Assessing the Impact of Variety, Irrigation, and Plant Distance on Predatory and Phytophagous Insects in Chili
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
- V1:
- TSBT
- V2:
- YSB
- V1PS1:
- Plant spacing of 60 cm
- V1PS2:
- Plant spacing of 40 cm
- V2PS1:
- Plant spacing of 40 cm
- V2PS2:
- Plant spacing of 30 cm
- I1:
- Irrigation daily, 40 min (7.33 L/m/day)
- I2:
- Irrigation every second day, 20 min (3.66 L/m/two days)
2.2. Characteristics of the Studied Varieties
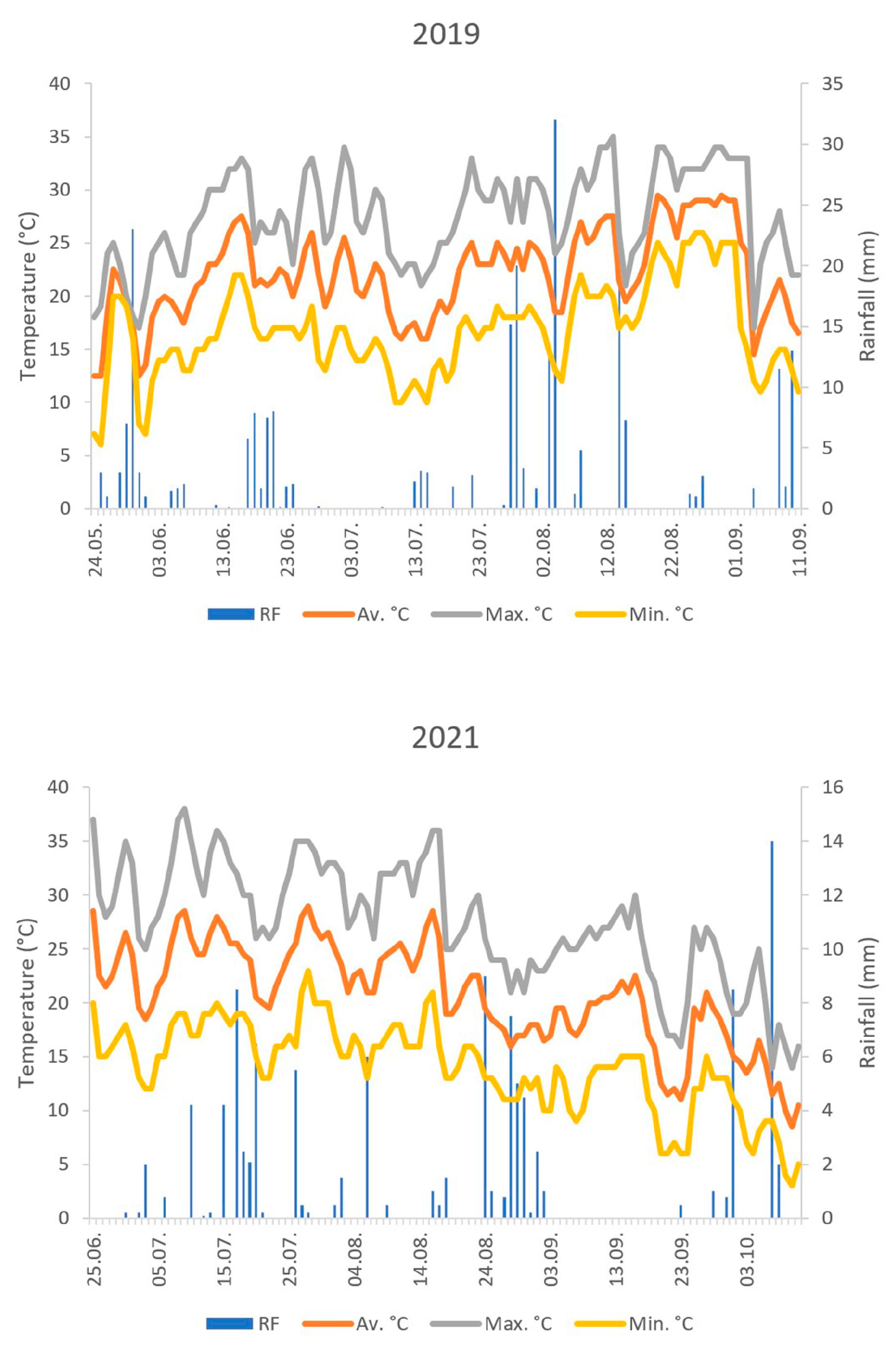
2.3. Meteorological Data
2.4. Flower Sample Collection
2.5. Statistical Analysis
3. Results
3.1. Abundance of Predatory and Phytophagous Insects by Treatments
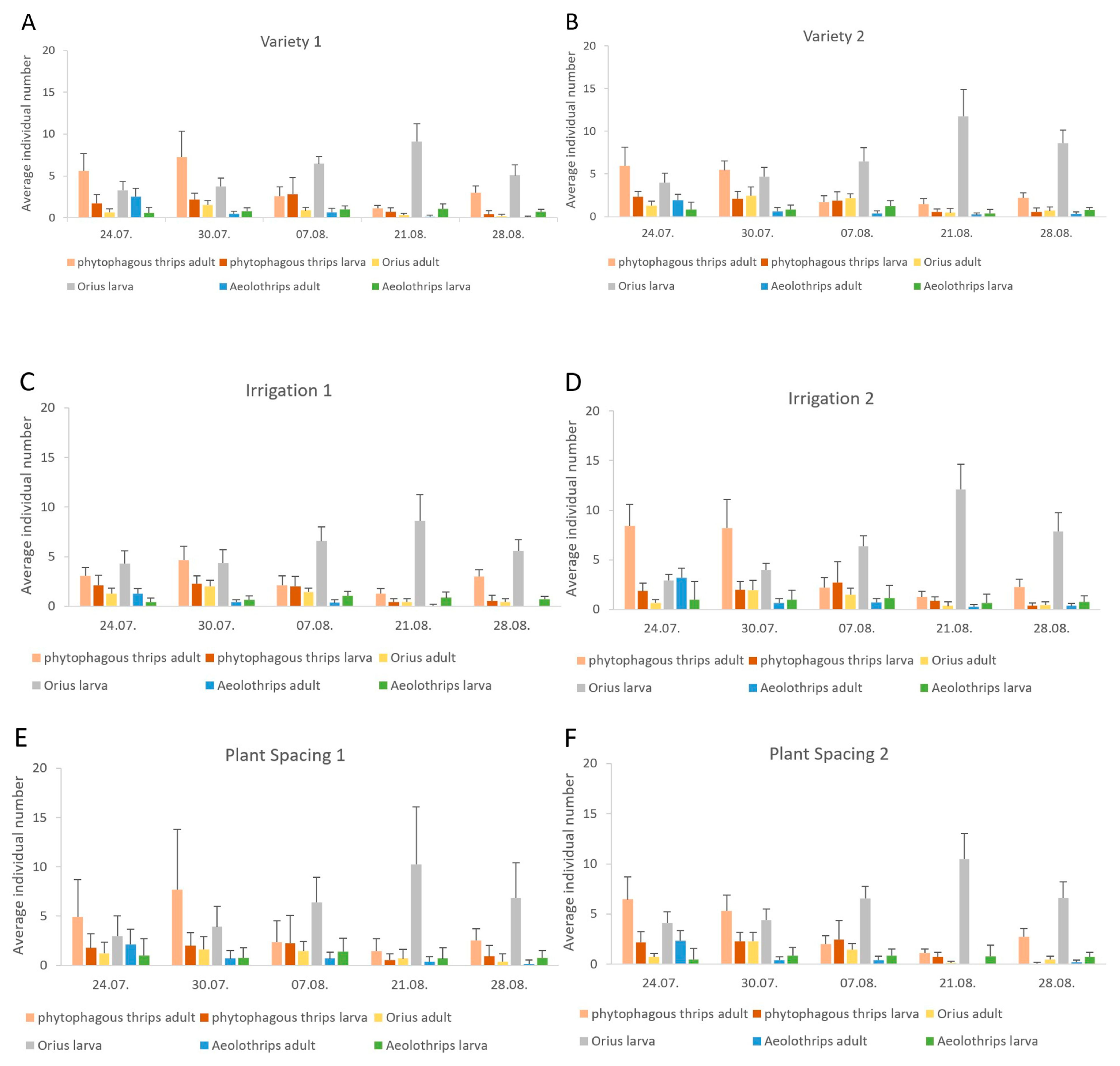
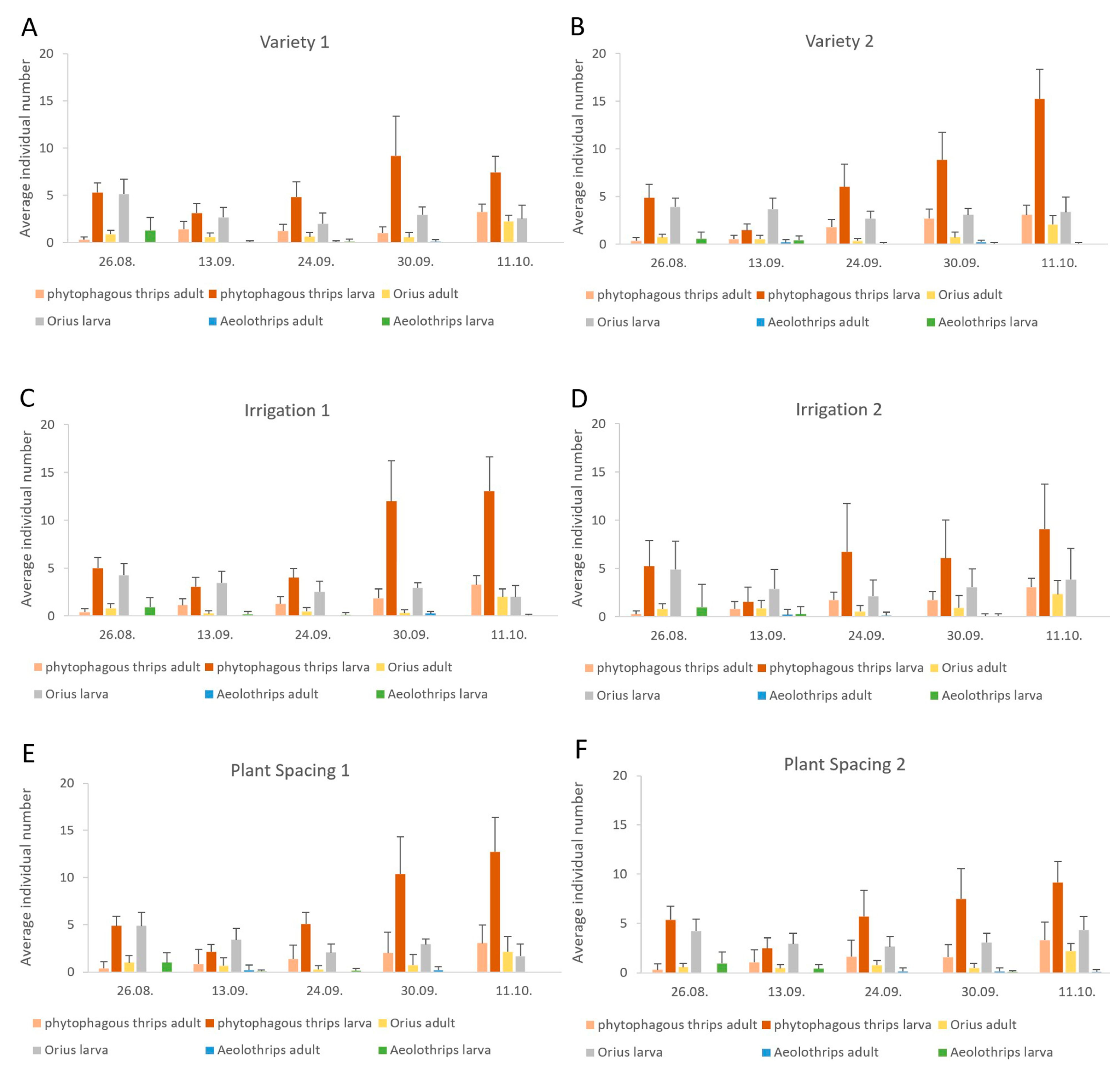
3.2. Seasonal Dynamics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcés-Claver, A.; Arnedo-Andrés, M.S.; Abadía, J.; Gil-Ortega, R.; Álvarez-Fernandez, A. Determination of capsaicin and dihydrocapsaicin in Capsicum fruits by liquid chromatography–electrospray/time-of-flight mass spectrometry. J. Agric. Food Chem. 2006, 54, 9303–9311. [Google Scholar] [CrossRef]
- Perry, L.; Dickau, R.; Zarrillo, S.; Holst, I.; Pearsall, D.M.; Piperno, D.R.; Berman, M.J.; Cooke, R.G.; Rademaker, K.; Ranere, A.J.; et al. Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science 2007, 315, 986–988. [Google Scholar] [CrossRef]
- FAOSTAT. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 1 July 2022).
- Omolo, M.A.; Wong, Z.Z.; Mergen, A.K.; Hastings, J.C.; Le, N.C.; Reiland, H.A.; Case, K.A.; Baumler, D.J. Antimicrobial properties of chili peppers. J. Infect. Dis. Ther. 2014, 2, 145. [Google Scholar] [CrossRef]
- Cordell, G.A.; Araujo, O.E. Capsaicin: Identification, nomenclature, and pharmacotherapy. Ann. Pharmacother. 1993, 27, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, I.; Bosland, P.W. Sensory properties of chile pepper heat—And its importance to food quality and cultural preference. Appetite 2017, 117, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, R.; Singh, J. Cayenne/American Pepper. In Handbook of Herbs and Spices, 1st ed.; Peter, K.V., Ed.; Woodhead Publishing: Cambridge, UK, 2006; Volume 3, pp. 299–312. [Google Scholar]
- Barzman, M.; Bàrberi, P.; Birch, A.N.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- González-Dugo, V.; Orgaz, F.; Fereres, E. Responses of pepper to deficit irrigation for paprika production. Sci. Hort. 2007, 114, 77–82. [Google Scholar] [CrossRef]
- Delfine, S.; Tognetti, R.; Loreto, F.; Alvino, A. Physiological and growth responses to water stress in field-grown bell pepper (Capsicum annuum L.). J. Hort. Sci. Biotechnol. 2002, 77, 697–704. [Google Scholar] [CrossRef]
- Singandhupe, R.B.; Rao, G.G.S.N.; Patil, N.G.; Brahmanand, P.S. Fertigation studies and irrigation scheduling in drip irrigation system in tomato crop (Lycopersicon esculentum L.). Eur. J. Agron. 2003, 19, 327–340. [Google Scholar] [CrossRef]
- Maisiri, N.; Senzanje, A. On farm evaluation of the effect of low cost drip irrigation on water and crop productivity compared to conventional surface irrigation system. Phys. Chem. Earth 2005, 30, 783–791. [Google Scholar] [CrossRef]
- Barkunan, S.R.; Bhanumathi, V.; Sethuram, J. Smart sensor for automatic drip irrigation system for paddy cultivation. Comput. Electr. Eng. 2019, 73, 180–193. [Google Scholar] [CrossRef]
- Das, S.; Rahman, M.; Dash, P.K.; Kamal, M. Suppression of chili leaf curl virus (ChLCV) incidence in chili (Capsicum annuum L.) across Bangladesh via manipulated planting date and spacing. J. Plant Dis. Prot. 2021, 128, 535–548. [Google Scholar] [CrossRef]
- Setiawati, W.; Muharam, A.; Hasyim, A.; Prabaningrum, L.; Moekasan, T.K.; Murtiningsih, R.; Lukman, L.; Mejaya, M.J. Growth, yield characters and pest and diseases severity of chili pepper under different plant density and pruning levels. Appl. Ecol. Environ. Res. 2022, 20, 543–553. [Google Scholar] [CrossRef]
- Boateng, E.; Adjei, E.A.; Osei, M.K.; Offei, K.O.; Olympio, N.S. Response of plant spacing on the morphology and yield of five hot pepper lines. Afr. J. Agric. Res. 2021, 7, 1281–1287. [Google Scholar] [CrossRef]
- Morse, J.G.; Hoddle, M.S. Invasion biology of thrips. Ann. Rev. Entomol. 2006, 51, 67–89. [Google Scholar] [CrossRef] [PubMed]
- ThripsWiki. ThripsWiki-Providing Information on the World’s Thrips. Available online: http://thrips.info/wiki/ (accessed on 29 June 2022).
- Maharijaya, A.; Vosman, B.; Steenhuis-Broers, G.; Harpenas, A.; Purwito, A.; Visser, R.G.F.; Voorrips, R.E. Screening of pepper accessions for resistance against two thrips species (Frankliniella occidentalis and Thrips parvispinus). Euphytica 2011, 177, 401–410. [Google Scholar] [CrossRef]
- Roggero, P.; Pennazio, S.; Masenga, V.; Tavella, L. Resistance to tospoviruses in pepper. In Proceedings of the 7th International Symposium on Thysanoptera, Thrips and Tospoviruses, Reggio Calabria, Italy, 2–7 July 2001; Marullo, R., Mound, L., Eds.; pp. 105–110. [Google Scholar]
- Whitfield, A.E.; Ullman, D.E.; German, T.L. Tospovirus–thrips interactions. Ann. Rev. Phytopathol. 2005, 43, 459–489. [Google Scholar] [CrossRef]
- Lewis, T.; Mound, L.A.; Nakahara, S.; Childers, C.C. Major crops infested by thrips with main symptoms and predominant injurious species. In Thrips as Crop Pests, 1st ed.; Lewis, T., Ed.; CAB Int.: Wallingford, UK, 1997; pp. 675–709. [Google Scholar]
- Reitz, S.R.; Yearby, E.L.; Funderburk, J.E.; Stavisky, J.; Momol, M.T.; Olson, S.M. Integrated management tactics for Frankliniella Thrips (Thysanoptera: Thripidae) in field-grown pepper. J. Econ. Entomol. 2003, 96, 1201–1214. [Google Scholar] [CrossRef]
- Cannon, R.J.C.; Matthews, L.; Collins, D.W. A review of the pest status and control options for Thrips palmi. Crop Prot. 2007, 26, 1089–1098. [Google Scholar] [CrossRef]
- Sahu, P.S.; Kumar, A.; Khan, H.H.; Naz, H. Seasonal incidence of chilli thrips, Scirtothrips dorsalis (Hood) (Thripidae: Thysanoptera): A review. J. Entomol. Zool. Stud. 2018, 6, 1738–1740. [Google Scholar]
- Jenser, G. A checklist of Thysanoptera of Hungary. Folia Entomol. Hung. 2011, 72, 31–46. [Google Scholar]
- zur Strassen, R. Die terebranten Thysanopteren Europas und des Mittelmeer-Gebietes. Die Tierwelt Deutschlands 74, 1st ed.; Verlag Goecke & Evers: Keltern, Germany, 2003; Volume 74, p. 46. [Google Scholar]
- Jenser, G. Tripszek—Thysanoptera. In Magyarország Állatvilága, Fauna Hungariae, 1st ed.; Academic Press: Budapest, Hungary, 1982; Volume 13, p. 19. [Google Scholar]
- Czencz, K.; Jenser, G. Microhabitats of Aeolothrips intermedius BAGNALL 1934 (Thysanoptera: Aeolothripidae). Cour. Forschungsinst. Senckenberg. 1994, 178, 51–55. [Google Scholar]
- Trdan, S.; Andjus, L.; Raspudić, E.; Kač, M. Distribution of Aeolothrips intermedius Bagnall (Thysanoptera: Aeolothripidae) and its potential prey Thysanoptera species on different cultivated host plants. J. Pest. Sci. 2005, 78, 217–226. [Google Scholar] [CrossRef]
- Trdan, S.; Rifelj, M.; Valič, N. Population dynamics of banded thrips (Aeolothrips intermedius Bagnall, Thysanoptera, Aeolothripidae) and its potential prey Thysanoptera species on white clover. Comm. App. Biol. Sci. Ghent Univ. 2005, 70, 753–758. [Google Scholar]
- Baez, I.; Reitz, S.R.; Funderburk, J.E.; Reitz, S.R.; Funderburk, J.E. Predation by Orius insidiosus (Heteroptera: Anthocoridae) onlife stages and species of Frankliniella flower thrips (Thysanoptera: Thripidae) in pepper flowers. Environ. Entomol. 2004, 33, 662–670. [Google Scholar] [CrossRef]
- Sabelis, M.W.; Van Rijn, P.C.J. Predation by insects and mites. In Thrips as Crop Pests, 1st ed.; Lewis, T., Ed.; CAB International: Wallingford, UK, 1997; pp. 259–354. [Google Scholar]
- Funderburk, J.; Stavisky, J.; Olson, S. Predation of Frankliniella occidentalis (Thysanoptera: Thripidae) in field peppers by Orius insidiosus (Hemiptera: Anthocoridae). Environ. Entomol. 2000, 29, 376–382. [Google Scholar] [CrossRef]
- Ramachandran, S.; Funderburk, J.; Stavisky, J.; Olson, S. Population abundance and movement of Frankliniella species and Orius insidiosus in field pepper. Agric. Forest Entomol. 2001, 3, 1–10. [Google Scholar] [CrossRef]
- Loomans, A. Parasitoids as Biological Control Agents of Thrips Pests. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2003. [Google Scholar]
- Blümel, S.; Fischer-Colbrie, P.; Höbaus, E. Nützlinge: Helfer im zeitgemässen Pflanzenschutz, 3rd ed.; Verlag Jugend & Volk Ges.m.b.H.: Wien, Austria, 1988; p. 111. [Google Scholar]
- Rácz, V. Poloskák (Heteroptera) Szerepe Magyarországi Kukoricások Életközösségében. Master’s Thesis, Hungarian Academy of Science, Budapest, Hungary, 1989. [Google Scholar]
- O’Keefe, D.A.; Palada, M.C. In-row plant spacing affects growth and yield of four hot pepper cultivars. In Proceedings of the Caribbean Food Crops Society, 38th Annual Meeting, Trois-Ilets, Martinique, France, 30 June–5 July 2002; Merlini, X., Jean-Baptiste, I., Mbolidi-Baron, H., Eds.; AMADEPA: Martinique, France; pp. 162–168. [Google Scholar]
- Karungi, J.; Obua, T.; Kyamanywa, S.; Mortensen, C.N.; Erbaugh, M. Seedling protection and field practices for management of insect vectors and viral diseases of hot pepper (Capsicum chinense Jacq.) in Uganda. Int. J. Pest. Manag. 2013, 59, 103–110. [Google Scholar] [CrossRef]
- Werner, J. Capsaicinoids—Properties and mechanisms of pro-health action. In Analytical Methods in the Determination of Bioactive Compounds and Elements in Food. Food Bioactive Ingredients; Jeszka-Skowron, M., Zgoła-Grześkowiak, A., Grześkowiak, T., Ramakrishna, A., Eds.; Springer: Cham, Switzerland, 2021; pp. 193–225. [Google Scholar] [CrossRef]
- Bosland, P.W.; Coon, D.; Reeves, G. ‘Trinidad Moruga Scorpion’ pepper is the world’s hottest measured chile pepper at more than two million Scoville heat units. Horttechnology 2012, 22, 534–538. [Google Scholar] [CrossRef]
- Trinidad Scorpion Butch, T. Available online: https://pepperhead.com/shop/trinidad-scorpion-butch-t/ (accessed on 25 July 2022).
- Maguire, K. Red Hot Chilli Grower, 1st ed.; Mitchell Beazley: London, UK, 2015; p. 110. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 20 January 2022).
- Fox, J. Using the R Commander: A Point-and-Click Interface for R; Chapman & Hall/CRC: Boca Raton, FL, USA, 2017; pp. 1–233. [Google Scholar]
- Faraway, J.J. Linear Models with R, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2014; pp. 1–270. [Google Scholar]
- Lewis, T. Thrips. Their Biology, Ecology and Economic Importance; Academic Press: London, UK, 1973; pp. 187–201. [Google Scholar]
- Maris, P.C.; Joosten, N.N.; Peters, D.; Goldbach, R.W. Thrips resistance in pepper and its consequences for the acquisition and inoculation of Tomato spotted wilt virus by the Western Flower Thrips. Phytopathology 2003, 93, 96–101. [Google Scholar] [CrossRef]
- Van Haperen, P.; Voorrips, R.E.; van Loon, J.J.A.; Vosman, B. The effect of plant development on thrips resistance in Capsicum. Arthropod Plant Interact. 2019, 13, 11–18. [Google Scholar] [CrossRef]
- Syamsudin, T.S.; Faizal, A.; Kirana, R. Dataset on antixenosis and antibiosis of chili fruit by fruit fly (Bactrocera dorsalis) infestation. Data Brief 2019, 23, 103758. [Google Scholar] [CrossRef] [PubMed]
- Syamsudin, T.S.; Kirana, R.; Karjadi, A.K.; Faizal, A. Characteristics of chili (Capsicum annuum L.) that are resistant and susceptible to oriental fruit fly (Bactrocera dorsalis Hendel) infestation. Horticulturae 2022, 8, 314. [Google Scholar] [CrossRef]
- Fery, R.L.; Thies, J.A. Evaluation of Capsicum chinense Jacq. Cultigens for resistance to the Southern Root-knot Nematode. Hort. Sci. 1997, 32, 923–926. [Google Scholar] [CrossRef]
- Kirk, W.D.J. Distribution, Abundance and Population Dynamics. In Thrips as Crop Pests; Lewis, T., Ed.; CAB Int.: Wallingford, UK, 1997; pp. 217–257. [Google Scholar]
- de Pedro, L.; López-Gallego, E.; Pérez-Marcos, M.; Ramírez-Soria, M.J.; Sanchez, J.A. Native natural enemies in Mediterranean melon fields can provide levels of pest control similar to conventional pest management with broad-spectrum pesticides. Biol. Control 2021, 164, 104778. [Google Scholar] [CrossRef]
- Fok, E.J.; Petersen, J.D.; Nault, B.A. Relationships between insect predator populations and their prey, Thrips tabaci, in onion fields grown in large-scale and small-scale cropping systems. BioControl 2014, 59, 739–748. [Google Scholar] [CrossRef][Green Version]
- El-Serwiy, S.A.; Razoki, I.A.; Ragab, A.S. Population density of Thrips tabaci (Lind.) and the predators Orius albidipennis (Reut.) and Aeolothrips fasciatus (L.) on onion. J. Agric. Water Resour. Res. 1985, 4, 57–67. [Google Scholar]
- Fathi, S.A.A.; Asghari, A.; Sedghi, M. 2008. Interaction of Aeolothrips intermedius and Orius niger in controlling Thrips tabaci on potato. Int. J. Agri. Biol. 2008, 10, 521–525. [Google Scholar]
- Abdala-Roberts, L.; Berny-Mier y Terán, J.C.; Moreira, X.; Durán-Yáñez, A.; Tut-Pech, F. Effects of pepper (Capsicum chinense) genotypic diversity on insect herbivores. Agric. Forest Entomol. 2015, 17, 433–438. [Google Scholar] [CrossRef]

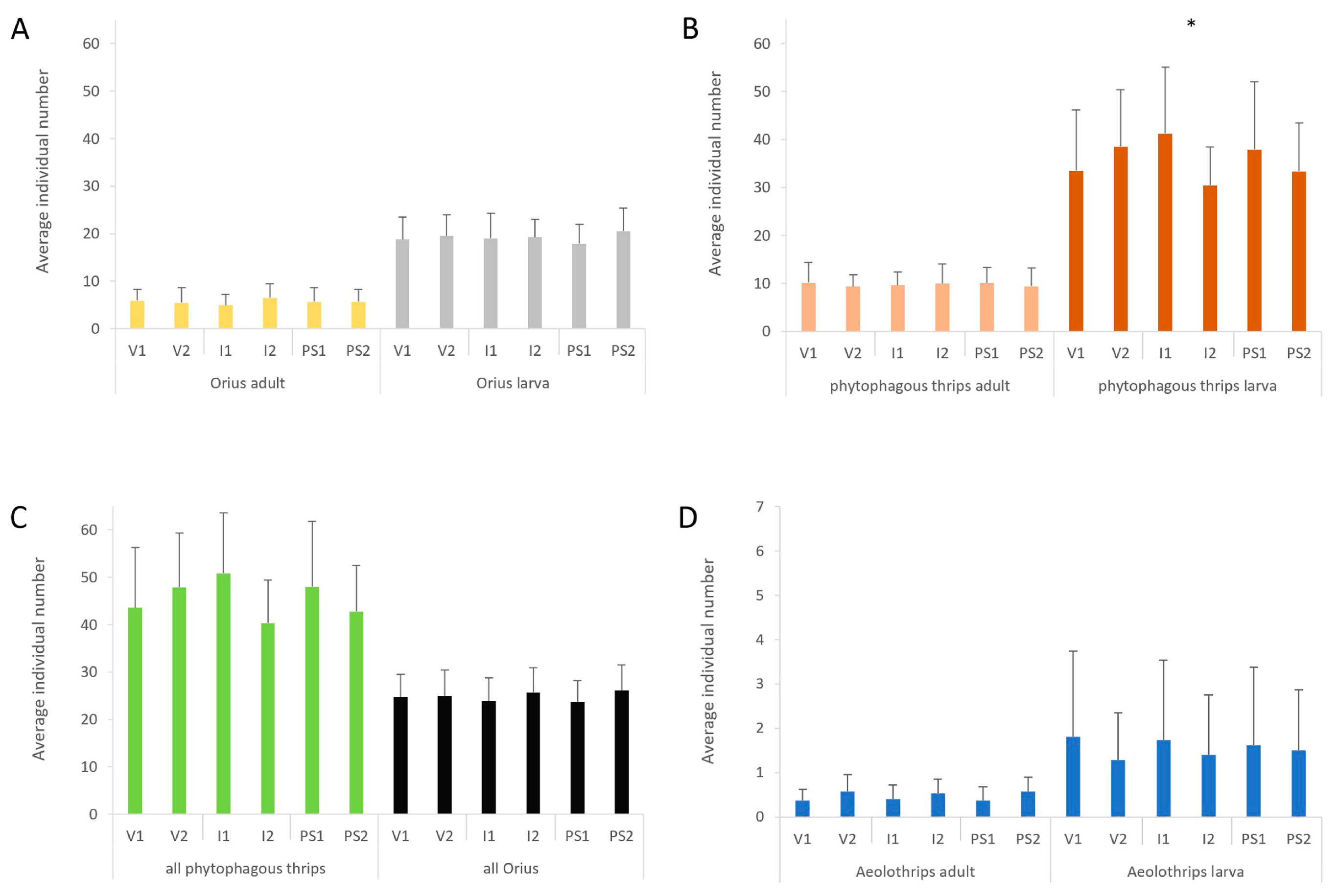
| Year | Aeolothrips Adults | Aeolothrips Larvae | Orius Adults | Orius Larvae | Phytophagous Thrips Adults | Phytophagous Thrips Larvae |
|---|---|---|---|---|---|---|
| 2019 | 103 | 116 | 146 | 877 | 512 | 213 |
| 2021 | 14 | 44 | 170 | 574 | 293 | 1030 |
| Variety | Irrigation | Plant Spacing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Insect Taxa | Effect Estimate | Test Statistics | p Value | Effect Estimate | Test Statistics | p Value | Effect Estimate | Test Statistics | p Value |
| Phytophagous thrips adults | 2.8307 | F = 0.8876 | 0.4160 | −8.2857 | F = 8.0624 | 0.0099 | 1.3333 | F = 0.4139 | 0.7030 |
| Phytophagous thrips larvae | 0.5589 | χ2 = 0.2866 | 0.8660 | −0.3571 | χ2 = 0.1174 | 0.3950 | −0.2717 | χ2 = 0.0677 | 0.4005 |
| All phytophagous thrips | 3.3897 | F = 1.1328 | 0.2189 | −8.6428 | F = 7.8629 | 0.0091 | 1.0615 | F = 0.2804 | 0.6013 |
| Orius adults | −3.4769 | χ2 = 16.190 | <0.001 | 0.7142 | χ2 = 0.6855 | 0.4080 | 0.3179 | χ2 = 0.1348 | 0.7130 |
| Orius larvae | −7.8717 | F = 0.0149 | 0.0135 | −3.78572 | F = 0.2915 | 0.4460 | −1.7487 | F = 0.2430 | 0.7550 |
| All Orius | −11.3487 | F = 12.5463 | 0.0011 | −3.0714 | F = 0.5030 | 0.4460 | −1.4307 | F = 0.1434 | 0.7550 |
| Aeolothrips adults | 0.4051 | χ2 = 0.3117 | 0.5810 | −3.0714 | χ2 = 18.513 | <0.001 | 0.7435 | χ2 = 1.0435 | 0.3080 |
| Aeolothrips larvae | 0.1230 | χ2 = 0.0255 | 0.8100 | −0.8571 | χ2 = 1.2436 | 0.2260 | 0.8820 | χ2 = 1.3037 | 0.2210 |
| All Aeolothrips | 0.5282 | χ2 = 0.2489 | 0.4441 | −3.9285 | χ2 = 13.962 | <0.001 | 1.6256 | χ2 = 2.3457 | 0.0714 |
| Variety | Irrigation | Plant Spacing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Insect Taxa | Effect Estimate | Test Statistics | p Value | Effect Estimate | Test Statistics | p Value | Effect Estimate | Test Statistics | p Value |
| Phytophagous thrips adults | 0.7678 | χ2 = 0.4517 | 0.3620 | −0.3333 | χ2 = 0.0853 | 0.7850 | 0.6339 | χ2 = 0.3078 | 0.4220 |
| Phytophagous thrips larvae | −5.0625 | F = 1.5248 | 0.4383 | 10.8000 | F = 6.9704 | 0.0266 | 4.5803 | F = 1.0643 | 0.2829 |
| All phytophagous thrips | −4.2946 | F = 1.1472 | 0.6198 | 10.4666 | F = 6.8442 | 0.0561 | 5.2142 | F = 1.5056 | 0.2637 |
| Orius adults | 0.4464 | χ2 = 0.2631 | 0.5150 | −1.6000 | χ2 = 3.3996 | 0.0608 | 0.0446 | χ2 = 0.0026 | 0.8250 |
| Orius larvae | −0.6875 | F = 0.1674 | 0.7960 | −0.2666 | F = 0.0253 | 0.8530 | −2.5625 | F = 2.4276 | 0.1770 |
| All Orius | −0.2411 | F = 0.0170 | 0.9330 | −1.8666 | F = 1.0233 | 0.3000 | −2.5178 | F = 1.8886 | 0.2800 |
| Aeolothrips adults | −0.1964 | χ2 = 0.6161 | 0.4240 | −0.1333 | χ2 = 0.2867 | 0.5820 | −0.1964 | χ2 = 0.6161 | 0.4240 |
| Aeolothrips larvae | 0.5267 | χ2 = 1.339 | 0.2098 | 0.3333 | χ2 = 0.5329 | 0.4552 | 0.1250 | χ2 = 0.0746 | 0.6571 |
| All Aeolothrips | 0.3303 | χ2 = 0.4027 | 0.4707 | 0.2000 | χ2 = 0.1476 | 0.6950 | −0.0714 | χ2 = 0.0187 | 0.9959 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, A.L.; Szalai, M.; Szénási, Á. Assessing the Impact of Variety, Irrigation, and Plant Distance on Predatory and Phytophagous Insects in Chili. Horticulturae 2022, 8, 741. https://doi.org/10.3390/horticulturae8080741
Juhász AL, Szalai M, Szénási Á. Assessing the Impact of Variety, Irrigation, and Plant Distance on Predatory and Phytophagous Insects in Chili. Horticulturae. 2022; 8(8):741. https://doi.org/10.3390/horticulturae8080741
Chicago/Turabian StyleJuhász, András Lajos, Márk Szalai, and Ágnes Szénási. 2022. "Assessing the Impact of Variety, Irrigation, and Plant Distance on Predatory and Phytophagous Insects in Chili" Horticulturae 8, no. 8: 741. https://doi.org/10.3390/horticulturae8080741
APA StyleJuhász, A. L., Szalai, M., & Szénási, Á. (2022). Assessing the Impact of Variety, Irrigation, and Plant Distance on Predatory and Phytophagous Insects in Chili. Horticulturae, 8(8), 741. https://doi.org/10.3390/horticulturae8080741






