Abstract
Over the past few decades, numerous studies investigated the vigor and productivity of fruit species depending on the rootstock on which they were grafted, but the exact size-controlling mechanism itself has not been fully elucidated, nor were the rapid rootstock selection methods defined. Thus, this study aimed to assess the root anatomical characteristics and their influence on the overall ‘Summit’ cherry tree vigor to confirm the size-controlling effect and establish an effective protocol for rapid rootstock selection. Plant material included three cherry species (Prunus cerasus, Prunus fruticosa, and Prunus mahaleb) and interspecific hybrid ‘Gisela 5′ (P. cerasus × Prunus canescens) as a control. The detailed anatomical analysis included root samples with the differentiated secondary structure taken from the sampling depth of 10–15 cm. Roots with percentages of vessels ≈40%, ≈50%, and ≈10% belonging to size-classes ˂700 µm2, 700–2000 μm2, and ˃2000 µm2 (respectively) are presumed to provide optimal amounts of water solution to the scion, without compromising plant vitality, drought tolerance, and size-controlling effect. Statistically significant correlations were determined between anatomical properties (the percentage of vessels, especially ˃2000 µm2, xylem porosity, and hydraulic conductivity, both per mm2 and total root) and vegetative growth in the juvenile vegetative phase, indicating direct vessel size influence on plant vigor and its employment in size-controlling cherry rootstock selection.
1. Introduction
Orchard establishment, in addition to choosing a variety with good biological and yielding characteristics, requires the appropriate rootstock selection. Although the ability to reduce vigor has been determined for many rootstocks, the exact size-controlling mechanism itself has not been fully elucidated. Over the past few decades, a number of authors have studied the vigor and productivity of fruit species depending on the rootstock on which they were grafted [1,2,3,4,5,6,7]. The rootstock influence on the water supply and transport of dissolved substances to the aboveground part, as well as the metabolism and translocation of plant hormones, are considered as potential reasons for vigor reduction in trees grafted on size-controlling rootstocks [2,8]. Secondary xylem characteristics, especially the vessel lumen area and number, as well as the closely related root hydraulic conductivity, are widely accepted parameters of low-vigorous rootstock selection [9,10,11,12,13,14,15,16]. Water conductance capacity through the plant can be estimated by calculating the theoretical hydraulic conductivity of participating tissues, which is directly related to the overall tree growth potential [13,17,18]. Solari et al. [19] found that water status affected the peach vegetative growth when grafted on different rootstocks. Similarly, Tombesi et al. [9,10] stated that phenotypic differences in the secondary wood characteristics, such as the vessel size and number in peach stems, trunk, and roots, significantly determine the capacity of hydraulic conductivity. A similar finding was obtained for cherry trees [11,20,21], emphasizing the application of anatomical properties such as vessel lumen area and number, the proportion of vessel area in the entire root/stem cross-sectional area, and portions of secondary wood and secondary cortex in predicting the overall grafted trees’ vigor. Recent findings determined lower vessel lumen areas in low-vigorous cultivar/rootstocks combinations compared to vigorous trees, as well as a positive correlation between average root vessel lumen area and length of annual branches, which confirmed the influence of anatomical parameters on apple and pears’ tree vigor [22]. In apple trees grafted on three rootstocks of a divergent size-controlling effect, different water requirements were determined [23], while a significant variation in apple leaf xylem properties was found due to grafting on rootstocks with different growth regulation capacities [24].
The rootstock influence on the yield and quality of cherry fruits was examined by a number of authors [25,26,27,28,29,30,31]. Trees on low-vigorous rootstocks are, in most cases, more productive than trees on vigorous ones [32]. The vigor reduction of grafted trees is often accompanied by a significant improvement in fruit quality, primarily skin color intensity, fruit size, and sugar content due to a lower degree of tree shading [33]. Tomaszevska and Nychnerewicz [34] proved the different influences of ‘Mazzard’, ‘Colt’, ‘PHL A’, and ‘Gisela 5′ rootstocks on the growth and fruiting of four sweet cherry varieties: ‘Burrlat’, ‘Cordia’, ‘Regina’, and ‘Van’. Low-vigorous rootstocks ‘PHL A’ and ‘Gisela 5′ reduced the tree vigor by 40–45% compared to the vigorous ones, with the highest vigor values and the lowest yielding determined for trees grafted on ‘Mazzard’. Sotirov [35] examined the rootstock influence on growth, productivity, and fruit quality of the ‘Summit’ cherry variety grafted on seven different rootstocks over 12 years. In the last vegetation, the highest tree vigor was determined on the ‘Ma × Ma 60′ rootstock, whilst ‘Gisela 5′ induced the weakest growth. Although the highest cumulative yield was achieved on the most dwarfing rootstocks, ‘Gisela 5′ and ‘Gisela 6′ negatively affected the achieved yield per tree, even in irrigation conditions. Due to such contradictory, unsatisfactory results, climate change, and the worldwide expansion of cherry production, constant work on the selection of dwarfing fruit rootstocks is necessary.
Due to different scion behavior after grafting on divergent rootstocks, even in the same climate and soil conditions, there is a need to examine the influence of different rootstocks on the growth and productivity of the same variety. Since each rootstock must go through a long time- and space-consuming selection period, cost-effective methods to shorten this procedure are being sought.
Thus, this study aimed to assess the root anatomical characteristics and their influence on the overall ‘Summit’ cherry tree vigor to confirm the size-controlling effect and establish an effective protocol for rapid rootstock selection.
2. Materials and Methods
2.1. Plant Material
Plant material included three cherry species and one interspecific hybrid of a complex ancestry: Prunus cerasus (‘Oblačinska’ sour cherry genotypes designated as PC), Prunus fruticosa (European ground cherry genotypes designated as PF), Prunus mahaleb (‘Mahaleb’ genotypes designated as PM), and ‘Gisela 5′ as a control (P. cerasus × P. canescens). The investigation included landraces and selections from natural populations, representing the autochthonous germplasm of Prunus species from Serbia. The origin of the rootstock candidates is designated with different locations and tier numbers (localities in Serbia), whereas for PC rootstock candidates 02 stands for Udovice, 04 stands for Rivica, and 06 stands for Nova Crvenka. Candidates of PF are designated with numbers 02 and 04 for location Fruška gora and 07 for Rimski Šančevi. The P. mahaleb rootstock candidates are designated with number 09 for Rimski Šančevi. After their in-situ characterization and collection of cuttings from mother trees in the listed localities, rootstock specimens were propagated by green, softwood cuttings (in high tunnels with a controlled environment, irrigated with the fogging system) and a new ex-situ collection was established in Autumn of 2015 for our investigation.
Further study was carried out at the experimental field of the Faculty of Agriculture, the University of Novi Sad, located in Rimski Šančevi, Northern Serbia (45°20′ N; 19°50′ E) at 80 m a.s.l, where ex-situ collection was established. This area is characterized as a continental climate with extremely warm summers and cold winters. During the experimental year 2021 (sixth orchard vegetation), the average annual temperature was 12.1 °C while the annual precipitation sum was 627 mm. Trees were distributed at a planting distance of 4 × 2 m. The experiment was established as a randomized complete block design which included five replicate plants per scion-rootstock combination. The field trial was not irrigated or pruned, and a minimal amount of herbicide was used to estimate the rootstock influence solely. Although the trees were planted on flat terrain exposed to the cold winds, frost protection was not implemented.
2.2. Anatomical Analysis
Anatomical measurements were performed on fully developed roots with secondary structure belonging to nine cherry genotypes (eight selections and one control). To preserve the structure of plant tissues, samples were fixed in 60% ethanol, with the addition of 10% of glycerin and 30% of water. Before cross-section preparation, plant material was immersed in 50% glacial acetic acid for 1–2 days to soften stems and facilitate sectioning. Cross-sections were obtained using a hand microtome and examined under a Motic Digital BA310 biological light microscope with a built-in digital camera. Images of cross-sections were taken at 40× and 400× magnifications and measurements were performed using the image analyzing system Motic Images Plus 2.0; Motic China Group Co., Ltd., Xiamen, Fujian, China. Cross-sections were obtained from approximately the middle of 1-year-old roots, with differentiated secondary structure as described by Zorić et al. [11]. On each root cross-section, anatomical characteristics were investigated on four radial segments, 90° apart. Anatomical analyses included both total cross-section and secondary wood measurements. On a total cross-section area, the percentage of the secondary wood (% SW), secondary cortex (% SC), and periderm (% PD) relative to the total area was calculated. Root cross-section was considered a regular circle [12]. At higher magnification, total vessel and ray areas, as well as xylem area per each visual field, were measured to calculate their percentages relative to the total secondary wood area. Based on their lumen area (VLA), vessels were classified into three classes: I—VLA < 700 μm2; II—VLA in the range 700–2000 μm2; and III—VLA > 2000 μm2 [11]. The number of vessels per class was expressed as a percentage of the total number of vessels (0–100%). Determined vessel lumen areas were employed for xylem porosity calculation (total vessel area relative to the total wood area). For further hydraulic conductance (kh) calculation, all captured vessel diameters (μm) were utilized. Based on the anatomical characterization, the theoretical axial hydraulic conductance (kh) per root mm2 and total root cross-section area were determined. The theoretical axial hydraulic conductance of roots was calculated according to the expression given by Tyree and Ewers [36], based on Hagen–Poisseuille’s law (Equation (1)):
where d was the diameter of the vessels in meters, ρ was the fluid density (assumed to be 103 kg × m−3 for water at 20 °C), and η was the viscosity (assumed to be 1.002 × 10−9 MPa × s for water at 20 °C).
2.3. Effective Crown Volume and Growth Rate Calculation
The investigated plants of all nine scion-rootstock combinations were measured to estimate the effective tree crown volume (Ve) for each tree. Crown height (m) and crown diameter (m) measurements were used in the tree crown volume calculation, according to the formula (Equation (2)) given by Changok [37]:
where CD represents crown diameter and CH represents crown height. A crown shape index was assigned to every plant as described by the same author.
CD2 × CH × crown shape index = tree crown volume (m3)
The effective crown volumes’ growth rates were determined for each vegetation period. Growth rates were calculated as a percentage of crown volume increase in the observed year in comparison to the values from the previous vegetation period. Additionally, the growth rate from the first to fifth experimental year (2017 to 2021) was determined. The average annual growth rate refers to the average percentage of crown volume enhancement on a 5-year basis.
During the crown analyses, besides quantitative measurements, plants were qualitatively screened for their vitality and presence of any signs of withering and cupped-leaves occurrence.
2.4. Statistical Methods
The obtained data were evaluated using Statistica 14 software (Tibco, Palo Alto, CA, USA). The experimental data were subjected to statistical analysis using Fisher’s factorial analysis of variance—ANOVA. The significance of differences in measured parameters between the samples determined by Duncan’s multiple range tests had a confidence limit p ≤ 0.05. Statistically significant correlation values were determined according to Pearson’s coefficients.
3. Results
3.1. Root Anatomical Characteristics of Investigated Cherry Rootstocks
Root anatomical characterization revealed significant differences between and within the species/groups of rootstock candidates (Figure 1). Great variability was found in terms of cross-sectional diameter, as root thickness ranged from 2.76 mm in PF_02_16 to 5.69 mm in PC_02_03/2 (Table 1). Observed between species, the average value of root diameter was the highest in the ‘Oblačinska’ cherry candidate (4.71 mm), while the lowest value was determined in the ‘Mahaleb’ candidate (3.95 mm).
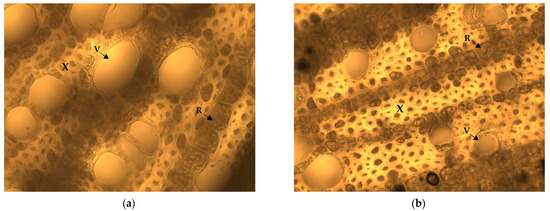
Figure 1.
Light micrographs of root cross-sections of investigated contrasting cherry species (a) PC_02_03/2; (b) PF_02_16. V—vessel, R—ray, X—xylem.

Table 1.
Root cross-section anatomical characteristics of investigated cherry rootstocks, belonging to Prunus cerasus (PC), Prunus fruticosa (PF), Prunus mahaleb (PM) species and control ‘Gisela 5′.
The percentage of secondary wood was the highest in the ‘Gisela 5′ rootstock, occupying more than half of the cross-sectional area (52.24%). The average values of this characteristic showed a higher percentage of secondary wood in ‘Mahaleb’ (45.86%) compared to both European ground and ‘Oblačinska’ sour cherries. The lowest secondary wood portion was determined in PC_06_12 roots (27.85%), where the secondary cortex portion reached 64.74%. The secondary cortex portion was the lowest in the control (38.11%), and above 50% in all three ‘Oblačinska’ cherry candidates as well as in the European ground cherry PF_07_08 genotype. The periderm percentage ranged from 4.87% in PC_02_03/2 to 12.54% in PF_02_16. A higher share of secondary cortex in a relation to the secondary wood was found in all three ‘Oblačinska’ cherry candidates, and the ratio of these two tissues was also less than 1, and in two European ground cherry genotypes—PF_04_09 and PF_07_08, as well as ‘Mahaleb’ candidate PM_09_01.
In the total secondary wood area, the largest vessel area portion (above 20%) was found in the ‘Oblačinska’ sour cherry candidate with a maximum of 28.51% (PC_04_01), as well as in one European ground cherry candidate (PF_07_08) as presented in Table 2. The lowest vessel portion was found in PM_09_01 (10.21%), which did not statistically significantly differ from the remaining two European ground cherry candidates. Ray area in the secondary wood ranged from 22.94% in PM_09_02 to 36.29% in PC_02_03/2. The lowest xylem portion was found in the ‘Oblačinska’ cherry candidate (below 48%), while both ‘Mahaleb’ candidates and PF_02_16 were distinguished with more than 60% of the xylem.

Table 2.
Vessel, ray, and xylem areas relative to the total root secondary wood area, in the investigated cherry rootstocks, belonging to Prunus cerasus (PC), Prunus fruticosa (PF), Prunus mahaleb (PM), species and control ‘Gisela 5′.
Different vessels’ distribution to size-classes was determined both within and between species (Table 3). ‘Oblačinska’ and European ground cherry candidates, as well as ‘Mahaleb’, were characterized by the highest percentage of vessels from size-class 700–2000 µm2, followed by the portion of the smallest vessels and the lowest portion of vessels larger than 2000 µm2. In this respect, all candidates differed from the control rootstock, in which a very large portion of vessels smaller than 700 µm2 was determined (88.34%), while no vessel larger than 2000 µm2 was found in the examined roots. In rootstock candidates, over 44% of vessels from size-class I (˂700 µm2) were found in PF_02_16 and PF_04_09, while only 13.61% of the smallest vessels were found in the roots of PM_09_02. The smallest differences among the candidates were observed in terms of the percentages of vessels from size-class II (700–2000 µm2), and the share of this vessel size-class ranged from 41.80% in PC_02_03/2 to 57.69% in PF_07_08. The percentage of vessels from size-class III in rootstock candidates was in the range of 4.93–33.36%, with the highest values in PM_09_02, PC_02_03/2 and PC_04_01 and the lowest values in PF_04_09, PF_07_08, and PM_09_01 (less than 10%).

Table 3.
Percentage of different vessel size-classes in the root secondary xylem of the examined cherry rootstocks, belonging to Prunus cerasus (PC), Prunus fruticosa (PF), Prunus mahaleb (PM), species and control ‘Gisela 5′.
Observed at the level of the entire cross-section, the root porosity was the highest in all three ‘Oblačinska’ sour cherry candidates and in PF_07_08, which corresponds to the determined values of the previous parameters (Table 4). Porosity ranged from 10.2–28.5%, of which the PM_09_01, PF_02_16, and PF_04_09 roots had the lowest porosity.

Table 4.
Root porosity and theoretical hydraulic conductance (kh) per mm2 and total root area of the examined cherry rootstocks, belonging to Prunus cerasus (PC), Prunus fruticosa (PF), Prunus mahaleb (PM), species and control ‘Gisela 5′.
On average, the hydraulic conductivity per mm2 ranged from 0.327 × 10−5 kg m/MPa s in ‘Gisela 5′ to 1.886 × 10−5 kg m/MPa s in ‘Oblačinska’ sour cherry. Among the specific candidates, the lowest value was found for PF_04_09 (0.530 × 10−5 kg m/MPa s) and the highest for PC_04_01 (2.640 × 10−5 kg m/MPa s).
Theoretical hydraulic conductance (kh) of a single root ranged from 1.628 × 10−5 kg m/MPa s in PF_02_16 to 20.51 × 10−5 kg m/MPa s in PC_02_03/2. The average kh value for ‘Oblačinska’ cherry candidates was 13.33 × 10−5 kg m/MPa s, which was up to 6 times higher than the conductance of the control rootstock, while the average conductance of the European ground cherry and ‘Mahaleb’ roots in a relation to the control was up to 2.5 times higher.
3.2. Effective Crown Volume Reduction
Effective crown volumes calculated for ‘Summit’ cherry trees grafted on the selections and control rootstock ranged from 1.12 m3 in PF_02_16 to 5.28 m3 in PM_09_02. Effective crown volume reduction compared to the control rootstock ‘Gisela 5′ (amounting 2.54 m3 and considered as zero) ranged from −56% in PF_02_16 and −44% in PF_04_09, over −35%, −25%, and −24% in PM_09_01, PC_04_01, and PC_06_12 (respectively) to only −7% in PC_02_03/2. One PF selection caused a slight increase in vigor (+5% in PF_07_08), whilst ‘Mahaleb’ selection PM_09_02 amplified vigor up to +108% (Figure 2). Besides quantitative measurements, during fieldwork plants were qualitatively scored for their vitality. Even when not irrigated, plants on candidate rootstocks showed no signs of withering or cupped leaves, while plants on control rootstocks did suffer during the late spring and summer months.
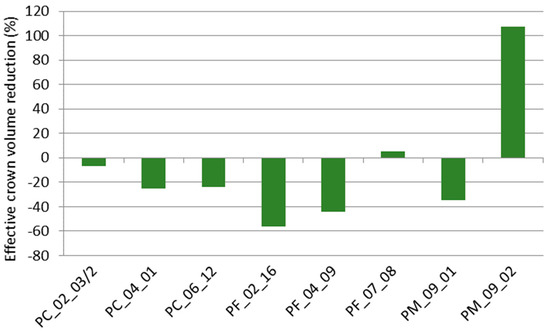
Figure 2.
Effective crown volume reduction of investigated ‘Summit’ cherry trees grafted on different size-controlling rootstocks (Prunus cerasus—PC, Prunus fruticosa—PF, Prunus mahaleb—PM) in relation to ‘Gisela 5′ considered as zero.
3.3. Correlation Analysis between Investigated Root Anatomical Characteristics and Crown Parameters
In addition to calculated crown volume reductions compared to control rootstock, a correlation analysis between investigated anatomical parameters and effective crown volume in 2021, as well as annual growth rates from 2017–2021, was performed (Table 5). Root anatomical characteristics did not show any statistically significant correlations with effective crown volumes measured in 2021, growth rates in 2021 relative to 2020, and growth rates in 2019 relative to 2018, while significant correlations were determined for the percentage of vessels and porosity of total secondary wood with growth rate in 2020 relative to 2019. Significant correlation coefficients emerged for growth rates in 2018 relative to 2017, growth rates in 2021 relative to 2017, as well as average annual growth rates and six anatomical characteristics: percentage of vessels in total secondary wood, percentage of xylem in total secondary wood, the porosity of total secondary wood, hydraulic conductivity per mm2 of secondary wood, hydraulic conductivity of total secondary wood, as well as the percentage of vessels > 2000 µm2.

Table 5.
Correlation coefficients between root anatomical characteristics and ‘Summit’ cherry crown parameters (underlined bold values were statistically significant at p < 0.05 according to Persons’ coefficients).
4. Discussion
A few decades ago, studies showed that the greatest vigor reduction of grafted cherry trees could be achieved by using rootstocks derived from sour and European ground cherries [38,39]. In addition to overall vigor reduction, sour cherry rootstocks alter fruit biochemical properties [40], drought stress tolerance [41], as well as productivity and yield efficiencies [42]. European ground cherry can be applied per se or contribute as a parent in hybridization during rootstock breeding [43,44] and is considered to be the most drought-resistant, transmitting this property both vegetatively and generatively [41]. During the selection process, each rootstock candidate must go through a long evaluation process, which requires significant space, theoretical and practical knowledge, as well as significant financial investments. A number of years are required to determine if there is a positive effect on scion precocity, vigor, yield, and finally fruit quality [45]. Even at nursery levels and semi-controlled conditions, evaluation of root systems is not easy to perform [46]. The definition of a reliable rootstock pre-selection method is of great importance since it shortens the time and effort requirements, concomitantly decreasing the cost of such research. On the other hand, such time- and resource savings enables a larger number of candidate testing, which increases the success chances of the entire process [13].
Although our previous study [18] has demonstrated that combined anatomical analysis, including fine and skeletal roots, rootstock stem, and scion stem, provides the most accurate vigor prediction, it is a demanding and invasive procedure that requires the destruction of investigated plants to obtain rootstock stem cross-sections. The pertinent methodology is not invasive, since 4 roots excavated from each side of the world shall not impair future root system growth, development, and proper functioning.
Root cross-sectional studies have shown that anatomical features differ significantly between and within species/groups of rootstock candidates. A higher portion of secondary wood on the total cross-section cannot be strictly associated with higher vigor, since the entire surface of secondary wood is not responsible for water conduction [11], i.e., roots with similar diameters may have very different roots tissue densities [47]. Roots of the control rootstock, where the low values of the grafted trees’ crown volumes were measured, were characterized by a very high secondary wood portion (˃50%). It seems that this property is more species-specific since crown volume reductions and wood percentages had the same trend ‘Mahaleb’ ˃ European ground cherry ˃ ‘Oblačinska’ sour cherry. Further analysis of vessel, ray, and xylem portion, in the root secondary wood, indicated a strong connection of these characteristics with the vegetative growth capacity of cherry trees. In terms of the vessel distribution to different size-classes, candidates of ‘Oblačinska’ sour and European ground cherries, as well as ‘Mahaleb’, had the largest share of vessels from 700 to 2000 µm2, a slightly lesser share of the smallest vessels, and the least share of vessels larger than 2000 µm2. Contrarily, the control rootstock ‘Gisela 5′ was characterized by the complete absence of the largest vessels and a very high share of vessels smaller than 700 µm2—albeit 88.34%. Based on the achieved crown volumes and plant vitality in the sixth vegetation (2021), it was shown that the pattern of vessel distribution observed in the candidate rootstocks, as opposed to the control ‘Gisela 5′, was more favorable for providing the optimal amount of water solution to the aboveground plant parts without compromising plant vitality, drought tolerance, and size-controlling effect. These findings are in accordance with the previous results regarding standard cherry rootstocks—‘Mahaleb’ and ‘Gisela 5′ [11], and slightly different to ones achieved for autochthonous cherry genotypes [18], confirming the anatomically-assisted selection reliability and further need for germplasm screening.
The complete absence of correlations between root anatomy and crown parameters in the years 2019–2021 is presumed to be due to early shifting from the juvenile–vegetative phase pronounced in 2017 and 2018 to the reproductive phase and very high yielding capacity of investigated rootstock candidates in the following years determined in the previous study [48]. In cases when comparison was performed in relation to 2017, highly significant correlations do appear, directly linking root anatomy and vegetative growth. Those correlations include the percentage of vessels (especially the largest ones), with tightly connected xylem porosity and hydraulic conductivity (both per mm2 and total root) all having negative precursors. This directly implies that larger xylem conduits are in a negative correlation, with stunted growth and low-vigor determined in a majority of investigated rootstock candidates (and vice versa). Keeping in mind that highly abundant small vessels like in ‘Gisela 5′ are not enough for drought tolerance, genotypes with intermediate xylem properties and the balanced portions of vessels from size-classes I and II are preferable (PC_04_01, PC_06_12, PF_04_09, and PM_09_01).
Unjustifiably root anatomical phenotypes are underutilized but important breeding goals for the development of the efficient, resilient crops urgently needed in global agriculture facing water scarcity [49]. Root anatomical studies in herbaceous and some perennial plants have been emerging over the past several years but are still very scarce in fruit species. There are even fewer studies when it comes to the correlation of root anatomical properties with aboveground plant growth and development. Recently, Jupa et al. [22] found a positive correlation between the mean vessel diameter of roots and the annual shoot length in apples and pears, confirming the root vessel-size-based predictions of scion growth intensity.
5. Conclusions
In the course of this research, differences in the root anatomical parameters in eight cherry rootstock candidates (belonging to ‘Oblačinska’ sour cherry, European ground cherry, and ‘Mahaleb’) were determined, which directly affected the water conductance and determined the overall ‘Summit’ sweet cherry vigor. Selection parameters—balanced percentage of vessels belonging to I and II size-class, wood porosity, the theoretical hydraulic conductance per root mm2 and total wood—and their correlation with juvenile tree growth provided a precision in the pre-selection of cherry rootstocks. The most favorable pattern of root vessel distribution by size-classes is a balanced participation (around 40–50%) of vessels from size class I (700 µm2) and II (700–2000 µm2), and minimal percentage of largest vessels from size-class III (around 10%). With such vessel distribution, even not irrigated, plants on candidate rootstocks were low-vigorous and showed no signs of withering or cupped leaves, while plants on control rootstocks (characterized by almost 90% of smallest vessels) did suffer during the late spring and summer months. Effective crown volume reduction compared to the control rootstock ‘Gisela 5′ was the most prominent in European ground cherry candidates, followed by one ‘Mahaleb’ candidate and ‘Oblačinska’ sour cherry candidates. Great intraspecific differences observed for ‘Mahaleb’ genotypes indicate the need for future broader study on selections belonging to this heterogeneous gene fund. The proposed methodology is not destructive and can be applied on a great number of rootstock candidates in a short period of time.
Author Contributions
Both T.N. and M.L. conceived and designed the experiments; performed the experiments; analyzed the data; wrote the paper and contributed to the paper discussion. All authors have read and agreed to the published version of the manuscript.
Funding
The research presented in this paper was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia, in a frame of ‘Program of scientific research work in 2021’, Faculty of Agriculture, University of Novi Sad, No. 451-03-9/2021-14/200117.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their gratitude to Dragan Kereši, for a great amount of assistance with the field investigation and data collection during the experimental years.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Atkinson, C.; Else, M. Understanding how rootstocks dwarf fruit trees. Compact Fruit Tree 2001, 34, 46–49. [Google Scholar]
- Webster, A.D. Vigour mechanisms in dwarfing rootstocks for temperate fruit trees. Acta Hortic. 2002, 658, 29–41. [Google Scholar] [CrossRef]
- Gjamovski, V.; Kiprijanovski, M. Influence of nine dwarfing apple rootstocks on vigour and productivity of apple cultivar ‘Granny Smith’. Sci. Hortic. 2011, 129, 742–746. [Google Scholar] [CrossRef]
- Marra, F.P.; Bianco, R.L.; La Mantia, M.; Caruso, T. Growth, yield and fruit quality of ‘Tropic Snow’ peach on size-controlling rootstocks under dry Mediterranean climates. Sci. Hortic. 2013, 160, 274–282. [Google Scholar] [CrossRef]
- Tworkoski, T.; Fazio, G. Hormone and growth interactions of scions and size-controlling rootstocks of young apple trees. Plant Growth Regul. 2016, 78, 105–119. [Google Scholar] [CrossRef]
- Yahmed, J.B.; Ghrab, M.; Mimoun, M.B. Eco-physiological evaluation of different scion-rootstock combinations of almond grown in Mediterranean conditions. Fruits 2016, 71, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Bujdosó, G.; Magyar, L.; Hrotkó, K. Long term evaluation of growth and cropping of sweet cherry (Prunus avium L.) varieties on different rootstocks under Hungarian soil and climatic conditions. Sci. Hortic. 2019, 256, 108613. [Google Scholar] [CrossRef]
- Hrotkó, K. Progress in cherry rootstock research. Acta Hortic. 2008, 795, 171–178. [Google Scholar] [CrossRef]
- Tombesi, S.; Johnson, R.S.; Day, K.R.; DeJong, T.M. Relationships between xylem vessel characteristics, calculated axial hydraulic conductance and size-controlling capacity of peach rootstocks. Ann. Bot. 2010, 105, 327–331. [Google Scholar] [CrossRef] [Green Version]
- Tombesi, S.; Almehdi, A.; DeJong, T.M. Phenotyping vigour control capacity of new peach rootstocks by xylem vessel analysis. Sci. Hortic. 2011, 127, 353–357. [Google Scholar] [CrossRef]
- Zorić, L.; Ljubojević, M.; Merkulov, L.; Luković, J.; Ognjanov, V. Anatomical characteristics of cherry rootstocks as possible preselecting tools for prediction of tree vigor. J. Plant Growth Regul. 2012, 31, 320–331. [Google Scholar] [CrossRef]
- Hajagos, A.; Végvári, G. Investigation of tissue structure and xylem anatomy of eight rootstocks of sweet cherry (Prunus avium L.). Trees 2013, 27, 53–60. [Google Scholar] [CrossRef]
- Ljubojević, M.; Ognjanov, V.; Zorić, L.; Maksimović, I.; Merkulov, L.; Bošnjaković, D.; Barać, G. Modeling of water movement trough cherry plant as preselecting tool for prediction of tree vigor. Sci. Hortic. 2013, 160, 189–197. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Rodriguez-Gamir, J.; Martínez-Cuenca, M.R.; Iglesias, D.J.; Primo-Millo, E.; Forner-Giner, M.A. Relationship between hydraulic conductance and citrus dwarfing by the flying dragon rootstock (Poncirus trifoliata L. Raft var monstruosa). Trees 2013, 27, 629–638. [Google Scholar] [CrossRef]
- Bruckner, C.H.; DeJong, T.M. Proposed pre-selection method for identification of dwarfing peach rootstocks based on rapid shoot xylem vessel analysis. Sci. Hortic. 2014, 165, 404–440. [Google Scholar] [CrossRef]
- Chen, B.; Wang, C.; Tian, Y.; Chu, Q.; Hu, C. Anatomical characteristics of young stems and mature leaves of dwarf pear. Sci. Hortic. 2015, 186, 172–179. [Google Scholar] [CrossRef]
- Solari, L.I.; Johnson, S.; DeJong, T.M. Hydraulic conductance characteristics of peach (Prunus persica) trees on different rootstocks are related to biomass production and distribution. Tree Physiol. 2006, 26, 1343–1350. [Google Scholar] [CrossRef] [Green Version]
- Ljubojević, M.; Zorić, L.; Maksimović, I.; Dulić, J.; Miodragović, M.; Barać, G.; Ognjanov, V. Anatomically assisted cherry rootstock selection. Sci. Hortic. 2017, 217, 197–208. [Google Scholar] [CrossRef]
- Solari, L.I.; Johnson, S.; DeJong, T.M. Relationship of water status to vegetative growth and leaf gas exchange of peach (Prunus persica) trees on different rootstocks. Tree Physiol. 2006, 26, 1333–1341. [Google Scholar] [CrossRef] [Green Version]
- Olmstead, M.A.; Lang, N.S.; Ewers, F.W.; Owens, S.A. Xylem vessel anatomy of sweet cherries grafted onto dwarfing and nondwarfing rootstocks. J. Am. Soc. Hortic. Sci. 2006, 131, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, B.; Correia, C.M.; Silva, A.P.; Bacelar, E.A.; Santos, A.; Ferreira, H.; Moutinho-Pereira, J.M. Variation in xylem structure and function in roots and stems of scion–rootstock combinations of sweet cherry tree (Prunus avium L.). Trees 2007, 21, 121–130. [Google Scholar] [CrossRef]
- Jupa, R.; Mészáros, M.; Plavcová, L. Linking wood anatomy with growth vigour and susceptibility to alternate bearing in composite apple and pear trees. Plant Biol. 2021, 23, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Naor, A. The effect of three rootstocks on water use, canopy conductance and hydraulic parameters of apple trees and predicting canopy from hydraulic conductance. Plant Cell Environ. 2002, 25, 17–28. [Google Scholar] [CrossRef]
- Hayat, F.; Asghar, S.; Yanmin, Z.; Xue, T.; Nawaz, M.A.; Xu, X.; Wang, Y.; Wu, T.; Zhang, X.; Qiu, C.; et al. Rootstock induced vigour is associated with physiological, biochemical and molecular changes in ‘Red Fuji’ apple. Int. J. Agric. Biol. 2020, 24, 1823–1834. [Google Scholar]
- Santos, A.; Santos-Ribeiro, R.; Cavalheiro, J.; Cordeiro, V.; Lousada, J.L. Initial growth and fruiting of ‘Summit’ sweet cherry (Prunus avium) on five rootstocks. N. Z. J. Crop Hortic. 2006, 34, 269–277. [Google Scholar] [CrossRef]
- Wociór, S. The effect of rootstock on the growth and yielding of ‘Regina’ cherry trees. Folia Hortic. 2008, 20, 15–22. [Google Scholar] [CrossRef] [Green Version]
- López-Ortega, G.; García-Montiel, F.; Bayo-Canha, A.; Frutos-Ruiz, C.; Frutos-Tomás, D. Rootstock effects on the growth, yield and fruit quality of sweet cherry cv. ‘Newstar’ in the growing conditions of the Region of Murcia. Sci. Hortic. 2016, 198, 326–335. [Google Scholar] [CrossRef]
- Dziedzic, E.; Błaszczyk, J.; Kaczmarczyk, E. Postharvest properties of sweet cherry fruit depending on rootstock and storage conditions. Folia Hortic. 2017, 29, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Pal, M.D.; Mitre, I.; Asănică, A.C.; Sestraș, A.F.; Peticilă, A.G.; Mitre, V. The influence of rootstock on the growth and fructification of cherry cultivars in a high density cultivation system. Not. Bot. Horti Agrobot. 2017, 45, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Balducci, F.; Capriotti, L.; Mazzoni, L.; Medori, I.; Albanesi, A.; Giovanni, B.; Giampieri, F.; Mezzetti, B.; Capocasa, F. The rootstock effects on vigor, production and fruit quality in sweet cherry (Prunus avium L.). J. Berry Res. 2019, 9, 249–265. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Lugli, S.; Tugnoli, A.; Boini, A.; Perulli, G.D.; Bresilla, K.; Venturi, M.; Grappadelli, L.C. Sweet cherry water relations and fruit production efficiency are affected by rootstock vigor. J. Plant Physiol. 2019, 237, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Webster, T. Dwarfing rootstocks: Past, present and future. Compact Fruit Tree 2002, 35, 67–72. [Google Scholar]
- Beckman, T.G.; Lang, G.A. Rootstock breeding for stone fruits. Acta Hortic. 2002, 622, 531–551. [Google Scholar] [CrossRef]
- Tomaszewska, Z.; Nychnerewicz, B. The effect of rootstock on growth and fruitage of sweet cherry. Sodininkystė Ir Daržininkystė 2006, 25, 224–229. [Google Scholar]
- Sotirov, D. Growth and yield characteristics of ‘Summit’ sweet cherry on different rootstocks. Acta Hortic. 2021, 132, 137–142. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Changok, L. Estimation of Urban Tree Crown Volume Based on Object-Oriented Approach and Lidar Data. Master’s Thesis, International Institute for Geoinformation Science and Earth Observation, Enschede, The Netherlands, 2007. [Google Scholar]
- Callesen, O. Recent developments in cherry rootstock research. Acta Hortic. 1998, 468, 219–228. [Google Scholar] [CrossRef]
- Wertheim, S.J. Rootstock Guide: Apple, Pear, Cherry, European Plum; Fruit Research Station: Wilhelminadorp, The Netherlands, 1998; pp. 85–114. [Google Scholar]
- Milošević, T.; Milošević, N.; Mladenović, J. Combining fruit quality and main antioxidant attributes in the sour cherry: The role of new clonal rootstock. Sci. Hortic. 2020, 265, 109236. [Google Scholar] [CrossRef]
- Solonkin, A.; Nikolskaya, O.; Seminchenko, E. The Effect of Low-Growing Rootstocks on the Adaptability and Productivity of Sour Cherry Varieties (Prunus cerasus L.) in Arid Conditions. Horticulturae 2022, 8, 400. [Google Scholar] [CrossRef]
- Cline, J.A. Planting density and size-controlling rootstocks influence the performance of Montmorency tart cherry (Prunus cerasus L.). Can. J. Plant Sci. 2019, 100, 16–28. [Google Scholar] [CrossRef]
- Barać, G.; Ognjanov, V.; Vidaković, D.O.; Dorić, D.; Ljubojević, M.; Dulić, J.; Miodragović, M.; Gašić, K. Genetic diversity and population structure of European ground cherry (Prunus fruticosa Pall.) using SSR markers. Sci. Hortic. 2017, 224, 374–383. [Google Scholar] [CrossRef]
- Ljubojević, M.; Sebolt, A.; Ognjanov, V.; Iezzoni, A. Heritability of Anatomical Characteristics in Cherry Interspecific Hybrids. J. Plant Growth Regul. 2022, 41, 965–982. [Google Scholar] [CrossRef]
- Hrotkó, K. Potentials in Prunus mahaleb L. for cherry rootstock breeding. Sci. Hortic. 2016, 205, 70–78. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean Ornamental Plants to Drought Stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Strock, C.F.; Lynch, J.P. Root secondary growth: An unexplored component of soil resource acquisition. Ann. Bot. 2020, 126, 205–218. [Google Scholar] [CrossRef]
- Narandžić, T.; Ljubojević, M.; Ostojić, J.; Barać, G.; Ognjanov, V. Investigation of stem anatomy in relation to hydraulic conductance, vegetative growth and yielding potential of ‘Summit’ cherry trees grafted on different rootstock candidates. Folia Hortic. 2021, 33, 248–264. [Google Scholar] [CrossRef]
- Lynch, J.P.; Strock, C.F.; Schneider, H.M.; Sidhu, J.S.; Ajmera, I.; Galindo-Castañeda, T.; Klein, S.P.; Hanlon, M.T. Root anatomy and soil resource capture. Plant Soil 2021, 466, 21–63. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).