Reusing Coir-Based Substrates for Lettuce Growth: Nutrient Content and Phytonutrients Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions and Substrates
2.2. Measurements
2.3. Data Analysis
3. Results and Discussion
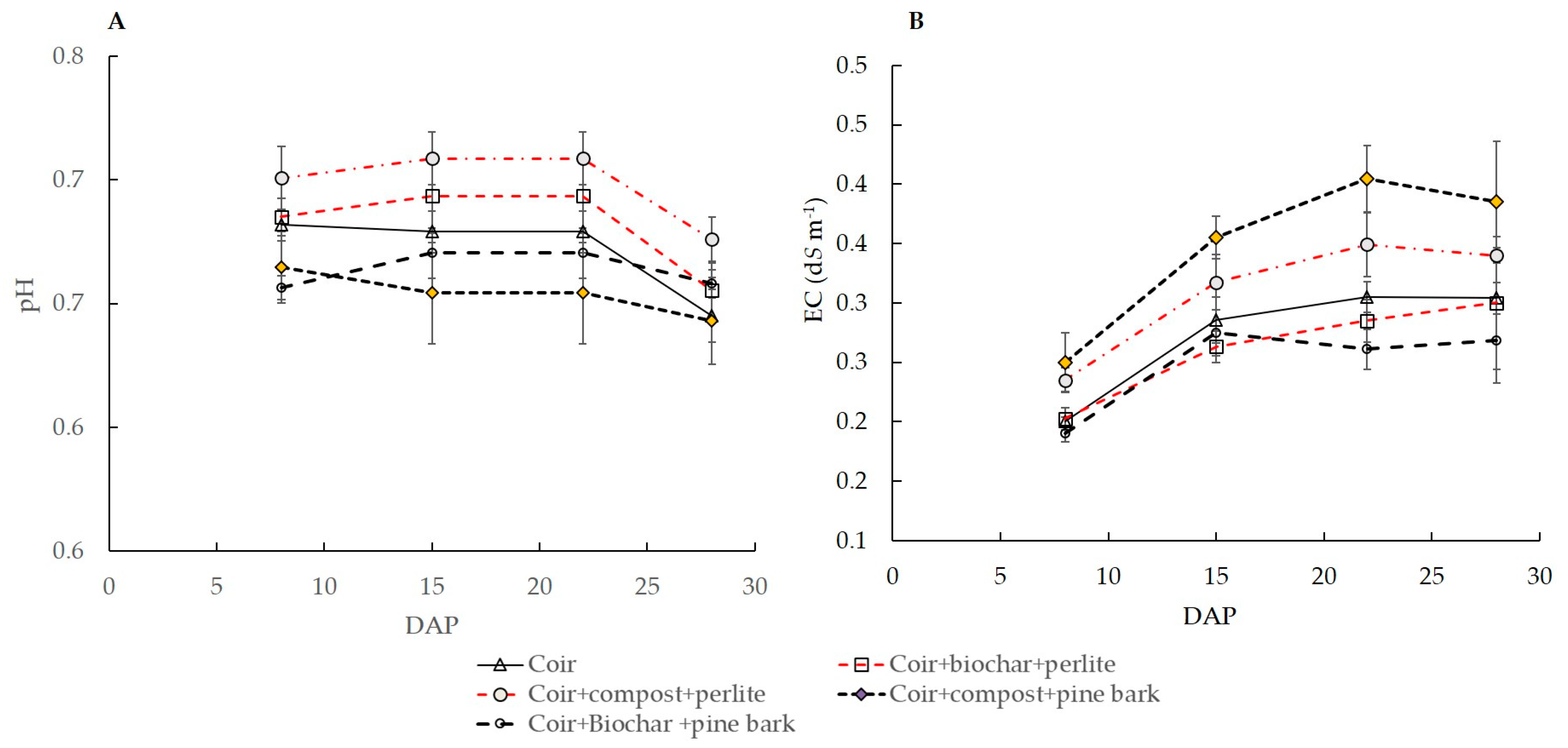
3.1. Leachate pH and EC
3.2. Photosynthetic Pigments
3.3. Shoot Nutrient Concentration
3.4. Plant Growth and Yield
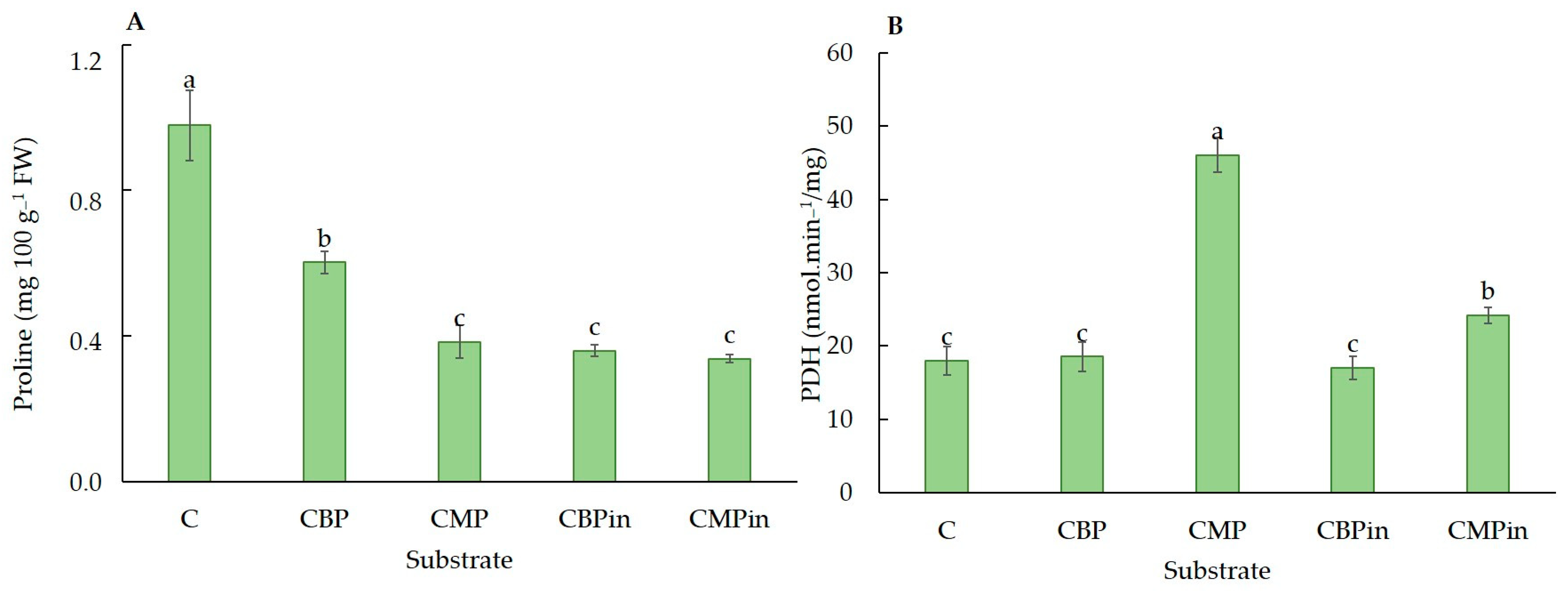
3.5. Phytonutrients Accumulation
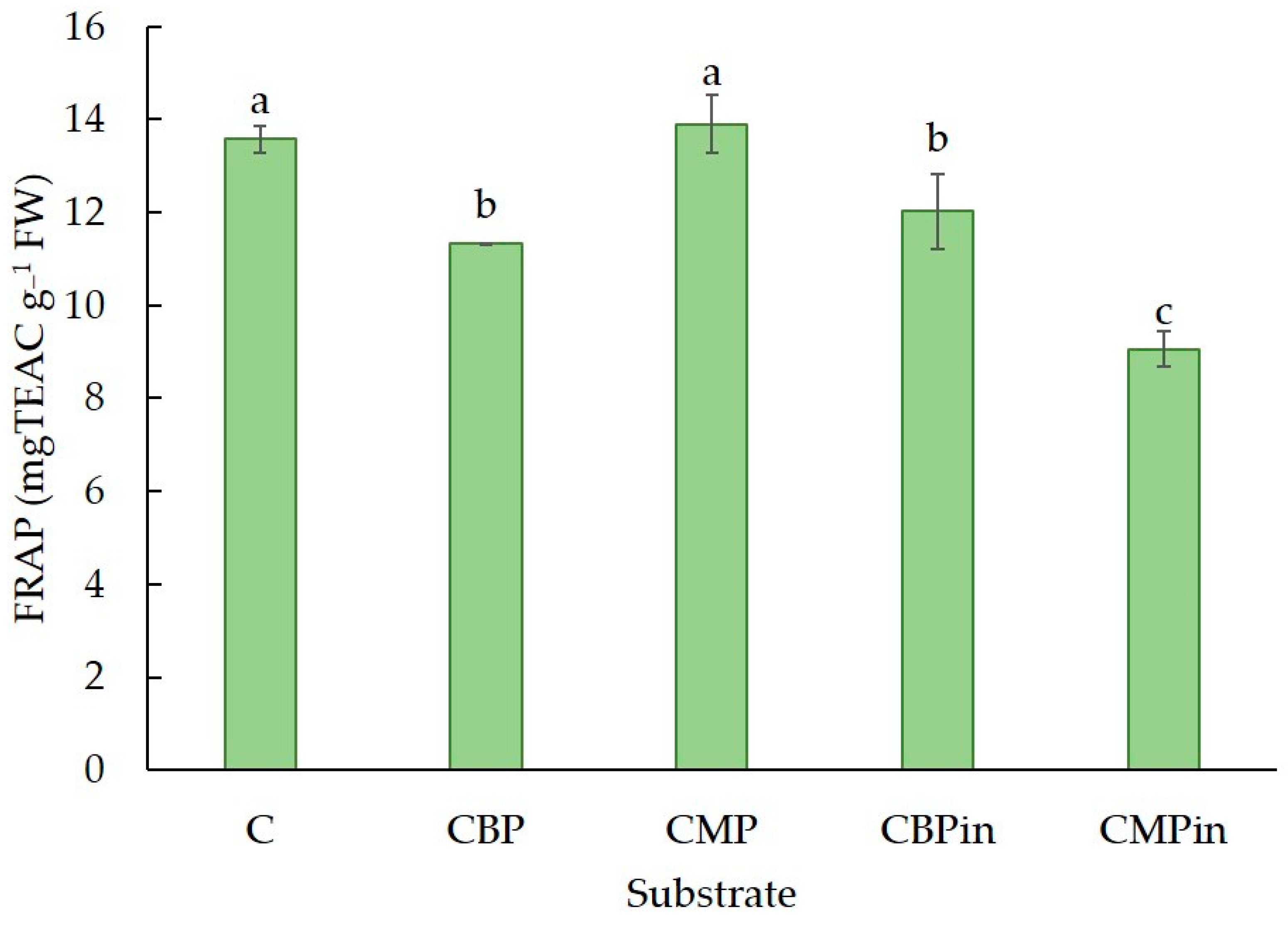
3.6. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Atzori, G.; Pane, C.; Zaccardelli, M.; Cacini, S.; Massa, D. The role of peat-free organic substrates in the sustainable management of soilless cultivations. Agronomy 2021, 11, 1236. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M. The Evolution of soilless systems towards ecological sustainability in the perspective of a circular economy. Is it really the opposite of organic agriculture? Agronomy 2021, 11, 950. [Google Scholar] [CrossRef]
- Tüzel, Y.; Bertschinger, L. Future direction and opportunities of horticultural research. Chron. Hortic. 2020, 60, 9–19. [Google Scholar]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Gruda, N.S. Advances in soilless culture and growing media in today’s horticulture—An Editorial. Agronomy 2022, 12, 2773. [Google Scholar] [CrossRef]
- Diara, C.; Incrocci, L.; Pardossi, A.; Minuto, A. Reusing greenhouse growing media. Acta Hortic. 2012, 927, 793–800. [Google Scholar] [CrossRef]
- Pardossi, A.; Carmassi, G.; Diara, C.; Incrocci, L.; Maggini, R.; Massa, D. Fertigation and Substrate Management in Closed Soilless Culture; University of Pisa: Pisa, Italy, 2011. [Google Scholar]
- Vandecasteele, B.; Blindeman, L.; Amery, F.; Pieters, C.; Ommeslag, S.; Loo, K.V.; de Tender, C.; De bode, J. Grow-Store-steam -repeat: Reuse of spent growing media for circular cultivation of Chrysanthemum. J. Clean. Prod. 2020, 276, 124128. [Google Scholar] [CrossRef]
- Caron, J.; Zheng, Y. Glossary of terms and basic characteristics to be reported in scientific publications on growing media. Acta Hortic. 2021, 1317, 55–64. [Google Scholar] [CrossRef]
- Kerloch, E.; Michel, J.-C. Pore tortuosity and wettability as main characteristics of the evolution of hydraulic properties of organic growing media during cultivation. Vadose Zone J. 2015, 14, vzj2014-11. [Google Scholar] [CrossRef]
- Machado, R.M.; Alves-Pereira, I.; Ferreira, R.; Gruda, N.S. Coir an alternative to peat—Effects on plant growth, phytochemical accumulation, and antioxidant power of spinach. Horticulturae 2021, 7, 127. [Google Scholar] [CrossRef]
- Barcelos, C.; Machado, R.M.; Alves-Pereira, I.; Ferreira, R.; Bryla, D.R. Effects of substrate type on plant growth and nitrogen and nitrate concentration in spinach. Int. J. Plant Biol. 2016, 7, 6325. [Google Scholar] [CrossRef]
- Machado, R.; Alves-Pereira, I.; Morais, C.; Alemão, A.; Ferreira, R. Effects of coir-based growing medium with municipal solid waste compost or biochar on plant growth, mineral nutrition, and accumulation of phytochemicals in spinach. Plants 2022, 11, 1893. [Google Scholar] [CrossRef] [PubMed]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Gruda, N.; Bragg, N. Developments in alternative organic materials as growing media in soilless culture systems. In Advances in Horticultural Soilless Culture; Gruda, N., Ed.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2021; ISBN 13 9781786764355. [Google Scholar]
- Gruda, N.S.; Hirschler, O.; Stuart, J. Peat reduction in horticulture—An overview of Europe. Acta Hortic. 2023; in print. [Google Scholar]
- Abad, M.; Noguera, P.; Noguera, V.; Roig, A.; Cegarra, J.; Paredes, C. Reciclado de residuos orgánicos y su aprovechamiento como sustratos de cultivo. Acta Hortic. 1997, 19, 92–109. [Google Scholar]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, K.; Han, L.F.; Chen, Y.L.; Liu, J.; Xing, B.S. Biochar stability and impact on soil organic carbon mineralization depend on biochar processing, aging and soil clay content. Soil Biol. Biochem. 2022, 169, 108657. [Google Scholar] [CrossRef]
- Prasad, M.; Tzortzakis, N.; McDaniel, N. Chemical characterization of biochar and assessment of the nutrient dynamics by means of preliminary plant growth tests. J. Environ. Manag. 2018, 216, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, F.; Dartigues, A.; Riviere, L.M. Properties of substrates with ground pine bark. Symp. Substr. Hortic. Other Soils Situ 1979, 99, 67–80. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Mazuela, P.C.; Martínez, G.A. Effect of substrate reutilization on yield and properties of melon and tomato crops. J. Plant Nutri. 2008, 31, 2031–2043. [Google Scholar] [CrossRef]
- Schnitzler, W.H. Pest and disease management of soilless culture. In Proceedings of the South Pacific Soilless Culture Conference-SPSCC, Palmerston North, New Zealand, 10–13 February 2003; pp. 191–203. [Google Scholar]
- Pascual, J.A.; Garcia, C.; Hernandez, T.; Lerma, S.; Lynch, J.M. Effectiveness of municipal waste compost and its humic fraction in suppressing Pythium Ultimum. Microb. Ecol. 2002, 44, 59–68. [Google Scholar] [CrossRef]
- Raviv, M. Recent advances in soil-borne disease control using suppressive media. Acta Hortic. 2007, 819, 125–134. [Google Scholar] [CrossRef]
- Neher, D.A.; Hoitink, H.A.; Biala, J.; Rynk, R.; Black, G. Compost use for plant disease suppression. In the Composting Handbook; Rynk, R., Black, G., Biala, J., Bonhotal, J., Cooperband, L., Gilbert, J., Schwarz, M., Eds.; Academic Press: London, UK, 2022; pp. 847–878. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Xiao, R.; Chen, X.; Zhang, T. A quantitative evaluation of the biochar’s influence on plant disease suppress: A global meta-analysis. Biochar 2022, 4, 43. [Google Scholar] [CrossRef]
- Lacomino, G.; Idbella, M.; Laudonia, S.; Vinale, F.; Bonanomi, G. The suppressive effects of biochar on above-and belowground plant pathogens and pests: A review. Plants 2022, 11, 3144. [Google Scholar] [CrossRef]
- Blok, C.; Van der Salm, C.; Hofland-Zijlstra, J.; Streminska, M.; Eveleens, B.; Regelink, I.; Fryda, L.; Visser, R. Biochar for horticultural rooting media improvement: Evaluation of biochar from gasification and slow pyrolysis. Agronomy 2017, 7, 6. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 1987, 4, 3.1–3.8. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar]
- Siegelman, H.W.; Hendricks, S.B. Photocontrol of anthocyanin synthesis. Apple Skin. Plant Physiol. 1958, 33, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Paiva, E.A.S.; Isaias, R.M.D.S.; Vale, F.H.A.; Queiroz, C.G.D.S. The influence of light intensity on anatomical structure and pigment contents of Tradescantia pallida (Rose) Hunt. cv. purpurea Boom (Commelinaceae) leaves. Braz. Arch. Biol. Technol. 2003, 46, 617–624. [Google Scholar] [CrossRef]
- Cai, W.M.; Tang, Z.C. Plant tolerance physiology. In Experimental Guide for Modern Plant Physiology, 1st ed.; Tang, Z.C., Ed.; Science Press: Beijing, China, 1999; pp. 315–316. [Google Scholar]
- Bates, L.S. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lake, B. Preparation and characterization of microsomal fractions for studies of xenobiotic metabolism. In Biochemical Toxi-Cology: A Practical Approach; Snell, K., Mullock, B., Eds.; IRL Press: Oxford, UK, 1987; pp. 183–215. [Google Scholar]
- Costilow, R.N.; Cooper, D. Identity of proline dehydrogenase and delta1-pyrroline-5carboxylic acid reductase in Clostridium sporogenes. J. Bacteriol. 1978, 134, 139–146. [Google Scholar] [CrossRef]
- Lowry, O.H.; Roseburg, N.J.; Farr, A.L.; Randell, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ozgen, S.; Sekerci, S. Effect of leaf position on the distribution of phytochemicals and antioxidant capacity among green and red lettuce cultivars. Span. J. Agric. Res. 2011, 9, 801–809. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Chatzieustratiou, E.; Constantopoulou, E.; Kapotis, G. Yield and quality of lettuce and rocket grown in floating culture system. Not. Bot. Horti. Agrobo. 2016, 44, 603–612. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Sapkota, S.; Liu, Z. Effects of nutrient composition and lettuce cultivar on crop production in hydroponic culture. Horticulturae 2019, 5, 72. [Google Scholar] [CrossRef]
- Tsouvaltzis, P.; Kasampalis, D.S.; Aktsoglou, D.C.; Barbayiannis, N.; Siomos, A.S. Effect of reduced nitrogen and supplemented amino acids nutrient solution on the nutritional quality of baby green and red lettuce grown in a floating system. Agronomy 2020, 10, 922. [Google Scholar] [CrossRef]
- Baslam, M.; Morales, F.; Garmendia, I.; Goicoechea, N. Nutritional quality of outer and inner leaves of green and red pigmented lettuces (Lactuca sativa L.) consumed as salads. Sci. Hortic. 2013, 151, 103–111. [Google Scholar] [CrossRef]
- Prasad, M.; Chrysargyris, A.; McDaniel, N.; Kavanagh, A.; Gruda, N.S.; Tzortzakis, N. Plant nutrient availability and pH of biochar and their fractions, with the possible use as a component in a growing media. Agronomy 2020, 10, 10. [Google Scholar] [CrossRef]
- Martins, T.C.; Machado, R.M.; Alves-Pereira, I.; Ferreira, R.; Gruda, N.S. Coir-Based GrowingMedia with municipal compostand biochar and their impacts on growth and some quality parameters in lettuce Seedlings. Horticulturae 2023, 9, 105. [Google Scholar] [CrossRef]
- Carlile, W.R.; Raviv, M.; Prasad, M. Organic soilless media components. In Soilless Culture—Theory and Practice, 2nd ed.; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Elsevier: London, UK, 2019; pp. 303–378. [Google Scholar]
- Toboso-Chavero, S.; Madrid-López, C.; Villalba, G.; Durany, X.G.; Hückstädt, A.B.; Finkbeiner, M.; Lehmann, A. Environmental and social life cycle assessment of growing media for urban rooftop farming. Int. J. Life Cycle Assess 2021, 26, 2085–2102. [Google Scholar] [CrossRef]
- Çelikel, G.; Caglar, G. The effects of reusing different substrates on the yield and earliness of cucumber on autumn growing period. Acta Hortic. 1997, 492, 259–264. [Google Scholar] [CrossRef]
- Çelikel, G. Influence of reusing substrates on the yield and earliness of eggplant in soilless culture. Acta Hortic. 1999, 491, 357–362. [Google Scholar] [CrossRef]
- Hernández, T.; Chocano, C.; Moreno, J.L.; García, C. Use of compost as an alternative to conventional inorganic fertilizers in intensive lettuce (Lactuca sativa L.) crops—Effects on soil and plant. Soil Till. Res. 2016, 160, 14–22. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and defense mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Kumar, M.; Tak, Y.; Potkule, J.; Choyal, P.; Tomar, M.; Meena, N.L.; Kaur, C. Phenolics as plant protective companion against abiotic stress. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; Volume 1, pp. 277–308. [Google Scholar] [CrossRef]
- Santander, C.; Vidal, G.; Ruiz, A.; Vidal, C.; Cornejo, P. Salinity eustress increases the biosynthesis and accumulation of phenolic compounds that improve the functional and antioxidant quality of red lettuce. Agronomy 2022, 12, 598. [Google Scholar] [CrossRef]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef]
- Sgherri, C.; Pérez-López, U.; Micaelli, F.; Miranda-Apodaca, J.; Mena-Petite, A.; Muñoz-Rueda, A.; Quartacci, M.F. Elevated CO2 and salinity are responsible for phenolics-enrichment in two differently pigmented lettuces. Plant Physiol. Biochem. 2017, 115, 269–278. [Google Scholar] [CrossRef]
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.F.; Muñoz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Bioch. 2018, 123, 233–241. [Google Scholar] [CrossRef]
- Cozzolino, E.; Giordano, M.; Fiorentino, N.; El-Nakhel, C.; Pannico, A.; di Mola, I.; Mori, M.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Appraisal of biodegradable mulching films and vegetal-derived biostimulant application as eco-sustainable practices for enhancing lettuce crop performance and nutritive value. Agronomy 2020, 10, 3. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Ferreira, C.F.R.; Barros, L. Phytochemicals in Vegetables: A Valuable Source of Bioactive Compounds; Bentham Science Publishers: Sharjah, United Arab Emirates, 2018; ISBN 10 1681087405. [Google Scholar]
- Jibril, S.A.; Hassan, S.A.; Ishak, C.F.; Wahab, P.E.M. Cadmium Toxicity Affects Phytochemicals and Nutrient Elements Composition of Lettuce (Lactuca sativa L.). Adv. Agric. 2017, 2017, 1236830. [Google Scholar] [CrossRef]
- Siomos, A.S.; Papadopoulou, P.P.; Dogras, C.C.; Vasiliadis, E.; Dosas, A.; Georgiou, N. Lettuce composition as affected by genotype and leaf position. II Balkan Symp. Veg. Potatoes 2000, 579, 635–639. [Google Scholar] [CrossRef]
- Viacava, G.E.; Gonzalez-Aguilar, G.; Roura, S.I. Determination of phytochemicals and antioxidant activity in butterhead lettuce related to leaf age and position. J. Food Biochem. 2014, 38, 352–362. [Google Scholar] [CrossRef]
- Trovato, M.; Mattioli, R.; Costantino, P. Multiple roles of proline in plant stress tolerance and development. Rend. Lincei. Sci. Fis. Nat. 2008, 19, 325–346. [Google Scholar] [CrossRef]
- Spormann, S.; Nadais, P.; Sousa, F.; Pinto, M.; Martins, M.; Sousa, B.; Fidalgo, F.; Soares, C. Accumulation of proline in plants under contaminated soils—Are We on the Same Page? Antioxidants 2023, 12, 666. [Google Scholar] [CrossRef]
- Gruda, N.; Schnitzler, W.H. The effect of water supply on bio-morphological and plant-physiological parameters of tomato transplants cultivated in wood fiber substrate. J. Appl. Bot. 2000, 74, 233–239. [Google Scholar]
- Gruda, N.; Schnitzler, W.H. Schnitzler: The effect of water supply of seedlings, cultivated in different substrates and raising systems on the bio-morphological and plant-physiological parameters of lettuce. In German: Einfluss der Wasserversorgung von Jungpflanzen angezogen in verschiedenen Substraten und Anzuchtsystemen auf biomorphologische und physiologische Merkmale von Kopfsalat. J. Appl. Bot. 2000, 74, 240–247. [Google Scholar]
- Machado, R.M.A.; Alves-Pereira, I.; Ferreira, R.M.A. Plant growth, phytochemical accumulation and antioxidant activity of substrate-grown spinach. Heliyon 2018, 4, e00751. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.M.A.; Alves-Pereira, I.; Faty, Y.; Perdigão, S.; Ferreira, R. Influence of nitrogen sources applied by fertigation to an enriched soil with organic compost on growth, mineral nutrition, and phytochemicals content of coriander (Coriandrum sativum L.) in two successive harvests. Plants 2022, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Szepesi, Á.; Szollosi, R. Mechanism of proline biosynthesis and role of proline metabolism enzymes under environmental stress in plants. In Plant Metabolites and Regulation under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; pp. 337–353. [Google Scholar] [CrossRef]
| Substrate | Photosynthetic Pigments (mg 100 g−1 FW) | ||||
|---|---|---|---|---|---|
| Total Chl | Chl a | Chl b | Cc | Chl a/Chl b | |
| Coir | 14.67 †a | 6.51 a | 8.17 ab | 5.51 | 0.80 a |
| Coir + biochar + perlite | 13.79 ab | 5.18 ab | 8.61 a | 3.51 | 0.60 b |
| Coir + compost 1 + perlite | 13.43 ab | 5.37 b | 8.06 ab | 3.64 | 0.68 ab |
| Coir + biochar + pine bark | 11.33 c | 3.86 c | 7.47 b | 5.07 | 0.52 b |
| Coir + compost + pine bark | 13.06 b | 4.64 bc | 8.42 ab | 5.45 | 0.54 b |
| Significance | ** | ** | ** | NS | ** |
| Substrate | Shoot Macronutrients (%) | Shoot Micronutrients (μg·g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | Fe | B | Mn | Zn | Na 1 | |
| Coir | 4.89 ab † | 0.73 | 5.37 a | 1.15 | 0.42 | 110.0 ab | 23.3 | 34.4 b | 59.4 | 0.62 |
| Coir + biochar + perlite | 4.93 a | 0.68 | 5.57 a | 1.07 | 0.38 | 107.5 ab | 23.6 | 50.0 a | 50.6 | 0.74 |
| Coir + Compost 2 + perlite | 4.72 ab | 0.68 | 5.45 a | 1.08 | 0.35 | 135.0 a | 21.0 | 37.5 b | 165.6 | 0.62 |
| Coir + biochar + pine bark | 4.78 b | 0.77 | 4.48 b | 1.20 | 0.42 | 91.3 b | 23.9 | 55.0 a | 84.4 | 0.74 |
| Coir + Compost + pine bark | 4.82 ab | 0.74 | 5.57 a | 1.25 | 0.41 | 58.8 c | 20.9 | 29.4 b | 45.6 | 0.62 |
| Significance | * | NS | * | NS | NS | * | NS | *** | NS | NS |
| Substrate | Shoot Dry Weight | Leaves | Leaf Area | Head Fresh Yield | |
|---|---|---|---|---|---|
| (g/Plant) | (%) | (nº/Plant) | (cm2/Plant) | (kg/m2) | |
| Coir | 11.0 | 3.7 | 25.0 | 5286.0 | 4.9 |
| Coir + biochar + perlite | 10.8 | 3.7 | 26.0 | 5155.5 | 4.8 |
| Coir + Compost 1 + perlite | 12.0 | 4.0 | 27.3 | 5182.2 | 4.7 |
| Coir + biochar + pine bark | 11.2 | 3.8 | 26.0 | 5122.4 | 4.6 |
| Coir + Compost + pine bark | 11.7 | 3.8 | 26.8 | 5246.3 | 4.8 |
| Significance | NS | NS | NS | NS | NS |
| Substrate | TPC (mg GAE 100 g−1 FW) 1 | Flavonoids (mg QE 100 g−1 FW) 3 | Anthocyanins (mg C3GE 100 g−1 FW) 2 | Ascorbic Acid (mg 100 g−1 FW) |
|---|---|---|---|---|
| Coir | 65.48 c† | 3.19 | 0.66 | 1.21 c |
| Coir + biochar + perlite | 138.9 a | 3.88 | 0.60 | 1.17 c |
| Coir + compost + perlite | 54.52 c | 3.41 | 0.65 | 1.82 b |
| Coir + biochar + pine bark | 65.38 c | 3.23 | 0.66 | 2.85 a |
| Coir + compost + pine bark | 92.25 b | 3.45 | 0.69 | 1.62 b |
| Significance | *** | NS | NS | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, R.M.A.; Alves-Pereira, I.; Alves, I.; Ferreira, R.M.A.; Gruda, N.S. Reusing Coir-Based Substrates for Lettuce Growth: Nutrient Content and Phytonutrients Accumulation. Horticulturae 2023, 9, 1080. https://doi.org/10.3390/horticulturae9101080
Machado RMA, Alves-Pereira I, Alves I, Ferreira RMA, Gruda NS. Reusing Coir-Based Substrates for Lettuce Growth: Nutrient Content and Phytonutrients Accumulation. Horticulturae. 2023; 9(10):1080. https://doi.org/10.3390/horticulturae9101080
Chicago/Turabian StyleMachado, Rui M. A., Isabel Alves-Pereira, Inês Alves, Rui M. A. Ferreira, and Nazim S. Gruda. 2023. "Reusing Coir-Based Substrates for Lettuce Growth: Nutrient Content and Phytonutrients Accumulation" Horticulturae 9, no. 10: 1080. https://doi.org/10.3390/horticulturae9101080
APA StyleMachado, R. M. A., Alves-Pereira, I., Alves, I., Ferreira, R. M. A., & Gruda, N. S. (2023). Reusing Coir-Based Substrates for Lettuce Growth: Nutrient Content and Phytonutrients Accumulation. Horticulturae, 9(10), 1080. https://doi.org/10.3390/horticulturae9101080








