Changes in Yield, Quality, and Morphology of Three Grafted Cut Roses Grown in a Greenhouse Year-Round
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Grafting Procedure
2.1.1. Plant Material
2.1.2. Cutting and Grafting Producing
2.2. Greenhouse and Environmental Control
2.3. Crop Management
2.4. Yield, Quality, Morphology, and Physiological Responses of Grafted Cut Roses
2.4.1. Yield and Quality
2.4.2. Root Activity
2.4.3. Photosynthesis Characteristics
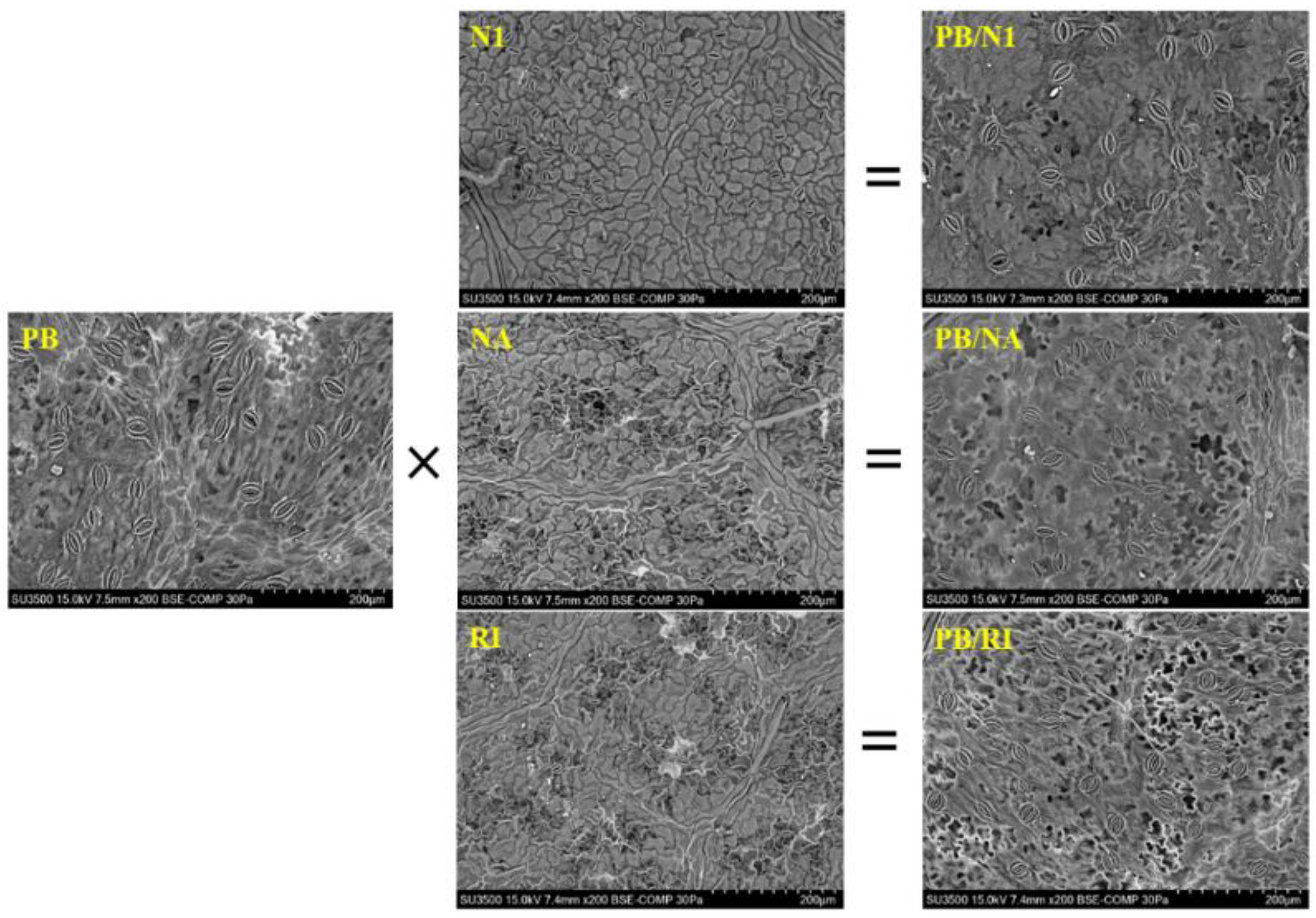
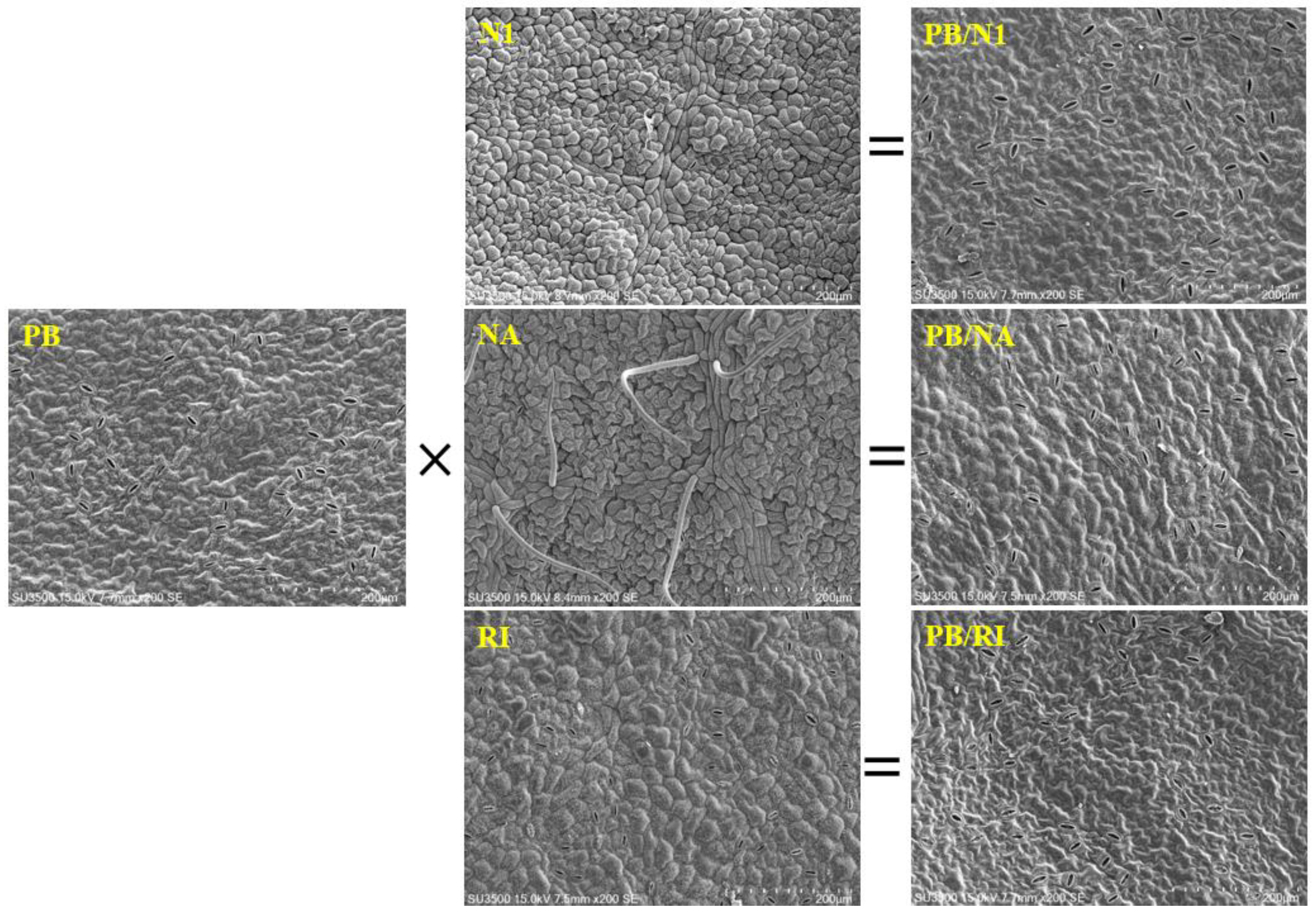
2.4.4. Scanning Electron Microscopy (SEM)
2.5. Experimental Design and Statistical Analysis
3. Results
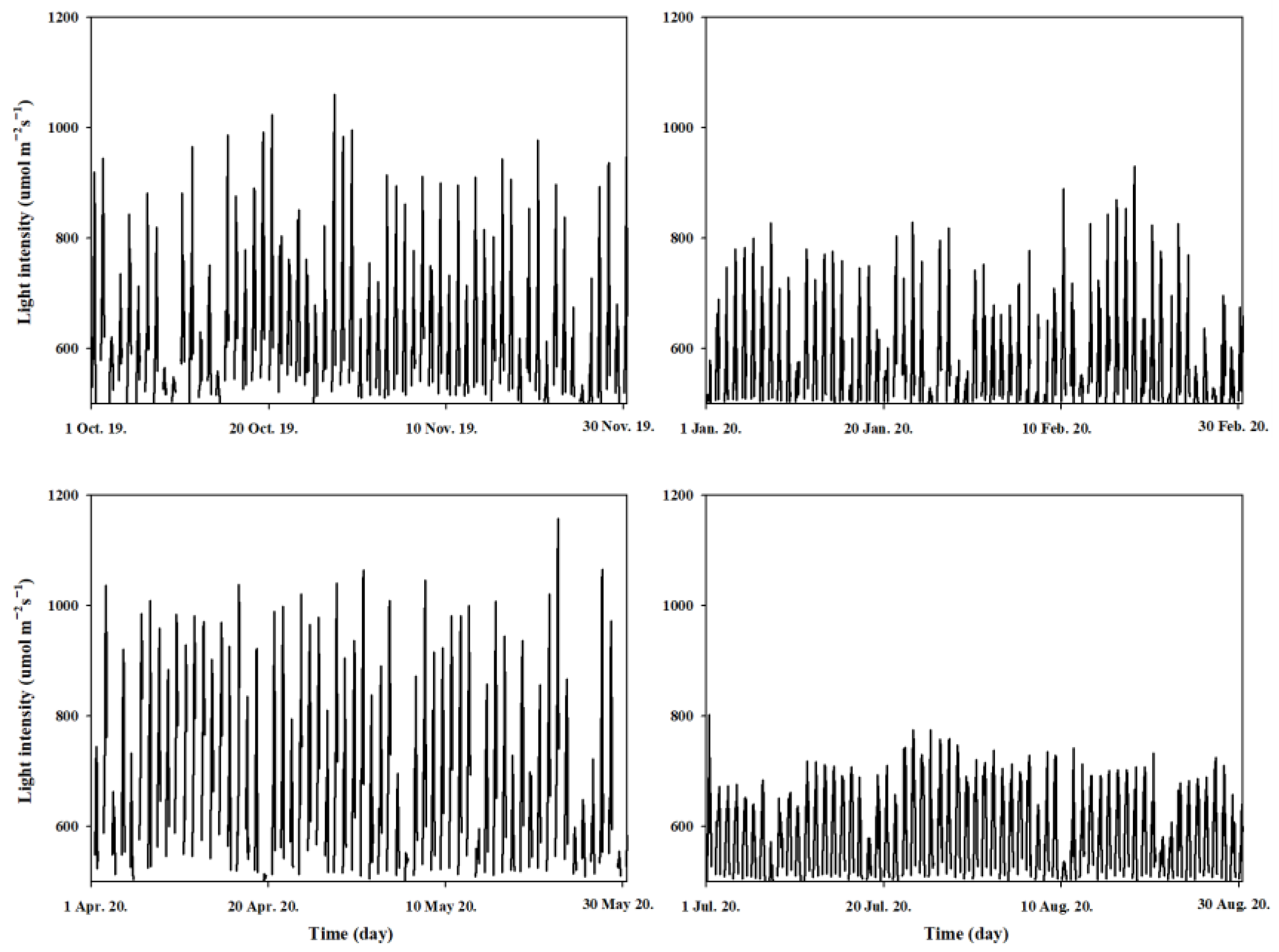
3.1. Temperature and Ambient Light Intensity in the Greenhouse
3.2. Root Activity of Cut Roses
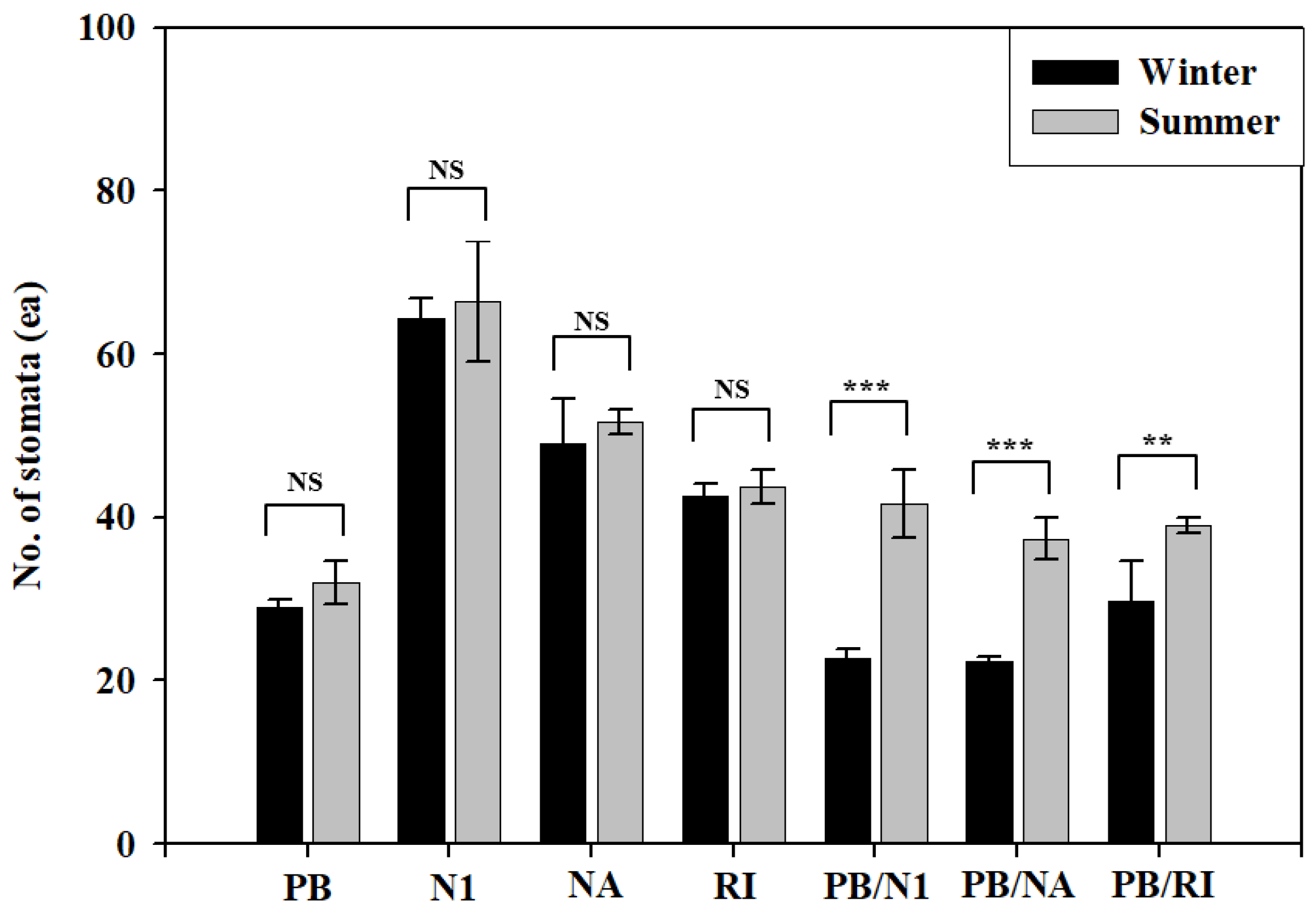
3.3. Morphological Characteristics of Leaves
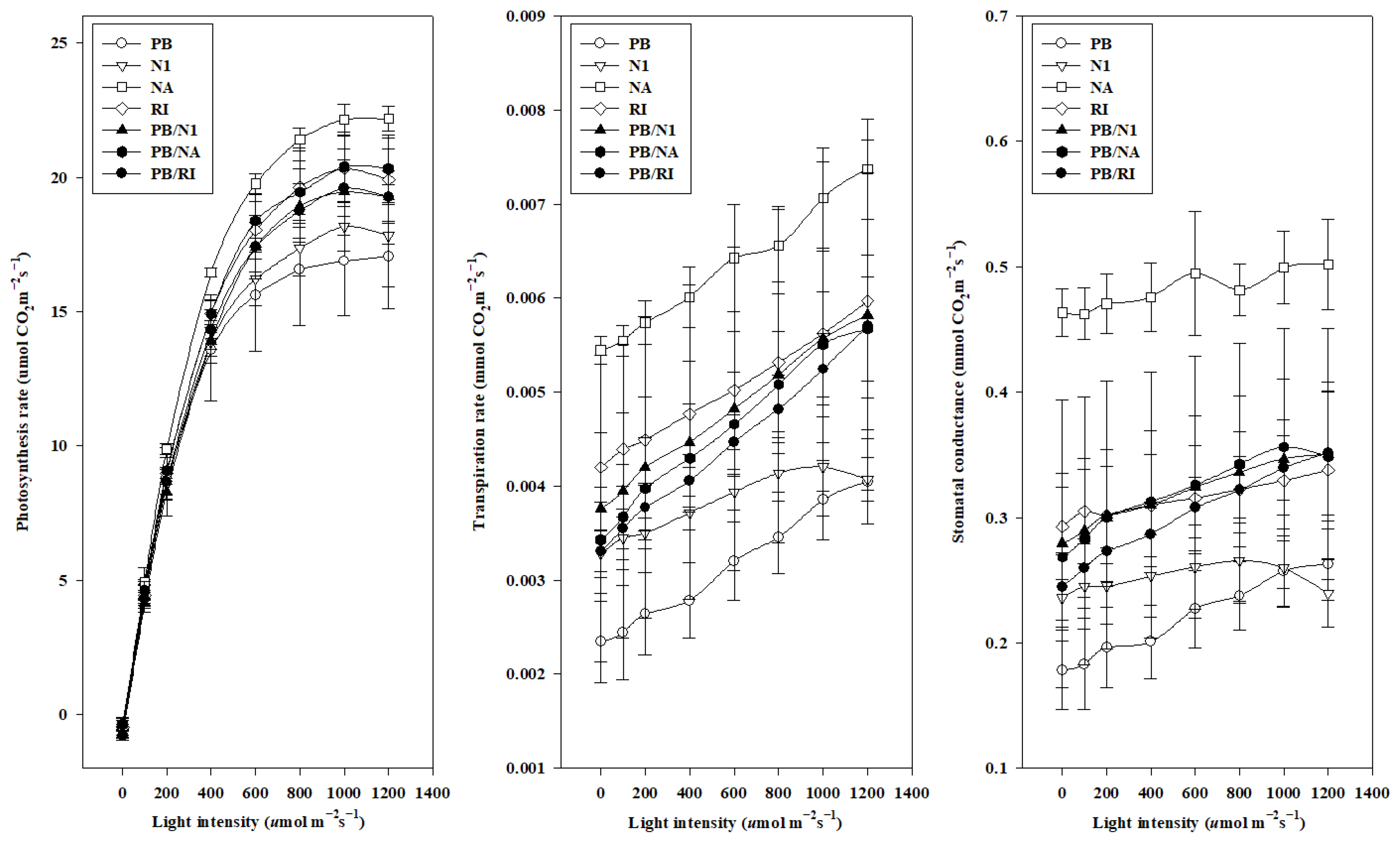
3.4. Photosynthesis of Grafted Cut Roses
3.4.1. Temperature Curve
3.4.2. Light Curve
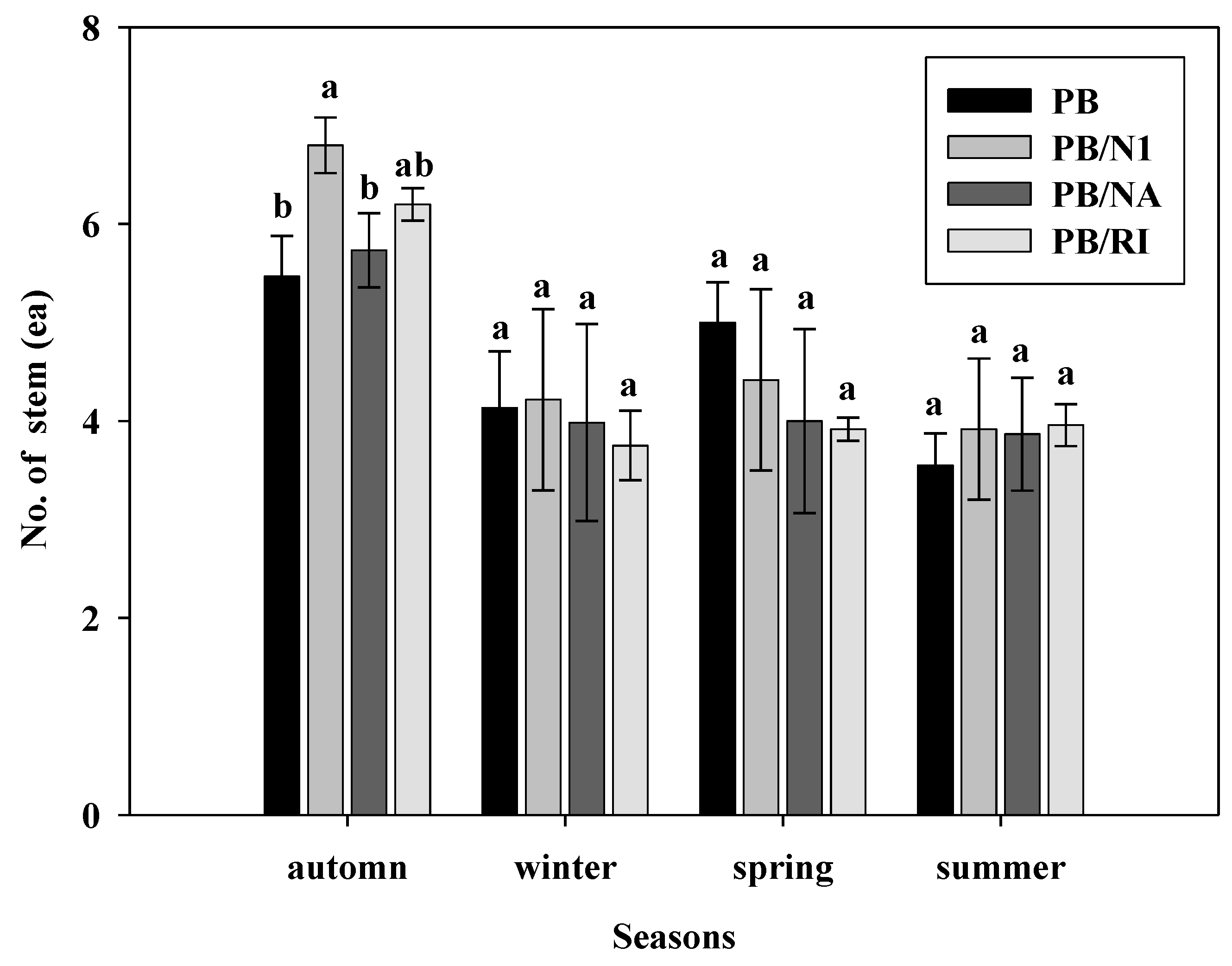
3.5. Yield and Quality of Cut Roses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.X.; Hussain, N.; Wang, Y.R.; Li, M.T.; Liu, L.; Qin, M.Z.; Ma, N.; Gao, J.P.; Sun, X.M. An ethylene-inhibited NF-YC transcription factor RhNF-YC9 regulates petal expansion in rose. Hortic. Plant J. 2020, 6, 419–427. [Google Scholar] [CrossRef]
- Shoor, M.; Pahnekolayi, M.D.; Tehranifar, A.; Samiei, L. Optimizing culture medium ingredients and micrografting devices can promote in vitro micrografting of cut roses on different rootstocks. Plant Cell Tissue Organ Cult. 2019, 137, 265–274. [Google Scholar]
- Singh, A.P.; Sane, A.P. Differential and reciprocal regulation of ethylene pathway genes regulates petal abscission in fragrant and non-fragrant roses. Plant Sci. 2019, 280, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Seo, J.H. Seasonal change in incidence bent peduncle phenomenon of flowering shoots of greenhouse-grown cut roses. Flower Res. J. 2013, 21, 74–77. [Google Scholar]
- Cheong, D.C.; Lee, J.J.; Choi, C.H.; Song, Y.J.; Kim, H.J.; Jeong, J.S. Growth and cut-flower productivity of spray rose as affected by shading method during high temperature period. Korean J. Hortic. Sci. Technol. 2015, 33, 227–232. [Google Scholar] [CrossRef]
- Lieth, J.H.; Pasian, C.C. Model for net photosynthesis of rose leaves as a function of photosynthetically active radiation, leaf temperature, and leaf age. J. Am. Soc. Hortic. Sci. 1990, 115, 486–491. [Google Scholar] [CrossRef]
- Zieslin, N.; Mor, Y. Light on roses. A review. Sci. Hortic. 1990, 43, 1–14. [Google Scholar] [CrossRef]
- Desta, B.; Tena, N.; Amare, G. Response of rose (Rosa hybrida L.) plant to temperature. Asian J. Plant Sci. 2022, 7, 93–101. [Google Scholar]
- Ueda, Y.; Nishihara, S.; Tomitab, H.; Oda, Y. Photosynthetic response of Japanese rose species Rosa bracteata and Rosa rugosa to temperature and light. Sci. Hortic. 2000, 84, 365–371. [Google Scholar] [CrossRef]
- Ushio, A.; Mae, T.; Makino, A. Effects of temperature on photosynthesis and plant growth in the assimilation shoots of a rose. J. Soil Sci. Plant Nutr. 2008, 54, 253–258. [Google Scholar] [CrossRef]
- Essemine, J.; Govindachary, S.; Ammar, S.; Bouzid, S.; Carpentier, R. Abolition of photosystem I cyclic electron flow in Arabidopsis thaliana following thermal-stress. Plant Physiol. Biochem. 2011, 49, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Lal, M.K.; Tiwari, R.K.; Gahlaut, V.; Mangal, V.; Kumar, A.; Singh, M.P.; Paul, V.; Kumar, S.; Singh, B.; Zinta, G. Physiological and molecular insights on wheat responses to heat stress. Plant Cell Rep. 2022, 41, 501–518. [Google Scholar] [CrossRef]
- Lal, M.K.; Sharma, N.; Adavi, S.B.; Sharma, E.; Altaf, M.A.; Tiwari, R.K.; Kumar, R.; Kumar, A.; Dey, A.; Paul, V.; et al. From source to sink: Mechanistic insight of photoassimilates synthesis and partitioning under high temperature and elevated [CO2]. Plant Mol. Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Lieth, J.H.; Kin, S.; Shin, H.K.; Kim, S.H.; Zieslin, N. Effect of temperature on leaf area and flower size in rose. Acta Hortic. 2001, 547, 185–191. [Google Scholar] [CrossRef]
- Jeong, B.R.; Park, Y.G. Effect of light intensity during stenting propagation on rooting and subsequent growth of two rose cultivars. Flower Res. J. 2012, 20, 228–232. [Google Scholar] [CrossRef]
- Yang, K.R.; Kim, W.H.; Kim, S.J.; Jung, H.H.; Yoo, B.S.; Lee, H.J.; Park, K.Y. Breeding of spray rose cultivar ‘Pink Shine’ with pink color and longer vase life. Flower Res. J. 2020, 28, 210–215. [Google Scholar] [CrossRef]
- An, D.C.; Kim, S.Y.; Chin, Y.D.; Park, H.G.; Bae, M.J.; Hwang, J.C. Yellow spray rose cultivar ‘Egg Tart’ with high productivity and suitable for export. Flower Res. J. 2020, 28, 220–227. [Google Scholar] [CrossRef]
- Cheong, D.C.; Lee, J.J.; Choi, C.H.; Kim, H.J. Breeding of standard rose ‘Pinky Luna’ with a fragrance, and pastel color of pale pink for cut flowers. Flower Res. J. 2020, 28, 93–98. [Google Scholar] [CrossRef]
- Younas, A.; Riaz, A. Effect of various hormones and different rootstocks on rose propagation. Caderno Pesquisa Sér. Bio. 2005, 17, 111–118. [Google Scholar]
- Venema, J.H.; Dijk, B.E.; Bax, J.M.; van Hasselt, P.R.; Elzenga, J.T.M. Grafting tomato (Solanum lycopersicum) onto the rootstock of a high-altitude accession of Solanum habrochaites improves suboptimal-temperature tolerance. Environ. Exp. Bot. 2008, 63, 359–367. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Mechanisms of tolerance differences in cucumber seedlings grafted on rootstocks with different tolerance to low temperature and weak light stresses. Turk. J. Bot. 2015, 39, 606–614. [Google Scholar] [CrossRef]
- Ximing, H. Growth and Productivity of Cut Rose as Related to the Rootstock. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2001. [Google Scholar]
- Liu, Y.F.; Qi, H.Y.; Bai, C.M.; Qi, M.F.; Xu, C.Q.; Hao, J.H.; Li, Y.; Li, T.L. Grafting helps improve photosynthesis and carbohydrate metabolism in leaves of muskmelon. Int. J. Biol. Sci. 2011, 7, 1161–1170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahn, S.J.; Im, Y.J.; Chung, G.C.; Cho, B.H.; Suh, S.R. Physiological responses of grafted-cucumber leaves and rootstock roots as affected by low root temperature. Sci. Hortic. 1999, 81, 397–408. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Reab, E.; Colla, G. Grafting of cucumber as a means to minimize copper toxicity. Environ. Exp. Bot. 2008, 63, 49–58. [Google Scholar] [CrossRef]
- Yetisir, H.; Kurt, F.; Sari, N.; Tok, F.M. Rootstock potential of Turkish Lagenaria siceraria germplasm for watermelon: Plant growth, graft compatibility, and resistance to Fusarium. Turk. J. Agric. For. 2007, 31, 381–388. [Google Scholar]
- Zheng, N.; Wang, M.L.; Wang, H.T.; Ai, X.Z. Effects of grafting on photosynthesis of sweet pepper seedlings under low temperature and weak light intensity. Ying Yong Sheng Tai Xue Bao 2009, 20, 591–596. [Google Scholar]
- Yang, Y.; Yu, L.; Wang, L.; Guo, S. Bottle gourd rootstock-grafting promotes photosynthesis by regulating the stomata and non-stomata performances in leaves of watermelon seedlings under NaCl stress. J. Plant Physiol. 2015, 186, 50–58. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.C.; Alcaraz-Lopez, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological aspects of rootstock–scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Yetisir, H.; Sari, N. Effect of different rootstock on plant growth, yield and quality of watermelon. Aust. J. Exp. Agric. 2003, 43, 1269–1274. [Google Scholar] [CrossRef]
- Fallahi, E.; Colt, W.M.; Fallahi, B.; Chun, I.J. The importance of apple rootstocks on tree growth, yield, fruit quality, leaf nutrition, and photosynthesis with an emphasis on ‘Fuji’. J. Am. Soc. Hortic. Sci. 2002, 12, 38–44. [Google Scholar] [CrossRef]
- National Agricultural Products Quality Management Service (NAQS). National Agricultural Products Quality Management. Available online: https://www.naqs.go.kr/eng/contents/contents.do?menuId=MN20674 (accessed on 14 October 2022).
- Ohkawa, K.; Suematsu, M. Arching cultivation techniques for growing cut-roses. Acta Hort. 1999, 482, 47–51. [Google Scholar] [CrossRef]
- Kang, S.B.; Lee, I.B.; Park, J.M.; Lim, T.J. Effect of waterlogging conditions on the growth, root activities and nutrient content of ‘Campbell Early’ grapevine. Korean J. Hortic. Sci. Technol. 2010, 28, 172–179. [Google Scholar]
- Clément, C.; Burrus, M.; Audran, J.C. Floral organ growth and carbohydrate content during pollen development in lilium. Am. J. Bot. 1996, 83, 459–469. [Google Scholar] [CrossRef]
- Toshiki, A. Hydroponics—A Standard Methodology for Plant Biological Researches; BoD-Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Dieleman, J.A.; Meine, E. Interacting effects of temperature integration and light intensity on growth and development of single-stemmed cut rose plants. Sci. Hortic. 2007, 113, 182–187. [Google Scholar] [CrossRef]
- Kim, H.K.; Oh, S.I.; Lee, A.K. Quality of cut spray roses grown in a seasonal cultivation environment of a smart farm in honam, Korea. Flower Res. J. 2020, 28, 285–293. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, C.; Wang, W.; Cao, Z.; Li, S.; Li, H.; Zhu, W.; Huang, Y.; Bao, M.; He, Y.; et al. Comparative transcriptome analysis of different heat stress responses between self-root grafting line and heterogeneous grafting line in rose. Hortic. Plant J. 2021, 7, 243–255. [Google Scholar] [CrossRef]
- Qi, W. The Mechanism of Improving the Heat Resistance of Rose by Grafting. M.D. Dissertation, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Lawson, T.; Silvere, V.C. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- John, A.R. Speedy small stomata? J. Exp. Bot. 2014, 65, 1415–1424. [Google Scholar]
- Özyigit, İ.İ.; Akınci, S. Effects of some stress factors (Aluminum, Cadmium and Drought) on stomata of roman nettle (Urtica pilulifera L.). Not. Bot. Hortic. Agrobot. Cluj-Napoca 2009, 37, 108–115. [Google Scholar]
- Sadras, V.O.; Montoro, A.; Moran, M.A.; Aphalo, P.J. Elevated temperature altered the reaction norms of stomatal conductance in field-grown grapevine. Agric. For. Meteorol. 2012, 165, 35–42. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Josef, U.; Miles, W.I.; McGuire, M.A.; Robert, O.T. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot. 2017, 68, 1757–1767. [Google Scholar]
- Rezaee, R.; Vahdati, K.; Grigoorian, V.; Valizadeh, M. Walnut grafting success and bleeding rate as affected by different grafting methods and seedling vigour. J. Hortic. Sci. Biotechnol. 2008, 83, 94–99. [Google Scholar] [CrossRef]
- Yagishita, N.; Hirata, Y. Graft-induced change in fruit shape in Capsicum annuum L. I. genetic analysis by crossing. Euphytica 1987, 36, 809–814. [Google Scholar] [CrossRef]
- Tsaballa, A.; Athanasiadis, C.; Pasentsis, K.; Ganopoulos, I.; Obeidat, I.N.; Tsaftaris, A. Molecular studies of inheritable grafting induced changes in pepper fruit shape. Sci. Hortic. 2013, 149, 2–8. [Google Scholar] [CrossRef]




| Treatment | Stem Length (cm) | Stem Diameter (cm) | No. of Leaves (/stem) | Dry Stem Weight (g/Plant) | No. of Tepal (/Stem) | Yield (No. of Stem) |
|---|---|---|---|---|---|---|
| N1 | 105.4 ± 3.1 b Z | 3.7 ± 0.2 b | 29.6 ± 0.4 c | 10.1 ± 5.0 b | 37.4 ± 2.2 a | 5.7 ± 1.2 a |
| NA | 140.6 ± 7.2 a | 5.4 ± 0.1 a | 32.7 ± 3.3 a | 35.6 ± 3.2 a | 28.5 ± 1.3 b | 4.3 ± 1.2 a |
| RI | 132.1 ± 14.6 a | 5.0 ± 0.2 a | 32.0 ± 1.4 b | 32.0 ± 1.4 a | 30.9 ± 0.6 b | 4.7 ± 2.1 a |
| Index | Spring (19 April–May) | Summer (19 July–August) | Autumn (19 October–November) | Winter (20 January–February) |
|---|---|---|---|---|
| EC (dS·m−1) | 1.5 | 1.2 | 1.5 | 1.8 |
| pH | 5.8~6.0 | 5.8∼6.0 | 5.8~6.0 | 5.8~6.0 |
| IANS (mL) | 800 | 1000 | 800 | 700 |
| Season | Winter | Summer | ||
|---|---|---|---|---|
| Index | Stomata Length (μm) | Stomata Width (μm) | Stomata Length (μm) | Stomata Width (μm) |
| PB | 36.7 ± 2.9 a Z | 16.7 ± 2.9 c | 22.6 ± 0.7 b | 9.1 ± 0.5 b |
| N1 | 21.7 ± 2.9 c | 13.3 ± 2.9 cd | 16.7 ± 0.3 c | 7.1 ± 0.4 c |
| NA | 15.8 ± 1.4 d | 10.7 ± 1.2 d | 14.4 ± 0.2 d | 7.2 ± 0.4 c |
| RI | 18.3 ± 2.9 cd | 10.8 ± 1.4 d | 17.5 ± 0.2 c | 7.4 ± 0.9 c |
| PS/N1 | 34.2 ± 1.4 ab | 25.8 ± 1.4 a | 27.6 ± 1.0 a | 10.2 ± 0.4 a |
| PS/NA | 31.3 ± 2.3 b | 21.7 ± 1.4 b | 23.6 ± 0.7 b | 8.9 ± 0.3 b |
| PS/RI | 31.6 ± 3.0 b | 26.7 ± 2.9 a | 22.9 ± 1.0 b | 9.1 ± 0.2 b |
| Season | Index | PB | PB/N1 | PB/NA | PB/RI |
|---|---|---|---|---|---|
| Spring | Flower height (cm) | 5.5 ± 0.1 b Z | 5.6 ± 0.2 b | 5.8 ± 0.1 ab | 5.9 ± 0.1 a |
| Flower diameter (mm) | 32.5 ± 1.2 ab | 33.6 ± 1.0 a | 31.4 ± 1.2 b | 34.4 ± 0.8 a | |
| No. of petals | 58.8 ± 0.2 a | 56.2 ± 1.3 b | 48.1 ± 2.1 d | 50.5 ± 0.8 c | |
| Summer | Flower height (cm) | 4.6 ± 0.2 a | 4.3 ± 0.1 a | 4.7 ± 0.2 a | 4.6 ± 0.4 a |
| Flower diameter (mm) | 23.1 ± 1.3 a | 22.7 ± 0.7 a | 23.4 ± 1.9 a | 23.1 ± 0.6 a | |
| No. of petals | 56.5 ± 2.4 b | 64.5 ± 2.3 a | 52.2 ± 0.5 c | 54.9 ± 2.2 bc | |
| Autumn | Flower height (cm) | 5.5 ± 0.4 a | 5.4 ± 0.0 a | 5.7 ± 0.3 a | 5.6 ± 0.1 a |
| Flower diameter (mm) | 31.4 ± 2.6 a | 31.6 ± 1.7 a | 32.9 ± 1.1 a | 33.1 ± 0.2 a | |
| No. of petals | 61.1 ± 7.2 a | 62.3 ± 0.8 a | 53.7 ± 4.2 a | 55.1 ± 2.6 a | |
| Winter | Flower height (cm) | 5.5 ± 0.1 c | 5.5 ± 0.2 bc | 6.0 ± 0.1 a | 5.8 ± 0.1 ab |
| Flower diameter (mm) | 33.2 ± 0.3 a | 32.5 ± 0.2 a | 32.2 ± 1.0 a | 33.2 ± 1.5 a | |
| No. of petals | 58.5 ± 1.0 a | 56.1 ± 2.0 a | 48.0 ± 2.3 b | 49.2 ± 0.3 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, O.-H.; Choi, H.-G.; Kim, S.-J.; Lee, Y.-R.; Jung, H.-H.; Park, K.-Y. Changes in Yield, Quality, and Morphology of Three Grafted Cut Roses Grown in a Greenhouse Year-Round. Horticulturae 2022, 8, 655. https://doi.org/10.3390/horticulturae8070655
Kwon O-H, Choi H-G, Kim S-J, Lee Y-R, Jung H-H, Park K-Y. Changes in Yield, Quality, and Morphology of Three Grafted Cut Roses Grown in a Greenhouse Year-Round. Horticulturae. 2022; 8(7):655. https://doi.org/10.3390/horticulturae8070655
Chicago/Turabian StyleKwon, O-Hyeon, Hyo-Gil Choi, Se-Jin Kim, Young-Ran Lee, Hyun-Hwan Jung, and Ki-Young Park. 2022. "Changes in Yield, Quality, and Morphology of Three Grafted Cut Roses Grown in a Greenhouse Year-Round" Horticulturae 8, no. 7: 655. https://doi.org/10.3390/horticulturae8070655
APA StyleKwon, O.-H., Choi, H.-G., Kim, S.-J., Lee, Y.-R., Jung, H.-H., & Park, K.-Y. (2022). Changes in Yield, Quality, and Morphology of Three Grafted Cut Roses Grown in a Greenhouse Year-Round. Horticulturae, 8(7), 655. https://doi.org/10.3390/horticulturae8070655






