Rootstocks for Commercial Peach Production in the Southeastern United States: Current Research, Challenges, and Opportunities
Abstract
1. Origin and Characteristics of Peach and Almond
2. Economic Importance of Peaches in the United States
3. Rootstocks for Stone Fruit Production
4. Production Problems in Southeast United States (Potentially Solved with Rootstocks Breeding)
4.1. Biotic Stress
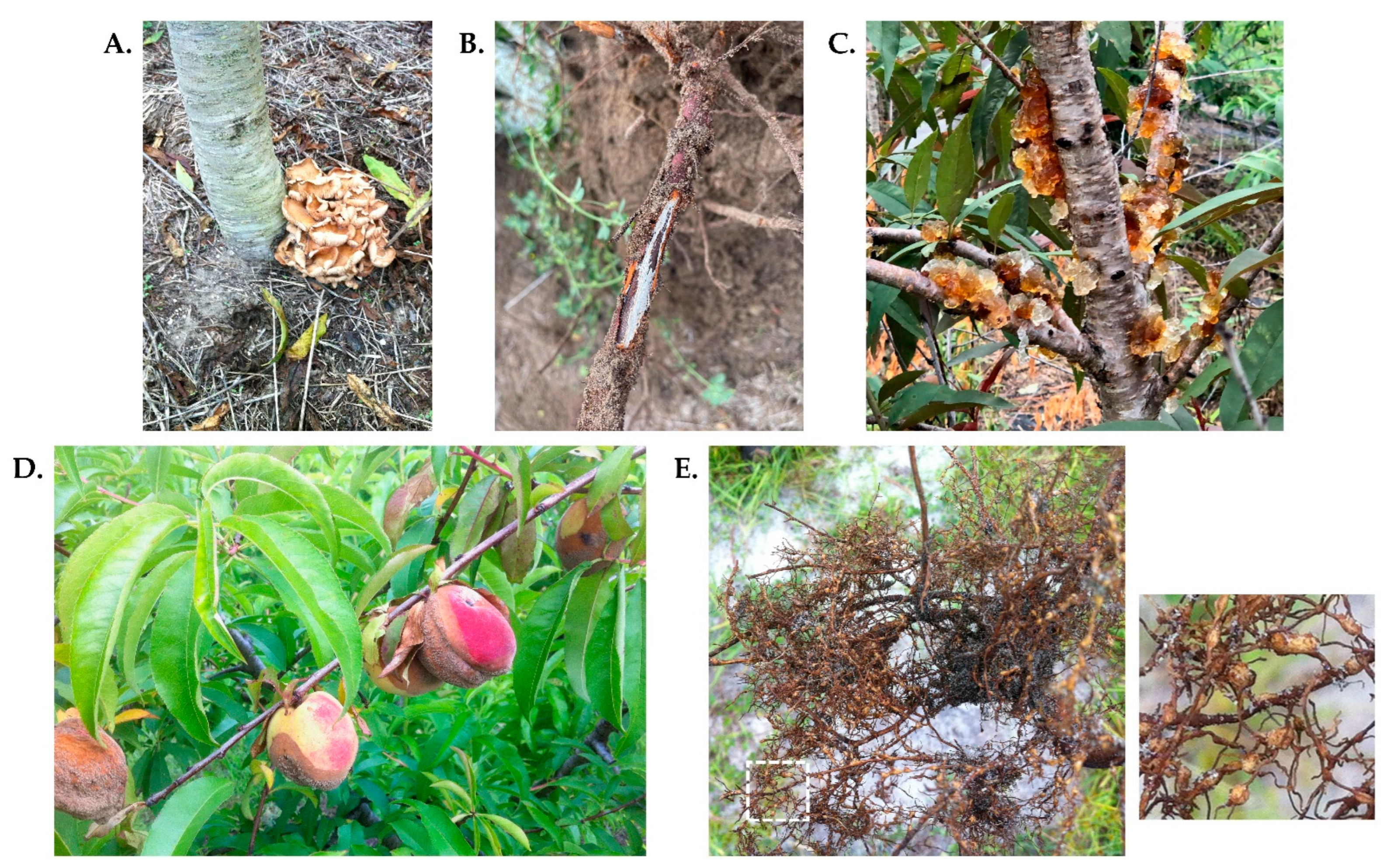
4.1.1. Peach Tree Short Life (PTSL)
4.1.2. Oak Root Rot (Armillaria mellea)
4.1.3. Root-Knot Nematodes (M. floridensis, M. arenaria, and M. javanica)
4.1.4. Peach Gummosis Caused by Botryosphaeria dothidea
4.1.5. Diseases Caused by Xylella fastidiosa
- Grapevine (Vitis vinifera): causes Pierce’s Disease (PD). In this species, X. fastidiosa was first identified at the end of the 19th century in the U.S. The first symptoms are sudden drying of large parts of green leaves that later become necrotic at the leaf margins before finally dropping. In general, these symptoms can be confused with salt toxicity or a deficiency of boron (B), copper (Cu), or phosphorus (P). Defoliation, shoot dwarfing, and cane stunting, as well as the dehydration of fruit clusters, may occur.
- Citrus spp.: causes Citrus Variegated Chlorosis (CVC) or Citrus X Disease. Symptoms can be observed, especially on sweet orange trees from the nursery up to 10 years old, as small interveinal chlorotic spots on leaves, similar to Zn deficiency. The fruits remain small, with a higher sugar content and a harder rind, and ripen earlier. Although the pathogen is considered not to be seedborne, the transmission from seeds to seedlings of sweet orange has been reported [55].
- Coffee (Coffea arabica): causes Coffee Leaf Scorch (CLS). The first symptoms appear on young shoots as large, scorched areas on the top or at the margins of mature leaves. The dwarf growth of new shoots, small, pale green to yellow leaves, shoot dieback, and overall plant stunting occurs. Fruit size and yield are impaired. The symptoms are severe under water stress conditions, but the trees generally do not die or only after some years [56].
- Prunus spp. (peaches, almonds, and plums): in peaches, causes Phony Peach Disease (PPD). In almonds and plums, it causes leaf scorch diseases: Almond Leaf Scorch (ALS) in P. amygdalus and Plum Leaf Scald (PLS) in P. domestica. The main symptoms of PPD include stunted young shoots, more numerous and darker green leaves, early blooming, and both leaves and flowers remain on the shoots longer than expected. There are shortened twig internodes and increased lateral branching, along with severely impaired fruit production, with smaller fruits and earlier ripening.
4.1.6. American Brown Rot Caused by Monilinia fructicola
4.2. Abiotic Stresses
4.2.1. Waterlogging
4.2.2. Alkalinity
4.2.3. Salinity
5. Advantages and Disadvantages of Commercial Rootstocks for Peaches
5.1. Peach Rootstocks
5.1.1. ‘Nemaguard’
5.1.2. ‘Nemared’
5.1.3. ‘Guardian’TM
5.1.4. ‘Lovell’ and ‘Halford’
5.1.5. ‘Bailey’
5.1.6. ‘Flordaguard’
5.2. Peach × Almond Rootstocks
5.2.1. ‘GF-677’
5.2.2. ‘Sirio’
5.2.3. ‘Castore’
5.2.4. ‘Polluce’
5.2.5. ‘Hansen 2168’ and ‘Hansen 536’
5.2.6. ‘Adafuel’
5.2.7. ‘Adarcias’
5.2.8. ‘Felinem’, ‘Garnem’, and ‘Monegro’
5.3. Peach × Plum Rootstocks
5.3.1. ‘Ishtara’
5.3.2. ‘Myran’
5.3.3. ‘MP-29’
5.3.4. ‘Controller 5’ and ‘Controller 9’
5.3.5. ‘Krymsk 86’
5.3.6. ‘Sharpe’
6. Rootstock Propagation
7. Wounding and Root Induction in Peach × Almond Hybrids
8. The Study of Root System Architecture (RSA) Traits
9. Final Remarks
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giovannini, D.; Liverani, A.; Sartori, A.; Cipriani, G. Botanical and pomological aspects of stone fruits physiology, agronomy and orchard management. In Agricultural and Food Biotechnologies of Olea Europaea and Stone Fruit; Muzzalupo, I., MuzzMicalialupo, S., Eds.; Bentham Science Publishers Ltd.: Sharjah, United Arab Emirates, 2014; pp. 161–242. [Google Scholar]
- Rehder, A. Manual of Cultivated Trees and Shrubs Hardy in North America: Exclusive of the Subtropical and Warmer Temperate Regions, 2nd ed.; MacMillan: New York, NY, USA, 1940. [Google Scholar]
- Hancock, J.F.; Scorza, R.; Lobos, G.A. Peaches. In Temperate Fruit Crop Breeding, Germoplasm to Genomics; Hancock, J.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 265–298. [Google Scholar]
- Potter, D. Basic Information on the Stone Fruit Crops. In Genetics, Genomics and Breeding of Stone Fruits; Kole, C., Abbott, A.G., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–21. [Google Scholar]
- Chin, S.W.; Shaw, J.; Haberle, R.; Wen, J.; Potter, D. Diversification of almonds, peaches, plums and cherries—Molecular systematics and biogeographic history of Prunus (Rosaceae). Mol. Phylogenet. Evol. 2014, 76, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, C.; Zhao, X.; Coe, R.; Li, Y.; Finn, D. India-Asia collision was at 24°N and 50 Ma: Palaeomagnetic proof from southernmost Asia. Sci. Rep. 2012, 2, 925. [Google Scholar] [CrossRef] [PubMed]
- FAO (United Nations Food and Agriculture Organization). FAOSTAT. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 2 July 2022).
- USDA-NASS. Noncitrus Fruits and Nuts—2018 Summary; United States Department of Agriculture, National Agricultural Statistics Service (USDA/NASS): Washington, DC, USA, 2019. [Google Scholar]
- Singerman, A.; Arouca, M.B.; Olmstead, M.A. Establishment and Production Costs for Peach Orchards in Florida: Enterprise Budget and Profitability Analysis; Food and Resource Economics Department, University of Florida: Florida, FL, USA, 2017. [Google Scholar] [CrossRef]
- Capstone, G. Disease Creates Challenges for Peach Farmers, Need for Change in Farming Patterns. Grady Newsource, 15 January 2020. Available online: https://gradynewsource.uga.edu/disease-creates-challenges-for-peach-farmers-need-for-change-in-farming-patterns/ (accessed on 2 July 2022).
- Crassweller, R.M.; Kime, L.F.; Harper, J.K. Agricultural Alternatives: Peach Production; PennState Extension: State College, PA, USA, 2017. [Google Scholar]
- USDA-NASS. Noncitrus Fruits and Nuts—2020 Summary; United States Department of Agriculture, National Agricultural Statistics Service (USDA/NASS): Washington, DC, USA, 2021. [Google Scholar]
- USDA. Fresh Peaches and Cherries: World Markets and Trade; United States Department of Agriculture (USDA): Washington, DC, USA, 2020. [Google Scholar]
- Harders, K.; Rumble, J.; Bradley, T.; House, L.; Anderson, S. Consumer Peach Purchasing Survey; University of Florida/IFAS Center for Public Issues Education, vol. PIE2016/17-02. 2016. Available online: https://edis.ifas.ufl.edu/publication/WC288 (accessed on 2 July 2022).
- Rumble, J.N.; Harders, K.; Stofer, K. Florida Peaches: A Perfect Snack; Department of Agricultural Education and Communication: Gainesville, FL, USA, 2017. [Google Scholar] [CrossRef]
- Olmstead, M.A.; Gilbert, J.L.; Colquhoun, T.A.; Clark, D.G.; Kluson, R.; Moskowitz, H.R. In Pursuit of the Perfect Peach: Consumer-assisted Selection of Peach Fruit Traits. HortScience 2015, 50, 1202–1212. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Olmstead, M.A.; Williamson, J.; Chaparro, J.X.; Popenoe, P. Alternative Opportunities for Small Farms: Peach and Nectarine Production Review; University of Florida IFAS Extension: Gainesville, FL, USA, 2018. [Google Scholar]
- Morgan, K.; Olmstead, M.A. A diversification strategy for perennial crops in Florida. HortTechnology 2013, 23, 482–489. [Google Scholar] [CrossRef]
- Sherman, W.B.; Lyrene, P.M.; Sharpe, R.H. Flordaguard’ peach rootstock. HortScience 1991, 26, 427–428. [Google Scholar] [CrossRef]
- Reighard, G.L.; Loreti, F. Rootstock development. In The Peach: Botany, Production and Uses; Layne, D., Bassi, D., Eds.; CAB International: Cambridge, MA, USA, 2008; pp. 193–215. [Google Scholar]
- Webster, A.D.; Wertheim, S.J.; Tromp, J. Rootstocks and interstems. In Fundamentals of Temperate Zone Tree Fruit Production; Tromp, J., Webster, A.D., Wertheim, S.J., Eds.; Backhuys: Leiden, The Netherlands, 2005; pp. 156–175. [Google Scholar]
- Webster, A.D.; Wertheim, S.J.; Tromp, J. Breeding. In Fundamentals of Temperate Zone Tree Fruit Production; Tromp, J., Webster, A.D., Wertheim, S.J., Eds.; Backhuys: Leiden, The Netherlands, 2005; pp. 136–155. [Google Scholar]
- Hrotkó, K. Advances and Challenges in Fruit Rootstock Research. Acta Hortic. 2007, 732, 33–42. [Google Scholar] [CrossRef]
- Moreno, M.A. Rootstocks for stone and pome fruit tree species in Spain. In Proceedings of the International Conference on Fruit Tree Rootstocks, Pisa, Italy, 26 June 2009; pp. 44–57. [Google Scholar]
- Reighard, G.L. Peach Rootstocks for the United States: Are Foreign Rootstocks the Answer? HortTechnology 2000, 10, 714–718. [Google Scholar] [CrossRef]
- Adaskaveg, J.E.; Schnabel, G.; Förster, H. Diseases of peach caused by fungi and fungal-like organisms: Biology, epidemiology and management. In The Peach: Botany, Production and Uses; Layne, D., Bassi, D., Eds.; CAB International: Cambridge, MA, USA, 2008; pp. 353–403. [Google Scholar]
- Adaskaveg, J.E.; Schnabel, G.; Förster, H. Nematodes. In The Peach: Botany, Production and Uses; Layne, D., Bassi, D., Eds.; CAB International: Cambridge, MA, USA, 2008; pp. 506–527. [Google Scholar]
- Nyczepir, A.; Okie, W.; Beckman, T. Creating a Short Life Site for Prunus Rootstock Evaluation on Land with No Innate Mesocriconema xenoplax Population. HortScience 2004, 39, 124–126. [Google Scholar] [CrossRef]
- Beckman, T.; Okie, W.; Nyczepir, A. Influence of Scion and Rootstock on Incidence of Peach Tree Short Life. Acta Hortic. 2002, 592, 645–648. [Google Scholar] [CrossRef]
- Downer, J.; Faber, B. Non-chemical control of Armillaria mellea infection of Prunus persica. J. Plant Sci. Phytopathol. 2019, 3, 50–55. [Google Scholar] [CrossRef]
- Morrison, D.J. Infection, Disease Development, Diagnosis, and Detection. In Armillaria Root Disease; USDA Forest Service Agricultural Handbook No. 691; Shaw, C.G., Kile, G., Eds.; United States Department of Agriculture Forest Service: Washington, DC, USA, 1991; pp. 62–75. [Google Scholar]
- Adaskaveg, J.E.; Förster, H.; Wade, L.; Thompson, D.F.; Connell, J.H. Efficacy of sodium tetrahthiocarbonate and propiconazole in managing Armillaria root rot of almond on peach rootstock. Plant Dis. 1999, 83, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Guillaumin, J.; Pierson, J.; Grassely, C. The susceptibility to Armillaria mellea of different Prunus species used as stone fruit rootstocks. Sci. Hortic. 1991, 46, 43–54. [Google Scholar] [CrossRef]
- Beckman, T.G.; Chaparro, J.X.; Sherman, W.B. ‘Sharpe’, a Clonal Plum Rootstock for Peach. HortScience 2008, 43, 2236–2337. [Google Scholar] [CrossRef]
- Beckman, T.G.; Chaparro, J.X.; Sherman, W.B. ‘MP-29’, a Clonal Interspecific Hybrid Rootstock for Peach. HortScience 2012, 47, 128–131. [Google Scholar] [CrossRef]
- Baumgartner, K.; Fujiyoshi, P.; Ledbetter, C.; Duncan, R.; Kluepfel, D.A. Screening Almond Rootstocks for Sources of Resistance to Armillaria Root Disease. HortScience 2018, 53, 4–8. [Google Scholar] [CrossRef]
- Elias-Roman, R.D.; Calderon-Zavala, G.; Guzman-Mendoza, R.; Vallejo-Perez, M.R.; Klopfenstein, N.B.; Mora-Aguilera, J.A. ‘Mondragon’: A clonal plum rootstock to enhance management of Armillaria root disease in peach orchards of Mexico. Crop Prot. 2019, 121, 89–95. [Google Scholar] [CrossRef]
- Abad, P.; Williamson, V.M. Plant–nematode interaction: A sophisticated dialogue. In Advances in Botanical Research; Kader, J.C., Delseny, M., Eds.; Academic Press Ltd.: London, UK, 2010; pp. 147–192. [Google Scholar]
- Handoo, Z.A.; Nyczepir, A.P.; Esmenjaud, D.; Van Der Beek, J.G.; Castagnone-Sereno, P.; Carta, L.K.; Skantar, A.M.; Higgins, J.A. Morphological, Molecular, and Differential-Host Characterization of Meloidogyne floridensis n. sp. (Nematoda: Meloidogynidae), a Root-Knot Nematode Parasitizing Peach in Florida. J. Nematol. 2004, 36, 20–35. [Google Scholar]
- Lecouls, A.C.; Salesses, G.; Minot, J.C.; Voisin, R.; Bonnet, A.; Esmenjaud, D. Spectrum of the Ma genes for resistance to Meloidogyne spp. in Myrobalan plum. Theor. Appl. Genet. 1997, 95, 1325–1334. [Google Scholar] [CrossRef]
- Rubio-Cabetas, M.J.; Lecouls, A.C.; Salesses, G.; Bonnet, A.; Minot, J.C.; Voisin, R.; Esmenjaud, D. Evidence of a new gene for high resistance to Meloidogyne spp. in Myrobalan plum, Prunus cerasifera. Plant Breed. 1998, 117, 567–571. [Google Scholar] [CrossRef]
- Lecouls, A.C.; Rubio-Cabetas, M.J.; Minot, J.C.; Voisin, R.; Bonnet, A.; Salesses, G.; Dirlewanger, E.; Esmenjaud, D. RAPD and SCAR markers linked to the Ma1 root-knot nematode resistance gene in Myrobalan plum (Prunus cerasifera Ehr.). Theor. Appl. Genet. 1999, 99, 328–335. [Google Scholar] [CrossRef]
- Dirlewanger, E.; Cosson, P.; Howad, W.; Capdeville, G.; Bosselut, N.; Claverie, M.; Voisin, R.; Poizat, C.; LaFargue, B.; Baron, O.; et al. Microsatellite genetic linkage maps of myrobalan plum and an almond-peach hybrid location of root-knot nematode resistance genes. Theor. Appl. Genet. 2004, 109, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Claverie, M.; Dirlewanger, E.; Bosselut, N.; Van Ghelder, C.; Voisin, R.; Kleinhentz, M.; Lafargue, B.; Abad, P.; Rosso, M.-N.; Chalhoub, B.; et al. The MaGene for Complete-Spectrum Resistance to Meloidogyne Species in Prunus is a TNL with a Huge Repeated C-Terminal Post-LRR Region. Plant Physiol. 2011, 156, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G. Plant Pathology; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Layne, R. Peach Rootstocks. In Rootstocks for Fruit Crops; Rom, R.C., Carlson, R.F., Eds.; John Wiley and Sons Inc.: New York, NY, USA, 1987; pp. 185–216. [Google Scholar]
- Esmenjaud, D.; Minot, J.C.; Voisin, R.; Pinochet, J.; Simard, M.H.; Salesses, G. Differential response to root-knot nematodes in prunus species and correlative genetic implications. J. Nematol. 1997, 29, 370–380. [Google Scholar] [PubMed]
- Beckman, T.G.; Reilly, C.C. Relative susceptibility of peach cultivars to fungal gummosis (Botryosphaeria dothidea). J. Am. Pomol. Soc. 2005, 59, 111–116. [Google Scholar]
- Beckman, T.; Pusey, P.; Bertrand, P. Impact of Fungal Gummosis on Peach Trees. HortScience 2003, 38, 1141–1143. [Google Scholar] [CrossRef]
- Pusey, P.L. Availability and dispersal of ascospores and conidia of botryosphaeria in peach orchards. Phytopathology 1989, 79, 635–639. [Google Scholar] [CrossRef]
- Moral, J.; Morgan, D.; Michailides, T.J. Management of Botryosphaeria canker and blight diseases of temperate zone nut crops. Crop Prot. 2019, 126, 104927. [Google Scholar] [CrossRef]
- Mancero-Castillo, D.; Beckman, T.G.; Harmon, P.F.; Chaparro, J.X. A major locus for resistance to Botryosphaeria dothidea in Prunus. Tree Genet. Genomes 2018, 14, 26. [Google Scholar] [CrossRef]
- Janse, J.; Obradovic, A. Xylella fastidiosa: Its biology, diagnosis, control and risks. J. Plant Pathol. 2010, 92, 35–48. [Google Scholar]
- Mizell, R.F.; Andersen, P.C.; Tipping, C.; Brodbeck, B. Xylella Fastidiosa Diseases and Their Leafhopper Vectors. Entomology and Nematology Department, UF/IFAS Extension, ENY-683. 2015. Available online: https://edis.ifas.ufl.edu/pdf/IN/IN17400.pdf (accessed on 2 July 2022).
- Li, W.-B.; Pria, W.D.; Lacava, P.M.; Qin, X.; Hartung, J.S. Presence of Xylella fastidiosa in Sweet Orange Fruit and Seeds and Its Transmission to Seedlings. Phytopathology 2003, 93, 953–958. [Google Scholar] [CrossRef][Green Version]
- De Lima, J.E.O.; Miranda, V.S.; Hartung, J.S.; Brlansky, R.H.; Coutinho, A.; Roberto, S.R.; Carlos, E.F. Coffee leaf scorch bacterium: Axenic culture, pathogenicity and comparison with Xylella fastidiosa of Citrus. Plant Dis. 1998, 82, 94–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dalbó, M.A.; Bruna, E.D.; de Souza, A.L.K. SCS 438 Zafira—A new plum cultivar resistant to leaf scald (Xylella fastidiosa). Crop Breed. Appl. Biotechnol. 2018, 18, 229–233. [Google Scholar] [CrossRef]
- Ledbetter, C.A.; Chen, J.; Livingston, S.; Groves, R.L. Winter curing of Prunus dulcis cv ‘Butte,’ P. webbii and their interspecific hybrid in response to Xylella fastidiosa infections. Euphytica 2009, 169, 113–122. [Google Scholar] [CrossRef]
- Krugner, R.; Ledbetter, C.A.; Chen, J.; Shrestha, A. Phenology of Xylella fastidiosa and Its Vector Around California Almond Nurseries: An Assessment of Plant Vulnerability to Almond Leaf Scorch Disease. Plant Dis. 2012, 96, 1488–1494. [Google Scholar] [CrossRef]
- Sholberg, P.; Kappel, F. Integrated management of stone fruit diseases. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Ciancio, A., Mukerji, K., Eds.; Springer: New Delhi, India, 2008; pp. 3–25. [Google Scholar]
- Beckman, T.; Lang, G. Rootstock Breeding for Stone Fruits. In Proceedings of the XXVI International Horticultural Congress: Genetics and Breeding of Tree Fruits and Nuts, Toronto, Canada, 31 August 2003; pp. 531–551. [Google Scholar] [CrossRef]
- Nimbolkar, P.K.; Shiva, B.; Rai, A.K. Rootstock breeding for abiotic stress tolerance in fruit crops. Int. J. Agric. Environ. Biotechnol. 2016, 9, 375–380. [Google Scholar] [CrossRef]
- Felipe, A.J. El Almendro: El Material Vegetal; Editorial Mira: Zaragoza, Spain, 2000. [Google Scholar]
- Loreti, F.; Massai, R. ’Castore’ and ‘Polluce’: Two new hybrid rootstocks for peach. Acta Hortic. 2006, 713, 275–278. [Google Scholar] [CrossRef]
- Amador, M.L.; Bielsa, B.; Aparisi, J.G.; Sancho, S.; Cabetas, M.J.R. Avances en el estudio de la tolerancia a la asfixia radicular en patrones de melocotonero. Rev. Frutic. 2010, 9, 48–55. [Google Scholar]
- Byrne, D.H.; Raseira Maria, C.B.; Bassi, D.; Piagnani, M.C.; Gasic, K.; Reighard, G.L.; Moreno, M.A.; Perez-Gonzalez, S. “Peach,” in Fruit Breeding; Badenes, M.L., Byrne, D., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Xiloyannis, C.; Dichio, B.; Tuzio, A.C.; Kleinhentz, M.; Salesses, G.; Gomez-Aparisi, J.; Esmenjaud, D. Characterization and selection of Prunus rootstocks resistant to abiotic stresses: Waterlogging, drought and iron chlorosis. Acta Hortic. 2007, 732, 247–251. [Google Scholar] [CrossRef]
- Mylavarapu, R.; Harris, W.; Hochmuth, G. Agricultural Soils of Florida; Department of Soil and Water Sciences: Gainesville, FL, USA, 2016. [Google Scholar]
- Chen, Y.; Barak, P. Iron Nutrition of Plants in Calcareous Soils; Department of Soil and Water Sciences: Gainesville, FL, USA, 1982; pp. 217–240. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition; IZA Publications, International Zinc Association: Brussels, Belgium, 2004. [Google Scholar]
- Läuchli, A.; Grattan, S.R. Soil pH Extremes. In Plant Stress Physiology; Shabala, S., Ed.; CAB International: Cambridge, MA, USA, 2012; pp. 194–197. [Google Scholar]
- Başar, H. Factors affecting iron chlorosis observed in peach trees in the Bursa region. Turk. J. Agric. For. 2000, 24, 237–245. [Google Scholar]
- Felipe, A.J. ‘Felinem’, ‘Garnem’, and ‘Monegro’ almond x peach hybrid rootstocks. HortScience 2009, 44, 196–197. [Google Scholar] [CrossRef]
- Tao, R.; Watari, A.; Hanada, T.; Habu, T.; Yaegaki, H.; Yamaguchi, M.; Yamane, H. Self-compatible peach (Prunus persica) has mutant versions of the S haplotypes found in self-incompatible Prunus species. Plant Mol. Biol. 2006, 63, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Kester, D.; Gradziel, T.; Grasselly, C. “Almonds (Prunus)”. Acta Hortic. 1990, 290, 699–758. [Google Scholar] [CrossRef]
- White, P. Ion uptake mechanisms of individual cells and roots: Short-distance transport. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2013; p. 448. [Google Scholar]
- Verbruggen, N.; Hermans, C. Root responses to trace metallic elements. In Plant Roots, the Hiden Half; Eshel, A., Beeckman, T., Eds.; CRC Press: New York, NY, USA, 2013; p. 341. [Google Scholar]
- Cinelli, F.; Viti, R.; Byrne, D.H.; Reed, D.W. Physiological characterization of two peach seedling rootstocks in bicarbonate nutrient solution. I. Root iron reduction and iron uptake. In Iron Nutrition in Soils and Plants; Abadía, J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 323–328. [Google Scholar]
- De la Guardia, M.D.; Felipe, A.; Alcantara, E.; Fournier, J.M.; Romera, F.J. Evaluation of Experimental Peach Rootstocks Grown in Nutrient Solutions for Tolerance to Iron Stress; Springer: Dordrecht, The Netherlands, 1995; pp. 201–205. [Google Scholar] [CrossRef]
- Bell, P.; Chaney, R.; Angle, J. Staining localization of ferric reduction on roots. J. Plant Nutr. 1988, 11, 1237–1252. [Google Scholar] [CrossRef]
- Longnecker, L.; Welch, R. Accumulation of apoplastic iron in plant roots. A factor in the resistance of soybeans to iron deficiency induced chlorosis? Plant Physiol. 1990, 92, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulos, T.E.; Kalfountzos, D.; Aleksiou, I.; Kotsopoulos, S.; Koutinas, N. Response of a clingstone peach cultivar to regulated deficit irrigation. Sci. Agric. 2010, 67, 164–169. [Google Scholar] [CrossRef]
- Tilbrook, J.; Roy, S. Salinity tolerance. In Plant Abiotic Stress; Jenks, M.A., Hasegawa, P.M., Eds.; Wiley: San Francisco, CA, USA, 2014; pp. 133–161. [Google Scholar]
- Ouraei, M.; Tabatabaei, S.J.; Falahi, E.; Imani, A. The effects of salinity stress and rootstock on the growth, photosynthetic rate, nutrient and sodium concentrations of almond (Prunus dulcis Mill.). J. Hortic. Sci. 2009, 23, 121–140. [Google Scholar]
- Hatami, E.; Shokouhian, A.A.; Ghanbari, A.; Naseri, L. Alleviating salt stress in almond rootstocks using of humic acid. Sci. Hortic. 2018, 237, 296–302. [Google Scholar] [CrossRef]
- Reighard, G.; Ouellette, D.; Brock, K. Performance of New Prunus Rootstocks for Peach in South Carolina. In Proceedings of the XXVII International Horticultural Congress-IHC2006: International Symposium on Enhancing Economic and Environmental, Seoul, Korea, 31 August 2008; pp. 237–240. [Google Scholar] [CrossRef]
- Zarrouk, O.; Gogorcena, Y.; Gómez-Aparisi, J.; Betrán, J.A.; Moreno, M.A. Influence of almond × peach hybrids rootstocks on flower and leaf mineral concentration, yield and vigour of two peach cultivars. Sci. Hortic. 2005, 106, 502–514. [Google Scholar] [CrossRef]
- Engels, C.; Kirkby, E.; White, P. Mineral nutrition, yield and source–sink relationships. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Zrig, A.; Mohamed, H.B.; Tounekti, T.; Ennajeh, M.; Valero, D.; Khemira, H. A comparative study of salt tolerance of three almond rootstocks. J. Agric. Sci. Technol. 2015, 17, 675–689. [Google Scholar]
- Gainza, F.; Opazo, I.; Guajardo, V.; Meza, P.; Ortiz, M.; Pinochet, J.; Muñoz, C. Rootstock breeding in Prunus species: Ongoing efforts and new challenges. Chil. J. Agric. Res. 2015, 75, 6–16. [Google Scholar] [CrossRef]
- Moore, J.; Ballington, J. International Society for Horticultural Science. Genetic Resources of Temperate Fruit and Nut Crops; Society for Horticultural Science: Wageningen, The Netherlands, 1994; Available online: https://catalog.hathitrust.org/Record/009627388 (accessed on 2 July 2022).
- Sarkhosh, A.; Olmstead, M.; Chaparro, J.; Beckman, T. Rootstocks for Florida Stone Fruit; University of Florida IFAS Extension: Gainesville, FL, USA, 2018. [Google Scholar]
- Kole, C.; Abbott, A.G. Diversity analysis. In Genetics, Genomics and Breeding of Stone Fruits; Kole, C., Abbott, A., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 55–75. [Google Scholar]
- Nyczepir, A.P.; Zehr, E.I.; Lewis, S.A.; Harshman, D.C. Short life of peach trees induced by Criconemella xenoplax. Plant Dis. 1983, 67, 507–508. [Google Scholar] [CrossRef]
- Zehr, E.I.; Miller, R.W.; Smith, F.H. Soil fumigation and peach rootstocks for protection against Peach Tree Short Life. Phytopathology 1976, 66, 689–694. [Google Scholar] [CrossRef]
- Blaauw, B.; Brannen, P.; Lockwood, D.; Schhnabel, G.; Ritchie, D. Southeastern Peach, Nectarine, and Plum Management Guide; University of Georgia: Athens, GA, USA, 2020. [Google Scholar]
- Beckman, T.; Okie, W.; Nyczepir, A.; Reighard, G.; Zehr, E.; Newall, W. History, Current Status and Future Potential of Guardiantm (By520–9) Peach Rootstock. In Proceedings of the VI International Symposium on Integrated Canopy, Rootstock, Environmental Physiology in Orchard Systems, Wenatchee, WA, USA, 31 August 2008; pp. 251–258. [Google Scholar] [CrossRef]
- Nyczepir, A.P.; Beckman, T.G.; Reighard, G.L. Reproduction and development of Meloidogyne incognita and M. javanica on ‘Guardian’ peach rootstock. J. Nematol. 1999, 31, 334–340. [Google Scholar] [PubMed]
- Nyczepir, A.P.; Beckman, T.G.; Reighard, G.L. Field evaluation of ‘Guardian’ peach rootstock to different root-knot nematode species. Acta Hortic. 2006, 713, 303–309. [Google Scholar] [CrossRef]
- Reighard, G.L.; Newall Jr, W.C.; Zehr, E.I.; Beckman, T.G.; Okie, W.R.; Nyczepir, A.P. Field Performance of Prunus Rootstock Cultivars and Selections on Replant Soils in South Carolina. In Proceedings of the VI International Symposium on Integrated Canopy, Rootstock, Environmental Physiology in Orchard Systems, Kelowna, BC, Canada, 1 November 1997; pp. 243–250. [Google Scholar] [CrossRef]
- Crossa-Raynaud, P.; Audergon, J.M. Apricot rootstocks. In Rootstocks for Fruit Crops; Rom, R.C., Carlson, R.F., Eds.; Wiley-Interscience Publications: Hoboken, NJ, USA, 1987; pp. 295–320. [Google Scholar]
- Sarkhosh, A.; Olmstead, M.; Chaparro, J.; Beckman, T. Rootstocks for Florida Stone Fruit; US Department of Agriculture: Washington, DC, USA, 2018; Volume 2018. [Google Scholar] [CrossRef]
- McGee, T.; Shahid, M.A.; Beckman, T.G.; Chaparro, J.X.; Schaffer, B.; Sarkhosh, A. Physiological and biochemical characterization of six Prunus rootstocks in response to flooding. Environ. Exp. Bot. 2021, 183, 104368. [Google Scholar] [CrossRef]
- Egilla, J.N.; Byrne, D. The search for peach rootstocks tolerant to alkalinity. Fruit Var. J. 1989, 43, 7–11. [Google Scholar]
- Pusey, P.L. Fungal gummosis. In Southeastern Peach Growers Handbook; University of Georgia Press: Athens, GA, USA, 2005. [Google Scholar]
- Rubio-Cabetas, M.J. Almond rootstocks: Overview. In Proceedings of the XVI GREMPA Meeting on Almonds and Pistachios, Zaragoza, Spain, 12–14 May 2015; pp. 133–143. [Google Scholar]
- Loreti, F.; Massai, R. Sirio: New Peach X Almond Hybrid Rootstock for Peach. In Proceedings of the IV International Peach Symposium, Bordeaux, France, 1 April 1998; pp. 229–236. [Google Scholar] [CrossRef]
- Moreno, M.; Cambra, R. Adarcias: An Almond × Peach Hybrid Rootstock. HortScience 1994, 29, 925. [Google Scholar] [CrossRef]
- Albás, E.; Jiménez, S.; Aparicio, J.; Betrán, J.; Moreno, M. Effect of Several Peach X Almond Hybrid Rootstocks on Fruit Quality of Peaches. In Proceedings of the I International Symposium on Rootstocks for Deciduous Fruit Tree Species, Zaragoza, Spain, 31 October 2004; pp. 321–326. [Google Scholar] [CrossRef]
- Marull, J.; Pinochet, J.; Felipe, A.; Cenis, J.L. Resistance verification in Prunus selections to a mixture of 13 Meloidogyne isolates and resistance mechanisms of a peach-almond hybrid to M. javanica. Fundam. Appl. Nematol. 1994, 16, 85–92. [Google Scholar]
- Pinochet, J.; Agles, M.; Dalmau, E.; Fernandez, C.; Felipe, A. Prunus rootstock evaluation to root-knot and lesion nematodes in Spain. J. Nematol. 1996, 28, 616–623. [Google Scholar]
- Cummins, J.N. Register of New Fruit and Nut Varieties. HortScience 1991, 26, 951–986. [Google Scholar] [CrossRef]
- Clark, J.R.; Finn, C.E. Register of New Fruit and Nut Cultivars List 43. HortScience 2006, 41, 1101–1133. [Google Scholar] [CrossRef]
- Okie, W. Register of New Fruit and Nut Varieties. HortScience 2004, 39, 1509–1523. [Google Scholar] [CrossRef]
- Webster, A.D. Temperate fruit tree rootstock propagation. N. Z. J. Crop Hortic. Sci. 1995, 23, 355–372. [Google Scholar] [CrossRef][Green Version]
- Hartmann, H.; Kester, D.; Davies, F.; Geneve, R. Principles of propagation by cuttings. In Hartmann & Kester’s Plant Propagation. Principles and Practices, 8th ed.; Hartmann, H., Kester, D., Eds.; Pearson: London, UK, 2014; pp. 293–360. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant growth regulators III: Gibberellins, ethylene, abscisic acid, their analogues and inhibitors; miscellaneous compounds. In Plant Propagation by Tissue Culture, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008; Volume 1, pp. 227–282. [Google Scholar]
- Gahan, P.; George, E. Adventitious regeneration. In Plant Propagation by Tissue Culture, 3rd ed.; George, E., Hall, M., Klerk, G., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 1, pp. 355–402. [Google Scholar]
- De Klerk, G.; Hanecakova, J. Ethylene and rooting of mung bean cuttings. The role of auxin induced ethylene synthesis and phase-dependent effects. Plant Growth Regul. 2008, 56, 203–209. [Google Scholar] [CrossRef]
- Soffer, H.; Burger, D.W. Studies on Plant Propagation Using the Aero-Hydroponic Method. In Proceedings of the Symposium on High Technology in Protected Cultivation, Hamamatsu, Japan, 1 September 1988; pp. 261–270. [Google Scholar] [CrossRef]
- Robbins, J.A.; Kays, S.J.; Dirr, M.A. Ethylene and its role in the rooting of wounded mung bean cuttings. HortScience 1981, 16, 401. [Google Scholar]
- Gonzalez, A.; Arigita, L.; Majada, J.; Sanchez-Tames, R. Ethylene involvement in in vitro organogenesis and plant growth of Populus tremula L. Plant Growth Regul. 1997, 22, 1–6. [Google Scholar] [CrossRef]
- Preece, J. Stock plant physiological factors affecting growth and morphogenesis. In Plant Propagation by Tissue Culture, 3rd ed.; George, E., Hall, M., Klerk, G., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 1, p. 403. [Google Scholar]
- Park, Y.G.; Son, S.H. In vitro organogenesis and somatic embryogenesis from punctured leaf of Populus nigra × P. maximowiezii. Plant Cell Tissue Organ Cult. 1988, 15, 95–105. [Google Scholar] [CrossRef]
- Debergh, P.; Read, P. Micropropagation. In Micropropagation: Technology and Application; Debergh, P., Zimmermann, R., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 1–14. [Google Scholar]
- Loreti, F.; Morini, S. Propagation Techniques. In The Peach: Botany, Production and Uses; Layne, D., Bassi, D., Eds.; CAB International: Wallingford, UK, 2008; pp. 221–243. [Google Scholar]
- Bartolini, G.; Fiorino, P. Gli interventi sulle piante madri per migliorare la radicazione delle talee. In Proceedings of the Estratto" Seminario Sul Vivaismo e Controllo della Rizogenesi Mediante Fitoregolatori, Pisa, Italy, 17 June 1978; pp. 9–25. [Google Scholar]
- Tworkoski, T.; Takeda, F. Rooting response of shoot cuttings from three peach growth habits. Sci. Hortic. 2007, 115, 98–100. [Google Scholar] [CrossRef]
- Blazich, F. Mineral Nutrition and Adventitious Rooting. In Adventitious Root Formation in Cuttings; Davis, T., Haissig, B., Sankhla, N., Eds.; Dioscorides Press: Portland, OR, USA, 1988; pp. 61–69. [Google Scholar]
- Loretti, F.; Morini, S.; Grilli, A. Rooting response of BS B2 and G.F. 677 rootstocks cutting. Acta Hortic. 1985, 173, 261–269. [Google Scholar] [CrossRef]
- Scalabrelli, G.; Couvillon, G. The Interaction Between Iba Treatment and Other Factors in Rooting and Establishment of Peach Hardwood Cuttings. In Proceedings of the V International Symposium on Growth Regulators in Fruit Production, Rimini, Italy, 1 July 1986; pp. 855–862. [Google Scholar] [CrossRef]
- Fiorino, P.; Vitagliano, C. Nuove tecniche per ottenere barbatelle di pesco: III ‘Ulteriori ricerche sulla nebulizzazione. Rivista di Ortoflorofrutticoltura Italiana 1968, 6, 779–795. [Google Scholar]
- Tsipouridis, C.; Thomidis, T.; Michailides, Z. Factors influencing the rooting of peach GF677 (peach × almond hybrid) hardwood cuttings in a growth chamber. N. Z. J. Crop Hortic. Sci. 2005, 33, 93–98. [Google Scholar] [CrossRef][Green Version]
- Lobet, G.; Pagès, L.; Draye, X. A novel image-analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiol. 2011, 157, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Chinnusamy, V.; Rathod, G.; Paul, V.; Jain, N. Evaluation of root growth and architecture. In Manual of ICAR Sponsored Training Programme on Physiological Techniques to Analyze the Impact of Climate Change on Crop Plants; Division of Plant Physiology IARI: New Delhi, India, 2017; pp. 16–25. [Google Scholar]
- Jiménez, S.; Pinochet, J.; Abadia, A.; Moreno, M.Á.; Gogorcena, Y. Tolerance response to iron chlorosis of Prunus selections as rootstocks. HortScience 2008, 43, 304–309. [Google Scholar] [CrossRef]
- Fernández, C.; Cunill, M.; Torrents, J.; Felipe, A.; López, M.M.; Lastra, B.; Pinochet, J. Response of new interspecific hybrids for peach to root-knot and lesion nematodes, and crown gall. Acta Hortic. 2002, 592, 707–716. [Google Scholar]
- De Dorlodot, S.; Forster, B.; Pagès, L.; Price, A.; Tuberosa, R.; Draye, X. Root system architecture: Opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 2007, 12, 474–481. [Google Scholar] [CrossRef]
| Rootstocks | Cultivar | Advantages | Disadvantages | Source |
|---|---|---|---|---|
| Peach (Prunus persica) | ‘Nemaguard’ |
|
| (Handoo et al. 2004; Nyczepir et al. 1983; Zehr et al. 1976) |
| Root-lesion nematode (Pratylenchus vulnus) | |||
| Fungal root rots | |||
| Meloidogyne incognita | Verticillium | |||
| M. javanica | Ring nematode (M. xenoplax) | |||
| M. arenaria |
| |||
| Crown gall (relatively) | Iron chlorosis | |||
| Root waterlogging | ||||
| ||||
| ‘Nemared’ |
|
| (Reighard & Loreti 2008) | |
| ||||
| ‘Guardian’TM |
|
| (Blaauw et al. 2020) | |
|
| |||
|
| |||
| M. incognita | Oak root rot (Desarmillaria tabescens). | |||
| M. javanica | Root-lesion nematode (P. vulnus) | |||
| M. arenaria | ||||
| ||||
| Bacterial canker | ||||
| Peach Tree Short Life (PTSL) | ||||
| ||||
| ‘Lovell’ |
|
| (Reighard & Loreti 2008) | |
|
| |||
| Oak root rot | |||
| Root-lesion nematode | |||
| Ring nematodes | Phytophthora spp. | |||
| Bacterial canker |
| |||
| PTSL | ||||
| ‘Halford’ |
|
| (Reighard & Loreti 2008) | |
| ||||
| ‘Bailey’ |
|
| (Reighard & Loreti, 2008) | |
| Root-knot nematodes | |||
| Fungal root rots | |||
| PTSL | |||
|
| |||
| ‘Flordaguard’ |
|
| ||
|
| |||
|
| |||
| ||||
| Peach’ almond rootstocks P. persica´ P. dulcis | ‘GF-677’ |
|
| (Loreti & Massai 2006) |
|
| |||
|
| |||
|
| |||
|
| |||
| Oak root rot | |||
| M. incognita | ||||
| A. tumefaciens | ||||
| Phytophthora cactorum | ||||
| Stereum purpureum | ||||
| ||||
| ‘Sirio’ |
|
| ||
|
| |||
| ||||
| ||||
| ||||
| ||||
| ‘Castore’ |
|
| ||
|
| |||
| ||||
| ||||
| ‘Polluce’ |
|
| ||
|
| |||
| ||||
| ||||
| ||||
| ‘Hansen 2168’ ‘Hansen 536’ |
|
| (Reighard & Loreti 2008) | |
|
| |||
| ||||
| M. incognita | ||||
| M. javanica | ||||
| ||||
| ‘Adafuel’ |
|
| (Reighard & Loreti 2008) | |
|
| |||
| ||||
| Powdery mildew (Sphaerotheca pannosa) | ||||
| Plum rust (Tranzschelia pruni-spinosae) | ||||
| Shot hole (Corineum beijerinckii) | ||||
| Phytophthora spp. | ||||
| ||||
| ||||
| ‘Adarcias’ |
| (Albás et al. 2004) | ||
| ||||
| ||||
| ||||
| ||||
| Colletotrichum beijerinckii Oud. | ||||
| T. pruni-spinosae (Pers.) Diet. | ||||
| ‘Felinem’ ‘Garnem’ ‘Monegro’ |
|
| ||
|
| |||
| Long vegetative period | Root-lesion nematode (P. vulnus) | |||
| Red-colored leaves | Crown gall caused by Agrobacterium tumefaciens | |||
| Low presence of feathers | ||||
| ||||
| ||||
| ||||
| M. incognita | ||||
| M. javanica | ||||
| Peach ´ plum rootstocks P. persica ´ P. cerasifera | ‘Ishtara’ |
|
| |
| ||||
| ||||
| ||||
| ||||
| ||||
| ‘Myran’ |
|
| ||
| ||||
| M. arenaria | ||||
| M. javanica | ||||
| M. incognita | ||||
| ||||
| ||||
| ||||
| ‘MP-29’ |
|
| (Beckman et al. 2012) | |
| ||||
| PTSL | ||||
| M. incognita | ||||
| M. floridensis | ||||
| ||||
| ||||
| ||||
| ||||
| ‘Controller 5’ |
|
| ||
| ‘Controller 9’ |
|
| ||
| ‘Krymsk 86’ |
| |||
| ||||
| ||||
| ||||
| ||||
| ‘Sharpe’ |
|
| ||
| ||||
| ||||
| Armillaria root rot (Desarmillaria tabescens) | ||||
| PTSL | ||||
| M. floridensis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesmes-Vesga, R.A.; Cano, L.M.; Ritenour, M.A.; Sarkhosh, A.; Chaparro, J.X.; Rossi, L. Rootstocks for Commercial Peach Production in the Southeastern United States: Current Research, Challenges, and Opportunities. Horticulturae 2022, 8, 602. https://doi.org/10.3390/horticulturae8070602
Lesmes-Vesga RA, Cano LM, Ritenour MA, Sarkhosh A, Chaparro JX, Rossi L. Rootstocks for Commercial Peach Production in the Southeastern United States: Current Research, Challenges, and Opportunities. Horticulturae. 2022; 8(7):602. https://doi.org/10.3390/horticulturae8070602
Chicago/Turabian StyleLesmes-Vesga, Ricardo A., Liliana M. Cano, Mark A. Ritenour, Ali Sarkhosh, José X. Chaparro, and Lorenzo Rossi. 2022. "Rootstocks for Commercial Peach Production in the Southeastern United States: Current Research, Challenges, and Opportunities" Horticulturae 8, no. 7: 602. https://doi.org/10.3390/horticulturae8070602
APA StyleLesmes-Vesga, R. A., Cano, L. M., Ritenour, M. A., Sarkhosh, A., Chaparro, J. X., & Rossi, L. (2022). Rootstocks for Commercial Peach Production in the Southeastern United States: Current Research, Challenges, and Opportunities. Horticulturae, 8(7), 602. https://doi.org/10.3390/horticulturae8070602








