Foliar Application of Some Macronutrients and Micronutrients Improves Yield and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Experimental Design
2.2. Measurements and Observations
2.2.1. Measurements and Analyses of Blueberry Leaves
- Chlorophyll a = (12.7 × A663 − 2.7 × A645) × V × (1000 × W)−1;
- Chlorophyll b = (22.9 × A645 − 4.7 × A663) × V × (1000 × W)−1;
- Sum of chlorophyll a + b = (20.2 × A645 + 8.02 × A663) × V × (10,000 × W)−1.
- Carotenoids = (1000 × A470 − 1.9 chlorophyll a − 63.14 chlorophyll b) × 214−1.
2.2.2. Macro- and Microelement Contents in Leaves and Fruits
2.2.3. Yielding and Quality of Fruits
2.3. Statistical Analysis
3. Results and Discussion
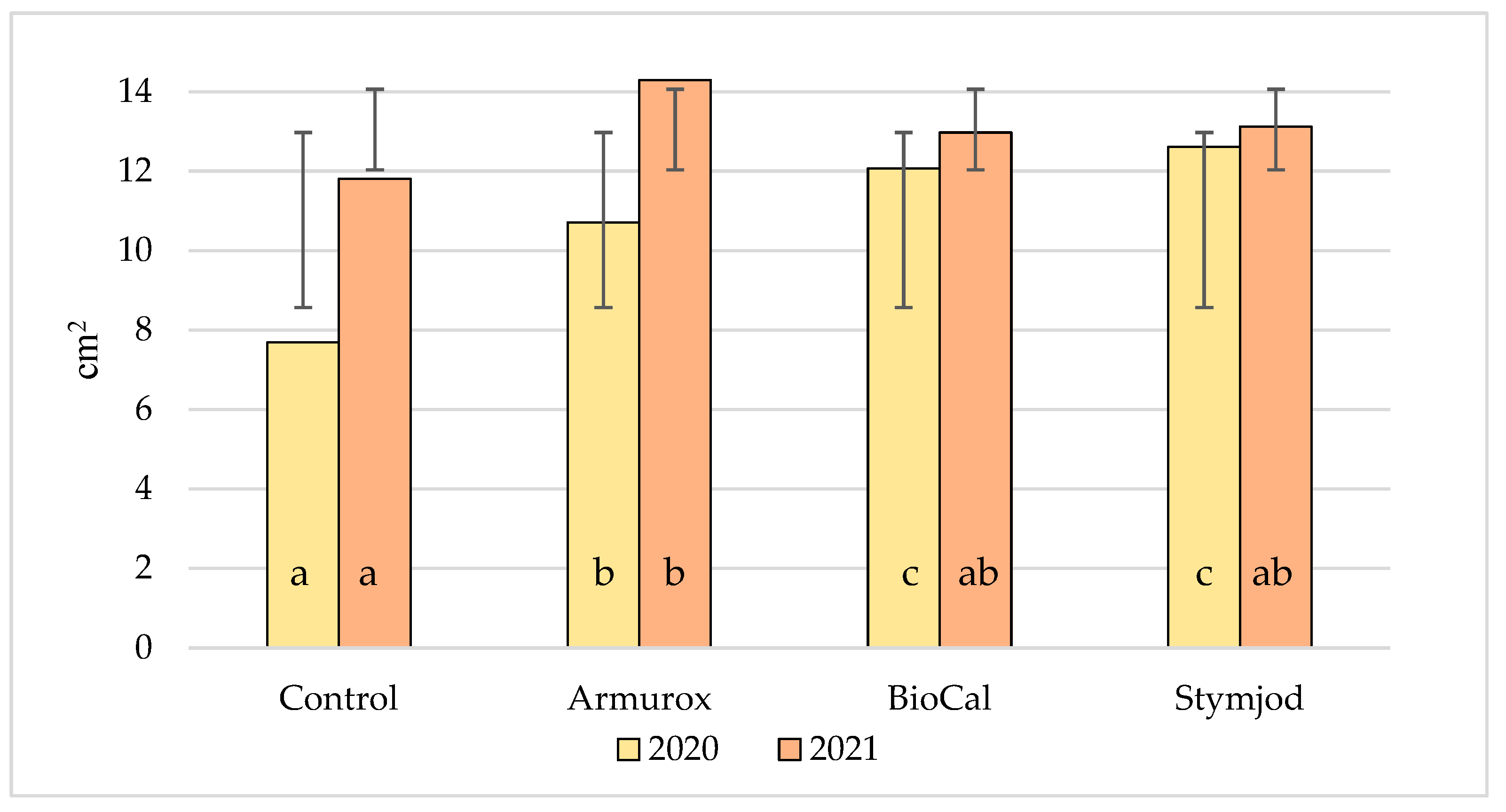
3.1. Parameters of Highbush Blueberry Leaves
3.2. Macroelements and Microelements Content of Leaves and Fruit
3.3. Yielding of Blueberry Bushes
3.4. Biometric and Quality Parameters of Highbush Blueberry Fruit
3.5. Sugar Content and Acidity of Highbush Blueberry Fruits
3.6. The Content of Health-Promoting Compounds in Highbush Blueberry Fruit
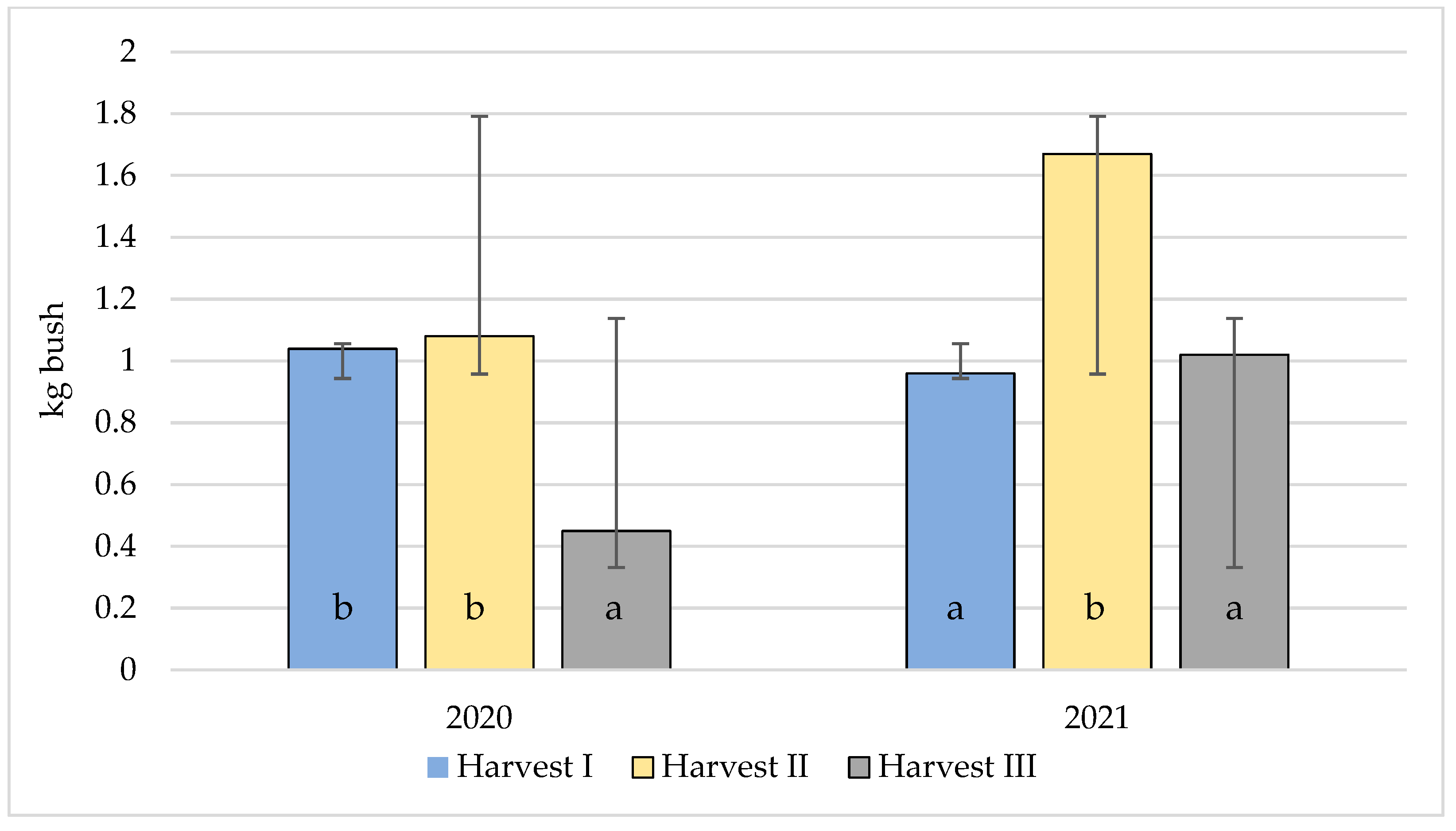
3.7. Impact of Harvesting Dates on the Tested Parameters of Highbush Blueberry
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Zmarlicki, K.; Brzozowski, P. Uwarunkowania w Produkcji Agrestu, Czarnej Porzeczki i Borówki Wysokiej; Instytut Ogrodnictwa: Skierniewice, Poland, 2016; 11p. [Google Scholar]
- Routray, W.; Orsat, V. Blueberries and their anthocyanins: Factors affecting biosynthesis and properties. Compr. Rev. Food Sci. Food Saf. 2011, 10, 303–320. [Google Scholar] [CrossRef]
- Norberto, S.; Silva, S.; Meireles, M.; Faria, A.; Pintado, M.; Calhau, C. Blueberry anthocyanins in health promotion: A metabolic overview. J. Funct. Foods 2013, 5, 1518–1528. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Guo, X.F.; Ruan, Y.; Li, Z.H.; Li, D. Flavonoid subclasses and type 2 diabetes mellitus risk: A meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 2850–2862. [Google Scholar] [CrossRef]
- Johnson, S.A.; Arjmandi, B.H. Evidence for anti-cancer properties of blueberries: A mini-review. Anti-Cancer Agents Med. Chem. 2013, 13, 1142–1148. [Google Scholar] [CrossRef]
- Ramata-Stunda, A.; Valkovska, V.; Borodušķis, M.; Livkiša, D.; Kaktiņa, E.; Silamiķele, B.; Borodušķe, A.; Pentjušs, A.; Rostoks, N. Development of metabolic engineering approaches to regulate the content of total phenolics, antiradical activity and organic acids in callus cultures of the highbush blueberry (Vaccinium corymbosum L.). Agron. Res. 2020, 18, 1860–1872. [Google Scholar] [CrossRef]
- Pormale, J.; Osvalde, A.; Nollendorfs, V. Comparison study of cultivated highbush and wild blueberry nutrient status in producing plantings and woodlands. Latv. J. Agron. 2009, 12, 80–87. [Google Scholar]
- Sas Paszt, L.; Sumorok, B.; Malusa, E.; Głuszek, S.; Derkowska, E. The influence of bioproducts on root growth and mycorrhizal occurrence in the rhizosphere of strawberry plants Elsanta. J. Fruit Ornam. Plant Res. 2011, 19, 13–34. [Google Scholar]
- Koszański, Z.; Rumasz-Rudnicka, E.; Friedrich, S. Anatomy, morphology and yield of highbush blueberry (Vaccinium corymbosum L.) under the influence of irrigation and mineral fertilisation. Acta Agrophys. 2008, 11, 677–684. [Google Scholar]
- Raj, A.B.; Raj, S.K. Zinc and boron nutrition in pulses: A review. J. Appl. Nat. Sci. 2019, 11, 673–679. [Google Scholar] [CrossRef]
- Wójcik, P. Uptake of mineral nutrients from foliar fertilization. J. Ornam. Plant Res. 2004, 12, 201–218. [Google Scholar]
- Jiang, Y.; Li, Y.; Zeng, Q.; Wei, J.; Yu, H. The effect of soil pH on plant growth, leaf chlorophyll fluorescence and mineral element content of two blueberries. Acta Hortic. 2017, 1180, 269–276. [Google Scholar] [CrossRef]
- Kleiber, T. The Role of Silicon in Plant Tolerance to Abiotic Stress. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Institution of Mechanical Engineers. Global Food. Waste Not, Want Not. Available online: http://www.imeche.org/docs/default-source/reports/Global_Food_Report.pdf?sfvrsn=0 (accessed on 15 May 2015).
- Glonek, J.; Komosa, A. Fertigation of highbush blueberry (Vaccinium corymbosum L.). Part I. The effect on growth and yield. Acta Sci. Pol. Hortorum Cultus 2013, 12, 47–57. [Google Scholar]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1978, 5, 1332–1334. [Google Scholar]
- Bozan, B.; Başer, K.H.C.; Kara, S. Quantitative determination of naphthaquinones of Arnebia densiflora (Nordm.) Ledeb. by an improved high-performance liquid chromatographic method. J. Chromatogr. A 1997, 782, 133–136. [Google Scholar] [CrossRef]
- Crisisto, C.H.; Slaughter, D.; Garner, D. Developing maximum maturity indices for “Tree ripe” fruit. Adv. Hort. Sci. 2004, 18, 29–32. [Google Scholar]
- Spanos, G.A.; Wrolstad, R.E.; Heatherbell, D.A. Influence of processing and storage on the phenolic composition of apple juice. J. Agric. Food Chem. 1990, 8, 1572–1579. [Google Scholar] [CrossRef]
- Wrolstad, R.E. Color and pigment analysis in fruit products. In Station Bulletin; University of Agricultural Experimental Station Oregon State: Corvallis, OR, USA, 1976; Volume 624, p. 16. [Google Scholar]
- Dragišic Maksimović, J.; Bogdanovic, J.; Maksimović, V.; Nikolic, M. Silicon modulates the metabolism and utilization of phenolic compounds in cucumber (Cucumis sativus L.) grown atexcess manganese. J. Plant Nutr. Soil Sci. 2007, 170, 739–744. [Google Scholar] [CrossRef]
- Silva Lobato, A.K.; Silva Guedes, E.M.; Marques, D.J.; de Oliveira Neto, C.F. Silicon: A benefic element to improve tolerance in plants exposed to water deficiency. In Responses of Organisms to Water Stress; Akıcnı, S., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Zydlik, Z.; Zydlik, P.; Kleiber, T. The effect of the mycorrhization on the content of macroelements in the soil and leaves of blueberry cultivated after replantation. Zemdirbyste-Agriculture 2019, 106, 345–350. [Google Scholar] [CrossRef]
- Pliszka, K. Borówka wysoka czyli amerykańska. Działkowiec 2001, 3, 34–35. [Google Scholar]
- Burkhard, N.; Lynch, D.; Percival, D.; Sharifi, M. Organic mulch impact on vegetation dynamics and productivity of highbush blueberry under organic production. HortScience 2009, 44, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Carroll, J.; Pritts, M.P.; Heidenreich, C. Production Guide for Organic Blueberries; N. Y. State Integrated Pest Manage Program: Ithaca, NY, USA, 2015. [Google Scholar]
- Ochmian, I.; Kozos, K. Influence of foliar fertilisation with calcium fertilisers on the firmness and chemical composition of two highbush blueberry cultivars. J. Elem. 2015, 20, 185–201. [Google Scholar] [CrossRef]
- Ochmian, I.; Oszmiański, J.; Jaśkiewicz, B.; Szczepanek, M. Fertilization of highbush blueberry with urea phosphate. Folia Hortic. 2018, 30, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Aliman, J.; Michalak, I.; Bušatlić, E.; Aliman, L.; Kulina, M.; Radovic, M.; Hasanbegovic, J. Study of the physicochemical properties of highbush blueberry and wild bilberry fruit in central Bosnia. Turk. J. Agric. For. 2020, 44, 156–168. [Google Scholar] [CrossRef]
- Correia, S.; Gonçalves, B.; Aires, A.; Silva, A.; Ferreira, L.; Carvalho, R.; Fernandes, H.; Freitas, C.; Carnide, V.; Silva, A.P. Effect of harvest year and altitude on nutritional and biometric characteristics of blueberry cultivars. J. Chem. 2016, 2016, 8648609. [Google Scholar] [CrossRef]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D. Changes in berry quality of northern highbush blueberry (Vaccinium corymbosum L.) during the harvest season. Turk. J. Agric. For. 2016, 40, 855–864. [Google Scholar] [CrossRef]
- Lanauskas, J.; Kviklienė, N.; Uselis, N.; Kviklys, D.; Buskienė, L.; Mažeika, R.; Staugaitis, G. The effect of calcium foliar fertilizers on cv. Ligol apples. Plant Soil Environ. 2012, 58, 466. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Rojas, M.E.; Mesa-Torres, P.A.; Grijalba-Rativa, C.M.; Pérez-Trujillo, M.M. Yield and fruit quality of the blueberry cultivars Biloxi and Sharpblue in Guasca, Colombia. Agron. Colomb. 2016, 34, 33–41. [Google Scholar] [CrossRef]
- Shevchuk, L.M.; Grynyk, I.V.; Levchuk, L.M.; Yareshcenko, O.M.; Tereshcenko, Y.Y.; Babenko, S.M. Biochemical contents of highbush blueberry fruits grown in the Western Forest-Steppe of Ukraine. Agron. Res. 2021, 19, 232–249. [Google Scholar] [CrossRef]
- Zenkova, М.; Pinchykova, J. Chemical composition of Sea-buckthorn and Highbush Blueberry fruits grown in the Republic of Belarus. Food Sci. Appl. Biotechnol. 2019, 2, 81–90. [Google Scholar] [CrossRef]
- Celik, F.; Bozhuyuk, M.R.; Ercisli, S.; Gundogdu, M. Physicochemical and Bioactive Characteristics of Wild Grown Bilberry (Vaccinium myrtillus L.) Genotypes from Northeastern Turkey. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Ersoy, N.; Kupe, M.; Gundogdu, M.; Gulce, I.; Ercisli, S. Phytochemical and antioxidant diversity in fruits of currant (Ribes spp.) cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Ayour, J.; Sagar, M.; Alfeddy, M.; Taourirte, M.; Benichou, M. Evolution of pigments and their relationship with skin color based on ripening in fruits of different Moroccan genotypes of apricots (Prunus armeniaca L.). Sci. Hort. 2016, 207, 168–175. [Google Scholar] [CrossRef]
- Echeverría, G.; Cantín, C.M.; Ortiz, A.; López, M.L.; Lara, I.; Graell, J. The impact of maturity, storage temperature and storage duration on sensory quality and consumer satisfaction of ‘Big Top®’ nectarines. Sci. Hort. 2015, 190, 179–186. [Google Scholar] [CrossRef]
- Milukic-Petkovsek, M.; Ivancic, A.; Schimitzer, V.; Veberic, R.; Stampar, F. Comparison of major taste compounds and antioxidative properties of fruits and flowers of different Sambucus species and interspecific hybrids. Food Chem. 2016, 200, 134–140. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Farcuh, M.; Cohen, Y.; Crisosto, C.; Sadka, A.; Blumwald, E. Non-climacteric ripening and sorbitol homeosta-556 sis in plum fruits. Plant Sci. 2015, 231, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Sun, J.; Jackson, A. Dynamic changes of enzymes involved in sugar and organic acid level modification during blueberry fruit maturation. Food Chem. 2020, 309, 125617. [Google Scholar] [CrossRef]
- Okan, O.T.; Deniz, I.; Yayli, N.; Şat, G.I.; Öz, M.; Serdar, G.H. Antioxidant activity, sugar content and phenolic profiling of blueberry cultivars: A comprehensive comparison. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 639–652. [Google Scholar] [CrossRef] [Green Version]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar Signaling During Fruit Ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Tosti, T.; Nedić, N.; Marković, M.; Ličina, V.; Milojković-Opsenica, D.; Tešić, Ž. Influence of frost damage on the sugars and sugar alcohol composition in quince (Cydonia oblonga Mill.) Floral nectar. Acta Physiol. Plant. 2015, 37, 1701. [Google Scholar] [CrossRef]
- Kirina, I.B.; Belosokhov, F.G.; Titova, L.V.; Suraykina, I.A.; Pulpito, V.F. Biochemical assessment of berry crops as a source of production of functional food products. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 548, p. 082068. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Cao, G.A.; Martin, A. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Rupasova, Z.; Pavlovskij, N.; Kurlovich, T.; Pyatnitsa, F.; Yakovlev, A.; Volotovich, A.; Pinchukova, Y. Variability of structure of biochemical composition of fruits of the highbush blueberry. Agron. Vestis Latv. J. Agron. 2009, 12, 103–107. [Google Scholar]
- Giacalone, M.; Di Sacco, F.; Traupe, I.; Pagnucci, N.; Forfori, F.; Giunta, F. Blueberry polyphenols and neuroprotection. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease, Prevention and Therapy, 1st ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 17–28. [Google Scholar] [CrossRef]
- Sadowska, A.; Dybkowska, E.; Rakowska, R.; Hallmann, E.; Świderski, F. Ocena zawartości składników bioaktywnych i właściwości przeciwutleniających proszków wyprodukowanych metodą liofilizacji z wybranych surowców roślinnych. Żywność Nauka Technol. Jakość 2017, 24, 59–75. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A.; Człapka-Matyasik, M. Evaluation of the antiradical potential of fruit and vegetable snacks. Acta Sci. Pol. Technol. Aliment. 2011, 10, 61–72. [Google Scholar]
- Gao, L.; Mazza, G. Quantitation and distribution of simple and acylated anthocyanins and other phenolics in blueberries. J. Food Sci. 1994, 59, 1057–1059. [Google Scholar] [CrossRef]
- Starast, M.; Karp, K.; Vool, E.; Moor, U.; Tonutare, T.; Paal, T. Chemical composition and quality of cultivated and natural blueberry fruit in Estonia. Veg. Crop. Res. Bull. 2007, 66, 143–153. [Google Scholar] [CrossRef]
- Sams, C.E. Preharvest factors affecting postharvest texture. Postharvest Biol. Technol. 1999, 15, 249–254. [Google Scholar] [CrossRef]
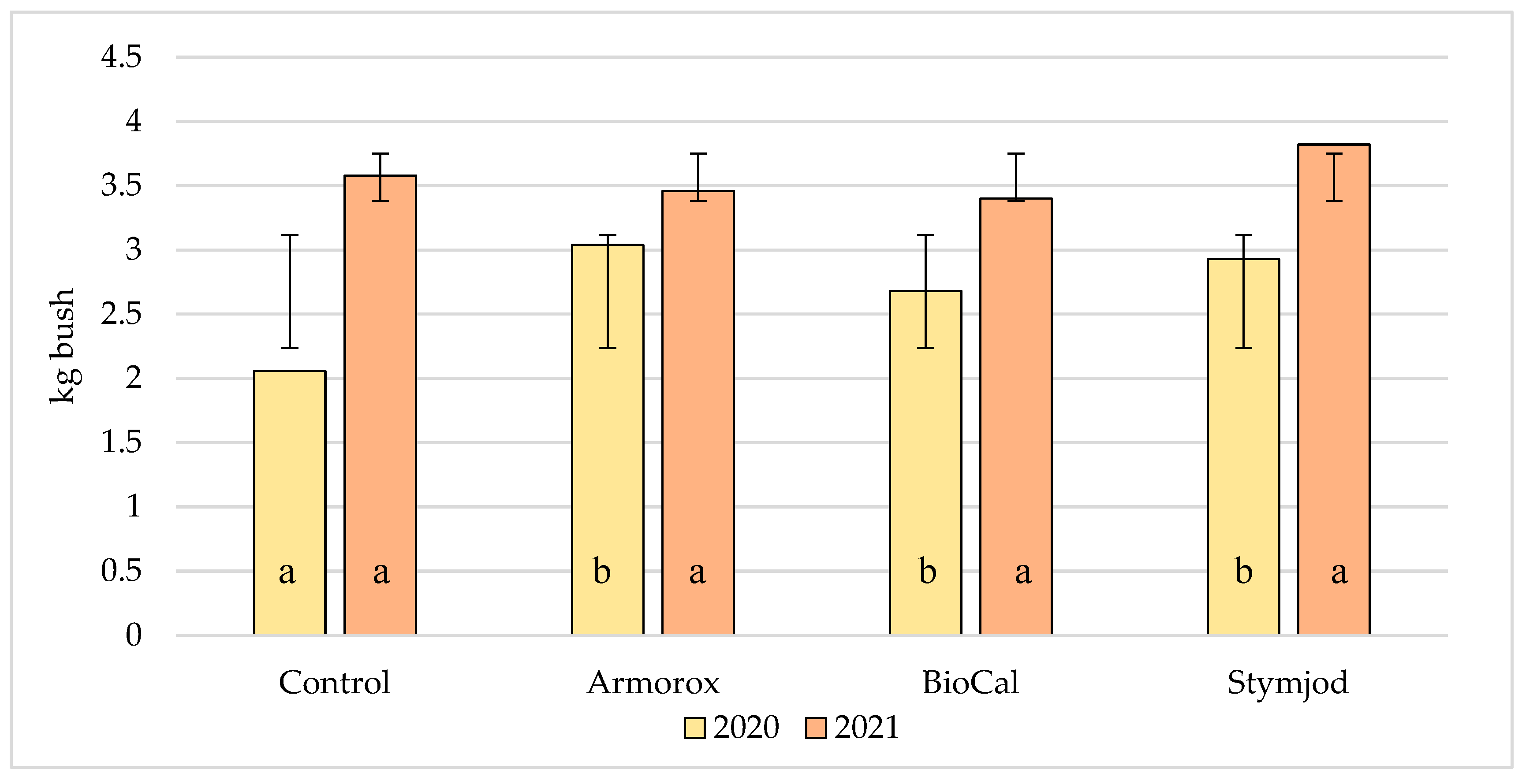
| Years | Months | Sum | ||||||
|---|---|---|---|---|---|---|---|---|
| IV | V | VI | VII | VIII | IX | X | ||
| Precipitation (mm) | ||||||||
| 2020 | 42.6 | 49.4 | 48.6 | 78.2 | 57.4 | 41.6 | 53.2 | 279.8 |
| 2021 | 29.2 | 36.8 | 78.6 | 96.7 | 43.8 | 60.7 | 32.4 | 378.2 |
| 1982–2012 1 | 28.0 | 48.0 | 63.5 | 78.8 | 61.9 | 41.0 | 32.0 | 353.2 |
| Air temperature (°C) | ||||||||
| 2020 | 8.9 | 11.6 | 18.2 | 18.5 | 20.3 | 15.1 | 10.5 | 103.1 |
| 2021 | 6.2 | 12.3 | 20.1 | 19.0 | 17.5 | 16.4 | 9.1 | 100.6 |
| 1982–2012 1 | 9.3 | 14.6 | 17.2 | 19.5 | 18.9 | 14.1 | 9.0 | 102.6 |
| Treatments | Chlorophyll a | Chlorophyll b | Chlorophyll a + b | Carotenoids |
|---|---|---|---|---|
| Control | 54.56 ± 0.10a 1 | 101.46 ± 0.54a | 156.13 ± 0.62ab | 750.30 ± 3.16a |
| Armurox | 54.60 ± 0.08a | 100.50 ± 1.03a | 155.20 ± 1.09a | 757.43 ± 6.61ab |
| BioCal | 54.85 ± 0.32ab | 101.65 ± 0.76ab | 156.61 ± 1.08bc | 790.34 ± 4.70b |
| Stymjod | 54.95 ± 0.12b | 102.76 ± 0.51b | 157.82 ± 0.14c | 997.42 ± 8.97c |
| Treatments | Macroelements (% d.m.) | Microelements (mg kg−1 d.m.) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ntot | P2O5 | K2O | CaO | MgO | Zn | Cu | Mn | B | |
| Control | 1.56 ± 0.011b | 0.26 ± 0.010a | 0.53 ± 0.012a | 1.04 ± 0.07c | 0.44 ± 0.017a | 7.75 ± 0.10a | 2.96 ± 0.36a | 213 ± 12.4d | 33.60 ± 2.6c |
| Armurox | 1.53 ± 0.019a | 0.26 ± 0.017a | 0.53 ± 0.009a | 0.92 ± 0.04a | 0.41 ± 0.021a | 8.86 ± 0.35a | 3.68 ± 0.54c | 173 ± 18.7a | 31.05 ± 1.9b |
| BioCal | 1.67 ± 0.013c | 0.25 ± 0.021a | 0.52 ± 0.017a | 1.12 ± 0.11d | 0.46 ± 0.011a | 32.0 ± 0.98c | 3.24 ± 0.31b | 204 ± 25.4c | 30.05 ± 3.8a |
| Stymjod | 1.70 ± 0.021d | 0.26 ± 0.019a | 0.53 ± 0.011a | 0.96 ± 0.09b | 0.49 ± 0.018a | 17.0 ± 0.67b | 7.67 ± 0.72d | 186 ± 17.6b | 49.30 ± 2.9d |
| Treatments | Macroelements (% d.m.) | Microelements (mg kg−1 d.m.) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N-NO3 | P2O5 | K2O | CaO | MgO | Zn | Cu | Mn | B | |
| Control | 0.74 ± 0.020c | 0.26 ± 0.030b | 0.80 ± 0.12c | 0.15 ± 0.07a | 0.08 ± 0.021a | 6.13 ± 0.45b | 2.94 ± 0.57b | 30.4 ± 2.45d | 5.28 ± 0.74c |
| Armurox | 0.64 ± 0.017b | 0.23 ± 0.027a | 0.69 ± 0.09a | 0.15 ± 0.03a | 0.08 ± 0.016a | 5.40 ± 0.26a | 2.48 ± 0.23a | 26.8 ± 1.98b | 4.18 ± 0.95a |
| BioCal | 0.76 ± 0.061d | 0.26 ± 0.019b | 0.74 ± 0.15b | 0.15 ± 0.02a | 0.08 ± 0.009a | 24.7 ± 1.34d | 2.97 ± 0.19c | 21.3 ± 0.86a | 4.43 ± 0.56b |
| Stymjod | 0.60 ± 0.019a | 0.26 ± 0.036b | 0.74 ± 0.17b | 0.15 ± 0.03a | 0.08 ± 0.010a | 6.52 ± 0.29c | 4.04 ± 0.36d | 28.9 ± 1.73c | 8.80 ± 1.03d |
| Treatments | Weight (g) | Height (mm) | Width (mm) | |||
|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | |
| Control | 1.39 ± 0.46a 1 | 1.63 ± 0.43a | 9.91 ± 1.12a | 9.98 ± 0.97a | 13.30 ± 1.44a | 14.29 ± 1.34a |
| Armurox | 1.92 ± 0.35b | 2.14 ± 0.37b | 10.85 ± 1.13c | 10.81 ± 0.80b | 15.46 ± 0.97d | 15.73 ± 1.09b |
| BioCal | 1.89 ± 0.45b | 2.19 ± 0.43b | 10.80 ± 0.85c | 11.17 ± 0.75c | 15.19 ± 1.38c | 15.92 ± 1.28b |
| Stymjod | 1.91 ± 0.39b | 2.14 ± 0.29b | 10.58 ± 0.86b | 11.32 ± 0.62c | 14.94 ± 1.23b | 15.75 ± 0.94b |
| Treatments | 2020 | 2021 | ||||
|---|---|---|---|---|---|---|
| Firmness | TSS | Firmness | TSS | |||
| H 1 | S 2 | H 1 | S 2 | |||
| Control | 176.00 ± 30.59a 3 | 14.29 ± 2.75a | 196.95 ± 21.29a | 176.6 ± 25.85a | 11.85 ± 1.51a | 12.75 ± 1.29a |
| Armurox | 232.53 ± 41.62b | 13.20 ± 1.62a | 264.20 ± 34.81b | 242.3 ± 26.35b | 14.43 ± 1.44c | 14.46 ± 1.43b |
| BioCal | 242.40 ± 34.66c | 13.88 ± 1.35a | 285.20 ± 41.92c | 241.4 ± 20.13b | 13.96 ± 1.73c | 13.90 ± 1.47a |
| Stymjod | 245.07 ± 36.42c | 14.19 ± 1.71a | 293.55 ± 38.02d | 249.10 ± 22.21c | 14.54 ± 1.94c | 16.46 ± 1.54b |
| Treatments | After Harvest | After Storage | ||||
|---|---|---|---|---|---|---|
| L | a | b | L | a | b | |
| Control | 28.08 ± 3.13a 1 | 0.86 ± 1.58b | 5.15 ± 0.44a | 27.76 ± 3.27c | 1.29 ± 0.25b | 4.79 ± 0.60a |
| Armurox | 27.38 ± 2.73a | 0.27 ± 1.36a | 4.93 ± 0.61a | 27.43 ± 3.56bc | 0.87 ± 0.17a | 4.16 ± 0.21a |
| BioCal | 28.08 ± 3.22a | 1.03 ± 1.66b | 5.38 ± 0.53a | 26.38 ± 3.11a | 1.14 ± 0.13ab | 4.68 ± 0.25a |
| Stymjod | 26.65 ± 2.69a | 0.12 ± 1.42a | 5.42 ± 0.61a | 26.65 ± 3.15ab | 0.99 ± 0.12ab | 4.54 ± 0.34a |
| Treatments | Fructose | Glucose | Sucrose | Total Sugar |
|---|---|---|---|---|
| Control | 6.03 ± 1.21b 1 | 9.48 ± 1.17b | 0.19 ± 0.02a | 15.22 ± 1.38b |
| Armurox | 4.89 ± 0.11a | 7.92 ± 0.70a | 0.18 ± 0.02a | 12.96 ± 1.03a |
| BioCal | 5.50 ± 0.61ab | 8.83 ± 0.13ab | 0.18 ± 0.07a | 14.51 ± 0.76b |
| Stymjod | 5.39 ± 0.79ab | 8.95 ± 0.86ab | 0.19 ± 0.42a | 14.53 ± 0.98b |
| Treatments | Ascorbic Acid | Citric Acid | Malic Acid |
|---|---|---|---|
| Control | 44.32 ± 6.16ab 1 | 9.12 ± 1.73a | 1.42 ± 0.39b |
| Armurox | 60.12 ± 3.36b | 8.75 ± 2.42a | 1.08 ± 0.16ab |
| BioCal | 36.58 ± 2.99a | 8.38 ± 2.46a | 1.06 ± 0.31ab |
| Stymjod | 51.99 ± 3.34b | 9.65 ± 2.37a | 1.00 ± 0.52a |
| Treatments | Total Phenolic Compounds (mg GA 100 g−1 d.m.) | Anthocyanins (mg 100 g−1 d.m.) |
|---|---|---|
| Control | 1716.25 ± 103.69a 1 | 38.76 ± 3.94b |
| Armurox | 1779.63 ± 139.09a | 32.46 ± 1.67a |
| BioCal | 1606.18 ± 220.86a | 32.32 ± 1.45a |
| Stymjod | 1631.84 ± 190.88a | 32.93 ± 1.94a |
| Harvest Dates | Weight (g) | Height (mm) | Width (mm) | |||
|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | |
| End of July (I) | 2.11 ± 0.37c 1 | 2.09 ± 0.31c | 11.27 ± 0.62b | 11.49 ± 0.65c | 15.57 ± 1.03c | 15.73 ± 1.05c |
| Beginning of August (II) | 1.69 ± 0.30b | 1.74 ± 0.29a | 10.20 ± 0.47a | 10.47 ± 0.67b | 14.50 ± 1.02b | 16.29 ± 0.98a |
| Mid-August (III) | 1,54 ± 0.29a | 1.88 ± 0.30b | 1013 ± 0.82a | 10.09 ± 0.68a | 14.16 ± 1.08a | 15.51 ± 15.51a |
| Harvest Dates | Firmness (g mm−1) | TSS (%) | ||
|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | |
| End of July (I) | 223.26 ± 37.67b 1 | 286.30 ± 25.50b | 12.81 ± 1.07a | 12.65 ± 0.96a |
| Beginning of August (II) | 211.80 ± 31.51a | 281.45 ± 40.61c | 14.73 ± 1.44b | 13.77 ± 1.36c |
| Mid-August (III) | 227.55 ± 25.02b | 225.50 ± 27.05a | 14.15 ± 1.37ab | 13.17 ± 1.44b |
| Studied Characteristic | Harvest I | Harvest II | Harvest III |
|---|---|---|---|
| Sugar content (mg 100 g −1) | |||
| Fructose | 5.97 ± 1.17b 1 | 5.64 ± 0.32b | 4.74 ± 0.58a |
| Glucose | 9.29 ± 1.16b | 8.57 ± 0.33a | 8.53 ± 0.74a |
| Sucrose | 0.19 ± 0.03ab | 0.17 ± 0.03a | 0.20 ± 0.06b |
| Total sugar | 15.08 ± 1.41c | 14.38 ± 1.01b | 13.45 ± 1.33a |
| Organic acids (mg 100 g−1) | |||
| Ascorbic acid | 28.86 ± 7.51a | 40.29 ± 26.92b | 68.12 ± 27.57c |
| Citric acid | 8.26 ± 1.33b | 6.12 ± 1.69a | 12.47 ± 2.06c |
| Malic acid | 1.01 ± 0.42a | 0.94 ± 0.29a | 1.47 ± 0.29b |
| Health-promoting compounds | |||
| Phenolic compounds | 1751.81 ± 92.77b | 1535.89 ± 199.21a | 1762.71 ± 134.34c |
| Anthocyanins | 34.06 ± 1.28b | 30.98 ± 3.99a | 37.32 ± 3.22c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zydlik, Z.; Zydlik, P.; Kafkas, N.E.; Yesil, B.; Cieśliński, S. Foliar Application of Some Macronutrients and Micronutrients Improves Yield and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.). Horticulturae 2022, 8, 664. https://doi.org/10.3390/horticulturae8070664
Zydlik Z, Zydlik P, Kafkas NE, Yesil B, Cieśliński S. Foliar Application of Some Macronutrients and Micronutrients Improves Yield and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.). Horticulturae. 2022; 8(7):664. https://doi.org/10.3390/horticulturae8070664
Chicago/Turabian StyleZydlik, Zofia, Piotr Zydlik, Nesibe Ebru Kafkas, Betul Yesil, and Szymon Cieśliński. 2022. "Foliar Application of Some Macronutrients and Micronutrients Improves Yield and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.)" Horticulturae 8, no. 7: 664. https://doi.org/10.3390/horticulturae8070664
APA StyleZydlik, Z., Zydlik, P., Kafkas, N. E., Yesil, B., & Cieśliński, S. (2022). Foliar Application of Some Macronutrients and Micronutrients Improves Yield and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.). Horticulturae, 8(7), 664. https://doi.org/10.3390/horticulturae8070664






