Characterization of Environmental Effects on Flowering and Plant Architecture in an Everbearing Strawberry F1-Hybrid by Meristem Dissection and Gene Expression Analysis
Abstract
:1. Introduction
2. Materials and Methods
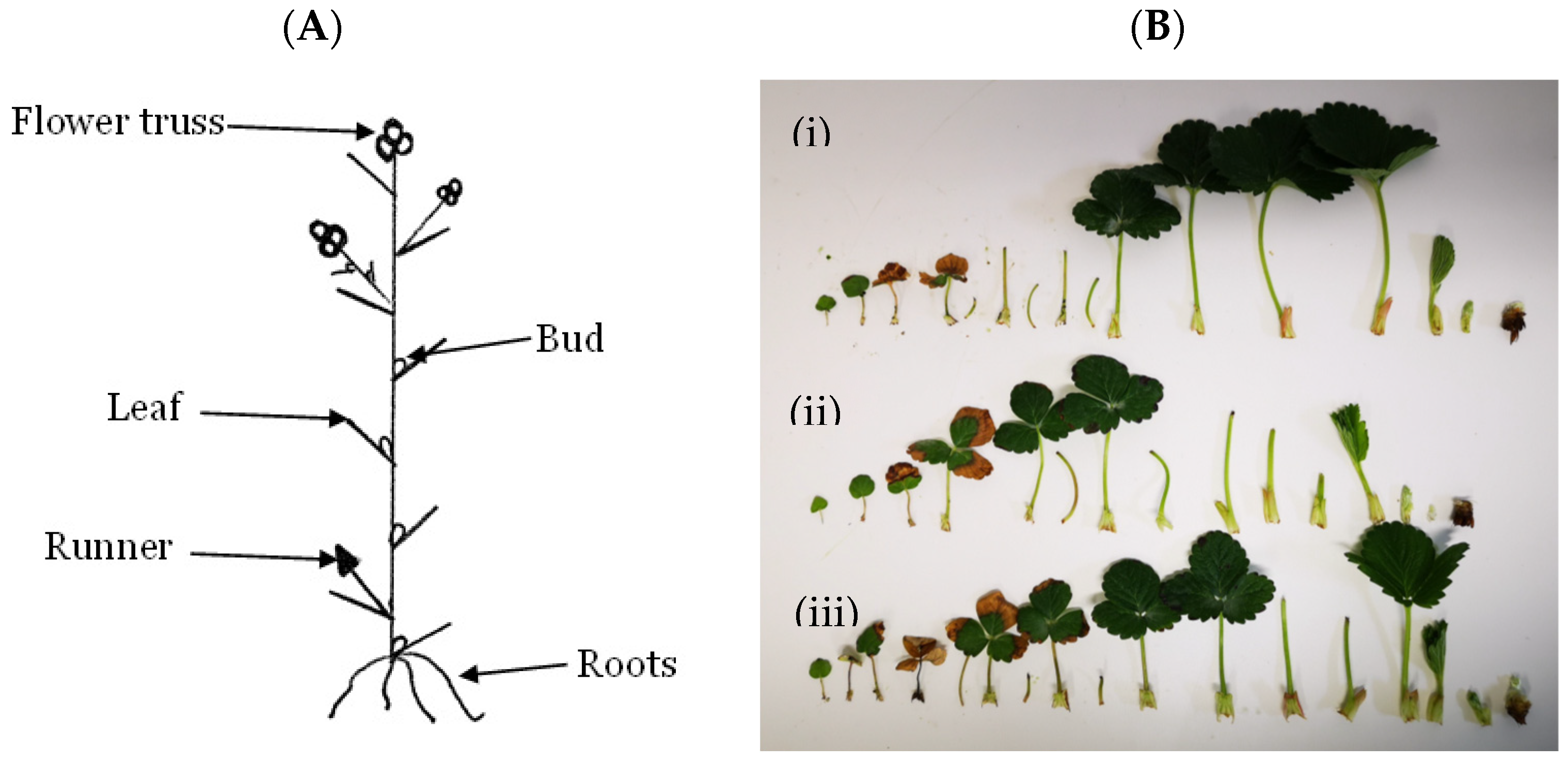
2.1. Plant Material, Growing Conditions and Sampling
2.2. Assessment of Flower Bud Initiation and Flowering Status
2.3. RNA Extraction and Quantitative RT-PCR
2.4. Statistical Analysis
3. Results
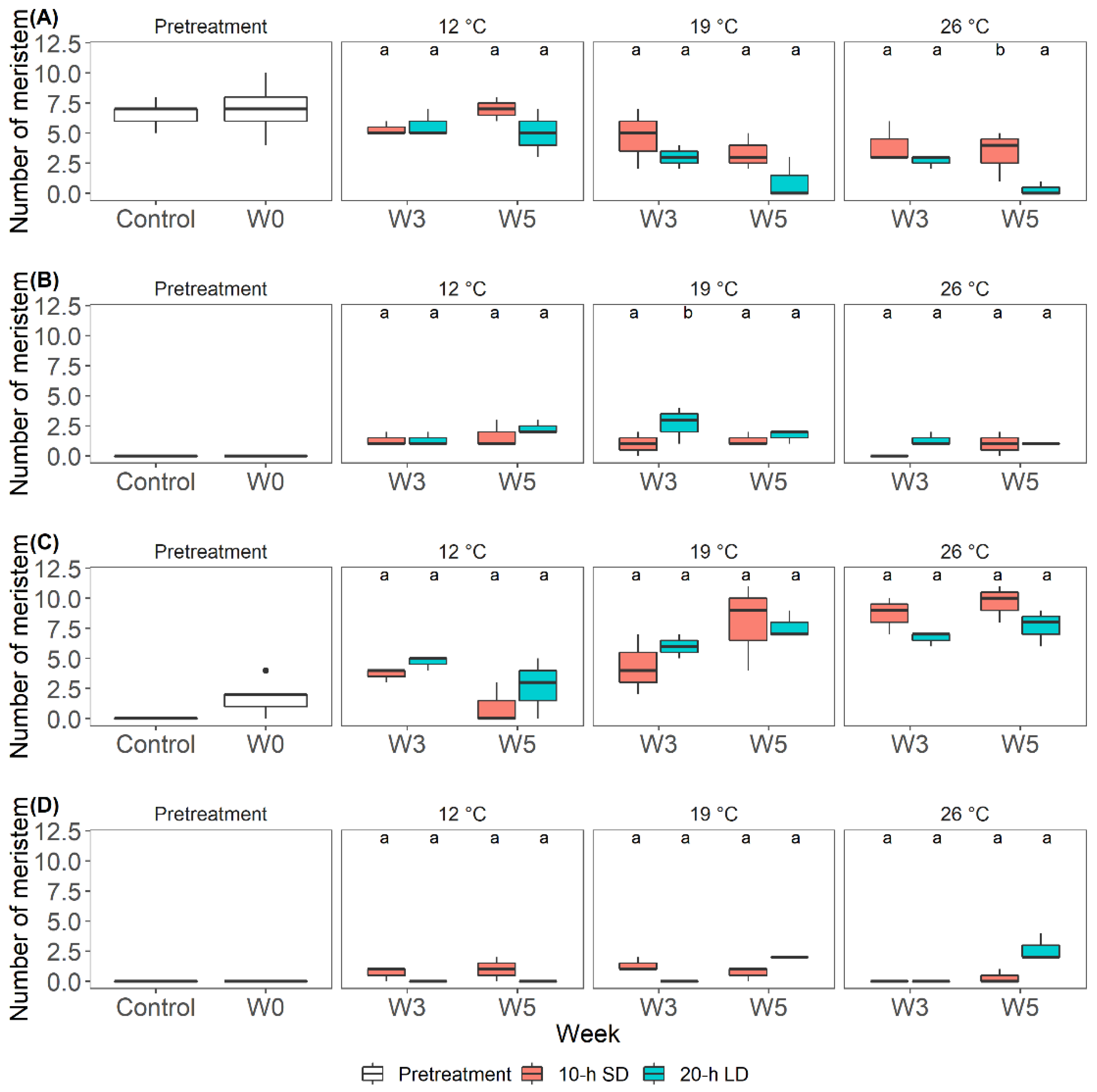
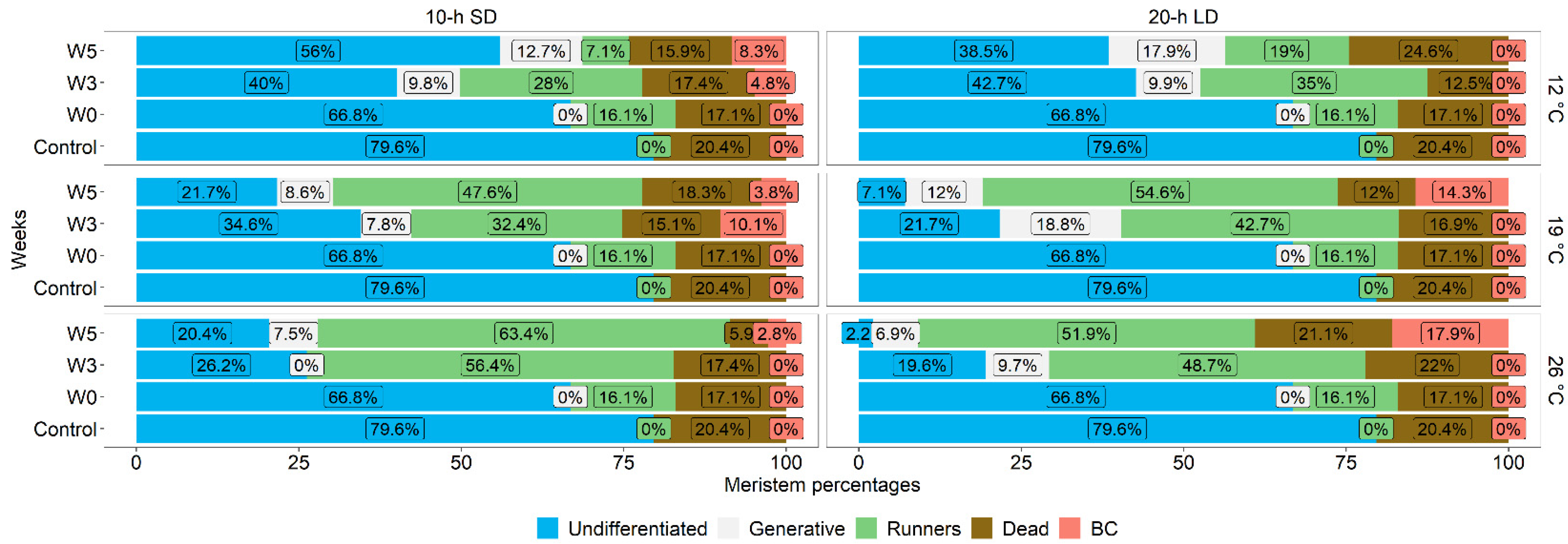
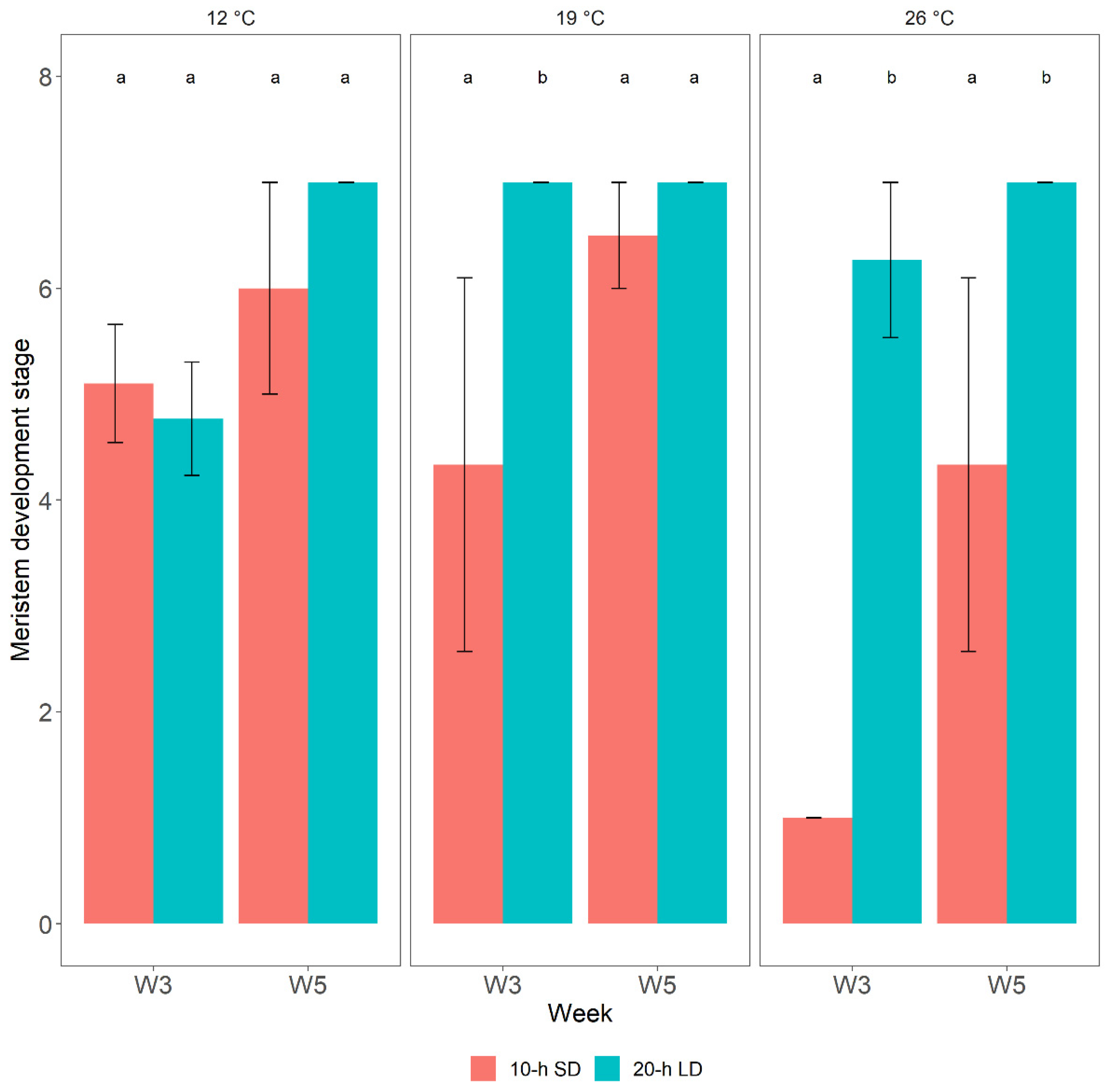
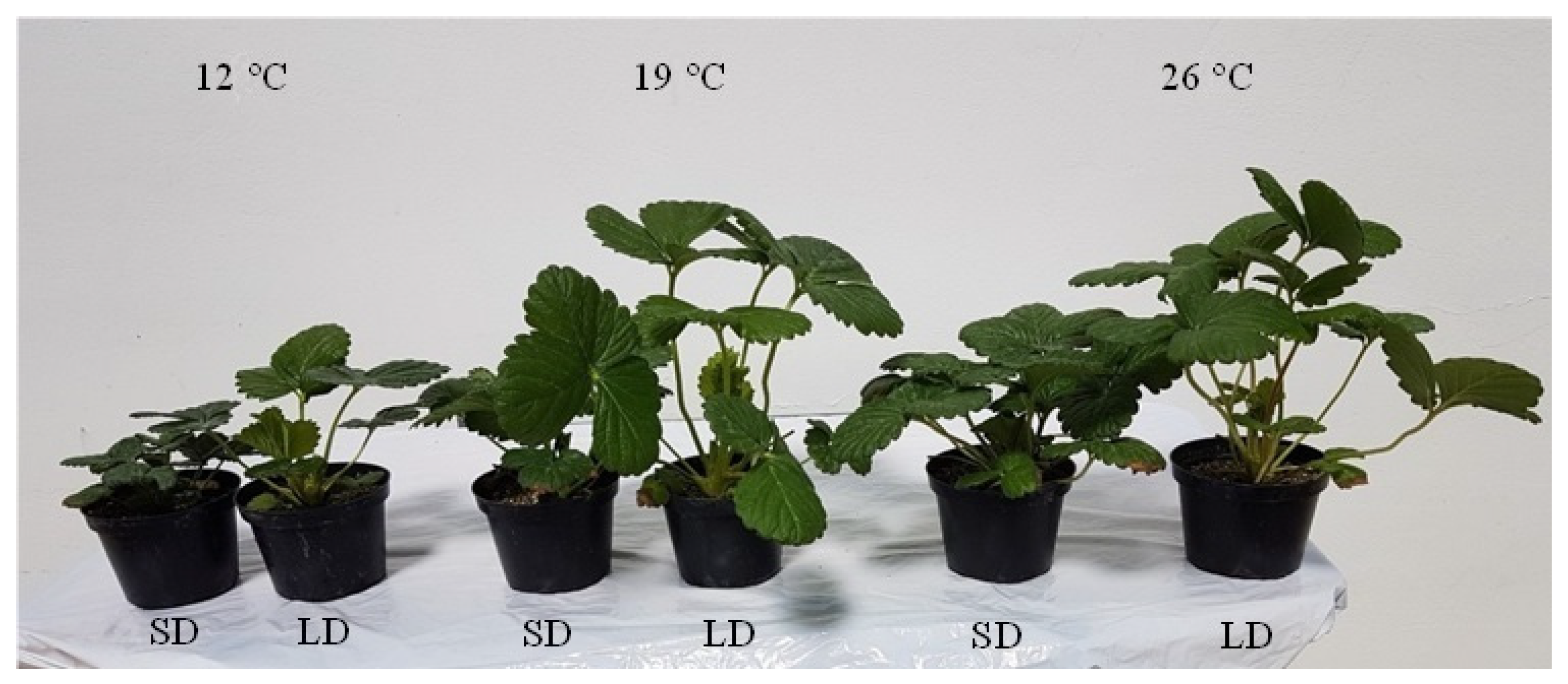
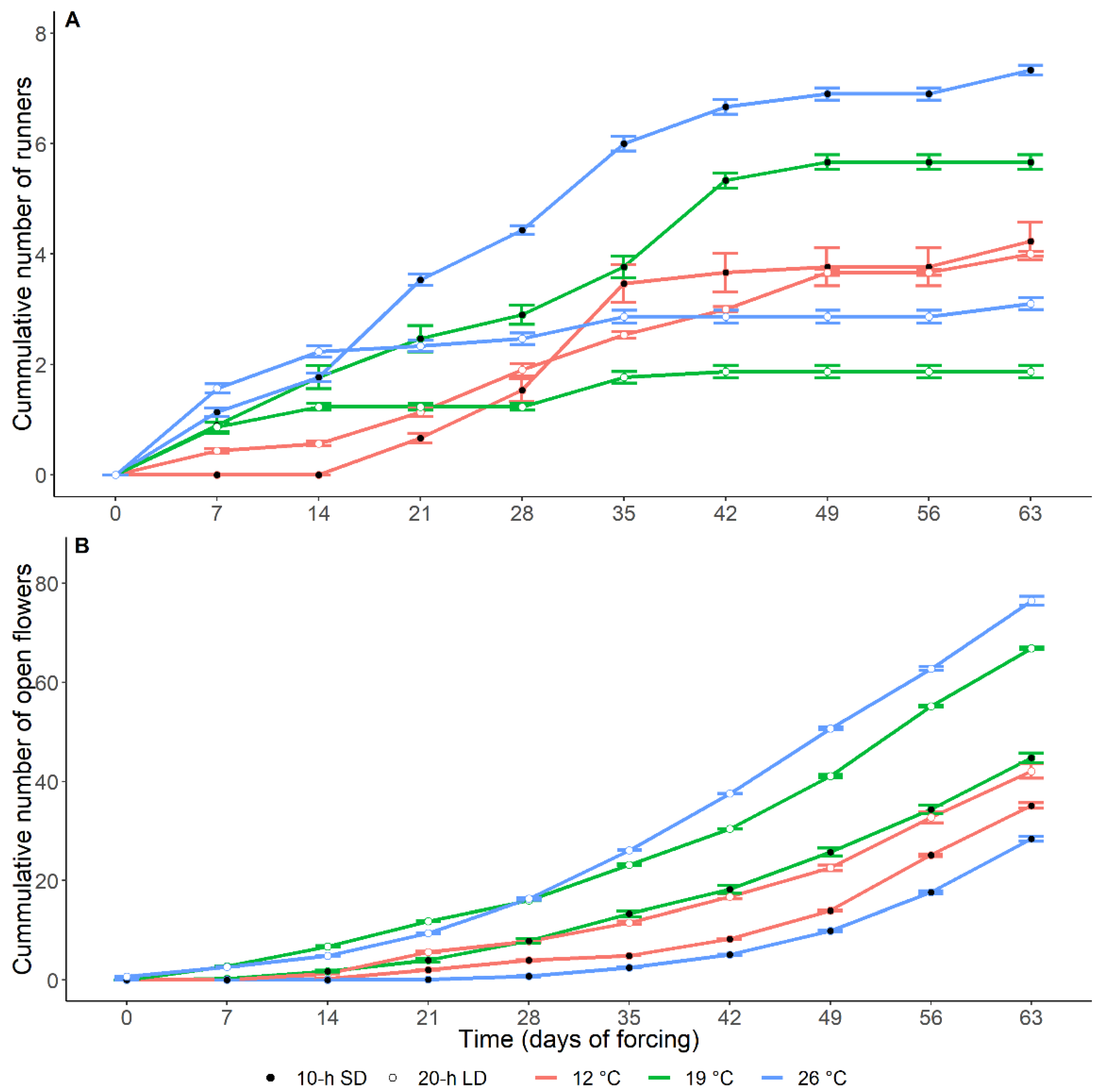
3.1. Effects of the Environment on Plant Growth and Development
3.2. Post-Treatment Plant Development
3.3. FaFT1 Expression in Leaves
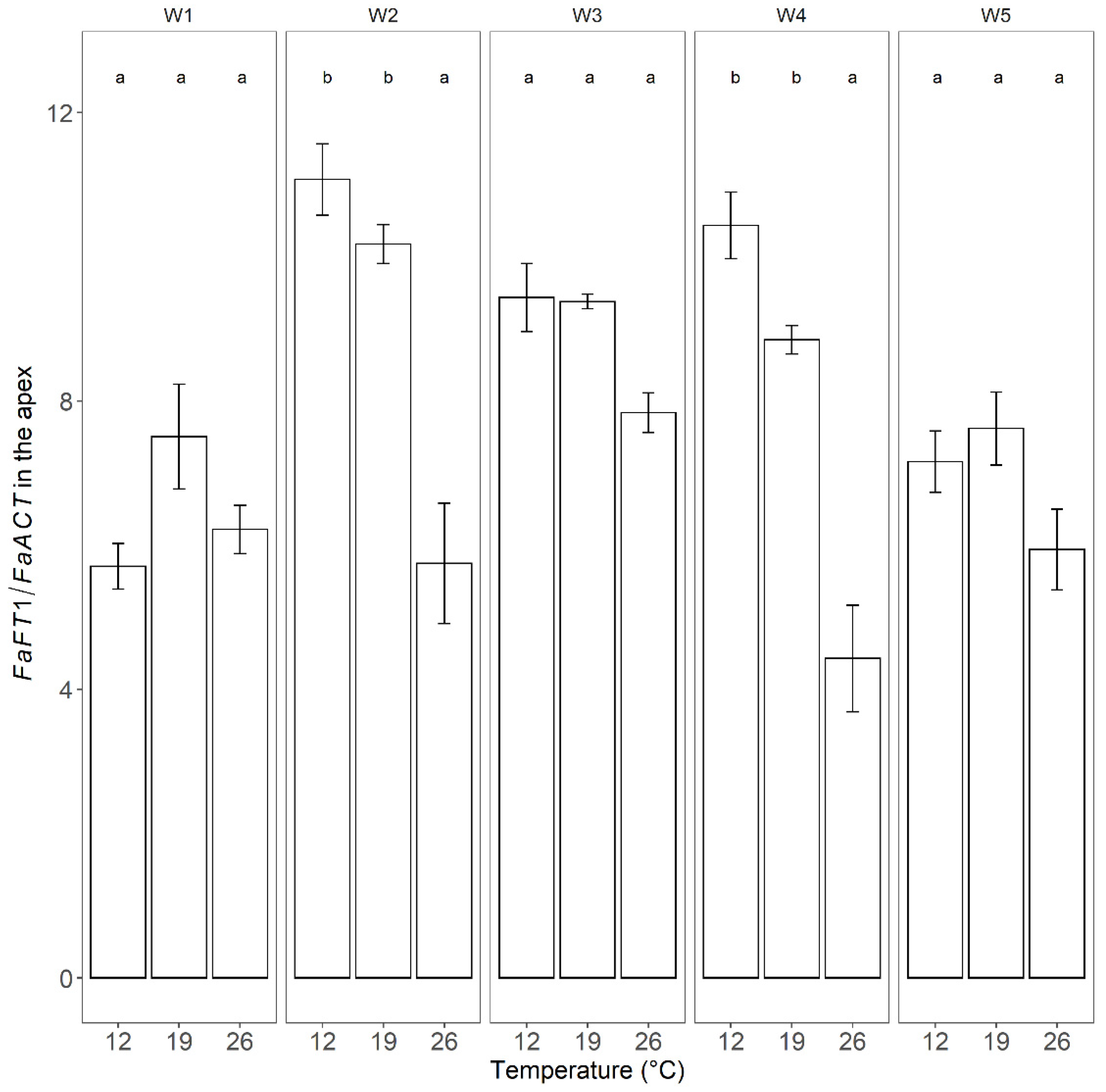
3.4. Effect of Photoperiod and Temperature on Flowering Genes in the Apex
4. Discussion
4.1. Environmental Effects on General Plant Growth and Development
4.2. Effects of the Environment on FaFT1 Expression
4.3. Post-Induction Floral Bud Initiation
4.4. Environmental Effects on FaFT2 and FaFT3 Expression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darrow, G.M.; Waldo, G.F. Responses of Strawberry Varieties and Species to Duration of the Daily Light Period; Bulletin Tech No. 1488-2016-124781; U.S. Department of Agriculture: Washington, DC, USA, 1934; pp. 1–31. [Google Scholar]
- Sønsteby, A.; Heide, O.M. Long-Day Control of Flowering in Everbearing Strawberries. J. Hortic. Sci. Biotechnol. 2007, 82, 875–884. [Google Scholar] [CrossRef]
- Heide, O.M.; Stavang, J.A.; Sønsteby, A. Physiology and Genetics of Flowering in Cultivated and Wild Strawberries—A Review. J. Hortic. Sci. Biotechnol. 2013, 88, 1–18. [Google Scholar] [CrossRef]
- Samad, S.; Butare, D.; Marttila, S.; Sønsteby, A.; Khalil, S. Effects of Temperature and Photoperiod on the Flower Potential in Everbearing Strawberry as Evaluated by Meristem Dissection. Horticulturae 2021, 7, 484. [Google Scholar] [CrossRef]
- Taylor, D.R. The Physiology of Flowering in Strawberry. Acta Hortic. 2002, 245–251. [Google Scholar] [CrossRef]
- Ito, H.; Saito, T. Studies on the Flower Formation in the Strawberry Plants. Tohoku J. Agric. Res. 1962, 13, 191–203. [Google Scholar]
- Guttridge, C.G. Fragaria x Ananassa. In CRC Handbook of Flowering; Halevy, A.H., Ed.; CRC Press: New York, NY, USA, 1985; Volume 3, pp. 16–33. [Google Scholar]
- Sønsteby, A.; Opstad, N.; Heide, O.M. Environmental Manipulation for Establishing High Yield Potential of Strawberry Forcing Plants. Sci. Hortic. 2013, 157, 65–73. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O.M. Flowering Performance and Yield of Established and Recent Strawberry Cultivars (Fragaria x Ananassa) as Affected by Raising Temperature and Photoperiod. J. Hortic. Sci. Biotechnol. 2017, 92, 367–375. [Google Scholar] [CrossRef]
- Rivero, R.; Remberg, S.F.; Heide, O.M.; Sønsteby, A. Environmental Regulation of Dormancy, Flowering and Runnering in Two Genetically Distant Everbearing Strawberry Cultivars. Sci. Hortic. 2021, 290, 110515. [Google Scholar] [CrossRef]
- Durner, E.F. Plant Architecture of “Albion” Strawberry (Fragaria × Ananassa Duch.) Is Not Influenced by Light Source during Conditioning. AIMS Agric. Food 2018, 3, 246–265. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O.M. Quantitative Long-Day Flowering Response in the Perpetual-Flowering F1 Strawberry Cultivar Elan. J. Hortic. Sci. Biotechnol. 2007, 82, 266–274. [Google Scholar] [CrossRef]
- Gallace, N.; Lieten, P.; Boonen, M.; Bylemans, D. Reduced Winter Chill as a Means to Improve the Production Potential of Late Day-Neutral Strawberry Cultivars. European J. Hortic. Sci. 2019, 84, 20–23. [Google Scholar] [CrossRef]
- Gallace, N.; Lieten, P. Winter Manipulation of Photoperiod and Chill to Enhance the Perpetual Flowering Nature of “Soprano". Acta Hortic. 2021, 1309, 333–340. [Google Scholar] [CrossRef]
- Savini, G.; Neri, D.; Mercadante, L.; Molari, G.; Magnani, D.; Capriolo, G. Meristem Analysis on Strawberry Plants during Propagation and Production. Acta Hortic. 2006, 708, 237–240. [Google Scholar] [CrossRef]
- van Delm, T.; Melis, P.; Stoffels, K.; van de Vyver, F.; Baets, W. Strawberry Plant Architecture and Flower Induction in Plant Production and Strawberry Cultivation. Acta Hortic. 2014, 1049, 489–494. [Google Scholar] [CrossRef]
- Koembuoy, K.; Hasegawa, S.; Otagaki, S.; Takahashi, H.; Nagano, S.; Isobe, S.; Shiratake, K.; Matsumoto, S. RNA-Seq Analysis of Meristem Cells Identifies the FaFT3 Gene as a Common Floral Inducer in Japanese Cultivated Strawberry. Hortic. J. 2020, 89, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Bosc, J.P.; Neri, D.; Massetani, F.; Bardet, A. Relationship between Plant Architecture and Fruit Production of the Short-Day Strawberry Cultivar Gariguette. J. Berry Res. 2012, 2, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Koskela, E.A.; Sønsteby, A.; Flachowsky, H.; Heide, O.M.; Hanke, M.-V.; Elomaa, P.; Hytönen, T. TERMINAL FLOWER1 Is a Breeding Target for a Novel Everbearing Trait and Tailored Flowering Responses in Cultivated Strawberry (Fragaria x Ananassa Duch.). Plant Biotechnol. J. 2016, 14, 1852–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, Y.; Higuchi, Y.; Yoshida, Y.; Hisamatsu, T. Environmental Responses of the FT/TFL1 Gene Family and Their Involvement in Flower Induction in Fragaria × Ananassa. J. Plant Physiol. 2015, 177, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Sabbadini, S.; Gaston, A.; Potier, A.; Denoyes, B.; Cappelletti, R.; Giovanetti, G.; Mezzetti, B. Isolation and Phenotypical Characterization of the FT-like Genes in Strawberry (Fragaria × Ananassa). Acta Hortic. 2021, 1309, 217–221. [Google Scholar] [CrossRef]
- Otagaki, S.; Koembuoy, K.; Takahashi, H.; Isobe, S.; Matsumoto, S. Gene Expression Profiling of Leaves in Conjunction with a Floral Bud Differentiation Process in Cultivated Strawberry “Akihime”. Acta Hortic. 2021, 1309, 19–24. [Google Scholar] [CrossRef]
- Koembuoy, K.; Nakajima, R.; Otagaki, S.; Kurokura, T.; Takahashi, H.; Nakazono, M.; Shiratake, K.; Matsumoto, S. Functional Analyses of Cultivated Strawberry FT and TFL1 Homologs. Acta Hortic. 2017, 1156, 95–102. [Google Scholar] [CrossRef]
- Nakajima, R.; Otagaki, S.; Yamada, K.; Shiratake, K.; Matsumoto, S. Molecular Cloning and Expression Analyses of FaFT, FaTFL, and FaAP1 Genes in Cultivated Strawberry: Their Correlation to Flower Bud Formation. Biol. Plant. 2014, 58, 641–648. [Google Scholar] [CrossRef]
- Mouhu, K.; Kurokura, T.; Koskela, E.A.; Albert, V.A.; Elomaa, P.; Hytönen, T. The Fragaria Vesca Homolog of Suppressor of Overexpression of Constans1 Represses Flowering and Promotes Vegetative Growth. Plant Cell 2013, 25, 3296–3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koskela, E.A.; Mouhu, K.; Albani, M.C.; Kurokura, T.; Rantanen, M.; Sargent, D.J.; Battey, N.H.; Coupland, G.; Elomaa, P.; Hytönen, T. Mutation in TERMINAL FLOWER1 Reverses the Photoperiodic Requirement for Flowering in the Wild Strawberry Fragaria Vesca. Plant Physiol. 2012, 159, 1043–1054. [Google Scholar] [CrossRef] [Green Version]
- Kurokura, T.; Samad, S.; Koskela, E.; Mouhu, K.; Hytönen, T. Fragaria Vesca CONSTANS Controls Photoperiodic Flowering and Vegetative Development. J. Exp. Bot. 2017, 68, 4839–4850. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, J.; Sun, H.; Wang, S.; Chen, K.; Liu, Y.; Li, H.; Ma, Y.; Zhang, Z. FveRGA1, Encoding a DELLA Protein, Negatively Regulates Runner Production in Fragaria Vesca. Planta 2017, 247, 941–951. [Google Scholar] [CrossRef]
- Wei, W.; Hu, Y.; Cui, M.-Y.; Han, Y.-T.; Gao, K.; Feng, J.-Y. Identification and Transcript Analysis of the TCP Transcription Factors in the Diploid Woodland Strawberry Fragaria Vesca. Front. Plant Sci. 2016, 7, 1937. [Google Scholar] [CrossRef] [Green Version]
- Rantanen, M.; Kurokura, T.; Jiang, P.; Mouhu, K.; Hytönen, T. Strawberry Homologue of TERMINAL FLOWER1 Integrates Photoperiod and Temperature Signals to Inhibit Flowering. Plant J. 2015, 82, 163–173. [Google Scholar] [CrossRef]
- Shulaev, V.; Sargent, D.J.; Crowhurst, R.N.; Mockler, T.C.; Folkerts, O.; Delcher, A.L.; Jaiswal, P.; Mockaitis, K.; Liston, A.; Mane, S.P.; et al. The Genome of Woodland Strawberry (Fragaria Vesca). Nat Genet. 2011, 43, 109–116. [Google Scholar] [CrossRef]
- Andrés, J.; Caruana, J.; Liang, J.; Samad, S.; Monfort, A.; Liu, Z.; Hytönen, T.; Koskela, E.A. Erratum to: Woodland Strawberry Axillary Bud Fate Is Dictated by a Crosstalk of Environmental and Endogenous Factors. Plant Physiol. 2022, 188, 674. [Google Scholar] [CrossRef]
- Ahmadi, H.; Bringhurst, R.S.; Voth, V. Modes of Inheritance of Photoperiodism in Fragaria. J. Amer. Soc. Hort. Sci. 1990, 115, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Castro, P.; Bushakra, J.M.; Stewart, P.; Weebadde, C.K.; Wang, D.; Hancock, J.F.; Finn, C.E.; Luby, J.J.; Lewers, K.S. Genetic Mapping of Day-Neutrality in Cultivated Strawberry. Mol. Breed. 2015, 35, 1–16. [Google Scholar] [CrossRef]
- Honjo, M.; Nunome, T.; Kataoka, S.; Yano, T.; Hamano, M.; Yamazaki, H.; Yamamoto, T.; Morishita, M.; Yui, S. Simple Sequence Repeat Markers Linked to the Everbearing Flowering Gene in Long-Day and Day-Neutral Cultivars of the Octoploid Cultivated Strawberry Fragaria × Ananassa. Euphytica 2016, 209, 291–303. [Google Scholar] [CrossRef]
- Liang, J.; Zheng, J.; Wu, Z.; Wang, H. Time-Course Transcriptomic Profiling of Floral Induction in Cultivated Strawberry. Int. J. Mol. Sci. 2022, 23, 6126. [Google Scholar] [CrossRef]
- Bradford, E.; Hancock, J.F.; Warner, R.M. Interactions of Temperature and Photoperiod Determine Expression of Repeat Flowering in Strawberry. J. Am. Soc. Hortic. Sci. 2010, 135, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.R.; Atkey, P.T.; Wickenden, M.F.; Crisp, C.M. A Morphological Study of Flower Initiation and Development in Strawberry (Fragaria x Ananassa) Using Cryo-Scanning Electron Microscopy. Ann. Appl. Biol. 1997, 130, 141–152. [Google Scholar] [CrossRef]
- Monte, D.; Somerville, S. Pine Tree Method for Isolation of Plant RNA. In DNA Microarrays: A Molecular Cloning Manual; Bowtell, D., Sambrook, J., Eds.; Cold Spring Harbour Laboratory Press: New York, NY, USA, 2002; pp. 124–126. [Google Scholar]
- RStudio Core Team. R: A Language and Environment for Statistical Computing; RStudio Core Team: Vienna, Austria, 2020. [Google Scholar]
- Dale, A.; Taghavi, T. Juvenile Runnering in F 1 Hybrid Strawberry and Its Effect on Inflorescence Numbers. Acta Hortic. 2021, 1309, 7–12. [Google Scholar] [CrossRef]
- Massetani, F.; Neri, D. Strawberry Plant Architecture in Different Cultivation Systems. Acta Hortic. 2016, 1117, 291–296. [Google Scholar] [CrossRef]
- Gianluca, S.; Letouzé, A.; Sabbadini, C.; Neri, D. Evaluation Of Tray-Plant Quality In The Propagation Phase. Acta Hortic. 2006, 708, 231–236. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O.M. Extreme Short-Day Induction Requirements for Flowering in the Late-Flowering Strawberry Cultivar “Malwina”. J. Hortic. Sci. Biotechnol. 2019, 95, 532–538. [Google Scholar] [CrossRef]
- Karapatzak, E.K.; Wagstaffe, A.; Hadley, P.; Battey, N.H. High-Temperature-Induced Reductions in Cropping in Everbearing Strawberries (Fragaria × Ananassa) Are Associated with Reduced Pollen Performance. Ann. Appl. Biol. 2012, 161, 255–265. [Google Scholar] [CrossRef]
- Lei, H.; Yuan, H.; Liu, Y.; Guo, X.; Liao, X.; Liu, L.; Wang, Q.; Li, T. Identification and Characterization of FaSOC1, a Homolog of Suppressor of Overexpression of Constans1 from Strawberry. Gene 2013, 531, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Perrotte, J.; Gaston, A.; Potier, A.; Petit, A.; Rothan, C.; Denoyes, B. Narrowing down the Single Homoeologous FaPFRU Locus Controlling Flowering in Cultivated Octoploid Strawberry Using a Selective Mapping Strategy. Plant Biotechnol. J. 2016, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sønsteby, A.; Heide, O.M. Long-Day Rather than Autonomous Control of Flowering in the Diploid Everbearing Strawberry Fragaria Vesca Ssp. Semperflorens. J. Hortic. Sci. Biotechnol. 2008, 83, 360–366. [Google Scholar] [CrossRef]

| Date | Days After Sowing | Week of Treatment | Leaf Sampling | Apex Sampling | Phenotyping |
|---|---|---|---|---|---|
| 14 April | 29 days | Control | x | x | x |
| 30 April | 43 days | W0 | x | x | |
| 7 May | 50 days | W1 | x | ||
| 14 May | 57 days | W2 | x | ||
| 20 May | 63 days | W3 | x | x | x |
| 28 May | 71 days | W4 | x | ||
| 4 June | 78 days | W5 | x | x | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samad, S.; Rivero, R.; Kalyandurg, P.B.; Vetukuri, R.R.; Heide, O.M.; Sønsteby, A.; Khalil, S. Characterization of Environmental Effects on Flowering and Plant Architecture in an Everbearing Strawberry F1-Hybrid by Meristem Dissection and Gene Expression Analysis. Horticulturae 2022, 8, 626. https://doi.org/10.3390/horticulturae8070626
Samad S, Rivero R, Kalyandurg PB, Vetukuri RR, Heide OM, Sønsteby A, Khalil S. Characterization of Environmental Effects on Flowering and Plant Architecture in an Everbearing Strawberry F1-Hybrid by Meristem Dissection and Gene Expression Analysis. Horticulturae. 2022; 8(7):626. https://doi.org/10.3390/horticulturae8070626
Chicago/Turabian StyleSamad, Samia, Rodmar Rivero, Pruthvi Balachandra Kalyandurg, Ramesh Raju Vetukuri, Ola M. Heide, Anita Sønsteby, and Sammar Khalil. 2022. "Characterization of Environmental Effects on Flowering and Plant Architecture in an Everbearing Strawberry F1-Hybrid by Meristem Dissection and Gene Expression Analysis" Horticulturae 8, no. 7: 626. https://doi.org/10.3390/horticulturae8070626
APA StyleSamad, S., Rivero, R., Kalyandurg, P. B., Vetukuri, R. R., Heide, O. M., Sønsteby, A., & Khalil, S. (2022). Characterization of Environmental Effects on Flowering and Plant Architecture in an Everbearing Strawberry F1-Hybrid by Meristem Dissection and Gene Expression Analysis. Horticulturae, 8(7), 626. https://doi.org/10.3390/horticulturae8070626






