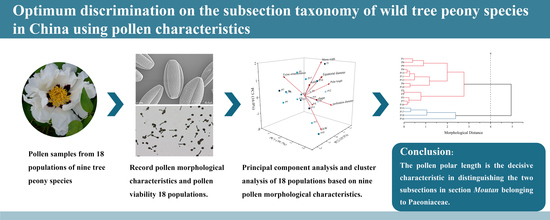
Optimum Discrimination on the Subsection Taxonomy of Wild Tree Peony Species in China Using Pollen Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Pollen Morphology
2.3. Pollen Viability
2.4. Statistical Analysis
3. Results
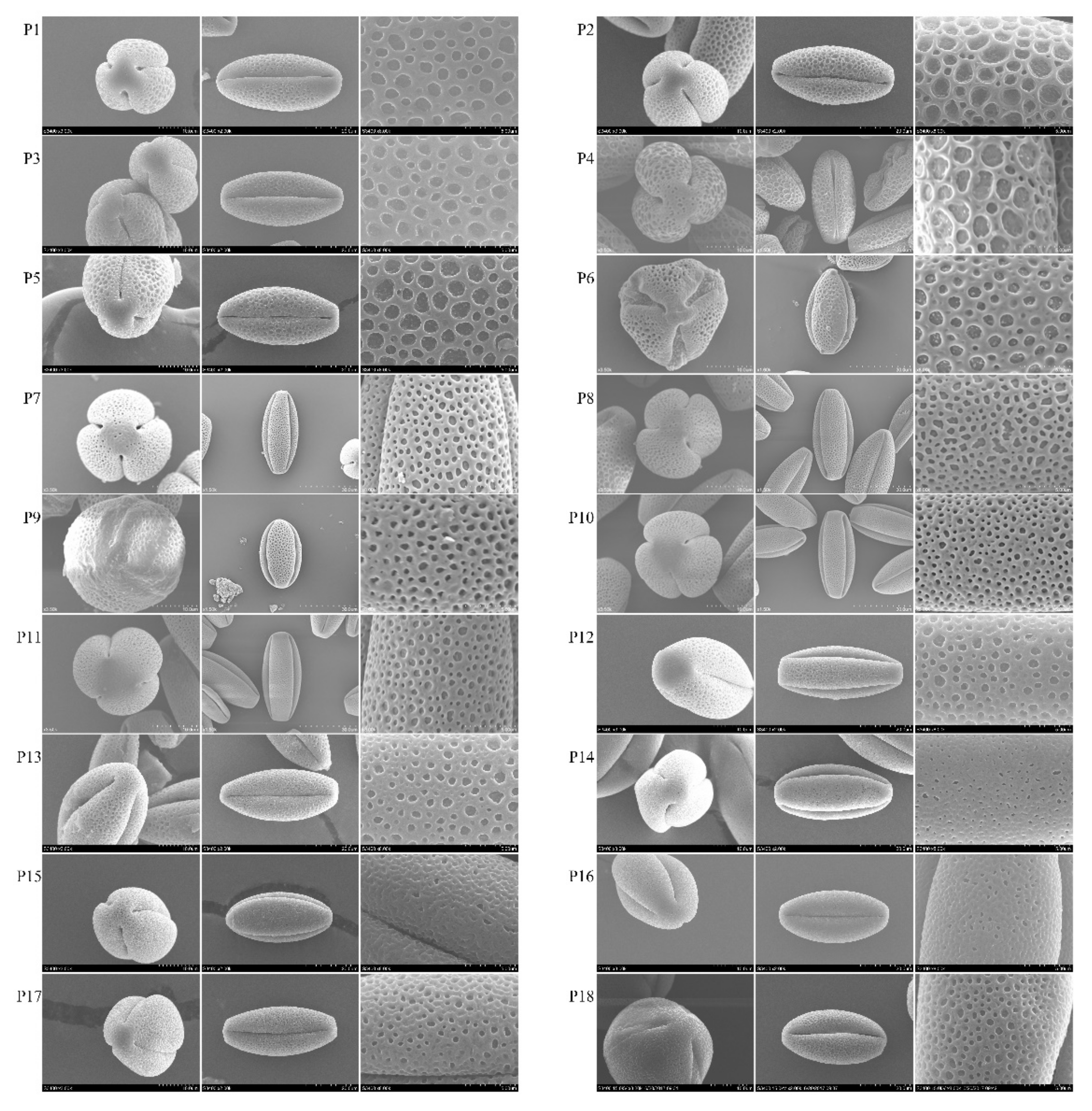
3.1. Morphology of Pollen Grains
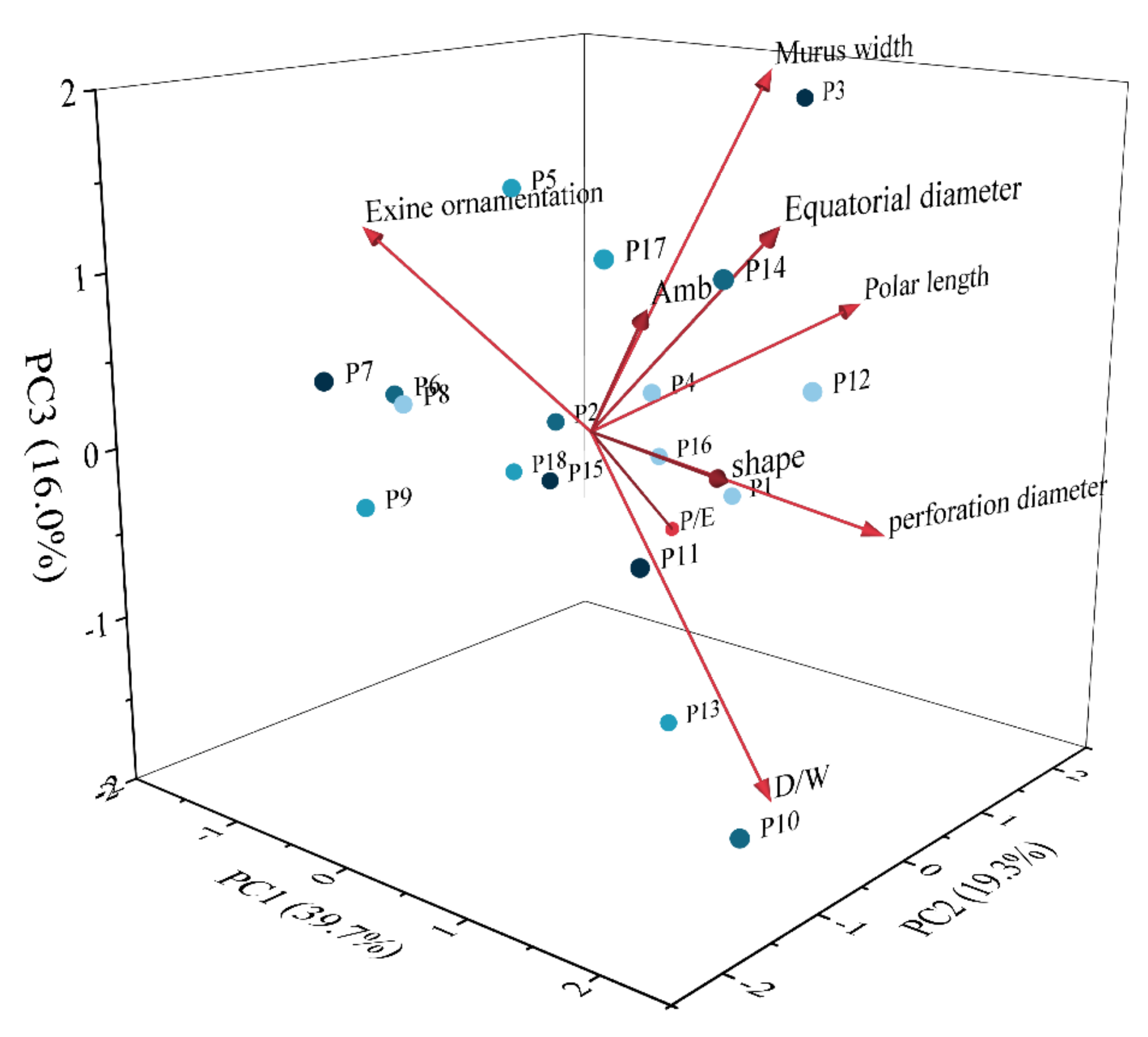
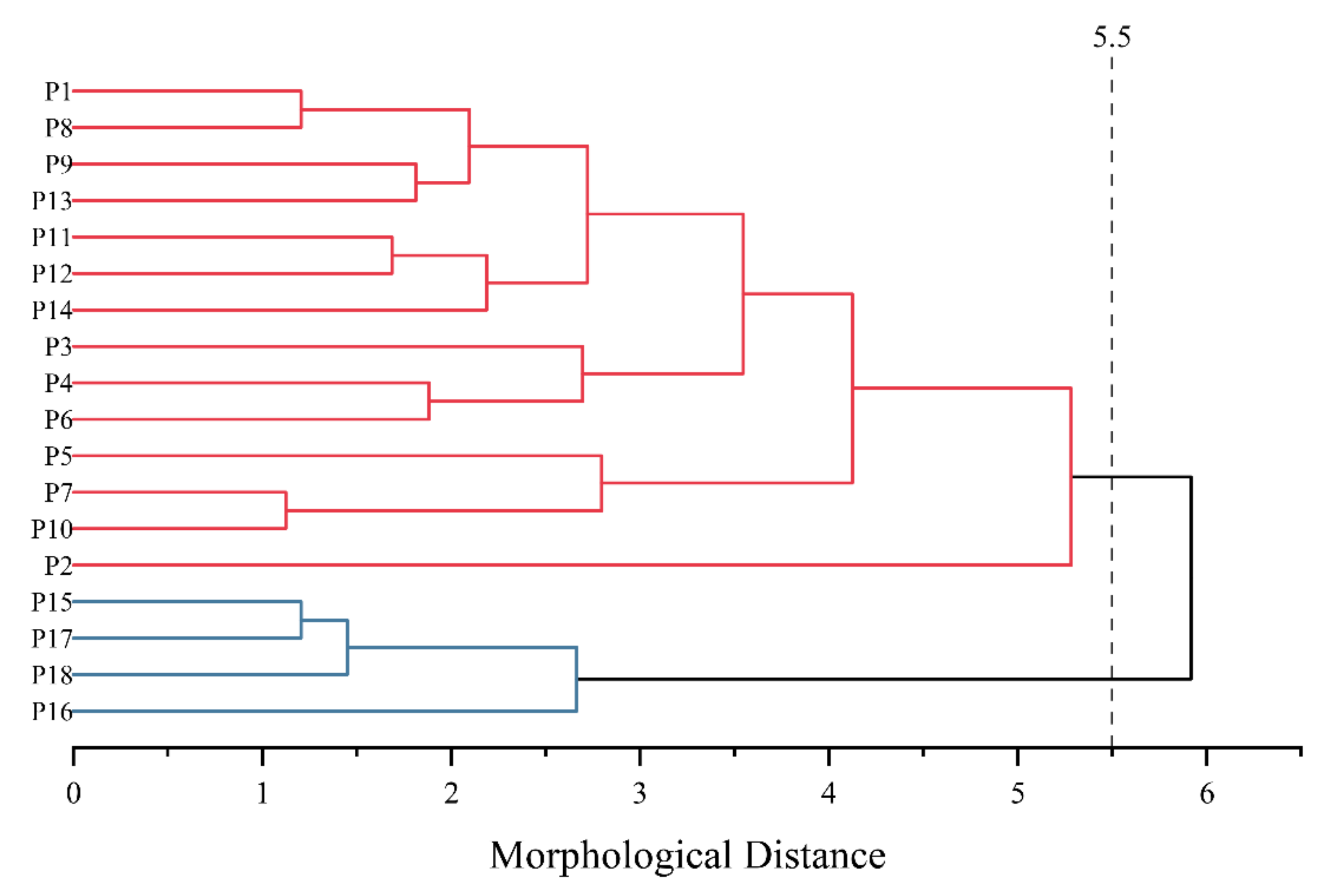
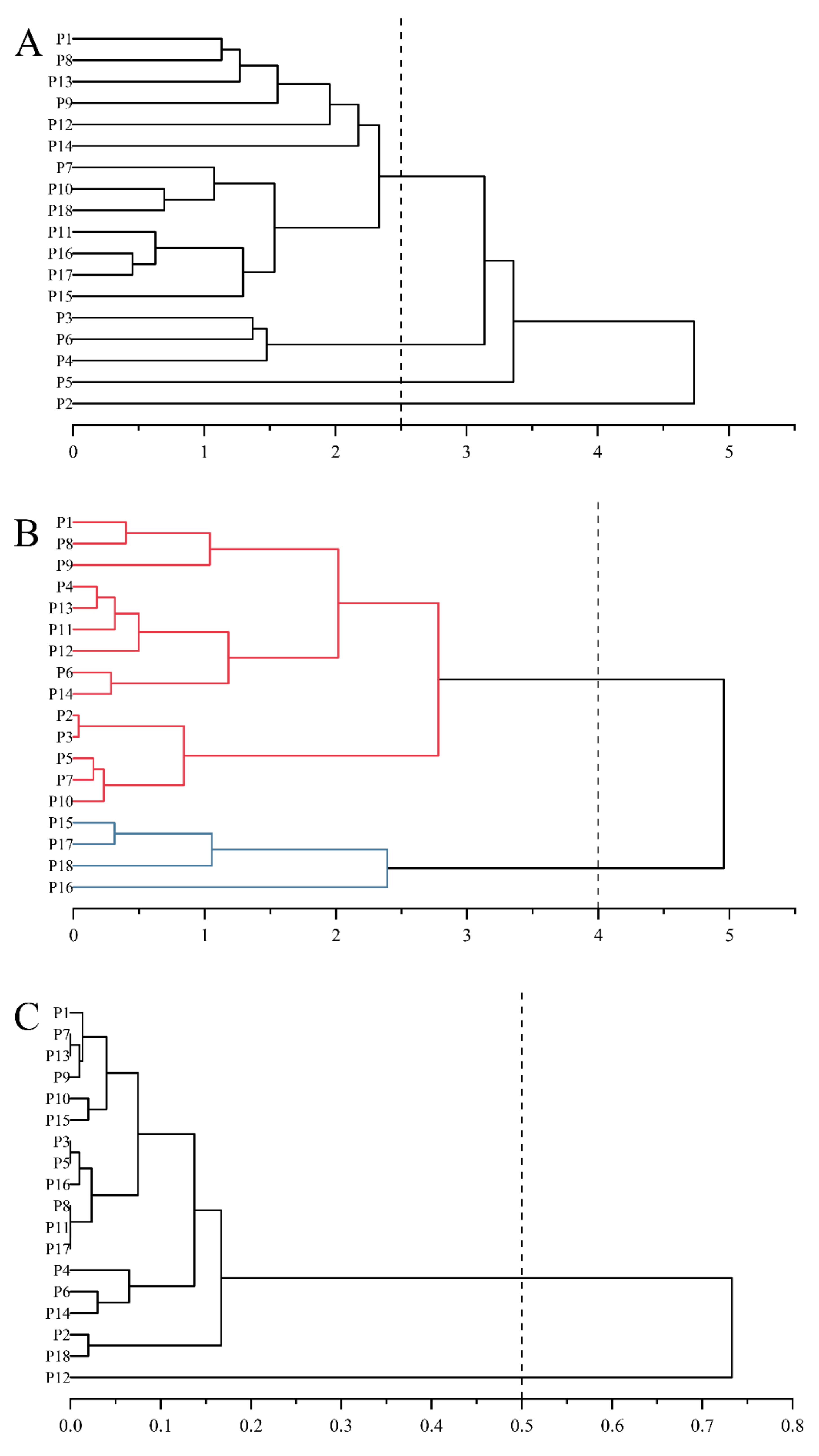
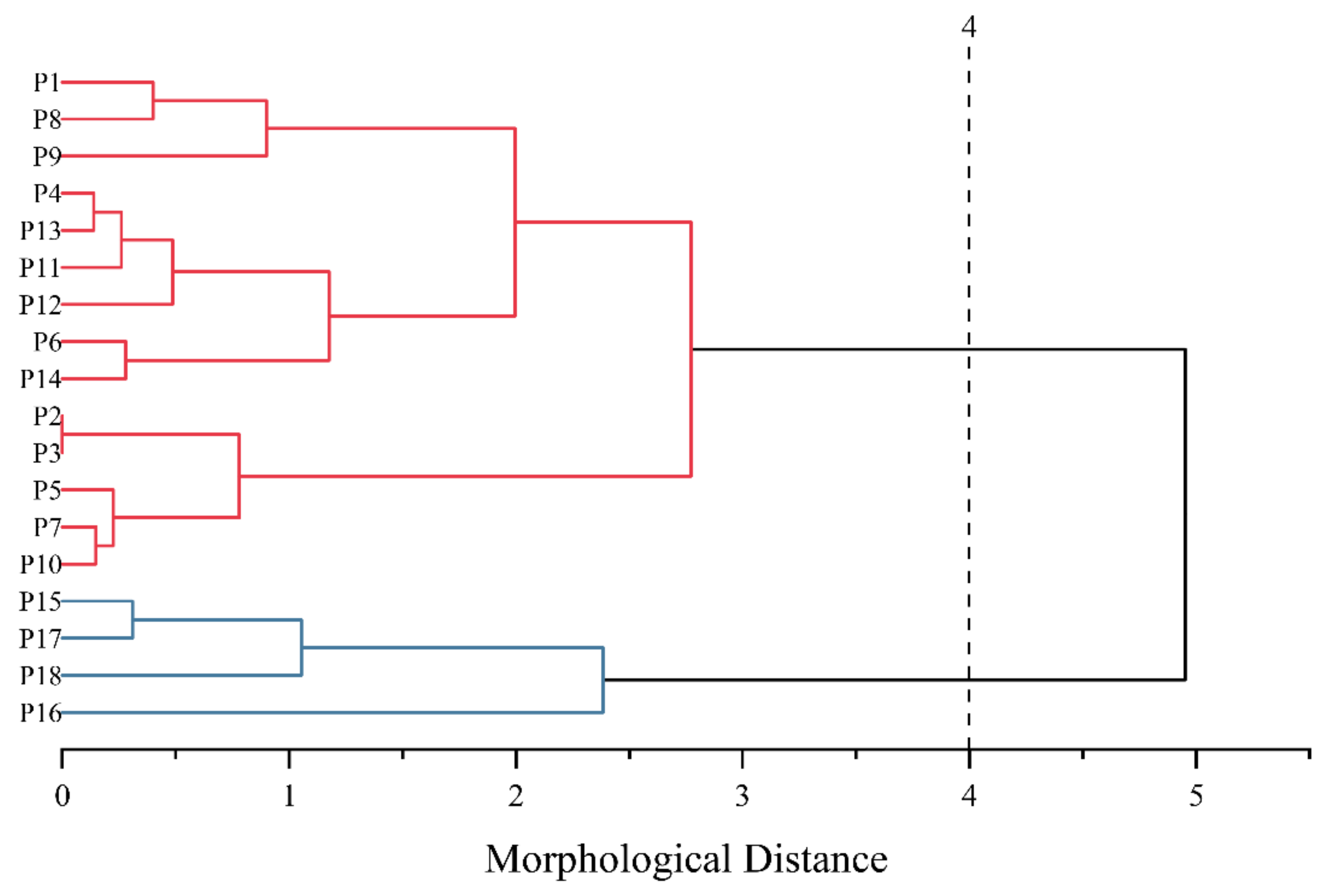
3.2. Principal Component Analysis
3.3. Pollen Viability and Germination
4. Discussion
4.1. Morphology of Pollen Grains
4.2. Principal Component Analysis
4.3. Pollen Viability and Germination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.X.; Shi, Q.; Ji, D.; Niu, L.; Zhang, Y. Determination of the Phenolic Content, Profile, and Antioxidant Activity of Seeds from Nine Tree Peony (Paeonia Section Moutan DC.) Species Native to China. Food Res. Int. 2017, 97, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Dai, Z. Study on the Chinese Wild Woody Peonies (III) new Taxa of Paeonia L. Sect. Moutan DC. Bull. Bot. Res. 1997, 17, 1–5. [Google Scholar]
- Li, J. Studies on the Origin of Chinese Mudan (Tree Peony). J. Beijing For. Univ. 1998, 20, 26–30. [Google Scholar]
- Hong, T. Gian Lupo Osti Study on the Chinese Wild Woody Peonies (II) new Taxa of Paeonia L. Sect. Moutan DC. Bull. Bot. Res. 1994, 14, 237–240. [Google Scholar]
- Hong, D.; Pan, K. Taxonomical History and Revision of Paeonia Sect. Moutan (Paeoniaceae). Acta Phytotaxon. Sin. 1999, 48–65. [Google Scholar]
- Stern, F.C. A Study of the Genus Paeonia, 1st ed.; Royal Horticultural Society: London, UK, 1946. [Google Scholar]
- Sun, J.; Wan, H.; Yuan, X.; Yang, Q. Advances in Research of Pollen Morphology of Paeonia Sect. Moutan. J. Hebei Agric. Sci. 2009, 13, 18–19. [Google Scholar] [CrossRef]
- Bravo-Hollis, H. Las Cactáceas de México; Universidad Nacional Autónoma de México: Mexico City, Mexico, 1978. [Google Scholar]
- Guinet, P.; Caccavari, M. Pollen Morphology of the Genus Stryphnodendron (Leguminosae, Mimosoideae) in Relation to Its Taxonomy. Grana 1992, 31, 101–112. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, L. Pollen Morphology of Several Tree Peony Wild Species and Discussion on Its Evolution and Taxonomy. J. Beijing For. Univ. 1999, 22–26. [Google Scholar]
- Aracely Aguilar-Garcia, S.; Maria Figueroa-Castro, D.; Castaneda-Posadas, C. Pollen Morphology of Pachycereus Weberi (Cactaceae): An Evaluation of Variation in Pollen Size. Plant Syst. Evol. 2012, 298, 1845–1850. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, C.; Zhao, Y.; Liu, J. Pollen Morphology of Aletris L. (Nartheciaceae) and Its Systematic Significance. Microsc. Res. Tech. 2019, 82, 2061–2071. [Google Scholar] [CrossRef]
- Xi, Y.Z. The Pollen Morphology and Exine Ultrastructure of Paeonia L. in China. Acta Bot. Sin. 1984, 26, 241–246. [Google Scholar]
- Wang, X.; Chen, J.; Gao, T.; Ju, Z. Recent Progress in Studies of Pollen of Paeonia. J. Jilin Agric. Sci. Technol. Univ. 2017, 26, 19–21+77. [Google Scholar]
- Santisuk, T. Palynological Study of the Tribe Ranunculeae (Ranunculaceae). Opera.Bot. 1979, 48, 1–74. [Google Scholar]
- Wang, Z.; Zhou, J. Pollen Morphology of Peach Germplsm. ACTA Hortic. Sin. 1990, 17, 161–168+241-244+247. [Google Scholar]
- Zhang, Z.; Xing, F.; Hou, X.; Wang, F.; QIAO, Q. Research Progress of Peony Reproductive Biology. Chin. Wild Plant Resour. 2021, 40, 41–47. [Google Scholar]
- Zhang, J.; Zhang, D.; Wei, J.; Shi, X.; Ding, H.; Qiu, S.; Guo, J.; Li, D.; Zhu, K.; Horvath, D.P.; et al. Annual Growth Cycle Observation, Hybridization and Forcing Culture for Improving the Ornamental Application of Paeonia Lactiflora Pall. in the Low-Latitude Regions. PLoS ONE 2019, 14, e0218164. [Google Scholar] [CrossRef]
- Li, T.; Qiao, Q.; Li, J.; Guo, X.; Hou, X. Advances in Determination Method and Storage of Paeonia Suffruticosa Pollen Activity. Guizhou Agric. Sci. 2020, 48, 123–126. [Google Scholar]
- Du, G.; Xu, J.; Gao, C.; Lu, J.; Li, Q.; Du, J.; Lv, M.; Sun, X. Effect of Low Storage Temperature on Pollen Viability of Fifteen Herbaceous Peonies. Biotechnol. Rep. 2019, 21, e00309. [Google Scholar] [CrossRef] [PubMed]
- Erdtman, G. Pollen Morphology and Plant Taxonomy: Angiosperms (An Introduction to Palynology I). Taxon 1952, 36, 779–780. [Google Scholar] [CrossRef]
- Peng, H.-Z.; Jin, Q.-Y.; Ye, H.-L.; Zhu, T.-J. A Novel in Vitro Germination Method Revealed the Influence of Environmental Variance on the Pecan Pollen Viability. Sci. Hortic. 2015, 181, 43–51. [Google Scholar] [CrossRef]
- Oliveira, E.J.; Oliveira Filho, O.S.; Santos, V.S. Classification of Cassava Genotypes Based on Qualitative and Quantitative Data. Genet. Mol. Res. GMR 2015, 14, 906–924. [Google Scholar] [CrossRef] [PubMed]
- Erdtman, G. Handbook of Palynology: Morphology-Taxonomy-Ecology: An Introduction to the Study of Pollen Grains and Spores; Hafner Publishing Co.: New York, NY, USA, 1969. [Google Scholar]
- Punt, W.; Hoen, P.P.; Blackmore, S.; Nilsson, S.; Le Thomas, A. Glossary of Pollen and Spore Terminology. Rev. Palaeobot. Palynol. 2007, 143, 1–81. [Google Scholar] [CrossRef]
- Hong, D. Peonies of the World: Taxonomy and Phytogeography, Illustrated edition; Royal Botanic Gardens, Kew: London, UK, 2010; ISBN 978-1-84246-392-5. [Google Scholar]
- Guo, X.; Wang, L.; Yuan, T. Study on Pollen Morphology of 4 Wild Herbaceous Peony. Sci. Silvae Sin. 2005, 41, 184–186+222-223. [Google Scholar]
- He, L.; Li, R.; Li, J.; Zhang, Y. Studies on Pollen Morphology of the Wild Peony. J. Lanzhou Univ. Sci. 2005, 43–49. [Google Scholar] [CrossRef]
- Xuan, Y.; Hu, Y.; Li, X.; Du, Y.; Yan, Y. Comparative Study on Different Provenance Oil Use and Ornamental Tree Peony Pollen Ultrastructure. North Hortic. 2015, 15, 99–103. [Google Scholar]
- Scotland, R. Pollen Morphology of Andrographideae (Acanthaceae). Rev. Palaeobot. Palynol. 1992, 72, 229–243. [Google Scholar] [CrossRef]
- Yang, Q.; Wan, H.; Sun, J.; Gong, S. Comparison of Pollen Morphology of Tree Peony Cultivar Groups. Sci. Silvae Sin. 2010, 46, 133–137. [Google Scholar]
- Hao, L.; Ma, H.; da Silva, J.A.T.; Yu, X. Pollen Morphology of Herbaceous Peonies with Different Ploidy Levels. J. Am. Soc. Hortic. Sci. 2016, 141, 275–284. [Google Scholar] [CrossRef]
- Yuan, J.H.; Cheng, F.Y.; Zhou, S.L. The Phylogeographic Structure and Conservation Genetics of the Endangered Tree Peony, Paeonia Rockii (Paeoniaceae), Inferred from Chloroplast Gene Sequences. Conserv. Genet. 2011, 12, 1539–1549. [Google Scholar] [CrossRef]
- Zhai, L.; Shi, Q.; Niu, L.; Zhang, Y. Research Progress of Wild Tree Peony Resources of Subsect.Vagintae. North Hortic. 2018, 10, 167–174. [Google Scholar]
- Li, T.; Qiao, Q.; Li, J.; Guo, X.; Hou, X. Cross Pollination of Different Peony Cultivars with “Feng Dan”. Cienc. Rural 2021, 51, e20190848. [Google Scholar] [CrossRef]
- Khatun, S.; Flowers, T.J. The Estimation of Pollen Viability in Rice. J. Exp. Bot. 1995, 151–154. [Google Scholar] [CrossRef]
- Lei, H.; Yu, Y.; Liu, Y.; Zhang, X.; Xing, L.; Lu, H.; Wu, J.; Guo, J. Study on TTC Method for Testing Seed Viability of Paeonia Lactiflora Pall. Seed 2019, 38, 41–44. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, F.; Peng, L.; Xian, H. Suggestions on Conservation and Utilization of Wild Tree Peony Resources of Subsect.Vagintae Based on Recent Investigation. J. Plant Genet. Resour. 2017, 18, 46–55. [Google Scholar] [CrossRef]
- Li, F.; Zhang, S.; Zhang, H.; Hu, H.; Tian, R.; Li, Q. Cluster Analysis for the Quantity and Germinating Characteristics of the Pollens from Different Pear Cultivars. J. Nanjing Agric. Univ. 2013, 36, 27–32. [Google Scholar]
- Liu, L.; Wang, J.; Liu, M.; Zhou, J. Pollen Number and Its Germination Rate of Different Chinese Jujube Cultivars. J. Plant Genet. Resour. 2006, 7, 3338–3341. [Google Scholar] [CrossRef]
- Li, J. Zhongguo Mudan Yu Shaoyao; China Forestry Publishing House: Beijing, China, 1999; ISBN 7-5038-2107-8. [Google Scholar]
- Liu, X.; Zhong, Y.; Cheng, F. Preliminary Exploration on Hybridization of Paeonia Qiui. In Proceedings of the Advances in Ornamental Horticulture of China 2016; Chinese Society for Horticultural Science, National Engineering Research Center for Floriculture: Changsha, Hunan, 2016; pp. 153–156. [Google Scholar]
- Jiang, Z.; Geng, X.; Zhu, Z.; Sheng, Q. Research Progress on Cross Breeding of Tree Peony. Mol. Plant Breed. 2021, 1, 1–29. [Google Scholar]
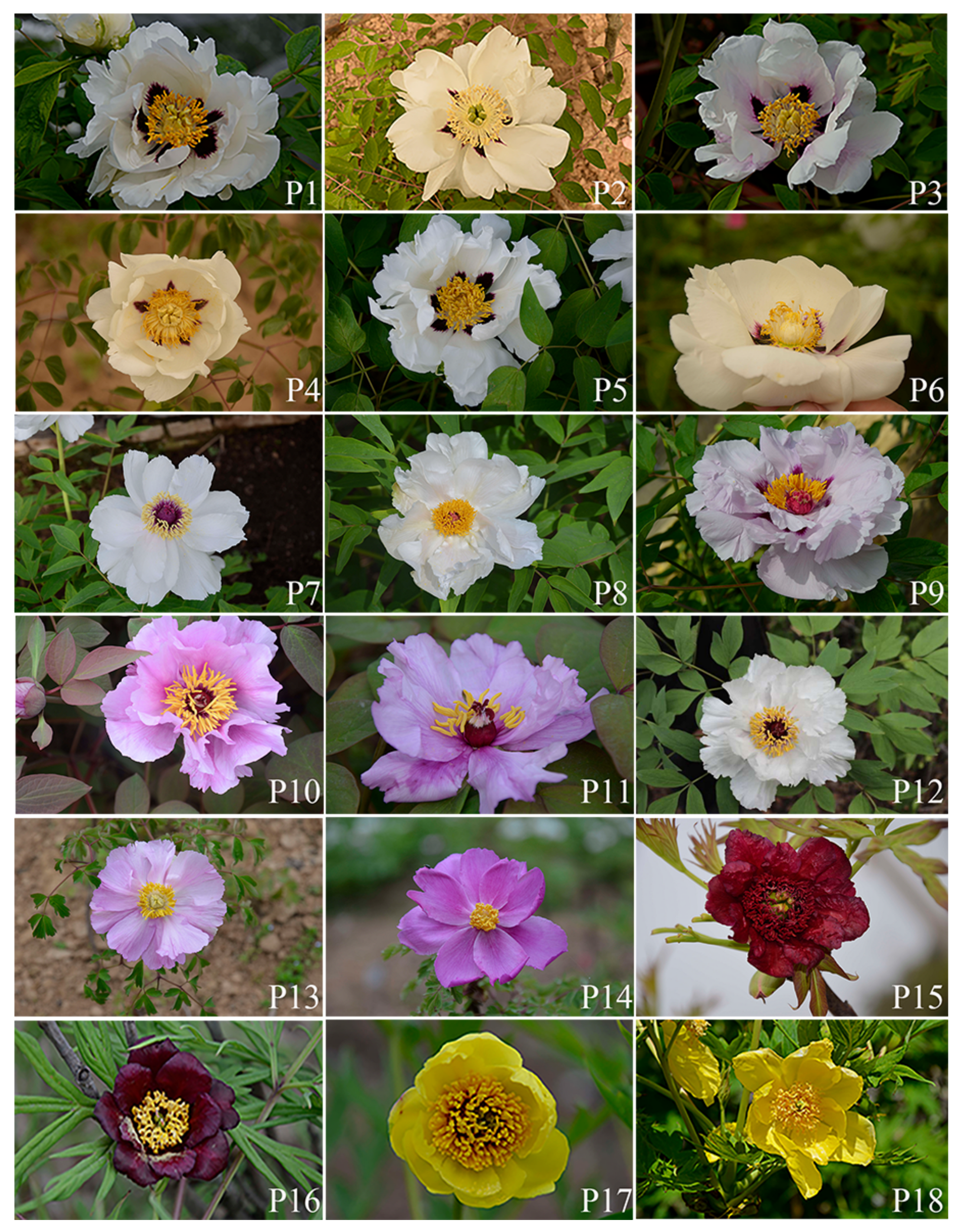
| Species | Population | Geographic Origin | Longitude E (°) and Latitude N (°) | Elevation (m) |
|---|---|---|---|---|
| P. rockii | P1 | Taibai County, Shaanxi | 107.52/33.82 | 1438 |
| P. rockii | P2 | Wudu District, Gansu | 105.24/33.32 | 2202 |
| P. rockii | P3 | Mei Co., Shaanxi | 107.70/34.08 | 1528 |
| P. rockii | P4 | Feng Co., Shaanxi | 106.46/33.94 | 1386 |
| P. rockii | P5 | Liuba Co., Shaanxi | 107.15/33.75 | 1250 |
| P. rockii | P6 | Ganquan Co., Shaanxi | 106.96/36.58 | 1412 |
| P. ostii | P7 | Mei Co., Shaanxi | 107.71/34.07 | 1563 |
| P. ostii | P8 | Shangnan Co., Shaanxi | 110.85/33.53 | 653 |
| P. qiui | P9 | Shennongjia, Hubei | 110.67/31.77 | 1976 |
| P. qiui | P10 | Xunyang Co., Shaanxi | 109.32/32.98 | 1558 |
| P. qiui | P11 | Shangnan Co., Shaanxi | 110.63/33.40 | 1141 |
| P. jishanensis | P12 | Jishan Co., Shanxi | 110.95/35.72 | 1163 |
| P. decomposita | P13 | Maerkang Co., Sichuan | 102.02/32.00 | 2504 |
| P. decomposita | P14 | Li Co., Sichuan | 103.45/31.58 | 2176 |
| P. delavayi | P15 | Lijiang City, Yunnan | 100.17/26.80 | 3015 |
| P. potaninii | P16 | Yajiang Co., Sichuan | 101.15/30.07 | 3127 |
| P. lutea | P17 | Chayu Co., Tibet | 96.79/28.72 | 2980 |
| P. ludlowii | P18 | Linzhi City, Tibet | 94.63/29.48 | 2958 |
| Code | Shape | Amb | Exine Ornamentation |
|---|---|---|---|
| 1 | Perprolate | Three-lobed circular | Reticulate |
| 2 | Prolate | Obtuse triangular | Microreticulate |
| 3 | - | - | Foveolate |
| 4 | - | - | Rugulate–reticulate |
| Subsection | Species | Population | P/μm | E/μm | P/E | D/μm | W/μm | D/W | Pollen Shape | Amb | Exine Ornamentation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| subsect. Vaginatae | P. rockii | P1 | 48.85 | 21.72 | 2.25 | 1.07 | 0.52 | 2.04 | Perprolate | Three-lobed circular | Reticulate |
| P. rockii | P2 | 44.18 | 22.72 | 1.94 | 1.82 | 0.33 | 5.48 | Prolate | Three-lobed circular | Reticulate | |
| P. rockii | P3 | 44.18 | 22.34 | 1.98 | 1.15 | 0.45 | 2.58 | Prolate | Three-lobed circular | Reticulate | |
| P. rockii | P4 | 47.04 | 23.76 | 1.98 | 1.67 | 0.66 | 2.51 | Prolate | Three-lobed circular | Reticulate | |
| P. rockii | P5 | 44.81 | 19.8 | 2.26 | 1.44 | 0.45 | 3.2 | Perprolate | Three-lobed circular | Reticulate | |
| P. rockii | P6 | 45.83 | 23.05 | 1.99 | 1.14 | 0.58 | 1.97 | Prolate | Obtuse triangular | Reticulate | |
| P. ostii | P7 | 44.96 | 20.02 | 2.25 | 0.59 | 0.53 | 1.11 | Perprolate | Three-lobed circular | Microreticulate | |
| P. ostii | P8 | 49.25 | 21.67 | 2.27 | 0.71 | 0.43 | 1.65 | Perprolate | Three-lobed circular | Microreticulate | |
| P. qiui | P9 | 48.15 | 22.28 | 1.75 | 0.89 | 0.54 | 1.02 | Perprolate | Three-lobed circular | Microreticulate | |
| P. qiui | P10 | 45.11 | 19.54 | 2.31 | 0.52 | 0.5 | 1.04 | Perprolate | Three-lobed circular | Foveolate | |
| P. qiui | P11 | 46.85 | 21.4 | 2.19 | 0.62 | 0.43 | 1.44 | Perprolate | Three-lobed circular | Foveolate | |
| P. jishanensis | P12 | 47.51 | 22.06 | 2.15 | 1.78 | 1.22 | 1.46 | Perprolate | Three-lobed circular | Foveolate | |
| P. decomposita | P13 | 47.18 | 22.58 | 2.09 | 0.71 | 0.53 | 1.35 | Perprolate | Three-lobed circular | Reticulate | |
| P. decomposita | P14 | 46.11 | 22.64 | 2.04 | 0.22 | 0.61 | 0.36 | Perprolate | Three-lobed circular | Foveolate | |
| subsect. Delavayanae | P. delavayi | P15 | 42.27 | 20.57 | 2.05 | 0.55 | 0.48 | 1.15 | Perprolate | Three-lobed circular | Rugulate–reticulate |
| P. potaninii | P16 | 39.69 | 21.5 | 1.85 | 0.43 | 0.46 | 0.95 | Perprolate | Three-lobed circular | Foveolate | |
| P. lutea | P17 | 42.58 | 21.06 | 2.02 | 0.37 | 0.43 | 0.86 | Perprolate | Three-lobed circular | Foveolate | |
| P. ludlowii | P18 | 41.37 | 20.22 | 2.05 | 0.39 | 0.35 | 1.11 | Perprolate | Three-lobed circular | Foveolate |
| Character | PC1 | PC2 | PC3 |
|---|---|---|---|
| Polar length (μm) | 0.391 | 0.723 | 0.193 |
| Equatorial diameter (μm) | 0.765 | −0.093 | 0.458 |
| P/E | −0.375 | 0.760 | −0.391 |
| perforation diameter | 0.814 | 0.328 | −0.158 |
| murus width | 0.154 | 0.573 | 0.649 |
| D/W | 0.708 | −0.010 | −0.622 |
| Pollen shape | 0.855 | −0.374 | 0.068 |
| Amb | 0.386 | −0.178 | 0.292 |
| Exine ornamentation type | −0.794 | −0.143 | 0.345 |
| Eigenvalue | 3.575 | 1.736 | 1.442 |
| Variance (%) | 39.772% | 19.288% | 16.026% |
| Cumulative variance (%) | 39.772% | 59.009% | 75.035% |
| Subsection | Population | Pollen Germination Rate (%) | Average (%) |
|---|---|---|---|
| subsect. Vaginatae | P1 P2 P3 P4 P5 P6 P7 P8 P9 P10 P11 P12 P13 P14 | 84.30 64.17 80.02 77.29 20.15 26.16 66.63 73.61 48.19 87.78 61.04 62.62 56.88 50.89 | 61.41 |
| subsect. Delavayanae | P15 P16 P17 P18 | 25.77 36.61 46.34 32.91 | 35.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, J.; Yan, Z.; Niu, L.; Zhang, X.; Zhang, Y. Optimum Discrimination on the Subsection Taxonomy of Wild Tree Peony Species in China Using Pollen Characteristics. Horticulturae 2022, 8, 736. https://doi.org/10.3390/horticulturae8080736
Wang Y, Wang J, Yan Z, Niu L, Zhang X, Zhang Y. Optimum Discrimination on the Subsection Taxonomy of Wild Tree Peony Species in China Using Pollen Characteristics. Horticulturae. 2022; 8(8):736. https://doi.org/10.3390/horticulturae8080736
Chicago/Turabian StyleWang, Yiting, Jiangnan Wang, Zhenguo Yan, Lixin Niu, Xiaoxiao Zhang, and Yanlong Zhang. 2022. "Optimum Discrimination on the Subsection Taxonomy of Wild Tree Peony Species in China Using Pollen Characteristics" Horticulturae 8, no. 8: 736. https://doi.org/10.3390/horticulturae8080736
APA StyleWang, Y., Wang, J., Yan, Z., Niu, L., Zhang, X., & Zhang, Y. (2022). Optimum Discrimination on the Subsection Taxonomy of Wild Tree Peony Species in China Using Pollen Characteristics. Horticulturae, 8(8), 736. https://doi.org/10.3390/horticulturae8080736