Abstract
Cross-pollination can improve the fruit set and quality of blueberry (Vaccinium spp.) for growers and consumers. However, the xenia effect in southern highbush blueberry remains unclear. Therefore, we selected eight cultivars of southern highbush blueberry (Vaccinium corymbosum L., interspecific hybrids) and applied pollination treatments (i.e., artificial self-pollination, artificial pollination with mixed pollen, or artificial pollination with individual cultivar pollen) to explore the xenia effects on the fruit set and quality of ‘O’Neal’ and ‘Emerald’. Pollen viability tests indicated that all of the cultivars are capable of fertilization. The highest fruit set came from ‘Bluerain’ pollen for ‘O’Neal’, while ‘Gulfcoast’ pollen increased fruit set the most in ‘Emerald’. Principal component analysis revealed that the cross combinations ‘Emerald’ × ‘Gulfcoast’ and ‘O’Neal’ × ‘Gulfcoast’ optimized the external and interior quality of the fruit. SSR was applied to determine which pollen source yielded the most seedlings. Results indicated that ‘Emerald’ × ‘Gulfcoast’ and ‘O’Neal’ × ‘Bluerain’ increased seedling production. Our results demonstrate that the xenia effects of ‘Gulfcoast’ pollen may increase ‘Emerald’ yields and promote fruit quality, while pollen from ‘Bluerain’ or ‘Jewel’ can improve ‘O’Neal’ fruit quality and seed number. Hence, these cross combinations may be utilized in blueberry production to increase fruit set, yield, and quality.
1. Introduction
The xenia effect is the influence of pollen on the resultant seed and fruit characteristics after fertilization. Xenia effects have been shown to improve the quality of apple (Malus sieversii Roem.), Korla fragrant pear (Pyrus sinkiangensis Yu), mango (Mangifera indica L.), litchi (Litchi chinensis Sonn.) [1,2,3,4], and macademia (Macadamia integrifolia) [5]. Changes in fruit ripening time and set, as well as quality characteristics including weight, size, color, shape, and flavor are also influenced by xenia [6]. Xenia also affects the size and color of seeds [7]. Certain peony (Paeonia ostii T. Hong and J. X. Zhang) crosses show increases in fruit set, seed volume, and seed weight due to xenia [8]. The pollen genotype changes the embryo and endosperm during fertilization, thus affecting seed germination through the double-fertilized tissue (embryo or endosperm), which has been termed “double-fertilization xenia” [9], the first type of xenia.
Blueberry (Vaccinium spp.) is a popular fruit with potential health benefits [10] and greater nutrient levels may be achieved by cross-pollination [11]. However, the topic of xenia in blueberries has received little research. To date, researchers have investigated xenia effects on blueberry fruit ripening time, fruit size, single-fruit weight, and external appearance [12,13,14]. Only in rabbiteye blueberry (‘Premier’) has xenia been found to affect interior quality attributes such as soluble solid content and anthocyanin content (ACY), with xenia effects manifesting at different stages after fertilization [15]. This specific type of xenia is classified as “non-double-fertilization xenia” [9], the second type of xenia. A third type of xenia is “combined xenia”, which refers to the phenomenon whereby the pollen transfers genetic information through the double-fertilized tissue and changes the resultant fruit quality attributes [9]. The information surrounding xenia effects on southern highbush blueberry (SHB) is lacking and could be utilized to inform planting designs for optimal pollination and fruit quality.
Important fruit quality attributes for blueberry are generally reflected in soluble solids’ content, fruit weight, and antioxidant capacity, and are generally represented through anthocyanin values [16]. Although the fruit quality of highbush blueberries can be changed through the adoption of organic practices [16], increased light quality [17], treatment with plant growth regulators, and exposure to ethylene postharvest [18,19], the pollinizer (i.e., pollen source) can potentially change fruits’ antioxidant content. In northern highbush blueberry (Vaccinium corymbosum L.), fruit size has been shown to be highly correlated with ACY [20]. Analysis of fruit from the northern highbush blueberry ‘Draper’ × southern highbush ‘Jewel’ showed that fruit color was positively correlated with ACY, while firmness was negatively correlated with ACY [21]. However, not all blueberry cross combinations produce better fruit. ‘O’Neal’ pollen may not improve fruit quality, even though it increases the number of mature seeds [22]. Interplanting with other cultivars sharing a similar bloom time promotes cross-pollination and has been found to increase yields among 13 SHB cultivars [14]. More cross combinations need to be designed in order to produce blueberries with an improved fruit quality.
Planting single cultivars in the same location or area facilitates self-pollination. Interplanting multiple cultivars improves the chances of cross-pollination but can make it difficult to determine the pollen donor for research studies. DNA markers, such as SSR (simple sequence repeat), can be used to identify pollen parents from progeny with desirable traits and have been used in blueberry genetic diversity assessments after intraspecific hybridization and open-pollination breeding [23,24,25]. Furthermore, EST–SSR, G–SSR, and EST–PCR markers have been used to test the correlation between genotype and antioxidant properties in blueberries [25].
The objective of this study was to categorize and characterize the xenia effects in two SHB cultivars (O’Neal and Emerald). Information from this study may inform which pollen source positively or negatively influences important production and quality characteristics for producers. A secondary objective was to screen the seedlings derived from mixed-pollen fertilization using SSR in order to determine which pollen is the optimal pollen donor.
2. Materials and Methods
Experiments outlined in this paper were conducted (2016, 2017, 2018) in the blueberry germplasm resource nursery of Zhejiang Normal University, Jinhua, Zhejiang Province, China.
2.1. Plant Materials
Eight representative SHB cultivars—O’Neal, Emerald, Sharpblue, Gulfcoast, Misty, Bluerain, Star, and Jewel—were selected for the study. We produced 18 combinations of self- and cross-pollination among the eight cultivars. Three-year-old plants were planted in soil and managed using standard cultivation practices [26].
2.2. Morphological Observation of Floral Organs
Photographs of longitudinal sections of the mature floral organs of each cultivar (preserving the style and part of the anther) were captured using a camera (Sony, DSC-HX400). Morphological characteristics were measured, including the flower bud length, corolla diameter, pistil length, and stamen length.
2.3. Pollen Viability and Stigma Receptivity Tests
Pollen collection started at the onset of bloom with 100 flower buds collected from each of the eight pollinizer cultivars. The flowers were bagged at the “Late pink bud” stage (https://www.canr.msu.edu/blueberries/growing_blueberries/growth-stages (accessed on 1 January 2022)) to avoid pollen contamination by nearby cultivars. When the flowers were at “Early bloom” stage, anthers were carefully removed from flowers in the lab, placed on paper, and dried at a constant temperature of 26 °C for 24 h. The next day, pollen was separated from the anthers with a 0.061 mm mesh screen and stored at 4 °C for 1 month. Following similar procedures, 50 flowers from each of the eight pollinizer cultivars were collected to make mixed pollen (abbreviated as “8 Mixed”).
Pollen viability was determined using the FDA (fluorescein diacetate; Coolaber, Beijing, China))–PI (propidium iodide; Coolaber, Beijing, China) test. Viable pollen grains emit green fluorescence, while nonviable pollen grains emit red fluorescence [27]. One drop of FDA–PI solution was placed on a slide and approximately 100–300 pollen grains were spread onto the slide. After 5 min, slides were observed with short blue light using a fluorescence microscope (10×) (Zeiss Axio Scope A1, Carl Zeiss Canada Ltd. Toronto, Canada and three randomly chosen areas (three replicates) on each slide were used to count pollen. Pollen grains that emitted yellow–green fluorescence were considered viable, while those that showed red fluorescence were nonviable. After pictures were taken, Image J software was used to calculate pollen viability. Images (JPG) were converted to RGB (8-bit) and the “Color Threshold” parameter was set for the RGB image. By adjusting the “Threshold” parameter, pollen grains could be determined as being round or oval. If there was a partial overlap of pollen, the “Watershed” function was used. Finally, the “Analyze Particles” function was used to count the pollen grains.
Stigma receptivity was checked using a benzidine–hydrogen peroxide [28] method (1% benzidine:3% hydrogen peroxide: water = 4:11:22). Blueberry styles were collected on the day of flowering, as well as at 1, 2 and 3 d after flowering, and placed in a benzidine–hydrogen peroxide solution. If two-thirds of the parts of a style were dark blue and accompanied by a large number of bubbles under the microscope (hydrogen peroxidase activity), the result indicated that the stigma was receptive.
2.4. Experimental Design and Pollination Treatments
We selected 20 O’Neal and 20 Emerald blueberry bushes as the maternal cultivars. The cultivars listed above (O’Neal, Emerald, Sharpblue, Gulfcoast, Misty, Bluerain, Star, and Jewel) were used as pollinizers. Three pollination treatments were applied—artificial self-pollination, mixed-pollen pollination or single cultivar pollination group. A total of 90 ‘Emerald’ or ‘O’Neal’ branches with inflorescences were selected based on uniformity, and 5–8 flower buds (“Late pink bud” to “Early bloom”, https://www.canr.msu.edu/blueberries/growing_blueberries/growth-stages (accessed on 1 June 2022)) from every branch were maintained. For each combination, 50 flowers were pollinated, and three replicates were conducted on other branches. The artificial self-pollination group was used as the control. This experiment was carried out over 3 years, with the same cross combination, the same operation methods and the same operators in the same field.
We selected 20 O’Neal and 20 Emerald blueberry bushes as the maternal cultivars. The cultivars listed above (O’Neal, Emerald, Sharpblue, Gulfcoast, Misty, Bluerain, Star, and Jewel) were used as pollinizers. Three pollination treatments were applied—artificial self-pollination, mixed-pollen pollination or single cultivar pollination group. A total of 90 ‘Emerald’ or ‘O’Neal’ branches with inflorescences were selected based on uniformity, and 5–8 flower buds (“Late pink bud” to “Early bloom”, https://www.canr.msu.edu/blueberries/growing_blueberries/growth-stages (accessed on 1 June 2022)) from every branch were maintained. For each combination, 50 flowers were pollinated, and three replicates were conducted on other branches. The artificial self-pollination group was used as the control. This experiment was carried out over 3 years, with the same cross combination, the same operation methods and the same operators in the same field.
Maternal flowers were emasculated during the balloon stage of bloom and bagged. Hand pollination treatments were applied by lightly touching the stigmatic surface several times with the head of a pencil bearing the specific pollen grains. After pollination, the stigmas were immediately covered with cheesecloth bags in order to avoid cross-pollination, and fruit set was calculated when the fruits were ripened. Fruit set was determined as the number of fruits divided by the number of pollinated flowers:
2.5. Evaluation of the Fruit Quality of ‘O’Neal’ and ‘Emerald’
Mature fruits were collected at harvest and 30 fruits from each pollination combination were randomly selected for further quality assessment. Mature blueberries were used to determine transverse and longitudinal diameters (a measure of berry size), weight, firmness, titratable acidity, anthocyanin content, soluble sugar content and soluble solids’ content. The transverse and longitudinal diameters of the fruits were directly measured with a Vernier caliper and averaged. Fruit Shape Index is the ratio of the longitudinal diameter to the transverse diameter. The fresh weight of the fruit was determined by an electronic analytical balance. Fruit firmness was measured using a handheld firmness tester (GY-2, HANDPI Co. Ltd., Yueqing, China). The titratable acidity content (TA) (citric acid) was evaluated using the acid–base titration method [29], titrated with 0.1 N NaOH to pH 8.2; the anthocyanin content was determined by retentate analysis with the pH value difference method [30]. A digital refractometer obtained the soluble sugar content through anthrone colorimetry [31]. The soluble solids’ content (SS) was determined using a digital handheld refractometer (ATAGO, PAL-1). The SS/TA was calculated as an indicator of overall sweetness.
2.6. Seed Number Determination
Seeds were extracted from mature fruits and washed to remove the pulp. Seed maturity was judged based on the color of the seed coat and the degree of fullness of the seeds, with a large and brown seed indicating maturity and others being immature [32]. Mature and total seed numbers in different treatments were recorded. Seed number refers to the average number of seeds per fruit.
2.7. DNA Extraction
Seeds were obtained from ‘Emerald’ and ‘O’Neal’ as the maternal cultivars pollinated by “8 Mixed”. Seeds were soaked in pure gibberellin (50 mg/L) for 12 h, followed by planting in a substrate with a mixture of Chilean moss and sterilized nutrient soil (1:2, v/v). When the plant leaves reached the size of a thumbnail, they were gently cut with scissors for the extraction of DNA using modified CTAB methods [33]. DNA concentrations were detected using a NanoDrop 2000. Electrophoresis was carried out on 1% agarose gels to check the quality and quantity of DNA. DNA content was 20 ng/μL, and it was stored at −20 °C when the value of OD260/OD280 was detected in the range of 1.8–2.0.
2.8. SSR
Twenty pairs of primers were screened using the SSR-PCR method detailed by Schuelke [34] and Zong Yu [35], and six pairs of primers (SSR11, SSR14, SSR19, SSR28, SSR35, SSR47) with high polymorphism were chosen. Primers were labeled with fluorescence dyes FAM (carboxyfluorescein) and HEX (hexachlorofluorescein). The universal M-13 sequence (TGTAAAACGACGGCCAGT) was added to the 5′ end of the forward primer, and was modified by FAM or HEX. The PCR reaction mix was prepared up to 20 μL, consisting of DNA (20 ng), 10 μL 2 × Taq PCR MasterMix (Aidlab, Beijing, China), 2 μM of forward primer, and 8 μM each of reverse and universal M13 primers. PCR amplification was carried out with an initial denaturing step at 94 °C for 5 min, followed by 30 cycles of 94 °C for 30 s, 60 °C for 45 s, and 72 °C for 45 s, and then 8 cycles of denaturation at 94 °C for 30 s, annealing at 53 °C for 45 s, and extension at 72 °C for 45 s. In the last cycle, primer extension was performed at 72 °C for 10 min and stored at 4 °C until electrophoresis. Amplicons were resolved by 2% agarose gel electrophoresis, then were visualized and documented under UV light using a gel documentation system (Tanon-1600). The amplified products were sent to Sangon Biotech (Shanghai) for capillary electrophoresis (CE).
2.9. Statistical Analysis
Experimental data are represented as the mean ± SD (standard deviation). All statistical analysis were analyzed using the SPSS 19.0 software. When the F-statistic was significant, a one-way ANOVA was used to compare the differences based on the Duncan’s multiple range test (p < 0.05). An analysis of correlation was realized with a Pearson two-tailed test. A p < 0.05 was considered statistically significant [36].
PCA (Principal Component Analysis) was conducted using the SPSS 19.0 software. The original data were standardized using the “description and statistics” function of SPSS 19.0. After putting the variables into the variables column, we chose “initial solution”, “coefficients”, “save as variables”, and “display factor score coefficient matrix” in sequence; the other parameters were set to default. The standardized data were multiplied by the eigenvectors and the products were added to obtain the scores of the principal components in different blueberry cultivars. Based on PCA, a loading diagram (loading) was also obtained, which is a representation of different factors contributing to the PCA results [37].
With the variance in the contribution rates of the factor weights of the three principal components, the comprehensive scores of the principal components were finally obtained. A score (comprehensive assessment index: FAC1-1 contribution rate % of PC1 + FAC2-1 contribution rate % of PC2 + FAC3-1 contribution rate % of PC3) was given to each fruit characteristic from each category; the higher the score, the better the fruit quality.
The GeneMarker V1.75 (Soft Genetics LLC, State College, PA, USA) software was used to read the capillary electrophoresis data returned by Sangon biotech company. Cervus 3.0 software was used to analyze the genetic data generated by codominant genetic markers. The primer sequences used for SSR-PCR analysis are listed in Supplementary Table S1. The number of alleles, observed heterozygosity, and expected heterozygosity were calculated using the GenALEx 6.501 software [38,39]. Paternal preference was performed by Cervus 3.0, with a simulation of parentage analysis [40]. Internal simulations were run (set to 10,000) to determine the significance of LOD scores.
3. Results
3.1. Flower Morphology
The blueberry inflorescences were compound racemes–panicles with four to nine bell-shaped, bisexual flowers in each inflorescence (see Figure 1I). The base of the petals formed a tubular corolla and the top of the petals split into five lobes. The petals completely wrapped the pistil at the “pink” bud stage. Among these eight cultivars, anthers were shorter than the stigma (Figure 1A–H). The distance between the stamen apex to the stigmatic surface was greatest in ‘O’Neal’, followed by ‘Sharpblue’ and ‘Misty’, while the smallest was in ‘Emerald’ (Figure 1J). The fruit set of the self-pollinated ‘Emerald’ was low in number after bagging at anthesis (Supplementary Table S2).
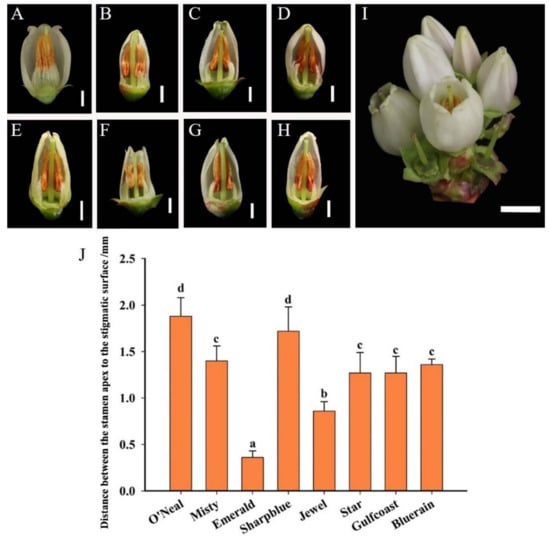
Figure 1.
Floral diversity of eight southern highbush blueberry cultivars. (A) ‘O’Neal’; (B) ‘Misty’; (C) ‘Emerald’; (D) ‘Sharpblue’; (E) ‘Jewel’; (F) ‘Star’; (G) ‘Gulfcoast’; (H) ‘Bluerain’; and (I) Flowers of blueberry. Bar: A to H = 2 mm; I = 0.5 cm. (J) The distance between the stamen apex to the stigmatic surface. The X axis indicates different blueberry cultivars. Bars with different letter superscripts are significantly different from each other at the p < 0.05 level using Duncan’s test.
3.2. Pollen Viability
Most of the pollen grains emitted yellow–green fluorescence (viable pollen grains). However, several pollen grains showed red fluorescence, indicating nonviable pollen (Figure 2A–H). The FDA–PI test revealed that the percentage of viable pollen differed among the cultivars (Figure 2I). The most viable pollen was that of ‘Sharpblue’, followed by ‘Gulfcoast’ and ‘Star’, while the least viable pollen was that of ‘O’Neal’. Stigma receptivity assessment indicated that the stigma was receptive (Supplementary Figure S1).
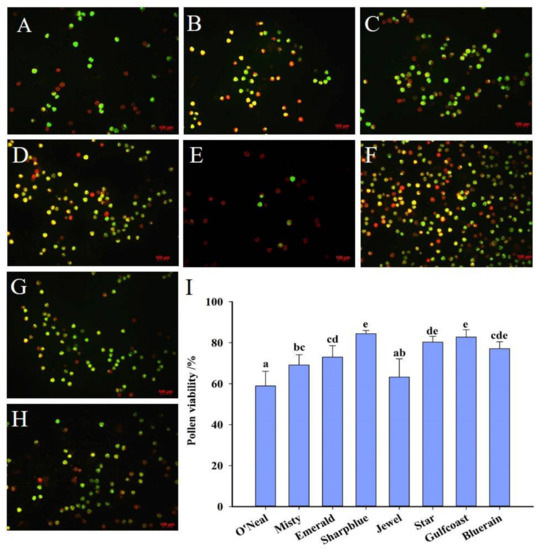
Figure 2.
Southern highbush blueberry (SHB) pollen viability tested by FDA–PI. (A) ‘O’Neal’; (B) ‘Misty’; (C) ‘Emerald’; (D) ‘Sharpblue’; (E) ‘Jewel’; (F) ‘Star’; (G) ‘Gulfcoast’; (H) ‘Bluerain’. Bar: A to H = 100 μm. (I) Pollen viability of SHB cultivars. The X-axis indicates different blueberry cultivars. Bars with different letter superscripts are significantly different from each other at the p < 0.05 level using the Duncan’s test.
3.3. Xenia Effect on Fruit Set
Fruit set varied greatly with the pollen donor (Figure S2). Fruit set increased in all combinations except for ‘Emerald’ × ‘Misty’. As shown in Figure 3, cross-pollination increased the fruit set rate when compared to artificial self-pollination in ‘O’Neal’ or ‘Emerald’. When ‘Emerald’ was the pollen recipient, the pollen from ‘Gulfcoast’ led to the highest set, followed by ‘Bluerain’ and “8 Mixed”; the pollen from ‘Misty’ had zero fruit set. When ‘O’Neal’ was the maternal cultivar, ‘Bluerain’ gave the highest fruit set at 73%, followed by “8 Mixed”. When comparing the setting rate using mixed pollen including ‘Bluerain’ pollen, pollination with pollen from ‘Bluerain’ alone promoted an increase in the fruit set of ‘O’Neal’ of more than 10% (Figure 3). A similar phenomenon was observed in ‘Emerald’, where single ‘Gulfcoast’ pollination was better than in “8 Mixed”. On the other hand, the lowest fruit set in both maternal cultivars was obtained when pollinating with ‘Misty’ pollen. Among these cross combinations, we found that mixed pollen increased the fruit set more than the self-pollination and other cross combinations, except for the special pollinizers ‘Bluerain’ and ‘Gulfcoast’.
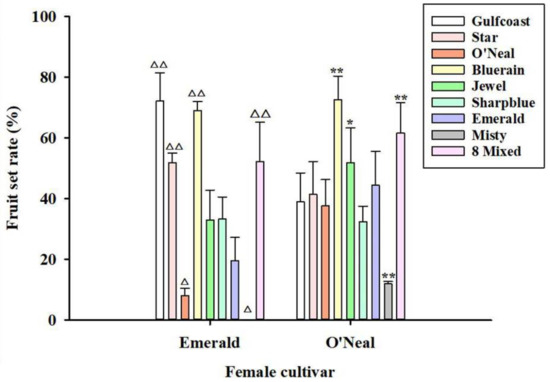
Figure 3.
Xenia effect on the fruit set of ‘O’Neal’ and ‘Emerald’ from SHB. Fruit set of ‘O’Neal’ and ‘Emerald’ were compared when pollinated with different pollen donors. The X axis indicates different pollen donors. Note: △ p < 0.05, △△ p < 0.01, compared to ‘Emerald’ × ‘Emerald’; * p < 0.05, ** p < 0.01, compared to ‘O’Neal’ × ‘O’Neal’. Bars sharing triangles or stars are significantly different from each other using the Duncan’s test.
3.4. Pollen Xenia Effects on the Fruit Quality
3.4.1. External Appearance
Different paternal pollen sources had different effects on the external appearance of blueberry fruits (Table 1, Supplementary Figure S3). For ‘Emerald’, pollen from ‘Gulfcoast’ led to the biggest fruits as measured by transverse and longitudinal diameters. Fruit weight was also greatest in this cross combination. Pollen from ‘O’Neal’ gave rise to the smallest fruit—even smaller than that with ‘Emerald’ self-pollination—indicating negative effects from cross-pollination. For ‘O’Neal’, the biggest fruit was produced with ‘Jewel’ pollen. Similarly, the smallest transverse and longitudinal diameters and the lightest weight of single fruits were obtained with artificial self-pollination. Pollination with mixed pollen raised the fruit weight and size (transverse an7d longitudinal diameters) in both maternal cultivars, in contrast to self-pollination. None of the crosspollination significantly altered the firmness of ‘Emerald’ (Table 1). On the other hand, when ‘O’Neal’ was the maternal cultivar, the “8 Mixed”, ‘Sharpblue’, ‘Bluerain’, and ‘Gulfcoast’ pollens increased the firmness of fruits.

Table 1.
Xenia effects on the appearance quality of SHB fruits.
3.4.2. Interior Fruit Quality
The interior quality of SHB fruits was influenced by the paternal pollen source (Table 2). ‘O’Neal’ self-pollination produced fruits with the highest soluble solids’ content. Pollen from ‘Bluerain’ had the greatest effect on soluble sugar content/titratable acidity (SS/TA) for both maternal cultivars. Cross combinations with “8 Mixed” and ‘Bluerain’ raised the anthocyanin concentration and reduced the titratable acidity significantly for both maternal cultivars, in contrast to the artificial self-pollination. When ‘O’Neal’ was the maternal cultivar, ‘Bluerain’ gave the highest SS/TA, soluble solids’, and anthocyanin content among all the individual pollinizers. Both the maternal cultivars showed similar tendencies after ‘Bluerain’, ‘Gulfcoast’, and ‘Jewel’ pollination: the soluble solids’, anthocyanin content, and soluble sugar of the fruit were increased (Table 2).

Table 2.
Xenia effects on the interior quality of SHB fruits.
3.4.3. Correlation Analysis between Fruit Set Rate and Fruit Quality
There was a strong correlation between the external and internal quality in both maternal cultivars, but the details were different (Tables S3 and S4). When ‘Emerald’ was the maternal cultivar, soluble sugar was negatively correlated with firmness (r = −0.763 *) but positively correlated with fruit size (transverse diameter, r = 0.826 *; longitudinal diameter r = 0.853 **) and weight (r = 0.869). However, when ‘O’Neal’ was the maternal cultivar, soluble solids were negatively correlated with fruit size and weight. In addition, the anthocyanin content was negatively correlated with the titratable acidity (r = −0.745 *). The fruit set rate of ‘Emerald’ was positively correlated with soluble sugar, fruit size, and weight (r = 0.985 **; Table S5). The fruit set rate of ‘O’Neal’ was positively correlated with soluble solids (Table S6).
3.4.4. Principal Component Analysis of the Blueberry Fruit Quality
Three components for which the eigenvalues were greater than one were identified and determined to be 91.27 and 86.34% of the fruit quality characteristics of ‘Emerald’ and ‘O’Neal’, respectively (Table S7). Furthermore, the first three principal components (PCs) accounted for most of the systematic variation in the data. These three PCs can represent all 10 traits when evaluating fruit quality, but they differed between ‘Emerald’ and ‘O’Neal’.
When ‘Emerald’ was the maternal cultivar, the factors contributing to the first principal component (PC1) were soluble sugars, transverse diameter, longitudinal diameter, and fruit weight. However, the titratable acidity content was grouped in the second principal component (PC2), and the third principal component (PC3) reflected anthocyanin content.
When ‘O’Neal’ was the maternal cultivar, the corresponding loading table showed that PC1 represented the transverse diameter, longitudinal diameter, and fruit weight (Supplementary Table S8). The second principal component (PC2) mainly represented soluble sugars and SS/TA, while the third (PC3) was determined by firmness. As shown in Table 3, based on the comprehensive evaluation of fruit quality, ‘Emerald’ × ‘Gulfcoast’ and ‘O’Neal’ × ‘Gulfcoast’ were considered to be the best combinations, with comprehensive scores of 1.08 and 0.53, respectively.

Table 3.
The comprehensive scores of the fruit quality evaluation for each cross combination in SHB.
3.4.5. Seed Number
The average number of seeds per fruit was affected by the pollen source. ‘Misty’ pollen did not produce seeds in either of the maternal cultivars (data not shown in Figure 4). As shown in Figure 4, we found that ‘Emerald’ and ‘O’Neal’ produced fewer seeds when self-fertilized. For ‘Emerald’, the seed number under different treatments varied from 8 (self-pollination) to 72 (‘Emerald’ × ‘Gulfcoast’). For ‘O’Neal’, we found that the pollinizers had a significant influence on the seed number, ranging from 15 (‘O’Neal’ × ‘Sharpblue’) to more than 84 (‘O’Neal’ × “8 Mixed”, ‘O’Neal’ × ‘Gulfcoast’). However, the seed number was not different between the ‘Bluerain’, ‘Jewel’, and ‘Emerald’ pollinizers. The results showed that ‘O’Neal’ × ‘Gulfcoast’ had the highest seed number (Figure 4). Pollen from ‘Gulfcoast’ produced more seeds than artificial self-pollination in both maternal cultivars.
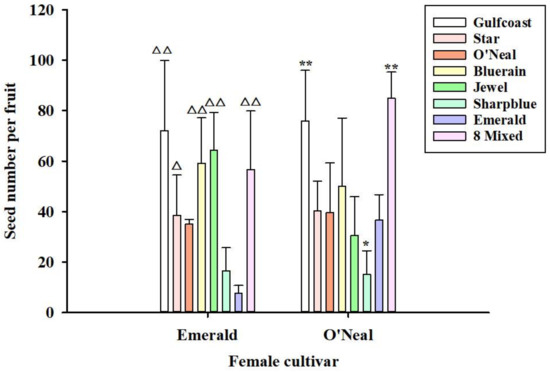
Figure 4.
Differences of average seed number per fruit when ‘Emerald’ and ‘O’Neal’ pollinated with different SHB pollen donors. Note: △ p < 0.05, △△ p < 0.01, compared to ‘Emerald’ × ‘Emerald’; * p < 0.05, ** p < 0.01, compared to ‘O’Neal’ × ‘O’Neal’. Bars sharing triangles or stars are significantly different from each other using the Duncan’s test.
3.5. Paternal Preference Based on the SSR Analysis of Seedlings
A total of 34 alleles (an average of 5.6 alleles per locus) were detected with six SSR markers. The observed heterozygosity (Ho) was 0.57, ranging from 0.14 to 0.87, and the expected heterozygosity (He) was 0.54 (Table 4). The range of polymorphic information was 0.17–0.62, with a mean value of 0.48 (Table 4). When ‘Emerald’ was the maternal cultivar (Figure 5A), ‘Emerald’ × ‘Gulfcoast’ had the largest number of seedlings. The highest progeny proportion was 37%. ‘Bluerain’ and ‘Jewel’ produced the same ratio of progeny (Figure 5A). Meanwhile, among the seedlings with ‘O’Neal’ as the maternal plant (Figure 5B), ‘Bluerain’ pollen led to the highest percentage of progeny (28%).

Table 4.
Diversity statistics based on six SSR makers in SHB resulting from cross pollinations.
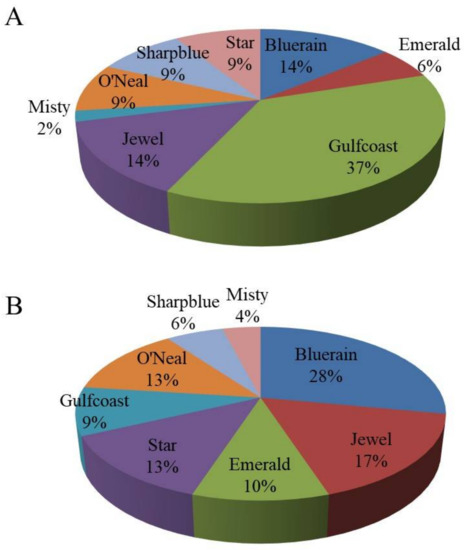
Figure 5.
Diagram of Paternity in SHB with ‘Emerald’ (A) or O’Neal’ (B) as the maternal cultivar. Paternal preference statistics were performed with CERVUS 3.0 based on an SSR analysis of 132 seedlings of ‘Emerald’ and 78 seedlings of ‘O’Neal’. Paternity was determined with a positive LOD score (>3.0), and rejected with a negative LOD score (−3.0 or less). Candidate fathers were then assigned to the clusters at 95% confidence.
4. Discussion
4.1. SHB Attains Higher Fruit Set, Better Fruit Quality, and More Seedlings with Optimal Cross Combination
In this study, we found substantial evidence that xenia occurs in SHB. Firstly, xenia effects were observed to influence the fruit set in SHB. The fruit set of ‘Emerald’ was increased up to 65% when pollinated with ‘Gulfcoast’. The fruit set of ‘O’Neal’ increased to 80% when pollinated with ‘Bluerain’ pollen. Overall, these two pollinizers are good pollen donors for the considered maternal cultivars. Furthermore, a single pollen source works better than mixed pollen, and that the pollen source should be selected individually for a particular maternal blueberry cultivar. Our results provide several new promising combinations for boosting blueberry yields through improved fruit set.
Fruit characteristics such as the fruit set and fruit quality demonstrated the potential to be positively influenced by xenia, although there was variation in the measures of appearance quality. When ‘O’Neal’ was cross-pollinated by ‘Misty’ pollen, fruit set was the lowest, but the fruit’s transverse diameter was large. This suggests that the appearance of fruit quality was mainly related to genotype, not xenia. ‘O’Neal’ self-pollination produced fruits with the highest soluble solids content and the lowest firmness of the fruit, indicating that it should be harvested and consumed as fresh fruit.
Our results demonstrated that the cross-pollination of multiple cultivars had significant effects on the fruit weight, fruit diameter, firmness, soluble solids, soluble sugar content, and acid content. Furthermore, given that cross-pollination of SHB affected the external appearance, interior fruit quality, and the seed number, we categorized SHB to experience “combined xenia”. This is distinct from the “non-double-fertilization” xenia of rabbiteye blueberry [9].
Specific to our study, ‘O’Neal’ and ‘Emerald’ produced better fruit after pollination with ‘Bluerain’, ‘Gulfcoast’, and ‘Jewel’ pollen (Table 2). Both maternal cultivars produced higher ACY and soluble sugar contents after pollination by the above three male cultivars. These findings indicate that anthocyanin and soluble sugar were altered simultaneously through xenia effects for SHB. Among the cultivars of northern highbush, southern highbush, and rabbiteye blueberries, fruit weight has been observed to be significantly negatively correlated with soluble solids, and soluble solids were positively correlated with the ferric-reducing ability of plasma (FRAP) and total monomeric anthocyanin content (TMAC) across the cultivars of all three blueberry types, suggesting that the fruit antioxidant capacity and sugar content can be improved simultaneously [41]. This result is consistent with those obtained in our study.
No significant correlations between soluble solids and other fruit quality features were detected in ‘Emerald’ fruits (Table S3; r < 0.6). In contrast, soluble solids displayed a negative correlation with fruit size and fruit weight in ‘O’Neal’ fruits. In a similar study, when rabbiteye cultivars were pollinated by ‘Bluegen’, the fruit diameter was significantly smaller than when pollinated by ‘Powderblue’ [42]. The soluble solids’ content was also lower with ‘Bluegen’ [42], implying that fruit size is positively correlated with soluble solids. This result is contrary to our data with ‘O’Neal’, as we found that soluble solids were negatively correlated with the transverse diameter and longitudinal diameter (r = −0.781 and r = −0.902, respectively; Table S8). These results indicate that there is no universal conclusion for the correlates of fruit quality in blueberry. Different cultivars should have unique correlations among their fruit qualities. Thus, it is important to set a unique comprehensive assessment index for cross combination screenings. Here, we used Principal Component Analysis (PCA) to determine which fruits had better quality and phytochemical characteristics. PCA can facilitate the selection of cross combinations in SHB, providing guidance for the blueberry industry.
The xenia effects on seed number were in agreement with those on fruit set rate and fruit quality when pollinated on ‘Emerald’, but this differed for ‘O’Neal’. For ‘O’Neal’, the pollinizers had a significant influence on the seed number, but with no difference between the ‘Bluerain’, ‘Jewel’, and ‘Emerald’ pollinizers. This result did not coincide with that of the fruit set, as the combination ‘O’Neal’× ‘Bluerain’ obtained the highest fruit set rate. According to the correlation analysis of fruit quality, fruit set, and seed number for both maternal cultivars (Tables S6 and S7), the seed number exhibited no correlation with the fruit set and fruit quality, with a coefficient range lower than 0.7. Although these results seemed to be unrelated to each other, ‘O’Neal’ × ‘Gulfcoast’ was still a good cross combination, with better fruit quality and a suitable number of seeds.
4.2. SSR Can Help to Find the Most Efficient Hybrid Combination after Mixed Pollination
The floral morphological structure of the eight cultivars reflects their low efficiency of self-pollination, thus requiring artificial or insect pollination to improve the fruit set. Overall, the pollen viability of all cultivars was greater than 50%, indicating that the pollen has strong vitality for pollination and fertilization. Based on our results, artificial pollination reliably promoted the fruit set (Table S3). Actually, literature also reported that the anatomical structure of SHB cultivar flowers raises difficulties for self-and cross-pollination, and it is necessary to increase pollination with insect pollinators [43]. These results suggest that planting these cultivars together, along with insect-mediated cross pollination, can improve fruit set. In addition, SSR can overcome the difficulties of paternity identification caused by interplanting.
For many plants, the amount of the pollen attached to the stigma far exceeds ovule number and the commercially acceptable levels of pollination. Especially in natural pollination, once the number of the pollen grains reaches the threshold, the competitiveness of the pollen becomes very important [44]. A large body of evidence has shown that the more intense the competition of pollen, the stronger the vitality of the seedling [45,46,47]. The competitiveness of the pollen not only increases the quality of seeds, but also reduces the variation in offspring progeny seedlings [48,49]. The use of SSR markers for paternity testing was found in this study to be a good method to infer the competitiveness of pollen as well as pollen identity. SSR is not affected by the plant growth environment, specific growth period, or cultivation conditions [50], further supporting its use for this application. SSR marker analysis also provides a high level of accuracy and is a fast and reliable means for pollen donor identification [51].
The results of this study indicated that combinations of ‘Emerald’ × ‘Gulfcoast’ and ‘O’Neal’ × ‘Bluerain’ had the highest proportions of seedlings. This finding was consistent with the fruit set data, which indicated that the ‘Gulfcoast’ and ‘Bluerain’ pollen were relatively competitive when compared with the other six cultivars. This may be attributed to the fact that the pollen of some cultivars has a more reproductive genotype. In this experiment, it should be noted that the pollination donors were all SHB cultivars and are closely related to each other. For example, ‘Star’ is a hybrid obtained from ‘O’Neal’ and ‘FB80-31′ [52]. The inbreeding coefficiency between all the combinations may be higher than the cross between wild germplasm, but can still help to attain better fruit quality and seedlings. Studies have reported that crosses resulting in high inbreeding coefficients might yield successful cultivars with high horticultural value, such as a high and early yield, and improved self-fruiting [53]. In fact, the effects of wide hybridization on the genetic diversity of cultivated blueberries are very low, which means that pedigree-based diversity measurements can result in an overestimation of the actual level of genetic diversity. Applying SSR markers provides a better method to assess the genetic relationships among SHB cultivars [54].
Although this study successfully identified more than 200 seedlings from the progeny of the cross combinations, there were some shortcomings. For example, the bands that were produced by some seedlings were from neither the maternal cultivar nor the paternal cultivar. Some seedlings lacked data with low LOD values, such that it was impossible to accurately determine the origin of the pollen donors. This is because the unequal exchange of chromosomes produces new fragments during the gamete formation process [55]. Furthermore, chromosomal exchange and modification of the DNA molecule bases may cause mutations, resulting in site deletion [56].
In conclusion, xenia effects were identified and categorized for SHB (‘O’Neal’ and ‘Emerald’). A broader outcome of the study is that a better understanding of xenia effects in fruit crops offers the potential benefit to improve commercially important traits related to yield and fruit quality. The application of SSR combined with an assessment of the fruit quality traits in blueberry cross-pollinated cultivars was also an effective approach to determine optimal pollen donors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8070659/s1, Figure S1: Stigma receptivity analysis of ‘O’Neal’, Figure S2: Fruit set status when ‘Emerald’ and ‘O’Neal’ pollinated with different SHB pollen donors, Figure S3: Comparisons of fruit sizes from different pollination combinations, Table S1: Primer sequence of 6 core SSR markers selected in this study, Table S2: Fruit setting ratio of two self-pollination methods, Table S3: The correlation of fruit quality for ‘Emerald’ as the maternal cultivar, Table S4: The correlation of fruit quality for ‘O’Neal’ as the maternal cultivar, Table S5: The correlation coefficient range of fruit quality, fruit setting ratio and seed number for ‘Emerald’ as the maternal cultivar, Table S6: The correlation coefficient range of fruit quality, fruit setting ratio and seed number for ‘O’Neal’ as the maternal cultivar, Table S7: Characteristic eigenvalue and accumulative contribution rate of each principal component, Table S8: Component loading table for ‘Emerald’ and ‘O’Neal’ as the maternal cultivar.
Author Contributions
Conceptualization, F.L. and W.G.; data curation, J.L. and Y.W.; formal analysis, J.L., J.X., K.L., L.Y. and F.L.; funding acquisition; F.L. and W.G.; writing—original draft, Y.W. and F.L.; writing—review and editing, F.L., W.C., Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Research Project of Science Technology Department of Zhejiang Province, grant number 2021C02066-9; the Key Research and Development Program of Zhejiang Science and Technology Department, grant number 2018C02007; and the Key Project for Science and Technology Research Program of Jinhua, grant number 2020-2-006.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Yongqiang Li (Zhejiang Normal University) and Youyin Zhu (Jinhua Polylechnic) for providing suggestions and help.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACY | Anthocyanin Content |
| SHB | Southern Highbush Blueberry |
| FDA–PI | Fluorescein Diacetate–Propidium Iodide |
| SS/TA | Soluble Sugar Content/Titratable Acidity |
| FAM | Carboxyfluorescein |
| HEX | Hexachlorofluorescein |
| PCA | Principal Component Analysis |
References
- Qiu, Y.P.; Dai, H.F.; Li, Z.Q.; Ou, L.X.; Xiang, X.; Chen, J.Z.; Wang, B.X. Effects of pollinator on fruit quality of Guiwei litchi cultivar. J. Fruit Sci. 2006, 23, 703–706. [Google Scholar]
- Xie, H.; Gao, Q.M.; Liu, Y.J.; Nan, X.; Zhang, W.; Li, S.Q.; Li, J. Effects of xenia on fruit quality of Korla fragrant pear cultivar. Acta Agric. Boreali-Occident. Sin. 2013, 22, 93–96. [Google Scholar]
- Yu, L.Y.; Zuo, L.H.; Zhang, J.; Yang, M.S. Effect of xenia on fruit quality of 4 Malus sieversii clones. Mol. Plant Breed. 2017, 15, 3667–3675. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yu, D.; Chen, Z.F.; Xu, L.; Wei, X.Q.; Xu, J.H. A preliminary discussion on pollen xenia of mango. Southeast Hortic. 2017, 5, 20–22. [Google Scholar]
- He, X.Y.; Tao, L.; Ni, S.B.; Chen, L.H.; Zhang, H.W.; Kong, G.H. Effects of pollen xenia on nut morphological characteristics and quality of ‘O.C’ cultivar in Macadamia spp. Nonwood For. Res. 2016, 34, 76–82. [Google Scholar] [CrossRef]
- Denney, J.O. Xenia includes metaxenia. HortScience 1992, 27, 722–728. [Google Scholar] [CrossRef]
- Pahlavani, M.H.; Abolhasani, K. Xenia effect on seed and embryo size in cotton (Gossypium hirsutum L.). J. Appl. Genet. 2006, 47, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Niu, L.; Zhang, Y.; Jin, M.; Ji, D.; Zhang, X. Pollen Sources Influence the Traits of Seed and Seed Oil in Paeonia ostii ‘Feng Dan’. HortScience 2017, 52, 700–705. [Google Scholar] [CrossRef]
- Yang, Q.; Fu, Y.; Liu, Y.; Zhang, T.; Peng, S.; Deng, J. Novel Classification Forms for Xenia. HortScience 2020, 55, 980–987. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2019, 11, 224–236. [Google Scholar] [CrossRef]
- Lang, G.A.; Danka, R.G. The influence of self-and cross-pollination on fruiting in southern highbush blueberries. HortScience 1991, 26, 486g. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Kramer, M. Self-fertility Evaluations of Northern-adapted Rabbiteye Blueberry Hybrids. HortScience 2012, 47, 1837–1842. [Google Scholar] [CrossRef]
- Miller, S.; Alspach, P.; Scalzo, J.; Meekings, J. Pollination of ‘Hortblue Petite’ Blueberry: Evidence of Metaxenia in a New Ornamental Home-garden Cultivar. HortScience 2011, 46, 1468–1471. [Google Scholar] [CrossRef]
- Taber, S.K.; Olmstead, J.W. Impact of cross- and self-pollination on fruit set, fruit size, seed number, and harvest timing among 13 southern highbush blueberry cultivars. HortTechnology 2016, 26, 213–219. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, J.L.; Luo, Y.; Liu, Y.L.; Zhang, T.T.; Peng, S. Effect of xenia on formation of key quality traits of premier rabbiteye. South China Fruits 2020, 49, 103–106. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.-T.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef]
- Cho, H.Y.; Kadowaki, M.; Che, J.; Horiuchi, N.; Ogiwara, I. Plant morphological characteristics for year-round production and its fruit quality in response to different light wavelengths in blueberry. In Proceedings of the XIII International Symposium on Plant Bioregulators in Fruit Production, Chiba, Japan, 27–31 August 2018; pp. 201–210. [Google Scholar]
- Wang, S.Y.; Zhou, Q.; Zhou, X.; Wei, B.D.; Ji, S.J. The effect of ethylene absorbent treatment on the softening of blueberry fruit. Food Chem. 2018, 246, 286–294. [Google Scholar] [CrossRef]
- Zhang, X.C.; Zhu, Y.Q.; Wang, Y.N.; Luo, C.; Wang, X. Effects of different plant growth regulators on blueberry fruit quality. IOP Conf. Ser. Earth Environ. Sci. 2017, 81, 012038. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, rubus, and ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Hancock, J.F.; Olmstead, J.W.; Itle, R.A.; Callow, P.W.; Neils-Kraft, S.; Wheeler, E.J.; Mangandi, J.; Sooriyapathirana, S.S.; Rowland, L.J.; Mackey, T.A. Performance of an elite, hybrid family of a northern × southern highbush cross (‘Draper’ × ‘Jewel’). Euphytica 2018, 214, 95. [Google Scholar] [CrossRef]
- Nagasaka, K.; Yamane, H.; Tao, R. Evaluation of the effects of pollination on fruit size and quality of highbush blueberry. Acta Hortic. 2021, 1312, 25–30. [Google Scholar] [CrossRef]
- Yang, B.; Ballington, J.; Raja, A.; Brouwer, C.; Reid, R.; Burke, M.; Wang, X.G.; Rowland, L.J.; Bassil, N.; Brown, A. Patterns of simple sequence repeats in cultivated blueberries (Vaccinium section Cyanococcus spp.) and their use in revealing genetic diversity and population structure. Mol. Breed. 2014, 34, 675–689. [Google Scholar] [CrossRef]
- Bassil, N.; Bidani, A.; Hummer, K.; Rowland, L.J.; Olmstead, J.; Lyrene, P.; Richards, C. Assessing genetic diversity of wild southeastern North American Vaccinium species using microsatellite markers. Genet. Resour. Crop Evol. 2018, 65, 939–950. [Google Scholar] [CrossRef]
- Bhatt, D.S.; Debnath, S.C. Genetic Diversity of Blueberry Genotypes Estimated by Antioxidant Properties and Molecular Markers. Antioxidants 2021, 10, 458. [Google Scholar] [CrossRef]
- Prodorutti, D.; Pertot, I.; Giongo, L.; Gessler, C. Highbush Blueberry: Cultivation, Protection, Breeding and Biotechnology. Eur. J. Plant Sci. Biotechnol. 2007, 1, 44–56. [Google Scholar]
- Shen, L.B.; Liang, W.Y.; Qu, J.H.; Xie, M.S.; Lei, P.J.; Liu, H.J. The viability determination of cyanobacteria double staining with fluorescein diacetate and propidium iodide. Environ. Chem. 2005, 24, 554–557. [Google Scholar]
- Dafni, A.; Maués, M.M. A rapid and simple procedure to determine stigma receptivity. Sex. Plant Reprod. 1998, 11, 177–180. [Google Scholar] [CrossRef]
- Wang, Y.S.; Luo, Z.S.; Du, R.X. Nitric oxide delays chlorophyll degradation and enhances antioxidant activity in banana fruits after cold storage. Acta Physiol. Plant. 2015, 37, 74–83. [Google Scholar] [CrossRef]
- Mtenga, A.B.; Kassim, N.; Lee, W.-G.; Shim, W.-B.; Yoon, Y.; Chung, D.-H. Resistance of Bacillus cereus and its enterotoxin genes in ready-to-eat foods to γ -irradiation. Food Sci. Biotechnol. 2012, 21, 443–452. [Google Scholar] [CrossRef]
- Guan, A.Q.; Feng, W.M.; Lu, Y.Y.; Chen, G.; Han, Y.Q. Research on related factors influencing content of souble solids in tomato. Acta Agric. Jiangxi 2017, 29, 27–31. [Google Scholar] [CrossRef]
- Dogterom, M.H.; Winston, M.L.; Mukai, A. Effect of pollen load size and source (self, outcross) on seed and fruit production in highbush blueberry cv. ‘Bluecrop’ (Vaccinium Corymboum; Ericaceae). Am. J. Bot. 2000, 87, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.S.; Li, F.; Xu, C.J.; Zhang, S.L.; Fu, C.X. An Efficient Macro-method of Genomic DNA Isolation from Actinidia chinensis Leaves. Hereditas 2004, 26, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M. An economic method for the fluorescent labeling of PCR fragments. Nat. Biotechnol. 2000, 18, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Wang, Y.; Zhu, Y.Y.; Shao, X.; Li, Y.Q.; Guo, W.D. Development and validation of SSR markers based on transcriptomic data of Chinese cherry (Prunus pseudocerasus). Acta Hortic. Sin. 2016, 43, 1566–1576. [Google Scholar] [CrossRef]
- Rêgo, E.R.d.; Rêgo, M.M.d.; Cruz, C.D.; Finger, F.L.; Casali, V.W.D. Phenotypic diversity, correlation and importance of variables for fruit quality and yield traits in Brazilian peppers (Capsicum baccatum). Genet. Resour. Crop Evol. 2011, 58, 909–918. [Google Scholar] [CrossRef]
- Cadima, J.; Jolliffe, I.T. Loading and correlations in the interpretation of principle compenents. J. Appl. Stat. 1995, 22, 203–214. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Jones, A.G.; Small, C.M.; Paczolt, K.A.; Ratterman, N.L. A practical guide to methods of parentage analysis. Mol. Ecol. Resour. 2010, 10, 6–30. [Google Scholar] [CrossRef]
- Gündüz, K.; Serçe, S.; Hancock, J.F. Variation among highbush and rabbiteye cultivars of blueberry for fruit quality and phytochemical characteristics. J. Food Compos. Anal. 2015, 38, 69–79. [Google Scholar] [CrossRef]
- Silveira, T.M.T.d.; Raseira, M.d.C.B.; Nava, D.E.; Couto, M. Blueberry pollination in southern Brazil and their influence on fruit quality. Rev. Bras. Frutic. 2011, 33, 081–088. [Google Scholar] [CrossRef][Green Version]
- Courcelles, D.M.M.; Button, L.; Elle, E. Bee visit rates vary with floral morphology among highbush blueberry cultivars (Vaccinium corymbosum L.). J. Appl. Entomol. 2013, 137, 693–701. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, Y.F.; Yu, Y.B.; Gao, H.Y.; Lai, C.C.; Luo, X.L.; Huang, X.G. Effects of cross-pollination by ‘Murcott’ tangor on the physicochemical properties, bioactive compounds and antioxidant capacities of ‘Qicheng 52′ navel orange. Food Chem. 2019, 270, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Björkman, T. The effect of pollen load and pollen grain competition on fertilization success and progeny performance in Fagopyrum esculentum. Euphytica 1995, 83, 47–52. [Google Scholar] [CrossRef]
- Mulcahy, D.L.; Mulcahy, G.B. The influence of gametophytic competition on sporophytic quality in Dianthus chinensis. Theor. Appl. Genet. 1975, 46, 277–280. [Google Scholar] [CrossRef]
- Schlichting, C.D.; Stephenson, A.G.; Small, L.E.; Winsor, J.A. Pollen loads and progeny vigor in cucurbita pepo: The next generation. Evolution 1990, 44, 1358–1372. [Google Scholar] [CrossRef]
- Hormaza, J.I.; Herrero, M. Male gametophytic selection as a plant breeding tool. Sci. Hortic. 1996, 65, 321–333. [Google Scholar] [CrossRef]
- Hormaza, J.I.; Herrero, M. Dynamics of pollen tube growth under different competition regimes. Sex. Plant Reprod. 1996, 9, 153–160. [Google Scholar] [CrossRef]
- Tabbener, H.E.; Cottrell, J.E. The use of PCR based DNA markers to study the paternity of poplar seedlings. For. Ecol. Manag. 2003, 179, 363–376. [Google Scholar] [CrossRef]
- Han, Z.Q.; Gao, P.; Geng, X.N.; Du, K.; Kang, X.Y. Identification of the male parent of superior half-sib Populus tomentosa individuals based on SSR markers. Mol. Breed. 2017, 37, 155. [Google Scholar] [CrossRef]
- Lyrene, P.M.; Sherman, W.B. ‘Star’ Southern Highbush Blueberry. Hortscience 2000, 35, 956–957. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K. The genetic composition and tetrasomic inbreeding coefficients of highbush blueberry cultivars. HortScienc 1994, 29, 1342–1345. [Google Scholar] [CrossRef]
- Brevis, P.A.; Bassil, N.V.; Ballington, J.R.; Hancock, J.F. Impact of wide hybridization on highbush blueberry breeding. J. Am. Soc. Hortic. Sci. 2008, 133, 427–437. [Google Scholar] [CrossRef]
- Wu, X.W.; Cui, G.F.; Wu, L.F.; Zhang, Y.P.; Ming, J.; Wang, J.; Wang, J.H. Identification of ISSR in lily hybrids. Acta Hortic. Sin. 2009, 36, 749–754. [Google Scholar] [CrossRef]
- Fang, J.G.; Zhang, Z.; Ma, Z.Q.; Liu, D.J.; Wang, S.H.; Lavi, U. The polymorphism and segregation patterns of AFLP markers in the F1 progenies from the cross of two mango cultivars. Sci. Agric. Sin. 2000, 33, 19–24. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).