Aroma Profile of Monovarietal Pét-Nat Ciders: The Role of Croatian Traditional Apple Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Location and Climatic Conditions
2.2. Apple Fruit Processing and Fermentation
2.3. Physicochemical Analysis
2.4. Malic Acid Analysis
2.5. Spectrofotometrical Analysis
2.5.1. Nitrogenous Compounds Analysis
2.5.2. Total Phenols
2.5.3. Color Parameters
2.6. Volatile Compounds Determination
2.7. Determination of Odor Activity Values
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Apple juice Analysis
3.2. Pét-Nat Ciders Basic Analysis
3.3. Volatile Compounds in Sparkling Pét-Nat Ciders
| Odor Active Value (OAV) | ||||
|---|---|---|---|---|
| Aromatic Compound | ‘Božićnica’ | ‘Bobovac’ | ‘Crvenka’ | Odor Descriptors |
| 1-Hexanol | 3.25 | 3.96 | 3.04 | Cutted grass [44] |
| 1-Decanol | 1.02 | 0.31 | 0.25 | Pear, waxy, violet [72] |
| 1-Propanol | 6.19 | 4.43 | 5.38 | Ripe fruit, alcohol [73] |
| (6Z)-Nonen-1-ol | 7.31 | 7.70 | 7.44 | Melon, green [74] |
| 1-Dodecanol | 5.64 | 1.96 | 2.22 | Waxy, coconut [75] |
| Hexanoic acid | 13.98 | 13.72 | 12.64 | Goaty, cheesy, oily [44] |
| Octanoic acid | 7.69 | 5.44 | 5.93 | Goaty, oily [44] |
| Isovaleric acid | 4.08 | 4.08 | 3.40 | Sweaty, cheesy [44] |
| Citronellol | 1.50 | 1.14 | 0.81 | Rose, citrus [44] |
| Ethyl hexanoate | 87.95 | 96.85 | 74.01 | Fruity, green apple [44] |
| Ethyl butanoate | 6.24 | 2.69 | 4.56 | Apple, fruity [44] |
| Ethyl 9-decenoate | 2.26 | 2.10 | 2.11 | Fruity, fatty [76] |
| Isoamyl acetate | 15.35 | 7.06 | 5.45 | Banana [44] |
| 4-Ethylguaiacol | 3.69 | Phenolic, smoke, clove [44] | ||
| Eugenol | 8.11 | 7.94 | 8.05 | Cinnamon, clove [44] |
| 4-Vinylguaiacol | 0.63 | 2.18 | 0.45 | Clove, curry, smoke [44] |
| 4-Ethyl-phenol | 7.16 | 0.28 | Leather, horse stable [44] | |
| Methionol | 23.75 | 9.60 | 17.55 | Potato, cauliflower [44] |
4. Sensory Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista Alcoholic Drinks, Rewenue Growth—Worldwide. Available online: https://www.statista.com/outlook/cmo/alcoholic-drinks/worldwide (accessed on 2 June 2022).
- AICV—European Cider and Fruit Wine Association European Cider Trends 2020; AICV–European Cider and Fruit Wine Association: Brussels, Belgium, 2020.
- Fior Markets. Cider Market by Product /Apple Flavored, Fruit Flavored, and Perry), Distribution Channel, Packaging, Regions, Global Industry Analysis, Narket Size, Share, Growth, Trends, and Forecast 2019 to 2026; Fior Markets: Pune, India, 2021. [Google Scholar]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of Apple Cultivar, Ripening Stage, Fermentation Type and Yeast Strain on Phenolic Composition of Apple Ciders. Food Chem. 2017, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qi, Y.; Zhang, J.; Liu, M.; Wei, X.; Fan, M. Effect of Reduced Glutathione on the Quality Characteristics of Apple Wine during Alcoholic Fermentation. Food Chem. 2019, 300, 125130. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.A.; Alexander, T.R.; Peck, G.; Galinato, S.P.; Gottschalk, C.; van Nocker, S. Growing Apples for Hard Cider Production in the United States—Trends and Research Opportunities. Horttechnology 2020, 30, 148–155. [Google Scholar] [CrossRef]
- Joshi, V.K.; Attri, B.L. Specific Features of Table Wine Production Technology. In Science and Technology of Fruit Wine Production; Kosseva, M.R., Joshi, V.K., Panesar, P.S., Eds.; Elsevier: Amsterdam, The Netherlands; ISBN 978-0-12-800850-8.
- Jarvis, B. Cider (Cyder; Hard Cider); Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; ISBN 9780123847331. [Google Scholar]
- AICV—European Cider and Fruit Wine Association European Cider Trends 2021; AICV–European Cider and Fruit Wine Association: Brussels, Belgium, 2021.
- HAPIH Croatian Plant Genetic Resources Database. Available online: https://cpgrd.hapih.hr/ (accessed on 2 June 2022).
- Jakobek, L.; Ištuk, J.; Matić, P.; Skendrović Babojelić, M. Interactions of Polyphenols from Traditional Apple Varieties ‘Bobovac’, ‘Ljepocvjetka’ and ‘Crvenka’ with β-Glucan during in Vitro Simulated Digestion. Food Chem. 2021, 363. [Google Scholar] [CrossRef]
- Jakobek, L.; Ištuk, J.; Buljeta, I.; Voća, S.; Žlabur, J.Š.; Babojelić, M.S. Traditional, Indigenous Apple Varieties, a Fruit with Potential for Beneficial Effects: Their Quality Traits and Bioactive Polyphenol Contents. Foods 2020, 9, 52. [Google Scholar] [CrossRef]
- Duralija, B.; Putnik, P.; Brdar, D.; Markovinović, A.B.; Zavadlav, S.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Kovačević, D.B. The Perspective of Croatian Old Apple Cultivars in Extensive Farming for the Production of Functional Foods. Foods 2021, 10, 708. [Google Scholar] [CrossRef]
- Jolicoeur, C. Acidity and PH of Apple Juice. Available online: http://cjoliprsf.ca/Documents/Acidity-pH.pdf (accessed on 27 May 2022).
- Kliks, J.; Kawa-Rygielska, J.; Gasiński, A.; Głowacki, A.; Szumny, A. Analysis of Volatile Compounds and Sugar Content in Three Polish Regional Ciders with Pear Addition. Molecules 2020, 25, 3564. [Google Scholar] [CrossRef]
- Downing, D.L. Processed Apple Products; Downing, D.L., Ed.; AVI Publishing Co.: New York, NY, USA, 1989. [Google Scholar]
- Lobo, A.P.; Tascón, N.F.; Madrera, R.R.; Valles, B.S. Sensory and Foaming Properties of Sparkling Cider. J. Agric. Food Chem. 2005, 53, 10051–10056. [Google Scholar] [CrossRef]
- Madrera, R.R.; Hevia, A.G.; García, N.P.; Valles, B.S. Evolution of Aroma Compounds in Sparkling Ciders. LWT Food Sci. Technol. 2008, 41, 2064–2069. [Google Scholar] [CrossRef]
- Cousin, F.J.; Le Guellec, R.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.M.; Cretenet, M. Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef]
- Keller, S.E.; Chirtel, S.J.; Merker, R.I.; Taylor, K.T.; Tan, H.L.; Miller, A.J. Influence of Fruit Variety, Harvest Technique, Quality Sorting, and Storage on the Native Microflora of Unpasteurized Apple Cider. J. Food Prot. 2004, 67, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Pando Bedrinana, R.; Querol Simon, A.; Suarez Valles, B. Genetic and Phenotypic Diversity of Autochthonous Cider Yeasts in a Cellar from Asturias. Food Microbiol. 2010, 27, 503–508. [Google Scholar] [CrossRef]
- Beech, F.W. 5—Yeasts in Cider-Making. In The Yeasts, 2nd ed.; Rose, A.H., Harrison, J.S., Eds.; Academic Press: San Diego, CA, USA, 1993; ISBN 978-0-12-596415-9. [Google Scholar]
- Sánchez, A.; Rodríguez, R.; Coton, M.; Coton, E.; Herrero, M.; García, L.A.; Díaz, M. Population Dynamics of Lactic Acid Bacteria during Spontaneous Malolactic Fermentation in Industrial Cider. Food Res. Int. 2010, 2101–2107. [Google Scholar] [CrossRef]
- Coton, E.; Coton, M.; Levert, D.; Casaregola, S.; Sohier, D. Yeast Ecology in French Cider and Black Olive Natural Fermentations. Int. J. Food. Microbiol. 2006, 108, 130–135. [Google Scholar] [CrossRef]
- Morrissey, W.F.; Davenport, B.; Querol, A.; Dobson, A.D. The Role of Indigenous Yeasts in Traditional Irish Cider Fermentations. 2004, 97, 647–655. J. Appl. Microbiol. 2004, 97, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Suarez Valles, B.; Bedrinana, R.P.; Tascon, N.F.; Simon, A.Q.; Madrera, R.R. Yeast Species Associated with the Spontaneous Fermentation of Cider. Food Microbiol. 2007, 24, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Machado dos Santos, T.P.; Ferreira Zielinski, A.A.; Eleutério dos Santos, C.M.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on Chemical Profile in Apple Juice and Cider Made from Unripe, Ripe and Senescent Dessert Varieties. LWT Food Sci. Technol. 2016, 65, 436–443. [Google Scholar] [CrossRef]
- Antón-Díaz, M.J.; Suárez Valles, B.; Mangas-Alonso, J.J.; Fernández-García, O.; Lobo, A.P. Impact of Different Techniques Involving Contact with Lees on the Volatile Composition of Cider. Food Chem. 2016, 190, 1116–1122. [Google Scholar] [CrossRef]
- Chassagne, D.; Guilloux-Benatier, M.; Hervé Alexandre, A.V. Sorption of Wine Volatile Phenols by Yeast Lees. Food Chem. 2005, 91, 39–44. [Google Scholar] [CrossRef]
- Pradelles, R.; Alexandre, H.; Ortiz-Julien, A.; Chassagne, D. Effects of Yeast Cell-Wall Characteristics on 4-Ethylphenol Sorption Capacity in Model Wine. J. Agric. Food Chem. 2008, 56, 11854–11861. [Google Scholar] [CrossRef]
- Maslov Bandić, L.; Mihaljević Žulj, M.; Fruk, G.; Skendrović Babojelić, M.; Jemrić, T.; Jeromel, A. The Profile of Organic Acids and Polyphenols in Apple Wines Fermented with Different Yeast Strains. J. Food Sci. Technol. 2019, 2, 599–606. [Google Scholar] [CrossRef]
- Ljevar, A.; Ćurko, N.; Tomašević, M.; Radošević, K.; Gaurina Srček, V.; Kovačević Ganić, K. Phenolic Composition, Antioxidant Capacity and in Vitro Cytotoxicity Assessment of Fruit Wines. Food Technol. Biotechnol. 2016, 54, 145–155. [Google Scholar]
- Jolicoeur, C. The Varietal Selection. In The New Cider Maker's Handbook: A Comprehensive Guide for Craft Producer; Chelsea Green Publishing: White River Junction, VT, USA, 2013. [Google Scholar]
- Resolution OIV 332A/2009; OIV Standard for International Wine and Spirituous Beverages of Viticultural Origin Competitions. OIV: Paris, France, 2009.
- Dukes, B.C.; Butzke, C.E. Rapid Determination of Primary Amino Acids in Grape Juice Using an O-Phthaldialdehyde/N-Acetyl-L-Cysteine Spectrophotometric Assay. Am. J. Enol. Vitic. 1998, 49, 125–134. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Glories, Y. La Couleur Des Vins Rouges II, Connaissance de La Vigne et Du Vin. Vigne vin 1984, 253–271. [Google Scholar]
- Falqué, E.; Fernández, E.; Dubourdieu, D. Differentiation of White Wines by Their Aromatic Index. Talanta 2001, 54, 271–281. [Google Scholar] [CrossRef]
- Allen, M.S.; Lacey, M.J.; Boyd, S. Determination of Methoxypyrazines in Red Wines by Stable Isotope Dilution Gas Chromatography–Mass Spectrometry. J. Agric. Food Chem. 1994, 42, 1734–1738. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining Wine Aroma from Compositional Data. Aust. J. GrapeWine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Williams, A.A. The Development of a Vocabulary and Profile Assessment Method for Evaluating the Flavour Contribution of Cider and Perry Aroma Constituents. J. Sci. Food Agric. 1975, 26, 567–582. [Google Scholar] [CrossRef]
- Dierings, L.R.; Braga, C.M.; Silva, K.M.; Wosiacki, G.; Nogueira, A. Population Dynamics of Mixed Cultures of Yeast and Lactic Acid Bacteria in Cider Conditions. Braz. Arch. Biol. Technol. 2013, 56, 837–847. [Google Scholar] [CrossRef]
- Su, Y.; Heras, J.M.; Gamero, A.; Querol, A.; Guillamón, M.J. Impact of Nitrogen Addition on Wine Fermentation by S. Cerevisiae Strains with Different Nitrogen Requirements. J Agric Food Chem. 2021, 69, 6022–6031. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons, Ltd: Chichester, UK, 2016. [Google Scholar]
- Ye, M.; Yue, T.; Yuan, Y. Changes in the Profle of Volatile Compounds and Amino Acids during Cider Fermentation Using Dessert Variety of Apples. Eur. Food Res. Technol. 2014, 239, 67–77. [Google Scholar] [CrossRef]
- Nogueira, A.; Guyot, S.; Marnet, N.; Luquere, J.M.; Drilleau, J.F.; Wosiacki, G. Effect of Alcoholic Fermentation in the Content of Phenolic Compounds in Cider Processing. Brazilian Arch. Biol. Technol. 2008, 51, 1025–1032. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Torello Marinoni, D.; Cerutti, A.K.; Canterino, S.; Bounous, G. Application of Sensory, Nutraceutical and Genetic Techniques to Create a Quality Profile of Ancient Apple Cultivars. J. Food Qual. 2012, 35, 169–181. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmianski, J.; Laskowski, P. Polyphenolic Compounds and Antioxidant Activity of New and Old Apple Varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef]
- Tehorbanov, B.; Mitchev, G.; Lazarova, G.; Popov, D. Studies on the Secondary Fermentation of Low Alcohol Sparkling Apple Wine. Am. J. Enol. Vitic. 1993, 44, 93. [Google Scholar]
- Ruppert, V.; Innerhofer, G.; Voit, J.; Hiden, P.; Siegmund, B. The Impact of the Fermentation Strategy on the Flavour Formation of Ilzer Rose (Malus Domestica Borkh.) Apple Wine. Foods 2021, 10, 2348. [Google Scholar] [CrossRef]
- Amerine, M.A.; Berg, H.W.; Kunkee, R.E.; Qugh, C.S.; Singleton, V.L.; Webb, A.D. The Technology of Wine Making, 4th ed.; AVI: Westport, CT, USA, 1980. [Google Scholar]
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X. Sen The Effects of Simultaneous and Sequential Inoculation of Yeast and Autochthonous Oenococcus Oeni on the Chemical Composition of Red-Fleshed Apple Cider. Lwt 2020, 124, 109184. [Google Scholar] [CrossRef]
- Mangas, J.J.; Cabranes, C.; Moreno, J.; Gomis, D.B. Influence of Cider-Making Technology on Cider Taste. LWT Food Sci. Technol. 1994, 27, 583–586. [Google Scholar] [CrossRef]
- Rapp, A.; Mandery, H. New Progress in Wine and Wine Research. Experientia 1987, 45, 873–884. [Google Scholar]
- Herrero, M. Influence Des Fermentations Alcoolique et Malolactique Sur La Composition Chimique Des Cidres À Distiller En Cours D’élaboaration; Université de Caen: Caen, France, 2011. [Google Scholar]
- Blanco-Gomis, D.; Mangas-Alonso, J.J.; Margolles-Cabrales, I.; Arias Abrodo, P. Characterization of Cider Apples on the Basis of Their Fatty Acid Profiles. J. Agric. Food Chem. 2002, 50, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.S.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, A.M. Volatile Composition of Baga Red Wine: Assessment of the Identification of the Would-Be Impact Odourants. Anal. Chim. Acta 2004, 51, 257–262. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, B.Q.; Wang, Y.H.; Lu, L.; Lan, Y.; Bin Reeves, M.J.; Duan, C.Q. Influence of Pre-Fermentation Cold Maceration Treatment on Aroma Compounds of Cabernet Sauvignon Wines Fermented in Different Industrial Scale Fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef]
- Antón, M.J.; Suárez Valles, B.; García Hevia, A.; Picinelli Lobo, A. Aromatic Profile of Ciders by Chemical Quantitative, Gas Chromatography-Olfactometry, and Sensory Analysis. J. Food Sci. 2014, 79. [Google Scholar] [CrossRef]
- Miranda-López, R.; Libbey, L.M.; Watson, B.T.; McDaniel, M.R. Identification of Additional Odor-Active Compounds in Pinot Noir Wines. Am. J. Enol. Vitic. 1992, 43, 90–92. [Google Scholar]
- Marais, J. Terpenes in the Aroma of Grape and Wines: A Review. South African J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Babot, E.D.; Aranda, C.; del Río, J.C.; Ullrich, R.; Kiebist, J.; Scheibner, K.; Hofrichter, M.; Martínez, A.T.; Gutiérrez, A. Selective Oxygenation of Ionones and Damascones by Fungal Peroxygenases. J Agric Food Chem 2020, 68, 5375–5383. [Google Scholar] [CrossRef] [PubMed]
- Azhu Valappil, Z.; Fan, X.; Zhang, H.Q.; Rouseff, R.L. Impact of Thermal and Nonthermal Processing Technologies on Unfermented Apple Cider Aroma Volatiles. J. Agric. Food Chem. 2009, 57, 924–929. [Google Scholar] [CrossRef]
- Diaz-Maroto, M.C.; Schneider, R.; Baumes, R. Formation Pathways of Ethyl Esters of Branched Short-Chain Fatty Acids during Wine Aging. J. Agric. Food Chem. 2005, 53, 3503–3509. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial Modulation of Aromatic Esters in Wine: Current Knowledge and Future Prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Garde-Cerdan, T.; Torrea-Goni, D.; Ancin-Azpilicueta, C. Accumulation of Volatile Compounds during Ageing of Two Red Wines with Different Composition. J. Food Eng. 2004, 65, 349–356. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, G.A.; Wang, L.P. Controlled Formation of Volatile Components in Cider Making Using a Combination of Saccharomyces Cerevisiae and Hanseniaspora Valbyensis Yeast Species. J. Ind. Microbiol. Biotechnol. 2006, 33, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Buron, N.; Coton, M.; Legendre, P.; Ledauphin, J.; Kientz-Bouchart, V.; Guichard, H.; Daniel Barillier, E.C. Implications of Lactobacillus Collinoides and Brettanomyces/Dekkera Anomala in Phenolic off-Flavour Defects of Ciders. Int. J. Food Microbiol. 2012, 153, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Buron, N.; Guichard, H.; Coton, E.; Jérôme Ledauphin, D.B. Evidence of 4-Ethylcatechol as One of the Main Phenolic off-Flavour Markers in French Ciders. Food Chem. 2011, 125, 542–548. [Google Scholar] [CrossRef]
- Pizarro, C.; Perez-Del-Notario, N.; Gonzalez-Saiz, J. Headspace Solid-Phase Microextraction for Direct Determination of Volatile Phenols in Cider. J. Sep. Sci. 2009, 32, 3746–3754. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Li, F.; Cui, L.; Guo, Y. Effects of Fermentation Temperature on Key Aroma Compounds and Sensory Properties of Apple Wine. J. Food Sci. 2015, 80, S2937–S2943. [Google Scholar] [CrossRef]
- Odor & Flavor Detection Thresholds in Water (In Parts per Billion). Available online: http://www.leffingwell.com/odorthre.htm (accessed on 10 May 2022).
- TGSC. Available online: http://www.thegoodscentscompany.com/data/rw1009211.html (accessed on 10 May 2022).
- Chemicalbook. Available online: https://www.chemicalbook.com/ (accessed on 10 May 2022).
- Perflavory. Available online: http://www.perflavory.com/docs/doc1014271.html (accessed on 10 May 2022).
- Pherobase. Available online: http://www.pherobase.com/database/kovats/kovats-detail-ethyl9-decenoate.php (accessed on 10 May 2022).
- Lea, A.G.H. Analysis of Phenolics in Oxidizing Apple Juice by HPLC Using a PH Shift Method. J. Chromatogr. A 1982, 238, 253. [Google Scholar] [CrossRef]

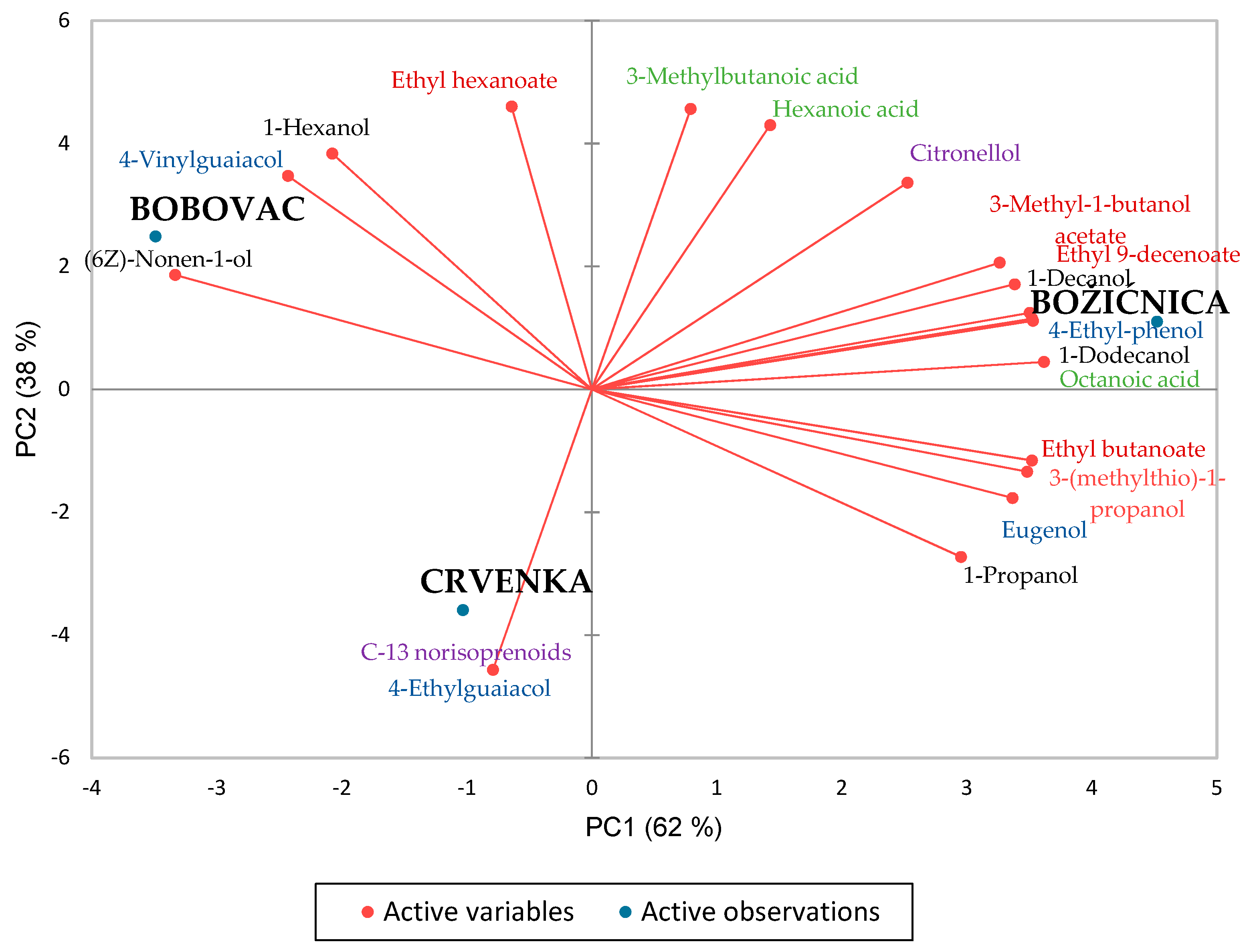
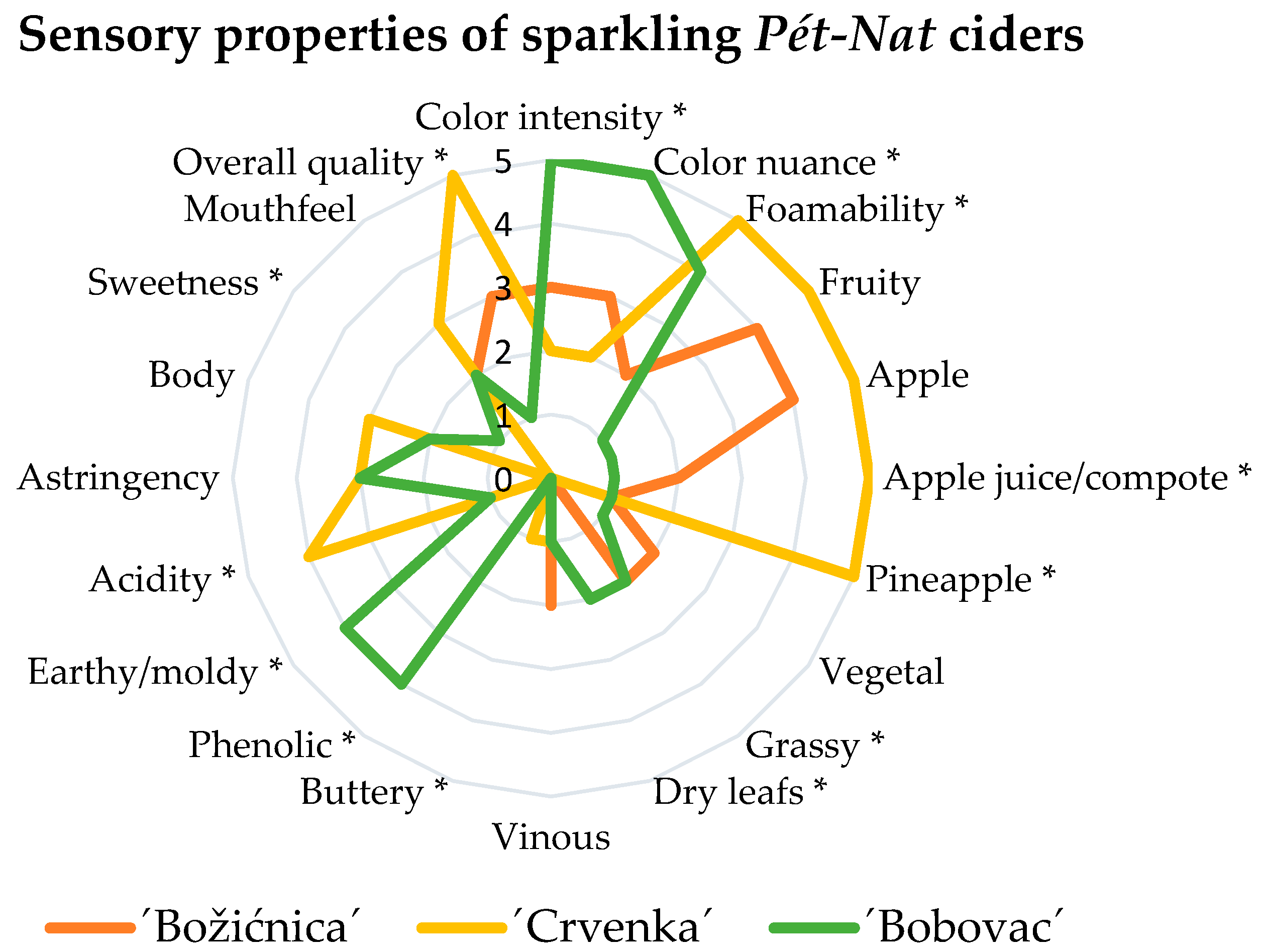
| Parameter | ‘Božićnica’ | ‘Bobovac’ | ‘Crvenka’ |
|---|---|---|---|
| Density (Oe°) | 56 ± 2 | 57 ± 1 | 55 ± 1 |
| pH | 3.38 ± 0.3 a | 3.33 ± 0.2 b | 3.24 ± 0.3 c |
| Total acidity, TA (g·L−1) * | 5.6 ± 0.05 c | 6.1 ± 0.1 b | 8.0 ± 0.05 a |
| Malic acid (g·L−1) | 4.96 ± 0.05 c | 5.18 ± 0.03 b | 6.41 ± 0.02 a |
| FAN (mg·L−1) | 16.7 ± 0.09 a | 12.7 ± 0.08 c | 14.72 ± 0.1 b |
| NH4+ (mg·L−1) | 97.2 ± 1.7 b | 94.6 ± 1.6 c | 124 ± 1.9 a |
| YAN (mg·L−1) | 113.9 ± 1.0 b | 107.3 ± 1.5 c | 138.7 ± 1.1 a |
| Parameter | ‘Božićnica’ | ‘Bobovac’ | ‘Crvenka’ |
|---|---|---|---|
| Specific gravity (20/20 °C) | 0.9989 ± 0.002 c | 1.0006 ± 0.003 a | 1.0001 ± 0.001 b |
| Alcohol (v/v, %) | 7.3 ± 0.06 a | 7.1 ± 0.03 b | 7.0 ± 0.03 b |
| Dry extract (g·L−1) | 23.2 ± 0.03 b | 27.1 ± 0.03 a | 27.1 ± 0.02 a |
| Residual sugar (g·L−1) | 1.4 ± 0.03 b | 5.8 ± 0.06 a | <1.0 ± 0.03 c |
| Total acidity 1 (g·L−1) | 3.5 ± 0.03 c | 5.2 ± 0.06 b | 8.6 ± 0.05 a |
| Volatile acidity 2 (g·L−1) | 0.43 ± 0.01 a | 0.35 ± 0.02 b | 0.26 ± 0.01 c |
| pH | 3.89 ± 0.05 a | 3.63 ± 0.01 b | 3.38 ± 0.01 c |
| Ash (g·L−1) | 2.51 ± 0.01 c | 2.56 ± 0.01 b | 2.60 ± 0.01 a |
| Parameter | ‘Božićnica’ | ‘Bobovac’ | ‘Crvenka’ |
|---|---|---|---|
| Total phenols (mg·L−1) * | 474 ± 2.64 b | 539 ± 4.58 a | 416 ± 4.36 c |
| A 420 nm | 0.646 ± 0.001 b | 0.778 ± 0.002 a | 0.415 ± 0.003 c |
| A 520 nm | 0.238 ± 0.003 b | 0.323 ± 0.003 a | 0.130 ± 0.003 c |
| A 620 nm | 0.123 ± 0.001 b | 0.184 ± 0.003 a | 0.055 ± 0.002 c |
| Color intensity (CI) | 1.007 ± 0.001 b | 1.285 ± 0.002 a | 0.600 ± 0.002 c |
| ‘Božićnica’ | ‘Bobovac’ | ‘Crvenka’ | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Compounds | Mean | SD | Mean | SD | Mean | SD | F | p |
| (6Z)-Nonen-1-ol | 7.31 ab | 0.10 | 7.70 a | 0.11 | 7.44 b | 0.11 | 7.01 | 0.07 |
| 1-Butanol | 3143.91 a * | 44.91 | 2212.90 c | 31.61 | 2402.54 b | 34.32 | 346.29 | 0.00 |
| Isoamyl alcohol | 12,825.40 a | 183.21 | 11,629.49 b | 166.13 | 11,360.83 b | 162.29 | 41.68 | 0.01 |
| 1-Decanol | 5119.46 a | 73.13 | 1542.46 b | 22.03 | 1246.84 c | 17.81 | 4532.56 | <0.0001 |
| 1-Dodecanol | 5648.33 a | 80.69 | 1965.21 c | 28.07 | 2224.13 b | 31.77 | 3052.26 | <0.0001 |
| 1-Hexanol | 8133.26 b | 116.18 | 9894.79 a | 141.35 | 7595.85 c | 108.51 | 191.75 | 0.00 |
| 1-Octanol | 96.27 a | 1.38 | 24.64 c | 0.35 | 28.60 b | 0.41 | 4457.47 | <0.0001 |
| 1-Octen-3-ol | 37.87 b | 0.54 | 39.74 a | 0.57 | 33.04 c | 0.47 | 85.50 | 0.00 |
| 1-Pentanol | 1936.05 b | 27.66 | 2317.47 a | 33.10 | 1811.43 c | 25.88 | 164.83 | 0.00 |
| 1-Propanol | 5136.21 a | 107.35 | 3679.34 c | 76.90 | 4466.41 b | 93.35 | 2311.56 | <0.0001 |
| 3-ethoxy-1-propanol | 1088.43 b | 22.75 | 1280.10 a | 26.75 | 1018.51 b | 21.29 | 65.27 | 0.00 |
| [S-(R *,R *)]-2.3-butanediol | 36.62 b | 0.77 | 40.22 a | 0.84 | 25.78 c | 0.54 | 214.14 | 0.00 |
| 2.3-Dehydro-4-oxo-ß-ionol | 7.69 b | 0.16 | 9.35 a | 0.20 | 6.36 c | 0.13 | 164.72 | 0.00 |
| 2-Furanmethanol | 101.82 c | 2.13 | 218.51 a | 4.57 | 121.92 b | 2.55 | 732.48 | <0.0001 |
| 2-Heptanol | 17.19 a | 0.36 | 5.80 c | 0.12 | 11.35 b | 0.24 | 972.62 | <0.0001 |
| (E)-2-Hexen-1-ol | 43.00 b | 0.90 | 51.85 a | 1.08 | 44.67 b | 0.93 | 46.42 | 0.01 |
| 2-Nonanol | 9.61 a | 0.20 | 9.04 a | 0.18 | 8.22 b | 0.17 | 27.62 | 0.01 |
| (E)-2-Octen-1-ol | 39.36 b | 0.82 | 44.95 a | 0.94 | 33.58 c | 0.70 | 94.55 | 0.00 |
| 3-Ethyl-4-methylpentan-1-ol | 1799.54 a | 37.61 | 1202.27 b | 25.13 | 896.37 c | 18.73 | 528.20 | 0.00 |
| (E)-3-Hexen-1-ol | 456.87 a | 9.55 | 258.10 c | 5.39 | 429.29 b | 8.97 | 346.54 | 0.00 |
| (Z)-3-Hexen-1-ol | 218.66 b | 4.57 | 267.90 a | 5.60 | 153.54 c | 3.21 | 315.73 | 0.00 |
| 3-Methylpentan-1-ol | 980.43 a | 27.19 | 816.09 b | 22.63 | 840.54 b | 23.31 | 26.29 | 0.01 |
| 3-Octanol | 43.30 a | 1.20 | 38.79 b | 1.08 | 32.68 c | 0.91 | 49.80 | 0.00 |
| 6-Methyl-5-hepten-2-ol | 9.57 b | 0.27 | 9.15 b | 0.25 | 11.99 a | 0.33 | 57.62 | 0.00 |
| (Z)-3-Octen-1-ol | 66.42 b | 1.84 | 151.95 a | 4.21 | 53.96 c | 1.50 | 730.13 | <0.0001 |
| 3-Methyl-3-pentanol | 79.90 c | 2.22 | 92.06 b | 2.55 | 123.05 a | 3.41 | 128.78 | 0.00 |
| 5-Hexen-1-ol | 108.89 a | 3.02 | 81.97 b | 2.27 | 50.47 c | 1.40 | 315.84 | 0.00 |
| (E)-5-Octen-1-ol | 90.21 a | 2.50 | 85.33 a | 2.37 | 67.98 b | 1.89 | 53.12 | 0.00 |
| (Z)-5-Octen-1-ol | 29.53 a | 0.82 | 30.96 a | 0.86 | 29.58 a | 0.82 | 1.90 | 0.29 |
| Benzyl alcohol | 118.64 a | 1.66 | 83.26 b | 1.17 | 81.95 b | 1.15 | 478.29 | 0.00 |
| Chavicol | 7.93 a | 0.17 | 6.47 b | 0.14 | 6.81 b | 0.15 | 49.75 | 0.01 |
| cis,cis-4.6-Octadienol | 72.74 a | 1.57 | 74.68 a | 1.61 | 66.41 b | 1.43 | 15.83 | 0.03 |
| Isobutanol | 86.49 a | 2.50 | 68.91 b | 1.99 | 89.60 a | 2.59 | 44.29 | 0.01 |
| Phenylethyl Alcohol | 2236.08 a | 48.16 | 2082.41 b | 44.85 | 2123.85 ab | 45.74 | 5.91 | 0.09 |
| Z-a-trans-Bergamotol | 6.13 a | 0.09 | 6.07 a | 0.08 | 5.55 b | 0.08 | 29.58 | 0.01 |
| Total higher alcohols | 49,839.15 a | 352.24 | 36,650.59 b | 363.63 | 37,511.14 b | 245.87 | 1031.30 | <0.0001 |
| Isovaleric acid | 1020.80 a | 28.31 | 1020.36 a | 28.29 | 849.29 b | 23.55 | 27.21 | 0.01 |
| Butanoic acid | 344.40 b | 4.82 | 384.76 a | 5.39 | 283.79 c | 3.97 | 227.67 | 0.00 |
| Decanoic acid | 875.43 a | 18.85 | 788.30 b | 16.98 | 695.97 c | 14.99 | 55.65 | 0.00 |
| Dodecanoic acid | 18.92 b | 0.41 | 22.34 a | 0.48 | 19.69 b | 0.42 | 33.40 | 0.01 |
| Ethyl trans-cinnamic acid | 61.99 a | 1.34 | 61.78 ab | 1.33 | 57.96 b | 1.25 | 6.06 | 0.09 |
| Heptanoic acid | 43.29 b | 1.25 | 56.46 a | 1.63 | 47.00 b | 1.36 | 45.68 | 0.01 |
| Hexanoic acid | 5872.22 a | 169.48 | 5761.55 ab | 166.29 | 5307.94 b | 153.20 | 6.72 | 0.08 |
| 2-ethyl-Hexanoic acid | 103.37 b | 2.98 | 114.44 a | 3.30 | 120.97 a | 3.49 | 14.83 | 0.03 |
| Nonanoic acid | 67.24 b | 1.94 | 75.77 a | 2.19 | 64.67 b | 1.87 | 16.83 | 0.02 |
| Octanoic acid | 3843.53 a | 110.93 | 2722.36 b | 78.57 | 2966.22 b | 85.61 | 80.83 | 0.00 |
| Propanoic acid | 73.99 b | 1.59 | 81.39 a | 1.75 | 74.62 b | 1.61 | 12.31 | 0.04 |
| 2-Methyl-propanoic acid | 322.07 b | 6.94 | 555.02 a | 11.95 | 249.58 c | 5.38 | 695.00 | <0.0001 |
| Undecanoic acid | 8.52 b | 0.18 | 10.04 a | 0.22 | 8.81 b | 0.19 | 33.40 | 0.01 |
| Total fatty acids | 12,655.79 a | 282.77 | 11,654.57 b | 251.01 | 10,746.50 c | 241.83 | 27.17 | 0.01 |
| 3-Octanone | 0.63 b | 0.02 | 3.94 a | 0.11 | 0.47 b | 0.01 | 1859.03 | <0.0001 |
| 1-Octen-3-one | n.d. | 5.28 a | 0.08 | 3.16 b | 0.05 | 5483.41 | <0.0001 | |
| Nonanal | 8.61 a | 0.25 | 8.37 b | 0.24 | 8.45 ab | 0.24 | 0.52 | 0.64 |
| Total carbonyls | 9.25 c | 0.23 | 17.59 a | 0.21 | 12.08 b | 0.28 | 625.37 | 0.00 |
| allo-Ocimene | n.d. | 13.76 a | 0.19 | n.d. | 10,201.00 | <0.0001 | ||
| a-Ocimene | n.d. | 11.42 a | 0.16 | n.d. | 10,201.00 | <0.0001 | ||
| a-Terpinene | 8.31 a | 0.12 | n.d. | n.d. | 10,201.00 | <0.0001 | ||
| Menthol | 6.42 b | 0.19 | 25.79 a | 0.74 | 6.10 b | 0.18 | 1232.21 | <0.0001 |
| cis-a-Bisabolene | 5.31 a | 0.11 | 5.16 a | 0.11 | 5.13 a | 0.11 | 1.42 | 0.37 |
| cis-ß-Farnesene | n.d. | 8.99 a | 0.19 | n.d. | 4312.11 | <0.0001 | ||
| Isocaryophyllene | 10.53 a | 0.30 | 9.64 ab | 0.28 | 8.78 b | 0.25 | 19.63 | 0.02 |
| (E,Z)-2.6-dimethyl-2.4,6-octatriene | 9.08 a | 0.19 | 4.52 b | 0.09 | 4.92 b | 0.10 | 688.92 | 0.00 |
| Citronellol | 59.81 a | 1.29 | 45.62 b | 0.98 | 32.55 c | 0.70 | 358.00 | 0.00 |
| D-Limonene | 5.95 b | 0.13 | 9.52 a | 0.20 | 6.19 b | 0.13 | 312.58 | 0.00 |
| Linalool | 5.49 a | 0.16 | 5.45 a | 0.16 | 5.46 a | 0.16 | 0.04 | 0.96 |
| a-Terpineol | 5.40 a | 0.12 | 5.22 a | 0.11 | 5.34 a | 0.12 | 1.34 | 0.38 |
| Linalyl butyrate | n.d. | 6.28 a | 0.18 | n.d. | 2401.00 | <0.0001 | ||
| Linalyl isobutyrate | 5.32 a | 0.15 | n.d. | n.d. | 2401.00 | <0.0001 | ||
| Geranyl formate | 33.82 a | 0.73 | 15.13 b | 0.33 | 3.85 c | 0.08 | 2136.64 | <0.0001 |
| Total terpenes | 155.45 b | 2.87 | 166.51 a | 2.84 | 78.33 c | 1.63 | 729.52 | <0.0001 |
| Vitispirane A | 7.48 b | 0.16 | 8.25 a | 0.18 | 5.31 c | 0.11 | 196.98 | 0.00 |
| Vitispirane B | 14.68 b | 0.32 | 9.85 c | 0.21 | 25.23 a | 0.54 | 843.29 | <0.0001 |
| 4-Hydroxy-ß-ionone | 5.08 b | 0.14 | 5.28 a | 0.15 | 5.04 b | 0.14 | 1.65 | 0.33 |
| Total C13 norisoprenoids | 27.25 b | 0.34 | 23.38 c | 0.24 | 35.58 a | 0.52 | 530.38 | 0.00 |
| Ethyl hexadecanoate | 37.95 a | 1.10 | 37.97 a | 1.10 | 37.55 a | 1.08 | 0.09 | 0.91 |
| Ethyl hexanoate | 1231.26 b | 35.54 | 1355.90 a | 39.13 | 1036.20 c | 29.91 | 42.24 | 0.01 |
| 3-Methylbutyl octanoate | 19.63 a | 0.57 | 13.65 b | 0.39 | 11.06 c | 0.32 | 200.78 | 0.00 |
| Ethyl octanoate | 392.03 a | 11.31 | 404.22 a | 11.67 | 344.43 b | 9.94 | 16.50 | 0.02 |
| Methyl octanoate | 17.89 b | 0.52 | 21.79 a | 0.63 | 14.10 c | 0.41 | 107.28 | 0.00 |
| Isopentyl decanoate | 9.00 a | 0.26 | 7.49 b | 0.22 | 5.45 c | 0.16 | 137.35 | 0.00 |
| Ehyl pentadecanoate | 6.30 b | 0.18 | 7.43 a | 0.21 | 5.92 b | 0.17 | 34.33 | 0.01 |
| Ethyl nonanoate | 48.66 a | 1.40 | 49.12 a | 1.42 | 48.65 a | 1.40 | 0.07 | 0.93 |
| Butyl ethyl succinate | 51.91 a | 1.12 | 46.77 b | 1.01 | 44.11 b | 0.95 | 29.78 | 0.01 |
| 2-Phenylethyl acetate | 167.75 b | 4.65 | 207.59 a | 5.76 | 93.13 c | 2.58 | 329.68 | 0.00 |
| Phenylethyl acetate | 49.42 b | 0.69 | 57.46 a | 0.80 | 27.82 c | 0.39 | 1103.41 | <0.0001 |
| Diethyl succinate | 948.24 a | 13.28 | 295.61 c | 4.14 | 492.43 b | 6.90 | 2790.58 | <0.0001 |
| 2-Methylpentyl butanoate | 22.18 c | 0.31 | 29.04 a | 0.41 | 25.79 b | 0.36 | 179.83 | 0.00 |
| Butyl 3-hydroxybutanoate | 96.04 b | 1.34 | 150.03 a | 2.10 | 70.24 c | 0.98 | 1383.47 | <0.0001 |
| Ethyl butanoate | 124.91 a | 1.75 | 53.82 c | 0.75 | 91.19 b | 1.28 | 1443.30 | <0.0001 |
| Diethyl malate | 63.87 c | 1.33 | 72.96 b | 1.52 | 79.98 a | 1.67 | 56.73 | 0.00 |
| Ethyl 4-hydroxybutanoate | 58.40 a | 1.26 | 50.58 c | 1.09 | 54.46 b | 1.17 | 22.12 | 0.02 |
| Ethyl 9-decenoate | 135.81 a | 2.92 | 126.11 b | 2.72 | 126.65 b | 2.73 | 7.63 | 0.07 |
| Ethyl 9-hexadecanoate | 5.02 a | 0.11 | 3.28 b | 0.07 | 2.96 c | 0.06 | 355.12 | 0.00 |
| Ethyl hydrogen succinate | 468.59 a | 10.09 | 171.52 b | 3.69 | 164.40 b | 3.54 | 1412.49 | <0.0001 |
| 3-Methylbutyl decanoate | 9.64 a | 0.27 | 7.40 b | 0.21 | 7.36 b | 0.20 | 65.66 | 0.00 |
| Isoamyl acetate | 460.65 a | 6.58 | 211.68 b | 3.02 | 163.66 c | 2.34 | 2633.37 | <0.0001 |
| Isoamyl propionate | n.d. | 24.61 a | 0.35 | n.d. | 9801.00 | <0.0001 | ||
| Total esters | 4581.28 a | 52.80 | 3528.43 b | 53.77 | 3010.30 c | 41.27 | 520.81 | 0.00 |
| 4-Ethyl-phenol | 3151.76 a | 87.40 | n.d. | 126.62 b | 3.51 | 2496.68 | <0.0001 | |
| Eugenol | 48.68 a | 1.05 | 47.62 a | 1.03 | 48.31 a | 1.04 | 0.53 | 0.63 |
| 4-Vinylguaiacol | 25.28 b | 0.70 | 87.23 a | 2.42 | 18.13 c | 0.50 | 1313.64 | <0.0001 |
| 4-Ethylguaiacol | n.d. | n.d. | 25.50 a | 0.55 | 4312.11 | <0.0001 | ||
| Total volatile phenols | 3225.73 a | 87.05 | 134.85 b | 1.39 | 218.56 b | 2.42 | 2452.52 | <0.0001 |
| Methionol | 4.74 a | 0.10 | 1.92 c | 0.04 | 3.51 b | 0.07 | 711.76 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagatić Korenika, A.-M.; Preiner, D.; Tomaz, I.; Skendrović Babojelić, M.; Jeromel, A. Aroma Profile of Monovarietal Pét-Nat Ciders: The Role of Croatian Traditional Apple Varieties. Horticulturae 2022, 8, 689. https://doi.org/10.3390/horticulturae8080689
Jagatić Korenika A-M, Preiner D, Tomaz I, Skendrović Babojelić M, Jeromel A. Aroma Profile of Monovarietal Pét-Nat Ciders: The Role of Croatian Traditional Apple Varieties. Horticulturae. 2022; 8(8):689. https://doi.org/10.3390/horticulturae8080689
Chicago/Turabian StyleJagatić Korenika, Ana-Marija, Darko Preiner, Ivana Tomaz, Martina Skendrović Babojelić, and Ana Jeromel. 2022. "Aroma Profile of Monovarietal Pét-Nat Ciders: The Role of Croatian Traditional Apple Varieties" Horticulturae 8, no. 8: 689. https://doi.org/10.3390/horticulturae8080689
APA StyleJagatić Korenika, A.-M., Preiner, D., Tomaz, I., Skendrović Babojelić, M., & Jeromel, A. (2022). Aroma Profile of Monovarietal Pét-Nat Ciders: The Role of Croatian Traditional Apple Varieties. Horticulturae, 8(8), 689. https://doi.org/10.3390/horticulturae8080689









