, the use of MeJA might potentially influence the inherent taste of the wine, requiring careful calibration to maintain the ideal equilibrium among sugar, acidity, and flavour. Due to the insufficient state of study on this topic, it is crucial to perform further investigation and achieve thorough knowledge.
Author Contributions
Conceptualization, E.E., A.E. and J.F.; methodology, E.E., A.E., Y.X., L.S. (Li Shaonan), L.S. (Lu Suwen), D.T. and J.F.; software, E.E. and A.E.; validation, E.E. and J.F.; formal analysis, E.E., A.E. and Y.X.; investigation, E.E., L.S. (Li Shaonan), L.S. (Lu Suwen) and D.T.; resources, E.E. and L.S. (Lu Suwen); data curation, E.E. and J.F.; writing—original draft preparation, E.E.; writing—review and editing, E.E., A.E., Y.X., L.S. (Li Shaonan), L.S. (Lu Suwen), D.T. and J.F.; visualization, E.E.; supervision, J.F.; project administration, J.F.; funding acquisition, J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by National Natural Science Foundation of China (32272647), the Fundamental Research Funds for the Central Universities (YDZX2023018), and Jiangsu Agricultural Industry Technology System (JATS[2022]457).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- FAO. Food and Agriculture Organization of the United Nations. Last Update 21 March 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 21 March 2021).
- Deng, Q.; Xia, H.; Lin, L.; Wang, J.; Yuan, L.; Li, K.; Zhang, J.; Lv, X.; Liang, D. SUNRED, a natural extract-based biostimulant, application stimulates anthocyanin production in the skins of grapes. Sci. Rep. 2019, 9, 2590. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Vazquez-Hernandez, M.; Maestro-Gaitan, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Table grapes during postharvest storage: A review of the mechanisms implicated in the beneficial effects of treatments applied for quality retention. Int. J. Mol. Sci. 2020, 21, 9320. [Google Scholar] [CrossRef] [PubMed]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.-T.; Ferrara, G.; Matarrese, A.M.S.; Gallotta, A.; Bellincontro, A. Grapevine quality: A multiple choice issue. Sci. Hortic. 2018, 234, 445–462. [Google Scholar] [CrossRef]
- Ruiz-García, L.; Hellín, P.; Flores, P.; Fenoll, J. Prediction of Muscat aroma in table grape by analysis of rose oxide. Food Chem. 2014, 154, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Serrano, M.; Martínez-Romero, D.; Valero, D. New approaches for postharvest quality retention of table grapes. Fresh Prod. 2010, 4, 103–110. [Google Scholar]
- Zhang, L.; Wang, L.; Zeng, X.; Chen, R.; Yang, S.; Pan, S. Comparative transcriptome analysis reveals fruit discoloration mechanisms in postharvest strawberries in response to high ambient temperature. Food Chem. X 2019, 2, 100025. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tu, M.; Yang, X.; Xu, J.; Yu, Z. Effect of cutting and storage temperature on sucrose and organic acids metabolism in postharvest melon fruit. Postharvest Biol. Technol. 2020, 161, 111081. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT Food Sci. Technol. 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, H.; Zhang, Y. Production of reactive oxygen species and antioxidant metabolism about strawberry leaves to low temperatures. J. Agric. Sci. 2011, 3, 89. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Q.; Hu, M.; Gao, Z.; An, F.; Li, M.; Jiang, Y. Low-temperature conditioning induces chilling tolerance in stored mango fruit. Food Chem. 2017, 219, 76–84. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, B.; Zhang, W.; Cao, J.; Jiang, W. Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biol. Technol. 2019, 147, 113–122. [Google Scholar] [CrossRef]
- An, X.; Xu, Y.; Jiang, L.; Huan, C.; Yu, Z. Effects of postharvest temperature on apoptosis-related enzyme activity and gene expression in peach fruits (Prunus persica L. cv. Xiahui 8). Sci. Hortic. 2019, 245, 178–184. [Google Scholar] [CrossRef]
- Bertini, L.; Palazzi, L.; Proietti, S.; Pollastri, S.; Arrigoni, G.; de Laureto, P.P.; Caruso, C. Proteomic analysis of MeJa-induced defense responses in rice against wounding. Int. J. Mol. Sci. 2019, 20, 2525. [Google Scholar] [CrossRef] [PubMed]
- Faizy, A.H.; Ozturk, B.; Aglar, E.; Yıldız, K. Role of methyl jasmonate application regime on fruit quality and bioactive compounds of sweet cherry at harvest and during cold storage. J. Food Process. Preserv. 2021, 45, e15882. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2018, 46, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Singh, Z.; Khurshid, T. Methyl jasmonate alleviates chilling injury and regulates fruit quality in “Midknight” Valencia orange. Postharvest Biol. Technol. 2018, 141, 58–62. [Google Scholar] [CrossRef]
- Chen, L.; Pan, Y.; Li, H.; Jia, X.; Guo, Y.; Luo, J.; Li, X. Methyl jasmonate alleviates chilling injury and keeps intact pericarp structure of pomegranate during low temperature storage. Food Sci. Technol. Int. 2021, 27, 22–31. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Sangprayoon, P.; Supapvanich, S.; Youryon, P.; Wongs-Aree, C.; Boonyaritthongchai, P. Efficiency of salicylic acid or methyl jasmonate immersions on internal browning alleviation and physicochemical quality of Queen pineapple cv. “Sawi” fruit during cold storage. J. Food Biochem. 2019, 43, e13059. [Google Scholar] [CrossRef]
- Seo, J.; Yi, G.; Lee, J.G.; Choi, J.H.; Lee, E.J. Seed browning in pepper (Capsicum annuum L.) fruit during cold storage is inhibited by methyl jasmonate or induced by methyl salicylate. Postharvest Biol. Technol. 2020, 166, 111210. [Google Scholar] [CrossRef]
- Liu, H.; Meng, F.; Miao, H.; Chen, S.; Yin, T.; Hu, S.; Shao, Z.; Liu, Y.; Gao, L.; Zhu, C. Effects of postharvest methyl jasmonate treatment on main health-promoting components and volatile organic compounds in cherry tomato fruits. Food Chem. 2018, 263, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Serrano, M.; Valero, D. Blood oranges maintain bioactive compounds and nutritional quality by postharvest treatments with γ-aminobutyric acid, methyl jasmonate or methyl salicylate during cold storage. Food Chem. 2020, 306, 125634. [Google Scholar] [CrossRef]
- Ozturk, A.; Yildiz, K.; Ozturk, B.; Karakaya, O.; Gun, S.; Uzun, S.; Gundogdu, M. Maintaining postharvest quality of medlar (Mespilus germanica) fruit using modified atmosphere packaging and methyl jasmonate. LWT 2019, 111, 117–124. [Google Scholar] [CrossRef]
- Modesti, M.; Petriccione, M.; Forniti, R.; Zampella, L.; Scortichini, M.; Mencarelli, F. Methyl jasmonate and ozone affect the antioxidant system and the quality of wine grape during postharvest partial dehydration. Food Res. Int. 2018, 112, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Baswal, A.; Dhaliwal, H.; Singh, Z.; Mahajan, B.; Gill, K. Postharvest application of methyl jasmonate, 1-methylcyclopropene and salicylic acid extends the cold storage life and maintain the quality of ‘Kinnow’mandarin (Citrus nobilis L. X C. deliciosa L.) fruit. Postharvest Biol. Technol. 2020, 161, 111064. [Google Scholar] [CrossRef]
- Buraphaka, H.; Putalun, W. Stimulation of health-promoting triterpenoids accumulation in Centella asiatica (L.) Urban leaves triggered by postharvest application of methyl jasmonate and salicylic acid elicitors. Ind. Crops Prod. 2020, 146, 112171. [Google Scholar] [CrossRef]
- Singh, U.B.; Malviya, D.; Singh, S.; Kumar, M.; Sahu, P.K.; Singh, H.; Kumar, S.; Roy, M.; Imran, M.; Rai, J.P. Trichoderma harzianum-and methyl jasmonate-induced resistance to Bipolaris sorokiniana through enhanced phenylpropanoid activities in bread wheat (Triticum aestivum L.). Front. Microbiol. 2019, 10, 1697. [Google Scholar] [CrossRef] [PubMed]
- Asghari, M. Impact of jasmonates on safety, productivity and physiology of food crops. Trends Food Sci. Technol. 2019, 91, 169–183. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Bodbodak, S. Physiological and biochemical mechanisms regulating chilling tolerance in fruits and vegetables under postharvest salicylates and jasmonates treatments. Sci. Hortic. 2013, 156, 73–85. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists International, Official Methods; Association of Officiating Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Wang, Y.; Gao, L.; Wang, Q.; Zuo, J. Low temperature conditioning combined with methyl jasmonate can reduce chilling injury in bell pepper. Sci. Hortic. 2019, 243, 434–439. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989; p. 1191. [Google Scholar]
- Sang, Y.; Yang, W.; Liu, Y.; Zhang, W.; Guo, T.; Shen, P.; Tang, Y.; Guo, M.; Chen, G. Influences of low temperature on the postharvest quality and antioxidant capacity of winter jujube (Zizyphus jujuba Mill. cv. Dongzao). LWT 2022, 154, 112876. [Google Scholar] [CrossRef]
- Ozturk, B.; Yildiz, M.; Yildiz, K.; Gun, S. Maintaining the postharvest quality and bioactive compounds of jujube (Ziziphus jujuba Mill. Cv. “Li”) fruit by applying 1-methylcyclopropene. Sci. Hortic. 2021, 275, 109671. [Google Scholar] [CrossRef]
- Mustafa, M.A.; Ali, A.; Seymour, G.; Tucker, G. Treatment of dragonfruit (Hylocereus polyrhizus) with salicylic acid and methyl jasmonate improves postharvest physico-chemical properties and antioxidant activity during cold storage. Sci. Hortic. 2018, 231, 89–96. [Google Scholar] [CrossRef]
- Mustafa, M.A.; Ali, A.; Seymour, G.; Tucker, G. Delayed pericarp hardening of cold stored mangosteen (Garcinia mangostana L.) upon pre-treatment with the stress hormones methyl jasmonate and salicylic acid. Sci. Hortic. 2018, 230, 107–116. [Google Scholar] [CrossRef]
- Ozturk, B.; Altuntas, E.; Yildiz, K.; Ozkan, Y.; Saracoglu, O. Effect of methyl jasmonate treatments on the bioactive compounds and physicochemical quality of “Fuji” apples. Cienc. Investig. Agrar. 2013, 40, 201–221. [Google Scholar] [CrossRef]
- Asghari, M.; Hasanlooe, A.R. Interaction effects of salicylic acid and methyl jasmonate on total antioxidant content, catalase and peroxidase enzymes activity in “Sabrosa” strawberry fruit during storage. Sci. Hortic. 2015, 197, 490–495. [Google Scholar] [CrossRef]
- Saavedra, G.M.; Figueroa, N.E.; Poblete, L.A.; Cherian, S.; Figueroa, C.R. Effects of preharvest applications of methyl jasmonate and chitosan on postharvest decay, quality and chemical attributes of Fragaria chiloensis fruit. Food Chem. 2016, 190, 448–453. [Google Scholar] [CrossRef]
- Fan, X.; Xi, Y.; Zhao, H.; Liu, B.; Cao, J.; Jiang, W. Improving fresh apricot (Prunus armeniaca L.) quality and antioxidant capacity by storage at near freezing temperature. Sci. Hortic. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Ge, Y.; Tang, Q.; Li, C.; Duan, B.; Li, X.; Wei, M.; Li, J. Acibenzolar-S-methyl treatment enhances antioxidant ability and phenylpropanoid pathway of blueberries during low temperature storage. LWT 2019, 110, 48–53. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Ali, M.R.; Darwish, O.S.; Rogers, H.J. Impact of salicylic acid, abscisic acid, and methyl jasmonate on postharvest quality and bioactive compounds of cultivated strawberry fruit. J. Berry Res. 2019, 9, 333–348. [Google Scholar] [CrossRef]
- Li, H.; Suo, J.; Han, Y.; Liang, C.; Jin, M.; Zhang, Z.; Rao, J. The effect of 1-methylcyclopropene, methyl jasmonate and methyl salicylate on lignin accumulation and gene expression in postharvest “Xuxiang” kiwifruit during cold storage. Postharvest Biol. Technol. 2017, 124, 107–118. [Google Scholar] [CrossRef]
- Chen, M.; Guo, H.; Chen, S.; Li, T.; Li, M.; Rashid, A.; Xu, C.; Wang, K. Methyl jasmonate promotes phospholipid remodeling and jasmonic acid signaling to alleviate chilling injury in peach fruit. J. Agric. Food Chem. 2019, 67, 9958–9966. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, X.; Hou, Y.; Pan, Y.; Shi, L.; Li, X. Effects of harvest maturity stage on postharvest quality of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit during cold storage. Sci. Hortic. 2021, 277, 109778. [Google Scholar]
- Huang, X.; Li, J.; Shang, H.; Meng, X. Effect of methyl jasmonate on the anthocyanin content and antioxidant activity of blueberries during cold storage. J. Sci. Food Agric. 2015, 95, 337–343. [Google Scholar] [CrossRef]
- Fagundes, C.; Moraes, K.; Pérez-Gago, M.B.; Palou, L.; Maraschin, M.; Monteiro, A. Effect of active modified atmosphere and cold storage on the postharvest quality of cherry tomatoes. Postharvest Biol. Technol. 2015, 109, 73–81. [Google Scholar] [CrossRef]
- Caleb, O.J.; Opara, U.L.; Mahajan, P.V.; Manley, M.; Mokwena, L.; Tredoux, A.G. Effect of modified atmosphere packaging and storage temperature on volatile composition and postharvest life of minimally-processed pomegranate arils (cvs. “Acco” and “Herskawitz”). Postharvest Biol. Technol. 2013, 79, 54–61. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Al-Otaibi, H.H.; Ali, M.R. A new approach for extending shelf-life of pomegranate arils with combined application of salicylic acid and methyl jasmonate. Horticulturae 2023, 9, 225. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Wang, J.; Wang, L.; Han, C.; Jin, P.; Zheng, Y. Methyl jasmonate enhances wound-induced phenolic accumulation in pitaya fruit by regulating sugar content and energy status. Postharvest Biol. Technol. 2018, 137, 106–112. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Jin, P.; Rui, H. Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit. Food Chem. 2009, 115, 1458–1463. [Google Scholar] [CrossRef]
- Meng, D.; Song, T.; Shen, L.; Zhang, X.; Sheng, J. Postharvest application of methyl jasmonate for improving quality retention of Agaricus bisporus fruit bodies. J. Agric. Food Chem. 2012, 60, 6056–6062. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhi, H.; Xu, J.; Zhang, L.; Liu, M.; Zong, W. Effect of methyl jasmonate on reactive oxygen species, antioxidant systems, and microstructure of Chinese winter jujube at two major ripening stages during shelf life. J. Hortic. Sci. Biotechnol. 2016, 91, 316–323. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Díaz, M.; Lobos, T.; Cardemil, L.; Nunes-Nesi, A.; Retamales, J.; Jaakola, L.; Alberdi, M.; Ribera-Fonseca, A. Methyl jasmonate: An alternative for improving the quality and health properties of fresh fruits. Molecules 2016, 21, 567. [Google Scholar] [CrossRef] [PubMed]
- Glowacz, M.; Rees, D. Using jasmonates and salicylates to reduce losses within the fruit supply chain. Eur. Food Res. Technol. 2016, 242, 143–156. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Participation of phenylalanine ammonia-lyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol. Technol. 2015, 104, 17–25. [Google Scholar] [CrossRef]
- Hodges, D.M.; Lester, G.E.; Munro, K.D.; Toivonen, P. Oxidative stress: Importance for postharvest quality. HortScience 2004, 39, 924. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid. Myzus Persicae Entomol. Exp. Appl. 2006, 120, 175–188. [Google Scholar] [CrossRef]
- Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.-Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef]
- Hu, Z.; Weijian, L.; Yali, F.; Huiquan, L. Gibberellic acid enhances postharvest toon sprout tolerance to chilling stress by increasing the antioxidant capacity during the short-term cold storage. Sci. Hortic. 2018, 237, 184–191. [Google Scholar] [CrossRef]
- Flores, G.; Blanch, G.P.; Del Castillo, M.L.R. Effect of postharvest methyl jasmonate treatment on fatty acid composition and phenolic acid content in olive fruits during storage. J. Sci. Food Agric. 2017, 97, 2767–2772. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhou, X.; Zhou, Q.; Liu, Z.Y.; Sheng, L.; Wang, L.; Cheng, S.C.; Ji, S.J. Proteomic analysis of peel browning of “Nanguo” pears after low-temperature storage. J. Sci. Food Agric. 2017, 97, 2460–2467. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).