Fruit Quality of Several Strawberry Cultivars during the Harvest Season under High Tunnel and Open Field Environments
Abstract
:1. Introduction
2. Materials and Methods
Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Total Anthocyanins
3.2. Total Soluble Solids
3.3. Acidity and Titratable Acidity
3.4. TSS/TA Ratio
3.5. TSS, Acidity, and TA over the Harvest Season
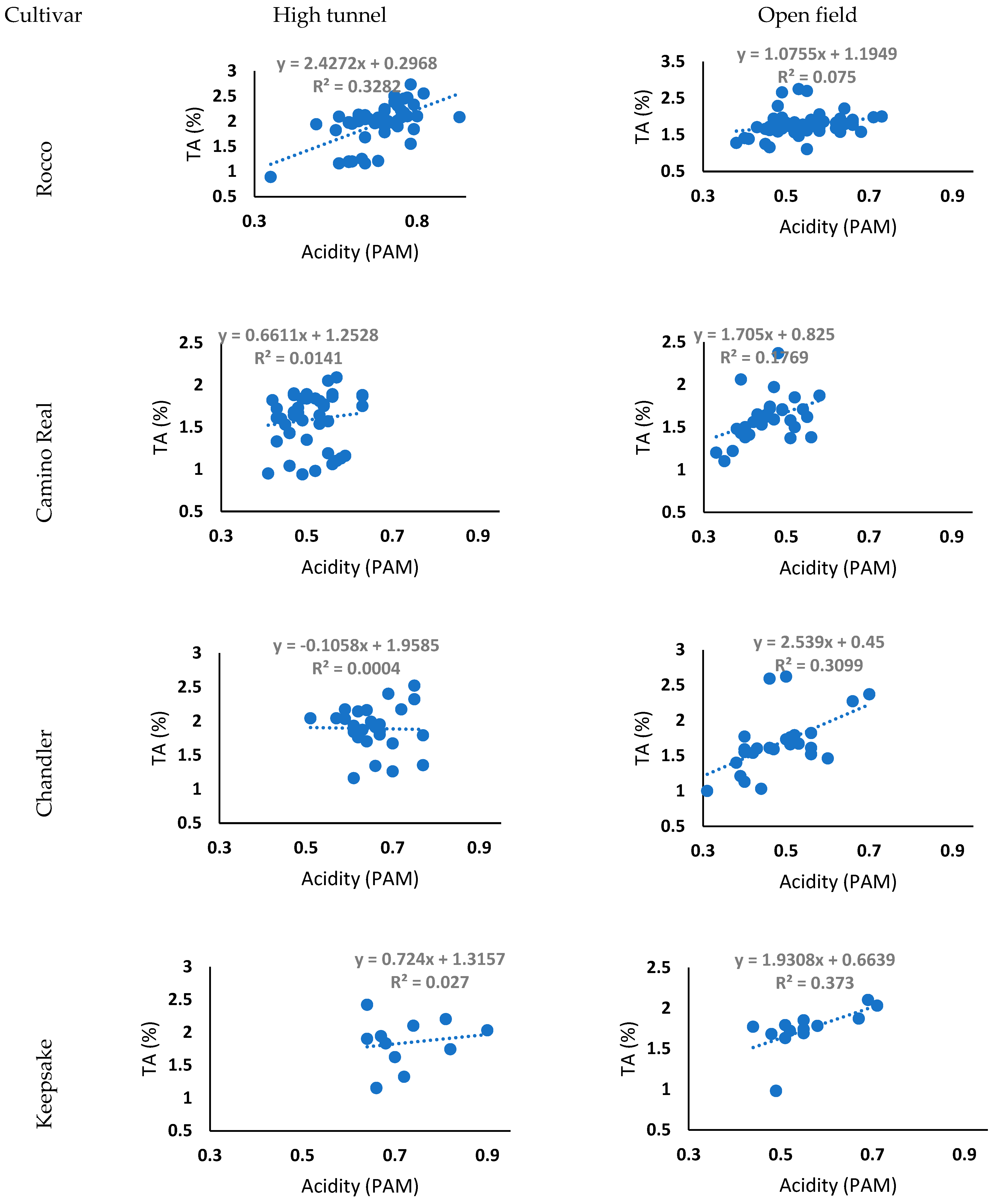
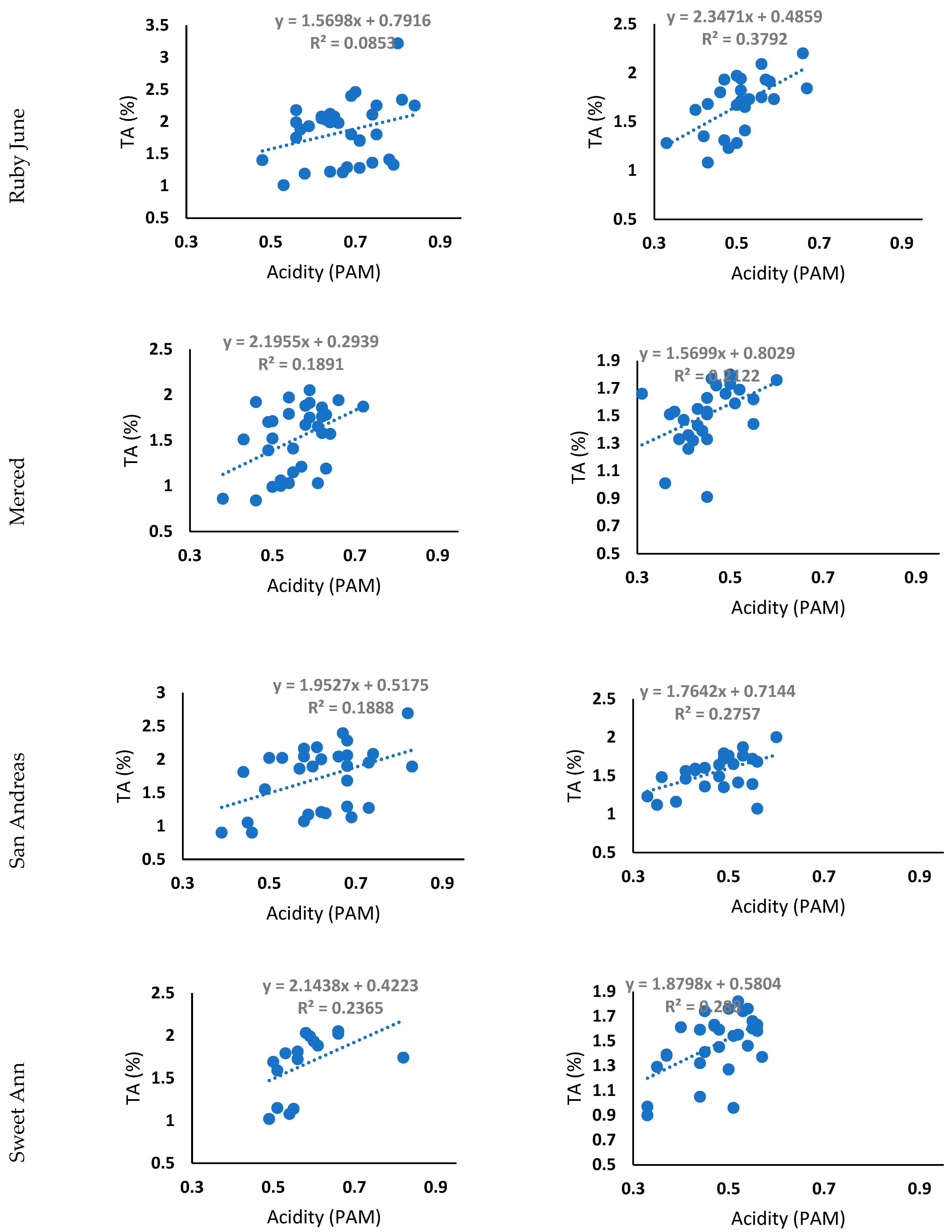
3.6. TA and Acidity (PAM Data) Regression
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahbandeh, M. Leading Strawberry Producing U.S. States, Strawberry Production in the United States in 2020 by State, 2020. Available online: https://www.statista.com/statistics/194235/top-10-strawbery-producing-us-states/ (accessed on 18 May 2021).
- Food and Agriculture Organization of the United Nations. 2015. Crops. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 28 November 2018).
- U.S. Department of Agriculture (USDA). World Strawberry Production, 1990–2011. 2013. Available online: http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1381 (accessed on 28 November 2018).
- Samtani, J.B.; Rom, C.R.; Friedrich, H.; Fennimore, S.A.; Finn, C.E.; Petran, A.; Wallace, R.W.; Pritts, M.P.; Fernandez, G.; Chase, C.A.; et al. The status and future of the strawberry industry in the United States. HortTechnology 2019, 29, 11–24. [Google Scholar] [CrossRef]
- Flanagan, R.D.; Samtani, J.B.; Manchester, M.A.; Romelczyk, S.; Johnson, C.S.; Lawrence, W.; Pattison, J. On-farm evaluation of strawberry cultivars in coastal Virginia. HortTechnology 2020, 30, 789–796. [Google Scholar] [CrossRef]
- Larson, K.D. Strawberry. In Handbook of Environmental Physiology of Fruit Crops; CRC Press: Boca Raton, FL, USA, 2018; pp. 271–297. [Google Scholar]
- Cervantes, L.; Ariza, M.T.; Miranda, L.; Lozano, D.; Medina, J.J.; Soria, C.; Martínez-Ferri, E. Stability of fruit quality traits of different strawberry varieties under variable environmental conditions. Agronomy 2020, 10, 1242. [Google Scholar] [CrossRef]
- Paroussi, G.; Voyiatzis, D.G.; Paroussis, E.; Drogoudi, P.D. Growth, flowering and yield responses to GA3 of strawberry grown under different environmental conditions. Sci. Horticult. 2002, 96, 103–113. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Rafie, R. Comparing pH differential and methanol-based methods for anthocyanin assessments of strawberries. Food Sci. Nutr. 2022, 10, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Voča, S.; Dobričević, N.; Dragović-Uzelac, V.; Duralija, B.; Družić, J.; Čmelik, Z.; Babojelić, M.S. Fruit Quality of New Early Ripening Strawberry Cultivars in Croatia. Food Technol. Biotechnol. 2008, 46, 292–298. [Google Scholar]
- Oregon Strawberry Commission. Product Development Guide, 2006; pp. 1–4. Available online: www.oregon-strawberries.com (accessed on 15 June 2022).
- Keutgen, A.J.; Pawelzik, E. Modifications of taste-relevant compounds in strawberry fruit under NaCl salinity. Food Chem. 2007, 105, 1487–1494. [Google Scholar] [CrossRef]
- Gündüz, K.; Özbay, H. The effects of genotype and altitude of the growing location on physical, chemical, and phytochemical properties of strawberry. Turk. J. Agric. For. 2018, 42, 145–153. [Google Scholar] [CrossRef]
- Pelayo-Zaldívar, C.L.A.R.A.; Ebeler, S.E.; Kader, A.A. Cultivar and harvest date effects on flavor and other quality attributes of California strawberries. J. Food Qual. 2005, 28, 78–97. [Google Scholar] [CrossRef]
- Chaiwong, S.; Bishop, C.F. Effect of vibration damage on the storage quality of ‘Elsanta’ strawberry. Aust. J. Crop Sci. 2015, 9, 859–864. [Google Scholar]
- Perkins-Veazie, P.; Ashrafi, H.; Fernandez, G.; Clark, J.R.; Threfall, R.; Worthington, M.; Taghavi, T. Multi User Testing of a Pocket Acidity Refractometer (PAM) as a Rapid Means to Determine Titratable Acidity in Small Fruits, Final Report for Southern Region Small Fruit Consortium Grant, 2019. Available online: https://smallfruits.org/files/2019/12/2019-SRFRC-titrmeter-study.pdf (accessed on 19 April 2022).
- Taghavi, T.; Patel, H.; Akande, O.E.; Galam, D.C.A. Total Anthocyanin Content of Strawberry and the Profile Changes by Extraction Methods and Sample Processing. Foods 2022, 11, 1072. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS/ACCESS®, 9.4; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Singh, A.; Syndor, A.; Deka, B.C.; Singh, R.K.; Patel, R.K. The effect of microclimate inside low tunnels on off-season production of strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2012, 144, 36–41. [Google Scholar] [CrossRef]
- Hanson, E.; Von Weihe, M.; Schilder, A.C.; Chanon, A.M.; Scheerens, J.C. High tunnel and open field production of floricane-and primocane-fruiting raspberry cultivars. HortTechnology 2011, 21, 412–418. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Hou, G.; Zhang, Y.; Chen, Q.; Lin, Y.; Li, M.; Wang, Y.; He, W.; Wang, X.; et al. Effect of Genotype and Harvest Date on Fruit Quality, Bioactive Compounds, and Antioxidant Capacity of Strawberry. Horticulturae 2022, 8, 348. [Google Scholar] [CrossRef]
- Herrington, M.E.; Chandler, C.K.; Moisander, J.A.; Reid, E.C. Rubygem strawberry. Hortscience 2007, 42, 1482–1483. [Google Scholar] [CrossRef]
- Saraçoğlu, O. Determination of Yield and Quality Performance of Some Neutral and Short Day Strawberry Cultivars in Kazova. Ph.D. Thesis, University of Gaziosmanpaşa, Tokat, Turkey, 2013. [Google Scholar]
- Andreotti, C.; Guerrero, G.; Zago, M. Quality of strawberry fruits cultivated in a highland area in South Tyrol (Italy). Acta Hort. 2014, 1049, 795–799. [Google Scholar] [CrossRef]
- Azodanlou, R. A Methodology for Assessing the Quality of Fruit and Vegetables. Ph.D. Thesis, Swiss Federal Institute of Technology, Zurich, Switzerland, 2011. Available online: https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/145336/eth-24231-02.pdf (accessed on 30 July 2023).
- Kallio, H.; Hakala, M.; Pelkkikangas, A.-M.; Lapveteläinen, A. Sugars and acids of strawberry varieties. Eur. Food Res. Technol. 2000, 212, 81–85. [Google Scholar] [CrossRef]


| Cultivar | Total Anthocyanin Content (A/gFW) | |
|---|---|---|
| Flavorfest | 289.4 | a * |
| Keepsake | 184.0 | d |
| Rocco | 222.4 | bc |
| Albion | 196.5 | cd |
| Ruby June | 151.8 | e |
| Merced | 187.2 | cd |
| Chandler | 254.1 | b |
| Camino Real | 219.9 | c |
| Sweet Ann | 141.7 | e |
| San Andreas | 212.2 | cd |
| LSD | 32.0 | |
| Environment | ||
| High tunnel | 246.5 | a |
| Open field | 197.7 | b |
| LSD | 16.6 | |
| Environment | Cultivar | Total Anthocyanin Content (A/gFW) | Standard Error |
|---|---|---|---|
| High Tunnel | Flavorfest | 300.0 a * | 10.16 |
| Keepsake | 212.7 bcd | 19.80 | |
| Rocco | 188.9 bcd | 23.66 | |
| Ruby June | 165.3 cd | 25.56 | |
| Merced | 172.9 cd | 25.56 | |
| Chandler | 193.7 bcd | 31.30 | |
| Sweet Ann | 211.7 bcd | 31.30 | |
| San Andreas | 228.7 abc | 62.60 | |
| Avg | 209.3 | ||
| Open Field | Flavorfest | 208.8 bcd | 28.00 |
| Keepsake | 161.9 cd | 17.36 | |
| Rocco | 229.7 abc | 11.07 | |
| Albion | 196.5 bcd | 11.63 | |
| Ruby June | 148.6 cd | 12.52 | |
| Merced | 190.1 bcd | 11.63 | |
| Chandler | 263.4 ab | 12.28 | |
| Camino Real | 219.9 bc | 11.63 | |
| Sweet Ann | 132.0 d | 11.63 | |
| San Andreas | 211.6 bcd | 11.63 | |
| Avg | 196.2 |
| Cultivar | TSS (°Brix) | Acidity (%, PAM) | TA (%) | TSS/TA |
|---|---|---|---|---|
| Flavorfest | 9.65 a * | 0.67 a | 1.94 a | 4.97 |
| Keepsake | 9.20 a | 0.63 ab | 1.79 ab | 5.14 |
| Rocco | 8.83 ab | 0.63 ab | 1.93 a | 4.58 |
| Albion | 8.82 ab | 0.58 cd | 1.77 abc | 4.98 |
| Ruby June | 8.79 ab | 0.60 bc | 1.77 abc | 4.97 |
| Merced | 8.22 bc | 0.51 e | 1.52 d | 5.41 |
| Chandler | 7.93 bcd | 0.57 cd | 1.79 ab | 4.43 |
| Camino Real | 7.59 cd | 0.49 e | 1.60 cd | 4.74 |
| Sweet Ann | 7.56 cd | 0.51 e | 1.54 d | 4.91 |
| San Andreas | 7.16 d | 0.55 d | 1.64 bcd | 4.37 |
| LSD | 0.87 | 0.04 | 0.16 | |
| Environment | ||||
| High Tunnel | 8.08 a | 0.62 a | 1.76 a | 4.59 |
| Open Field | 8.30 a | 0.50 b | 1.64 b | 5.06 |
| LSD | 0.32 | 0.14 | 0.06 |
| Cultivar | °Brix-Avg | °Brix-Harvest1 | °Brix-Harvest2 | °Brix-Harvest3 | °Brix-Harvest4 | °Brix-Harvest5 |
|---|---|---|---|---|---|---|
| Flavorfest | 9.98 a * | 9.76 a | 9.10 a | 10.13 a | - b | - |
| Keepsake | 9.22 b | 8.93 b | 8.90 a | 9.28 b | 11.23 a | - |
| Rocco | 8.80 b | 8.55 bc | 8.35 b | 8.23 cd | 8.63 b | 8.79 ab |
| Albion | 8.70 bc | 7.30 e | 7.48 c | 8.47 bc | 7.93 cd | 8.53 b |
| Ruby June | 8.79 b | 8.15 cd | 7.49 c | 9.31 b | 8.89 b | 9.28 a |
| Merced | 8.21 cd | 8.12 cd | 7.60 c | 8.09 d | 8.41 bc | 8.68 b |
| Chandler | 7.94 de | 7.84 d | 7.65 c | 7.32 e | 7.23 e | 8.51 b |
| Camino Real | 7.70 de | 7.27 e | 7.20 c | 7.31 e | 7.89 cd | 7.76 c |
| Sweet Ann | 7.59 ef | 7.08 e | 7.27 c | 7.88 de | 7.68 de | 7.47 c |
| San Andreas | 7.14 f | 7.06 e | 7.11 c | 6.61 f | 7.78 de | 7.32 c |
| LSD | 0.52 | 0.44 | 0.50 | 0.61 | 0.59 | 0.52 |
| Environment | ||||||
| High Tunnel | 8.42 a | 7.99 a | 7.88 a | 8.16 a | 8.13 a | 8.34 a |
| Open Field | 8.30 a | 7.93 a | 7.56 b | 8.03 a | 8.11 a | 8.24 a |
| LSD | 0.23 ns | 0.20 ns | 0.20 ** | 0.22 ns | 0.26 ns | 0.25 ns |
| Cultivar | Acidity-Avg | Acidity-Harvest1 | Acidity-Harvest2 | Acidity-Harvest3 | Acidity-Harvest4 | Acidity-Harvest5 |
|---|---|---|---|---|---|---|
| Flavorfest | 0.71 a * | 0.72 a | 0.55 c | 0.65 a | - b | - |
| Keepsake | 0.64 b | 0.69 a | 0.62 ab | 0.56 bc | 0.71 a | - |
| Rocco | 0.63 b | 0.63 b | 0.67 a | 0.58 b | 0.61 b | 0.58 b |
| Albion | 0.59 c | 0.56 cde | 0.55 c | 0.60 ab | 0.56 bc | 0.59 ab |
| Ruby June | 0.59 c | 0.60 bcd | 0.58 bc | 0.61 ab | 0.55 c | 0.63 a |
| Merced | 0.51 fg | 0.53 ef | 0.47 d | 0.50 cd | 0.45 de | 0.53 c |
| Chandler | 0.56 cd | 0.60 bc | 0.59 bc | 0.58 b | 0.53 cd | 0.50 c |
| Camino Real | 0.48 g | 0.49 f | 0.44 d | 0.48 d | 0.47 e | 0.50 c |
| Sweet Ann | 0.53 ef | 0.54 def | 0.54 c | 0.51 cd | 0.44 e | 0.51 c |
| San Andreas | 0.55 de | 0.57 cde | 0.56 bc | 0.55 bcd | 0.56 bc | 0.51 c |
| LSD | 0.03 | 0.05 | 0.06 | 0.06 | 0.05 | 0.04 |
| Environment | ||||||
| High Tunnel | 0.65 a | 0.65 a | 0.64 a | 0.63 a | 0.62 a | 0.63 a |
| Open Field | 0.50 b | 0.52 b | 0.48 b | 0.48 b | 0.45 b | 0.47 b |
| LSD | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Cultivar | TA-Avg | TA-Harvest1 | TA-Harvest2 | TA-Harvest3 | TA-Harvest4 | TA-Harvest5 |
|---|---|---|---|---|---|---|
| Flavorfest | 2.04 a * | 2.00 a | 1.85 ab | 1.85 ab | - b | - |
| Keepsake | 1.80 c | 1.91 ab | 1.68 bc | 1.80 ab | 1.68 bc | - |
| Rocco | 1.93 b | 1.86 abc | 1.97 a | 1.74 abc | 2.10 a | 1.81 a |
| Albion | 1.80 c | 1.85 abc | 1.77 ab | 1.85 ab | 1.52 bcd | 1.82 a |
| Ruby June | 1.77 c | 1.67 bcd | 1.85 ab | 2.04 a | 1.59 bcd | 1.71 ab |
| Merced | 1.52 e | 1.62 cd | 1.47 c | 1.36 c | 1.59 bcd | 1.53 bc |
| Chandler | 1.78 c | 1.95 a | 1.77 ab | 1.70 abc | 1.72 b | 1.60 abc |
| Camino Real | 1.60 de | 1.69 bcd | 1.68 bc | 1.73 abc | 1.37 cd | 1.36 c |
| Sweet Ann | 1.57 de | 1.57 d | 1.70 abc | 1.53 bc | 1.31 d | 1.63 ab |
| San Andreas | 1.65 d | 1.75 abcd | 1.82 ab | 1.52 bc | 1.76 b | 1.60 abc |
| LSD | 0.10 | 0.22 | 0.24 | 0.34 | 0.31 | 0.24 |
| Environment | ||||||
| High Tunnel | 1.83 a | 1.84 | 1.86 a | 1.75 a | 1.70 a | 1.74 a |
| Open Field | 1.64 b | 1.72 | 1.64 b | 1.64 a | 1.56 b | 1.54 b |
| LSD | 0.04 | 0.10 | 0.10 | 0.12 ns | 0.13 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, H.; Taghavi, T.; Samtani, J.B. Fruit Quality of Several Strawberry Cultivars during the Harvest Season under High Tunnel and Open Field Environments. Horticulturae 2023, 9, 1084. https://doi.org/10.3390/horticulturae9101084
Patel H, Taghavi T, Samtani JB. Fruit Quality of Several Strawberry Cultivars during the Harvest Season under High Tunnel and Open Field Environments. Horticulturae. 2023; 9(10):1084. https://doi.org/10.3390/horticulturae9101084
Chicago/Turabian StylePatel, Hiral, Toktam Taghavi, and Jayesh B. Samtani. 2023. "Fruit Quality of Several Strawberry Cultivars during the Harvest Season under High Tunnel and Open Field Environments" Horticulturae 9, no. 10: 1084. https://doi.org/10.3390/horticulturae9101084
APA StylePatel, H., Taghavi, T., & Samtani, J. B. (2023). Fruit Quality of Several Strawberry Cultivars during the Harvest Season under High Tunnel and Open Field Environments. Horticulturae, 9(10), 1084. https://doi.org/10.3390/horticulturae9101084






