Abstract
Urban heat islands (UHIs) and global warming will unavoidably have a negative impact on human health in urban areas, making urban forests much more susceptible to the risk of heat waves than forests. It is pivotal for urban forest management to understand tree species’ adaptation mechanisms by focusing on the species-dependent variability of polyamines (PAs), significant players in the amelioration of biotic and abiotic stress in plants, to mitigate the negative effects of UHIs and global warming on human health. Based on this background, the content of major polyamines (PAs) (putrescine, spermidine, and spermine) and total phenolics and the corresponding antioxidant capacities were determined and analyzed in the 24 most prevalent deciduous and coniferous tree species found in urban areas, namely Futoški Park in Novi Sad (Serbia). High-performance liquid chromatography (HPLC) coupled with fluorometric detection (HPLC-FD) was used to separate and quantify major PAs from tree species. Results showed a species-specific level variation in polyamines, total phenolic, and antioxidant capacity in coniferous and deciduous woody plant species in inspected urban areas. In terms of total PA content, the most notable deciduous tree species were Betula pendula, Junglans regia, and Quercus rubra, while the coniferous tree species Thuja occidentalis, Taxodium distichum, Pinus nigra, and Abies concolor stand out. The most dominant foliar PA in most of the inspected species was putrescine (ranging from 527.67 to 10,049.3 nmol g−1 DW), followed by spermidine (from 250.56 to 2015.92 nmol g−1 DW) and spermine (from 168.8 to 718.41 nmol g−1 DW). Furthermore, significant intra-genus variability in terms of PA content was recorded within the genera Pinus, Thuja, and Picea. This study demonstrated that the PA and phenolic compounds, in combination with antioxidant assays, can serve as reliable and trustworthy criteria and descriptors for the selection of adaptable tree species in the context of urban climate–smart forestry.
1. Introduction
Urban forests will undoubtedly be far more vulnerable to risk from heat events and global warming than forests due to the presence of phenomena like ‘urban heat islands’ (UHI), which unavoidably affect human health in urban areas [1,2,3,4]. The sustainability of ecosystem services that urban tree species provide to people who live in urban environments, such as air purification, noise reduction, shades, urban cooling, and runoff mitigation, is severely impacted by heat conditions as well as pollution, which pose a threat to the survival of the urban trees [5,6]. Therefore, the ability of different tree species to adapt to future altered climate conditions is crucial for both the stability of urban forest ecosystems and the sustainability of these ecosystem services, as well as the understanding of the underpinning mechanism of adaptation in order to identify the climate-ready tree species [7]. Trees in urban surroundings have to adapt to significantly changed environments [8,9,10]. On the other hand, ongoing climate change evidently negatively affects whole vegetation and, in synergy with intensive drought periods, significantly reduces their growth and physiological properties, as well as vitality status [11,12,13].
To adapt to ever-changing environmental conditions, whether they are caused by abiotic or biotic factors, all plants developed a whole range of sophisticated machinery of adaptive mechanisms during the course of their evolution to counteract these stressful events and survive [14]. One of these mechanisms is the accumulation of various compatible solutes with osmoprotective and antioxidant properties, such as quaternary ammonium compounds (glycine betaine-GB and proline-PRO), tertiary sulphonium compounds (e.g., dimethylsulfoniopropionic acid-DMSP), or sugars and sugar alcohols (polyols), which are all grouped together under the general term ‘osmolytes’ [15]. These osmolytes are of great importance not only for crop plants’ tolerance but also for trees’ ability to withstand a wide range of biotic and abiotic stress, including the presence of heavy metals [9] and organic pollutants [16], but also drought [17] and heat stress [18]. For example, different oak species have been discovered to differentiate in response to heat and drought stress by accumulating GB, PRO, and tertiary sulphonium compounds (such as DMSP) in an organ- and species-dependent manner [19].
Despite being effective osmolytes and antioxidants, also acknowledged as significant indicators of both abiotic and biotic stress, woody plant species have not been well investigated with regard to the species-dependent accumulation of polyamines (PAs). PAs are ubiquitous, aliphatic amines that control plant growth and development and display a multitude of functions in plants, creating their own ‘multiverse’ that acts as an interface in cross-kingdom communication [20]. PAs are involved in nearly all physiological processes in plants, including cell division, embryogenesis, organogenesis, flowering, fruit formation, ripening, transcription, RNA and histone modification, and protein biosynthesis acting as a multifunctional ‘Swiss army knife’ in the process [21,22]. At physiological pH, PAs are positively charged, which determines some of their functions in the stabilization of negatively charged biomolecules such as nucleic acids and phospholipids on thylakoid membranes and modulation of different enzyme activities via chaperones-like activity [23,24]. On the other hand, PAs are well known as strong antioxidants since they are capable of scavenging different Reactive Oxygen Species (ROS), but they are also involved in the generation of Nitric Oxide (NO) and, via their catabolism, the generation of hydrogen peroxide as a secondary messenger, also exhibiting prooxidative properties [21]. The three main PAs found in plants are diamine putrescine (PUT), triamine spermidine (SPD), and tetraamine spermine (SPM), whereas putrescine is synthesized via the catalytic activity of either Arginine Decarboxylase (ADC) or Ornithine Decarboxylase (ODC), while “higher” PAs such as SPM and PUT are synthesized via the activities of SPD synthase and SPM synthase by addition of two aminopropyl moiety, respectively [20]. Foliar levels of particular PAs depend on their biosynthesis, conjugation, and transport, as well as their catabolism [22]. PAs in woody plant species are already examined in plant tolerance against biotic stress [25] but also against abiotic stress such as drought [17] and heat stress [18] but have not been examined so far in the context of urban areas and species-specific variability and its contribution to total antioxidant activities estimated by different antioxidant assays.
Therefore, the main aim of the study was to estimate the potential adaptability of prevalent deciduous and coniferous tree species present in Futoški Park, Novi Sad (Serbia), to heat stress imposed during dry summer conditions in urban area by tracking levels of major PAs (putrescine, spermidine, and spermine), as well as total phenolics as biochemical indicators of abiotic stress in plants. Furthermore, another objective of this research was to estimate species-specific variability of PA profiles in various tree species and to establish a correlation between particular PAs and the total antioxidant activities of leaf extracts. Finally, the authors aimed to determine whether biochemical parameters such as individual PAs and total phenolics could serve as a reliable criterion for the selection of tree species that are more resilient to heat stress as potential climate-ready candidates for cultivation in urban areas.
2. Materials and Methods
2.1. Site Characteristics and Sampling Strategy
Climate conditions in the sampling area are defined as temperate continental to modified continental, fully humid with warm summers [26]. The ten-year average annual temperature is 11.2 °C, with an annual rank of 22.1 °C and an average precipitation of 603.1 mm m−2 [27]. The altitudes of the selected trees are located within ranges from 78 to 80 m a.s.l. Futoški Park were chosen as a representative site for our research (N: 45°14′ 59.16″; E: 19°49′ 37.80″). This is a large city park (~8 ha) that was established in the 20th century and located in the urban core. The soil can be characterized as urbisol type, which is compacted, polluted, and structurally deteriorated by anthropogenic activities. Soil profile descriptions and heavy metals allocation via the soil profile were presented in [28,29].
Based on analyzing of cadaster of urban greenery, 24 common tree urban species were chosen—12 deciduous (sycamore maple (Acer pseudoplatanus L.); silver birch (Betula pendula Roth.); European nettle tree (Celtis australis L.); Turkish hazel (Corylus colurna L.); ginkgo (Ginkgo biloba L.); English walnut (Junglans regia L.); white mulberry (Morus alba L.); white poplar (Populus alba L.); red oak (Quercus rubra L.); white willow (Salix alba L.); Chinese scholar tree (Sophora japonica L.); large-leaved linden (Tilia grandiflora Ehrh.)) and 12 conifers (White fir (Abies concolor (Gordon) Lindley ex Hildebrand); Spanish fir (Abies pincapo Boiss.); Atlas cedar (Cedrus atlantica (Endl.) Manetti ex Carrière); spruce (Picea abies (L.) H. Karst.); Serbian spruce (Picea omorika (Pančić) Purk.); blue spruce (Picea pungens Engelm.); black pine (Pinus nigra J.F.Arnold); Himalayan pine (Pinus wallichiana A. B. Jacks.); bald cypress (Taxodium distichum (L.) Rich.); English yew (Taxus baccata L.); Northern White cedar (Thuja occidentalis L.); and oriental thuja (Thuja orientalis L.). All analyzed specimens were adult trees and characteristically shaped with a diameter at breast height and heights enlisted in Supplementary Material (Table S1 in Supplementary Material).
Rigorous tree selection and leaf sampling were realized in the first week of September 2022 in the morning hours (10–12 h) in sunny and dry weather. All potential trees were evaluated following Kostić et al. [30] adapted criteria for urban forestry. Only mature solitary trees in excellent fitness conditions were included in our study. Only fully developed, characteristic, undamaged, and full-sunned leaves, as well as one-year-old needles, were collected. Only healthy leaves, with no obvious pathological symptoms, were collected. Three tree specimens per species were selected, and about 100 g of fresh weight leaves/needles were collected. The collected leaves were stored in a transport fridge to the laboratory. After that, leaves were frozen with liquid nitrogen and stored at −80 °C, lyophilized, powdered, and stored in a cold and dark place up to the analysis.
Microclimate Characteristics
Continuous measurements exhibited significant variations among local climate zones (LCZ) in the Novi Sad metropolitan areas [26]. The warmest summer months, July and August, in the examined urban area showed an increased value of average temperatures of more than 2 °C compared to natural areas based on four-year-long measurements (2014–2018; Table 1), while the temperature extremes in some highly urbanized LCZs indicated occurrence of the UHIs in Novi Sad. Annual averages between mean daily temperatures in urban and natural areas vary by 1.68 °C (urban: 14.09; natural: 12.41 °C). Likewise, 35 tropical nights were noted in urban areas, while in natural areas, they were significantly reduced (4 nights only). On the other hand, tropical days were similar (about 55 days). Air relative humidity caused a negative impact on the tree’s health and had a synergistic effect with air warming. Average annual temperatures in the sampling year were 13.4 °C measured out of urban areas, which is ~1 °C higher than reported values in a four-year-long timespan and the sampling year can be characterized as an extremely warm year.

Table 1.
Microclimate characteristics in urban and natural areas from 2014 to 2018 timespan [26].
2.2. Polyamine Analysis
Plant tissues (approximately 20 mg dry weight (DW) of freeze-dried material) were subjected to extraction using 10 times the volume of a 4% perchloric acid (PCA) solution. The resulting homogenate was left on ice for 1 h and then underwent centrifugation at 15,000× g for 30 min. Subsequently, samples of the supernatants, along with standard solutions containing putrescine (PUT), spermidine (SPD), and spermine (SPM), were treated with dansyl-chloride according to the method outlined by Scaramagli et al. [31]. The resulting dansylated derivatives were then extracted using toluene, dried, and reconstituted in acetonitrile before being subjected to analysis using high-performance liquid chromatography (HPLC). The separation and quantification of PAs were carried out using HPLC equipment from Jasco, Tokyo, Japan, employing a reverse phase C18 column (Spherisorb ODS 2,5-μm particle diameter, 4.6 × 250 mm, Waters, Wexford, Ireland) and a programmed acetonitrile-water gradient. The results of the PAs quantification were expressed as nanomoles per gram of dry weight (nmol g−1 DW).
2.3. Determination of Antioxidant Capacity and Total Phenolic and Flavonoid Contents
- The ABTS assay was employed to assess total antioxidant capacity, which relies on monitoring the conversion of the cationic radical 2,2′-azinobis(3-ethylbenzothiozoline-6-sulfonic acid), ABTS•+ from a blue-green color to its neutral and colorless form at 734 nm. This procedure followed the methodology outlined by Miller and Rice-Evans [32].
- The DPPH-scavenging activity was assessed using the method described by Arnao [33]. This method relies on the transformation of purple DPPH• (2,2-diphenyl-1-picrylhydrazyl radical) into its reduced yellow form, DPPH-H, following a 30 min incubation at 30 °C in the absence of light. The absorbance was subsequently measured at 515 nm.
- The inhibition of the nitric oxide radical (NO•) was assessed via the Griess diazotization process, following the methodology devised by Hensley et al. [34]. The extent of inhibition was determined by measuring the absorbance of the resulting chromophore at 546 nm. This approach allowed for the quantification of the NO• inhibitory effects.
- The ferric reducing antioxidant power (FRAP) assay involves the reduction in FeIII –TPTZ (Iron (II)-2,4,5-tripyridyl-S-triazine) under low pH conditions, resulting in the formation of a blue-colored FeII–TPTZ complex. The measurement involves reading the absorbance of an intense blue complex at 593 nm, as detailed in a prior description published by Benzie and Strain [35].
- The total phenolic content (TPC) was quantified according to the Folin–Ciocalteu (FC) method described by Singleton et al. [36]. The method is based on the spectrophotometric detection of phenols forming a colored complex with a FC reagent. Absorbance was measured spectrophotometrically at 760 nm, and TPC was expressed as milligrams of gallic acid equivalents (GAE) per gram of DW (mg GAE g−1 DW), calculated according to the standard calibration curve.
- The total flavonoid content (TFC) was quantified spectrophotometrically, employing aluminum chloride (AlCl3) as the complexing reagent for flavonoids. Absorbance readings were taken at 415 nm, and quercetin was used as a standard for the calibration curve. The TFC value of the extract is presented as milligrams of quercetin equivalents (QE) per gram of DW (mg QE g−1 DW) [37].
The radical scavenging capacity (RSC) against ABTS, DPPH, and NO radicals, as well as FRAP, were determined by constructing a standard curve. The results were quantified and expressed as nanomoles of Trolox equivalents per gram of plant material, either fresh or dry weight (mmol TEAC g−1 DW), depending on the specific extract used in the assay.
2.4. Statistical Analysis
Descriptive statistics, one-way Analysis of Variance (ANOVA), t-test, Principal Component Analysis (PCA), dendrogram hierarchical clustering, and Pearson correlation statistical techniques were employed. In one-way ANOVA, the tree group (coniferous/deciduous) was used as a factor, which was interpreted using the Fisher (F) test and their statistical significance levels (p). The t-test results were visually represented on a box-plot diagram. The R programming environment was used for all statistical data processing. The “rstatix” R package was used to calculate descriptive statistics and run two-way ANOVA and t-tests, the “dendextend” R package for dendrogram clustering, while the “ggplot2” R package [38] was used for other visual representations.
3. Results
Following one-way ANOVA, statistically significant differences between coniferous and deciduous tree species were detected for six of the nine analyzed parameters for p ≤ 0.05. Only TFC, SPD, and SPM did not depend on the tree type. In 24 different tree species, including 12 coniferous and 12 deciduous tree species, major PAs were isolated using HPLC technology and quantified using a fluorescence detector. Overall, the highest mean concentration of all PAs was noted in coniferous tree species (Figure 1d), which were statistically significantly different (F = 5.01, p = 0.036). PUT was the most prevalent PA in all the species that were examined. Amounts of PUT were followed by SPD and SPM in the majority of the species. The mean value of PUT was generally higher in coniferous than deciduous tree species. The PUT level in coniferous species varied from 1569.9 to 10,049.3 nmol g−1 DW (Figure 1a). In terms of PUT content, Quercus rubra and Thuja orientalis were the most prevalent deciduous and conifer species, respectively. The coniferous species Abies pinsapo and the deciduous species Celtis australis both had the lowest levels of PUT, respectively.
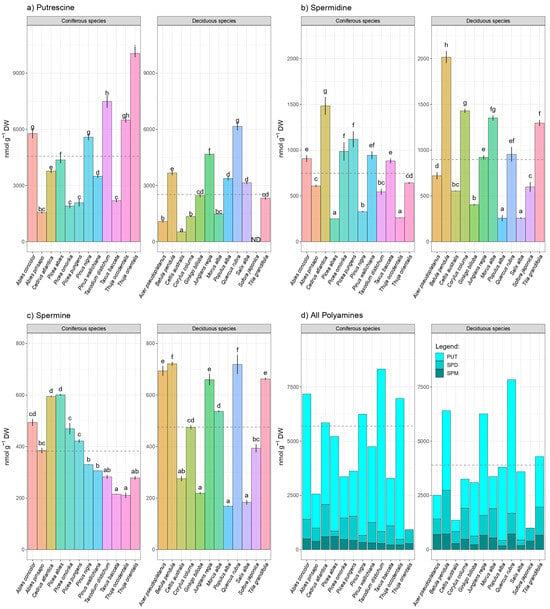
Figure 1.
Species-specific level variation in major PAs: (a) putrescine (PUT), (b) spermidine (SPD), (c) spermine (SPM), as well as (d) total PAs within 24 coniferous and deciduous tree species collected in urban area. Different small letters indicate significant differences among different species; Tukey’s honestly significant difference (HSD) post hoc test (p ≤ 0.05). Data represent the mean ± standard deviation (SD).
The putrescine-derived second-dominant major PA, SPD, showed considerable species diversity while variation between tree groups is absent (F = 0.62; p = 0.437). SPD levels fluctuated from 258.7 nmol g−1 DW in Salix alba up to 2015.9 nmol g−1 DW were found in Betula pendula. Unlike PUT, the mean value of PUT was generally higher in deciduous tree species. Coniferous species with relatively high amounts of spermidine were Cedrus atlantica, Picea omorica, Picea pungens, Pinus wallichiana, and Taxus baccata, while species like Picea abies, Pinus nigra, and Thuja orientalis exhibited low amounts of foliar SPM. On the other hand, among deciduous species, Betula pendula, Corylus colurna, Morus alba, and Tilia gradifolia were the most abundant with SPM, while Populus alba, Salix alba, and Gingko biloba were characterized by low amounts of spermidine (Figure 1b).
Like SPD, mean values of tetraamine SPM, were higher in deciduous species compared to conifers. SPM levels in deciduous species ranged from 168.8 to 720.7 nmol g−1 DW, while within coniferous species, SPM levels ranged between 211.16 and 601.0 nmol g−1 DW. Although high inter-species variability has been noted, the variations between deciduous and coniferous species were not detected (F = 1.56, p = 0.224). The most abundant deciduous species in terms of SPM content were Betula pendula and Quercus rubra, while among the coniferous species, Picea alba and Cedrus atlantica were the most prominent.
In terms of total PA content, the coniferous ornamental tree species Thuja occidentalis, Taxodium distichum, Pinus nigra, and Abies concolor stand out, while the most notable deciduous tree species were Betula pendula, Junglans regia, and Quercus rubra. Coniferous tree species had a greater mean value for total PA content than deciduous tree species (Figure 1d). The major PAs were found to be in the following sequence based on abundance PUT > SPD > SPM, with exceptions of Picea alba and Pinus nigra whereas SPM was found higher than SPD. Another exception to the PUT > SPD abundance pattern was Corylus colurna, which had more SPD than PUT.
The species Picea abies, Picea omorika, and Picea pungens, as well as Pinus nigra and Pinus wallichiana, Thuja occidentalis, and Thuja orientalis, all showed an intra-genus variation in all individual PAs (PUT, SPM, and SPD), and total PA content. Picea abies was the most prevalent species in the genus Picea in terms of PUT and SPM content, while Picea pungens was notable in terms of SPD concentrations. Compared to Thuja occidentalis, Thija orientalis showed higher levels of each individual PA. Moreover, the genus Pinus also showed intra-genus variation in PA content; Pinus nigra had higher amounts of PUT and SPM, whilst Pinus wallichiana had higher levels of SPD.
There is a high species specificity regarding flavonoid content within deciduous and coniferous tree species. Total flavonoid content ranged from 16.44 mg QE g−1 DW, which was found in Abies pinsapo, up to 165.96 mg QE g−1 DW, that was quantified in Acer pseudoplatanus. Higher variations were noted among species and compared between tree groups following ANOVA. Mean values of flavonoids were higher in deciduous species, while mean values of total phenolics were higher in coniferous species. Deciduous species that stand out for their flavonoid content were Acer pseudoplatanus, Betula pendula, and Junglans regia, while Taxodium distichum and Thuja orientalis were the most prominent coniferous tree species regarding total flavonoid content (Figure 2a).
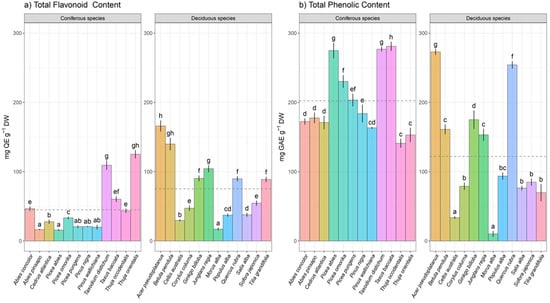
Figure 2.
Species-specific level variation in (a) total flavonoids and (b) total phenolics contents within coniferous and deciduous tree species collected in urban areas. Different small letters indicate significant differences among different species; Tukey’s honestly significant difference (HSD) post hoc test (p ≤ 0.05). Data represent the mean ± standard deviation (SD).
Moreover, Picea omorika and Taxodium distichum were the most abundant coniferous species regarding total phenolics contents with 274.9 and 276.9 mg GAE g−1 DW, respectively, while within deciduous species Acer pseudoplatanus and Betula pendula stand out containing 165.96 and 139.9 mg GAE g−1 DW, respectively. Very low amounts of total phenolic content were recorded in deciduous species such as Celtis australis and Morus alba. The genera Pinus, Picea, and Thuja showed statistically significant intra-genus and inter-species heterogeneity in terms of total flavonoid and phenolic contents.
Overall, all the biochemical assays used to measure total antioxidant capacity showed significant species-specific heterogeneity. Coniferous species had statistically significant higher mean values for all applied radical scavenger capacity assays (DPPH, FRAP, ABTS, and RSC NO) than deciduous species following ANOVA F test and p values (Figure 3). There is a great similarity between ABTS, FRAP, and DPPH assays in the sequence of antioxidant activities (Figure 3a–c). The order of the antioxidant activity of examined coniferous and deciduous tree species estimated by different assays, such as ABTS, FRAP, and DPPH assays, are very comparable. The most potent antioxidant tree species among coniferous species, according to these antioxidant assays, are Taxodium distichum, Taxus baccata, Abies pinsapo, and Abies concolor. Within deciduous tree species, Acer pseudoplatanus, Ginkgo billoba, and Quercus rubra stand out as the plants with the most potent antioxidant capacity. The total antioxidant capacity estimated by the DPPH assay ranged from 2.35 mmol TE g−1 DW, which was found in Betula pendula, up to 50.25 mmol TE g−1 DW, which was quantified in Abies pinsapo. Additionally, according to the ABTS and FRAP assays, the total antioxidant capacity of all inspected species ranged from 46.73 mmol TE g−1 DW (Celtis australis) up to 177.58 mmol TE g−1 DW (Acer pseudoplatanus) and between 9.57 mmol TE g−1 DW (Morus alba) and 127.16 mmol TE g−1 DW (Acer pseudoplatanus), respectively.
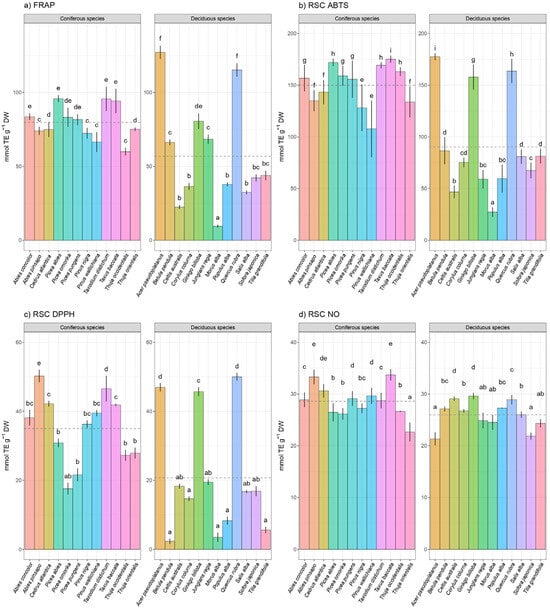
Figure 3.
Total antioxidant capacity (mmol TEAC g−1 DW) measured by (a) FRAP, (b) ABTS, (c) DPPH assay and radical scavenger capacity against NO radical, and (d) RSC NO in leaves of 24 coniferous and deciduous tree species collected in urban area. Different small letters indicate significant differences among different species; Tukey’s honestly significant difference (HSD) post hoc test (p ≤ 0.05). Data represent the mean ± standard deviation (SD).
Hierarchical Claster Analysis, Principal Component Analysis (PCA), and Correlation Matrix
Biochemical data of PA and phenolics content and antioxidant capacities estimated in coniferous and deciduous tree species were analyzed using principal component analysis (PCA) and Pearson correlation’s matrix for p < 0.05. According to the PCA analysis, the first two principal components (PCs) described 66.07% of total species variation, whereas PC1 describes 42.92% and PC2 23.15% of the variation (Figure 4a). Parameters such as RSC ABTS, PUT, TPC, and FRAP were generally associated with PC1, while parameters such as TFC, SPM, SPD, and RSC NO were accompanied by PC2. Therefore, the variability of species such as Taxodium distichum, Picea abies, Abies concolor, Cedrus atlantica, and Sofora japonica that were placed across PC1 is based on the parameters associated with the PC1 axes (e.g., RSC ABTS, PUT, TPC, and FRAP), while species such as Abies pinsapo, Thuja occidentalis, Pinus nigra, Pinus wallichiana, Thuja orientalis, Junglans regia, and Betula pendula were distributed in proximity of PC2 axis that is described by parameters such as TFC, SPM, SPD, and RSC NO. Different PCAs and correlation matrix patterns that define different relations among analyzed parameters indicate species-specific responses to urban environments. PCA graph clearly demonstrates intra-genus variability since Pinus wallichiana and Pinus nigra as well as Thuja orinetalis and Thuja occidentalis, were separated across the PC2 axis; therefore, their heterogeneity is based on the variability of parameters such as TFC, SPM, SPD, and RSC NO. The different patterns of inter-genus variability were shown by Picea abies and Picea pungens displaying across PC1, while Picea pungens and Picea omorica were separated via the PC2 axis.
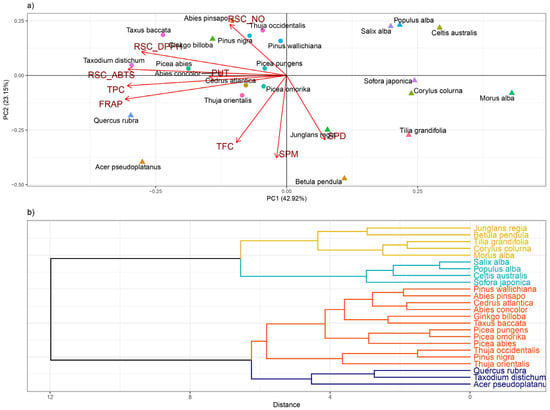
Figure 4.
PCA analysis of all examined parameters in coniferous and deciduous tree species collected in urban areas (a) and hierarchical clustering of 24 analyzed tree species (b). The following abbreviations examined parameters: TPC: total phenolic content; TFC-total flavonoid content FRAP: ferric reducing antioxidant power; SPD: spermidine; SPM: spermine; PUT: putrescine; RSC DPPH: radical scavenger capacity against 2,2-Diphenyl-1-picrylhydrazyl radical, RSC NO: radical scavenger capacity against NO radical, RSC ABTS: radical scavenger capacity against 2,2′-azinobis(3-ethylbenzothiozoline)-6-sulfonic acid, ABTS•+.
The most important finding from PCA analysis is that, with the exception of Acer pseudoplatanus and Quercus rubra, all deciduous tree species (marked with a triangle in the PCA graph) were displayed on the right side of the PC2 axis, whereas all coniferous species (marked with a circle in PCA) were displayed on the left.
According to all inspected biochemical parameters, hierarchical cluster analysis divided examined species into four clusters. All coniferous species were separated in one cluster. As expected, Salix alba and Populus alba species as members of the same genera were closely grouped within the same cluster, as well as three members of the Picea genera (Picea abies, Picea pungens, and Picea omorika). On the other hand, Acer pseudoplatanus, as well as Junglans regia and Betula pendula, distinguished themselves from other inspected species (Figure 4b).
4. Discussion
To the best of the authors’ knowledge, species-specific level variation in PAs in tree species has never been thoroughly explored, yet there is a great embodiment of research supporting PAs’ critical role in plants’ ability to withstand abiotic and biotic stress [21].
The prevailing opinion is that constitutional PA levels are genetically determined and that higher PA concentrations make a species more resilient to environmental challenges, emphasizing the protective role of PAs in plants. This protective role has been additionally confirmed on crop species by exogenous application of PAs, which resulted in increased tolerance to drought, cold, salt, or heat stress [21,39,40]. PAs are enrolled in a variety of physiological processes, exhibiting different modes of action in the plant cell.
Due to their small size and unique polycationic and aliphatic chemical structure, PAs are highly protonated and positively charged molecules at physiological pH and are bound to form strong electrostatic linkages with negatively charged molecules like nucleic acids or phospholipids in cellular or thylakoid membranes, cell walls, and thus modify and stabilize their structure and modulate their properties [41]. On the other hand, via covalent bounding to transglutaminases (TGs), PAs regulate intra- and inter-molecular cross linking of proteins, including cytoskeleton proteins [42]. PA catabolism presents another key mechanism of action for PAs, considering the fact that hydrogen peroxide, a reactive oxygen species (ROS) and important secondary messenger and key activator of stress-related transduction pathways, is produced as a by-product of the enzymatic degradation of PAs by polyamine oxidase (PAO), which causes the stomatal closure, which is crucial for preventing water loss during drought and heat stress [43]. Furthermore, H2O2 regulates the expression of transcription factors and regulative genes that are involved in osmotic and stress response but also stimulates the expression of genes that encode enzymes enrolled in antioxidant response [44,45]. Intriguingly, PAs and hydrogen peroxide both function as “double-edged swords” in plant abiotic stress responses because PAs occasionally display their antioxidant properties while during their catabolism, they generate hydrogen peroxide with high prooxidative capacity, which is prone to form the most damaging hydroxyl radical in Fenton-like reactions, but on the other hand, also act as an important secondary messenger of the stress to activate antioxidant response [43]. Both of them play crucial roles as redox regulators and signaling molecules that switch on to the stress response and control crosstalk with hormonal signaling cascades [20]. The most significant mechanism of PA action is associated with PA-induced stimulation of nitric oxide (NO) generation [46], a key molecule in plant thermoregulation [47,48] but also one that triggers the hypersensitive reaction (HR) under biotic stress [49].
While there are many abiotic stress factors that can affect the biochemical characteristics of trees in urban environments, given that the leaf samples were collected after a particularly hot summer, we assumed that heat stress was the most significant stress in the urban area of Futoški Park. Since the overall goal of this study was to track variance of individual polyamines and phenolics and its species determined heterogeneity, and considering the fact that leaf material was collected from all tree species that were grown under same microclimate conditions at the same soil type, composition and characteristics, we disregarded abiotic stresses resulting from potentially present air pollutants, such as polycyclic aromatic hydrocarbons (PAHs), because their amounts in the examined urban area had already been established to be at low levels [50].
Studies on pears have shown that the over- and under-expression of PA biosynthetic genes directly affect PAs accumulation [51,52]. Furthermore, heat stress dramatically increased PUT levels in pedunculate oak seedlings that were not mycorrhized but had no impact on spermidine or spermine levels. In contrast, heat stress markedly increased all inspected PA levels in oak seedlings that had been inoculated with ectomycorrhizal inoculum [18]. Similar to this, when pedunculate oak was subjected to progressive drought, the levels of SPD and SPM were dramatically increased while PUT remained unaltered [17]. Trifoliate orange seedlings (Poncirus trifoliata L.) exposed to drought stress for 15 days were also shown to have a considerable increase in total PA content [53]. Regarding coniferous species, moderate drought stress induced by PEG during the vegetative propagation method of somatic embryogenesis affected PA metabolism in Scots pine (Pinus sylvestris L.) [54]. The ability of PAs to promote root prolongation and growth by enhancing root cell division in regenerated Virginia pine plantlets may be related to one of the putative processes by which PAs ameliorate drought stress [55]. Another mechanism that PAs may use to reduce the effects of drought stress is their modulation of the expression patterns of several aquaporins (AQs), intrinsic plasma membrane proteins that enable transcellular passive conduction of water and stress signals [56,57].
Along with genetic predisposition, the distribution of polyamines in free and conjugated forms also influences total foliar polyamine content [22]. Free polyamines, in particular, are prone to attach to phenylpropanoids and form conjugates known as phenolamides or hydroxycinnamic acid amides (HCAAs) as a result of the activity of hydroxycinnamoyltransferases [22]. Sometimes, these conjugates are even more potent antioxidants than their free forms [22]. Recently, the general hypothesis that “the higher the polyamine level, the better” was speculated, emphasizing that this notion cannot be applied universally since it was proven that major polyamines are interconvertible within the “polyamine cycle”, but these changes do not affect the total amounts in different species [58]. Additional data on PA levels in various tree species in the context of heat and water deprivation in urban areas are dearth. This study represents a pioneering investigation on the species-specificity of urban tree species regarding polyamine metabolic profiles.
A high positive correlation between TFC and TPC parameters and the antioxidant capacity parameters (FRAP, DPPH, and ABTS) (Figure 5) suggests that these parameters use the same mechanism of antioxidant action, an electron transfer (ET) mechanism, which is thoroughly explained previously [59].
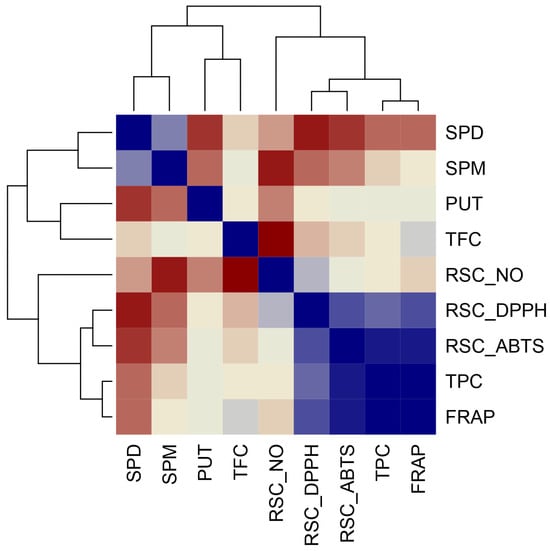
Figure 5.
Pearson’s coefficient of the correlation matrix of the examined parameters in 24 examined coniferous and deciduous tree species collected in urban areas. Blue squares represent a highly significant correlation of inspected parameters, while red squares present low interaction assessed according to the corresponding Pearson’s coefficient. The following abbreviations examined parameters: TPC: total phenolic content; TFC: total flavonoid content FRAP: ferric reducing antioxidant power; SPD: spermidine; SPM: spermine; PUT: putrescine; RSC DPPH: radical scavenger capacity against 2,2-Diphenyl-1-picrylhydrazyl radical, RSC NO: radical scavenger capacity against NO radical, RSC ABTS: radical scavenger capacity against 2,2′-azinobis(3-ethylbenzothiozoline)-6-sulfonic acid, ABTS•+.
Evidently, the PCA’s spatial arrangement was supported by the Pierson correlation matrix. All total radical scavenger capacity assays (RSC ABTS, RSC DPPH, and FRAP) were highly positively correlated with each other and were positively correlated with total phenolic compounds (TPC). SPD was negatively correlated to all inspected total antioxidant capacities (RSC DPPH, RSC ABTS, and FRAP) as well as with TPC. Other PAs (PUT and SPM) show a mild correlation with total antioxidant capacities (RSC DPPH, RSC ABTS, and FRAP), while SPM exhibited a highly negative correlation with RSC NO. Furthermore, TFC showed a highly negative correlation with RSC NO and a highly positive correlation with TFC.
Since there is only a weak correlation between individual polyamines and total antioxidant power, the correlation matrix suggests that phenolic compounds contribute more than polyamines to the total antioxidant power of the extracts of the tree species estimated by all three assays (FRAP, DPPH, and ABTS) (Figure 5). A negative correlation between spermine and spermidine and radical scavenger capacity against NO radical indicates that polyamines contribute to the generation of NO radical rather than NO neutralization.
5. Conclusions
A high degree of species-specificity for polyamine content was found in both deciduous and coniferous tree species, including a total of 24 different tree species. Regarding polyamine content, the most notable coniferous tree species are Thuja occidentalis, Taxodium distichum, Pinus nigra, and Abies concolor, whereas the most prominent deciduous tree species were Betula pendula, Junglans regia, and Quercus rubra. It can be generally concluded that in most of the species, the PUT is the most dominant PA in the leaves, followed by SPD and SPM. The PCA analysis clearly separated coniferous and deciduous species in separated quadrants. In the context of urban climate-smart forestry, this study showed that according to quantified levels of polyamine and phenolics and associated antioxidant activities, adaptable tree species can be distinguished and that these parameters can serve as a trustworthy indicator and descriptor for the selection of adaptable species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9101157/s1, Table S1: Diameter at breast height and height of selected tree specimens.
Author Contributions
Conceptualization, M.K. and S.K.; methodology, M.R. and M.K.; software, S.K.; formal analysis, M.R. and M.K.; resources, S.K.; data curation, S.K., M.R. and D.V.S.; writing—original draft preparation, M.K.; writing—review and editing S.K., M.R., S.O., S.S. and D.V.S.; visualization, S.K.; supervision, S.O.; funding acquisition, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Provincial Secretariat for Higher Education and Scientific Research via the project ‘Influence of specific factors in urban environment on alley trees vitality’ (Contract No. 142-451-2558/2021-01/2).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Esperon-Rodriguez, M.; Rymer, P.D.; Power, S.A.; Challis, A.; Marchin, R.M.; Tjoelker, M.G. Functional adaptations and trait plasticity of urban trees along a climatic gradient. Urban For. Urban Green. 2020, 54, 126771. [Google Scholar] [CrossRef]
- Coumou, D.; Robinson, A. Historic and future increase in the global land area affected by monthly heat extremes. Environ. Res. Lett. 2013, 8, 034018. [Google Scholar] [CrossRef]
- Sharma, A.; Andhikaputra, G.; Wang, Y.C. Heatwaves in South Asia: Characterization, Consequences on Human Health, and Adaptation Strategies. Atmosphere 2022, 13, 734. [Google Scholar] [CrossRef]
- Ulpiani, G. On the linkage between urban heat island and urban pollution island: Three-decade literature review towards a conceptual framework. Sci. Total Environ. 2021, 751, 141727. [Google Scholar] [CrossRef] [PubMed]
- Pukowiec-Kurda, K. The urban ecosystem services index as a new indicator for sustainable urban planning and human well-being in cities. Ecol. Indic. 2022, 144, 109532. [Google Scholar] [CrossRef]
- Rahmonov, O.; Pukowiec-Kurda, K.; Banaszek, J.; Brom, K. Floristic diversity in selected city parks in southern Poland. Environ. Prot. Nat. Resour. 2020, 30, 8–17. [Google Scholar] [CrossRef]
- McPherson, E.G.; Berry, A.M.; van Doorn, N.S. Performance testing to identify climate-ready trees. Urban For. Urban Green. 2018, 29, 28–39. [Google Scholar] [CrossRef]
- Kesić, L.; Vuksanović, V.; Karaklić, V.; Vaštag, E. Variation of Leaf Water Potential and Leaf Gas Exchange Parameters of Seven Silver Linden (Tilia tomentosa Moench) Genotypes in Urban Environment. Topola 2020, 205, 15–24. [Google Scholar] [CrossRef]
- Kebert, M.; Rapparini, F.; Neri, L.; Bertazza, G.; Orlović, S.; Biondi, S. Copper-Induced Responses in Poplar Clones are Associated with Genotype-and Organ-Specific Changes in Peroxidase Activity and Proline, Polyamine, ABA, and IAA Levels. J. Plant Growth Regul. 2017, 36, 131–147. [Google Scholar] [CrossRef]
- De la Sota, C.; Ruffato-Ferreira, V.J.; Ruiz-García, L.; Alvarez, S. Urban green infrastructure as a strategy of climate change mitigation. A case study in northern Spain. Urban For. Urban Green. 2019, 40, 145–151. [Google Scholar] [CrossRef]
- Vastag, E.; Kesić, L.; Karaklić, V.; Zorić, M.; Vuksanović, V.; Stojnić, S. Physiological performance of sweetgum (Liquidambar stryraciflua L.) and norway maple (Acer platanoides L.) under drought condition in urban environment. Topola 2019, 204, 17–27. [Google Scholar]
- Esperon-Rodriguez, M.; Tjoelker, M.G.; Lenoir, J.; Baumgartner, J.B.; Beaumont, L.J.; Nipperess, D.A.; Power, S.A.; Richard, B.; Rymer, P.D.; Gallagher, R.V. Climate change increases global risk to urban forests. Nat. Clim. Chang. 2022, 12, 950–955. [Google Scholar] [CrossRef]
- Dale, A.G.; Frank, S.D. Warming and Drought Combine to Increase Pest Insect Fitness on Urban Trees. PLoS ONE 2017, 12, e0173844. [Google Scholar] [CrossRef]
- Calfapietra, C.; Peñuelas, J.; Niinemets, Ü. Urban plant physiology: Adaptation-mitigation strategies under permanent stress. Trends Plant Sci. 2015, 20, 72–75. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant. Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Tanwir, K.; Amna; Javed, M.T.; Shahid, M.; Akram, M.S.; Ali, Q. Antioxidant Defense Systems in Bioremediation of Organic Pollutants. In Handbook of Bioremediation: Physiological; Mirza, H., Majeti, N.V.P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 505–521. ISBN 9780128193822. [Google Scholar]
- Kebert, M.; Kostić, S.; Stojnić, S.; Čapelja, E.; Markić, A.G.; Zorić, M.; Kesić, L.; Flors, V. A Fine-Tuning of the Plant Hormones, Polyamines and Osmolytes by Ectomycorrhizal Fungi Enhances Drought Tolerance in Pedunculate Oak. Int. J. Mol. Sci. 2023, 24, 7510. [Google Scholar] [CrossRef]
- Kebert, M.; Kostić, S.; Čapelja, E.; Vuksanović, V.; Stojnić, S.; Markić, A.G.; Zlatković, M.; Milović, M.; Galović, V.; Orlović, S. Ectomycorrhizal Fungi Modulate Pedunculate Oak’s Heat Stress Responses through the Alternation of Polyamines, Phenolics, and Osmotica Content. Plants 2022, 11, 3360. [Google Scholar] [CrossRef]
- Kebert, M.; Vuksanović, V.; Stefels, J.; Bojović, M.; Horák, R.; Kostić, S.; Kovačević, B.; Orlović, S.; Neri, L.; Magli, M.; et al. Species-Level Differences in Osmoprotectants and Antioxidants Contribute to Stress Tolerance of Quercus robur L., and Q. cerris L. Seedlings under Water Deficit and High Temperatures. Plants 2022, 11, 1744. [Google Scholar] [CrossRef] [PubMed]
- Biondi, S.; Antognoni, F.; Marincich, L.; Lianza, M.; Tejos, R.; Ruiz, K.B. The polyamine “multiverse” and stress mitigation in crops: A case study with seed priming in quinoa. Sci. Hortic. 2022, 304, 111292. [Google Scholar] [CrossRef]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Gondor, O.K.; Janda, T. Unfinished story of polyamines: Role of conjugation, transport and light-related regulation in the polyamine metabolism in plants. Plant Sci. 2021, 308, 110923. [Google Scholar] [CrossRef] [PubMed]
- Aktar, F.; Islam, M.S.; Milon, M.A.-A.; Islam, N.; Islam, M.A. Polyamines: An Essentially Regulatory Modulator of Plants to Abiotic Stress Tolerance: A Review. Asian J. Appl. Sci. 2021, 9, 195–204. [Google Scholar] [CrossRef]
- Sobieszczuk-Nowicka, E.; Paluch-Lubawa, E.; Mattoo, A.K.; Arasimowicz-Jelonek, M.; Gregersen, P.L.; Pacak, A. Polyamines—A new metabolic switch: Crosstalk with networks involving senescence, crop improvement, and mammalian cancer therapy. Front. Plant Sci. 2019, 10, 460298. [Google Scholar] [CrossRef] [PubMed]
- Kebert, M.; Kostić, S.; Zlatković, M.; Stojnic, S.; Čapelja, E.; Zorić, M.; Kiprovski, B.; Budakov, D.; Orlović, S. Ectomycorrhizal Fungi Modulate Biochemical Response against Powdery Mildew Disease in Quercus robur L. Forests 2022, 13, 1491. [Google Scholar] [CrossRef]
- Milošević, D. Application and Evaluation of Classification System of Local Climate Zones Using Automatic Model and Bioclimate Analysis. Ph.D. Thesis, University of Novi Sad, Novi Sad, Serbia, 2018. [Google Scholar]
- Bajsanski, I.; Stojakovic, V.; Jovanovic, M. Effect of tree location on mitigating parking lot insolation. Comput. Environ. Urban Syst. 2016, 56, 59–67. [Google Scholar] [CrossRef]
- Pekeč, S.; Marković, M.; Kebert, M.; Karaklić, V. Osobine zemljišta na području Futoškog parka u Novom Sadu. Šumarstvo 2020, 2020, 111–118. [Google Scholar]
- Kostić, S.; Kebert, M.; Todorović, H.; Pekeč, S.; Zorić, M.; Stojanović, D.; Orlović, S. Soil horizon-dependent heavy metals, and micro-and macro-elements distributions: A case study of Futoški park (Novi Sad, Serbia). Topola 2022, 9, 15–27. [Google Scholar] [CrossRef]
- Kostić, S.; Čukanović, J.; Orlović, S.; Ljubojević, M.; Mladenović, E. Allometric Relations of Sycamore Maple (Acer pseudoplatanus) and its Red Leaf Cultivar (A. pseudoplatanus “ Atropurpureum ”) in Street and Park Habitats of Novi Sad (Serbia, Europe). J. For. 2019, 117, 114–127. [Google Scholar] [CrossRef]
- Scaramagli, S.; Blondi, S.; Torrigiani, P. Methylglyoxal(bis-guanylhydrazone) inhibition of organogenesis is not due to S-adenosylmethionine decarboxylase inhibition/polyamine depletion in tobacco thin layers. Physiol. Plant. 1999, 107, 353–360. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Factors Influencing the Antioxidant Activity Determined by the ABTS•+ Radical Cation Assay. Free. Radic. Res. 2009, 26, 195–199. [Google Scholar] [CrossRef]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Hensley, K.; Floyd, R.A.; Hensley, K.; Mou, S.; Pye, Q.N. Nitrite Determination by Colorimetric and Fluorometric Griess Diazotization Assays. In Methods in Biological Oxidative Stress; Hensley, K., Floyd, R.A., Eds.; Humana Press: Totowa, NJ, USA, 2003; pp. 185–193. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Wimalasekera, R.; Tebartz, F.; Scherer, G.F. Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci. 2011, 181, 593–603. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Dziurka, M.; Hura, K.; Ostrowska, A.; Dziurka, K. Free and cell wall-bound polyamines under long-term water stress applied at different growth stages of × Triticosecale Wittm. PLoS ONE 2015, 10, e0135002. [Google Scholar] [CrossRef]
- Del Duca, S.; Aloisi, I.; Parrotta, L.; Cai, G. Cytoskeleton, transglutaminase and gametophytic self-incompatibility in the Malinae (Rosaceae). Int. J. Mol. Sci. 2019, 20, 209. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Sengupta, A.; Chakraborty, M.; Gupta, B. Hydrogen peroxide and polyamines act as double edged swords in plant abiotic stress responses. Front. Plant Sci. 2016, 7, 1343. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Bhardwaj, R.; Gupta, B.D.; Dutt, P.; Gupta, R.K.; Kanwar, M.; Biondi, S. Enhancing effects of 24-epibrassinolide and putrescine on the antioxidant capacity and free radical scavenging activity of Raphanus sativus seedlings under Cu ion stress. Acta Physiol. Plant. 2011, 33, 1319–1333. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Tun, N.N.; Santa-Catarina, C.; Begum, T.; Silveira, V.; Handro, W.; Floh, E.I.S.; Scherer, G.F. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 2006, 47, 346–354. [Google Scholar] [CrossRef]
- Sánchez-Vicente, I.; Lorenzo, O. Nitric oxide regulation of temperature acclimation: A molecular genetic perspective. J. Exp. Bot. 2021, 72, 5789–5794. [Google Scholar] [CrossRef]
- Parankusam, S.; Adimulam, S.S.; Bhatnagar-Mathur, P.; Sharma, K.K. Nitric oxide (NO) in plant heat stress tolerance: Current knowledge and perspectives. Front. Plant Sci. 2017, 8, 1582. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef]
- Škrbić, B.; Đurišić-Mladenović, N.; Živančev, J.; Tadić, Đ. Seasonal occurrence and cancer risk assessment of polycyclic aromatic hydrocarbons in street dust from the Novi Sad city, Serbia. Sci. Total Environ. 2019, 647, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.P.; Pang, X.M.; Matsuda, N.; Kita, M.; Inoue, H.; Hao, Y.J.; Honda, C.; Moriguchi, T. Over-expression of the apple spermidine synthase gene in pear confers multiple abiotic stress tolerance by altering polyamine titers. Trans. Res. 2008, 17, 251–263. [Google Scholar] [CrossRef]
- Wen, X.P.; Ban, Y.; Inoue, H.; Matsuda, N.; Kita, M.; Moriguchi, T. Antisense inhibition of a spermidine synthase gene highlights the role of polyamines for stress alleviation in pear shoots subjected to salinity and cadmium. Environ. Exp. Bot. 2011, 72, 157–166. [Google Scholar] [CrossRef]
- Zou, Y.-N.; Zhang, F.; Srivastava, A.K.; Wu, Q.-S.; Kuča, K. Arbuscular Mycorrhizal Fungi Regulate Polyamine Homeostasis in Roots of Trifoliate Orange for Improved Adaptation to Soil Moisture Deficit Stress. Front. Plant Sci. 2021, 11, 600792. [Google Scholar] [CrossRef]
- Salo, H.M.; Sarjala, T.; Jokela, A.; Häggman, H.; Vuosku, J. Moderate stress responses and specific changes in polyamine metabolism characterize Scots pine somatic embryogenesis. Tree Physiol. 2016, 36, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Newton, R.J. Polyamines promote root elongation and growth by increasing root cell division in regenerated Virginia pine (Pinus virginiana Mill.) plantlets. Plant Cell Rep. 2005, 24, 581–589. [Google Scholar] [CrossRef]
- Sharma, K.; Gupta, S.; Thokchom, S.D.; Jangir, P.; Kapoor, R. Arbuscular Mycorrhiza-Mediated Regulation of Polyamines and Aquaporins During Abiotic Stress: Deep Insights on the Recondite Players. Front. Plant Sci. 2021, 12, 642101. [Google Scholar] [CrossRef] [PubMed]
- Tailor, A.; Bhatla, S.C. Polyamine homeostasis modulates plasma membrane-and tonoplast-associated aquaporin expression in etiolated salt-stressed sunflower (Helianthus annuus L.) seedlings. Protoplasma 2021, 258, 661–672. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).