Magnetized Water and Proline to Boost the Growth, Productivity and Fruit Quality of ‘Taifi’ Pomegranate Subjected to Deficit Irrigation in Saline Clay Soils of Semi-Arid Egypt
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Treatments
2.3. Leaf Analysis
2.4. Vegetative Growth
2.5. Yield
2.6. Consumptive Water Use (CWU), WUE, and Water Productivity (WP)
2.7. Fruit Physiochemical Characteristics
2.8. Fruit Physiological Disorders
2.9. Soil Chemical Characteristics after the Experiment
2.10. Feasibility Study
- (1)
- Treatment cost ($500.31, $59.54, and $559.85, respectively) taking into account that irrigation water is free.
- (2)
- Cost of the regular agricultural practices: electricity for irrigation = $298·ha−1; fertilizers (N, P, K, Ca and micronutrients) = $745·ha−1; pesticides = $447·ha−1; and labor = $296.12·ha−1.
- (3)
- Average fruit price (USD·kg−1) was determined per fruit weight; 200–299 g = $0.25, 300–399 g = $0.31, and ≥400 g = $0.34.
2.11. Statistical Analysis
3. Results
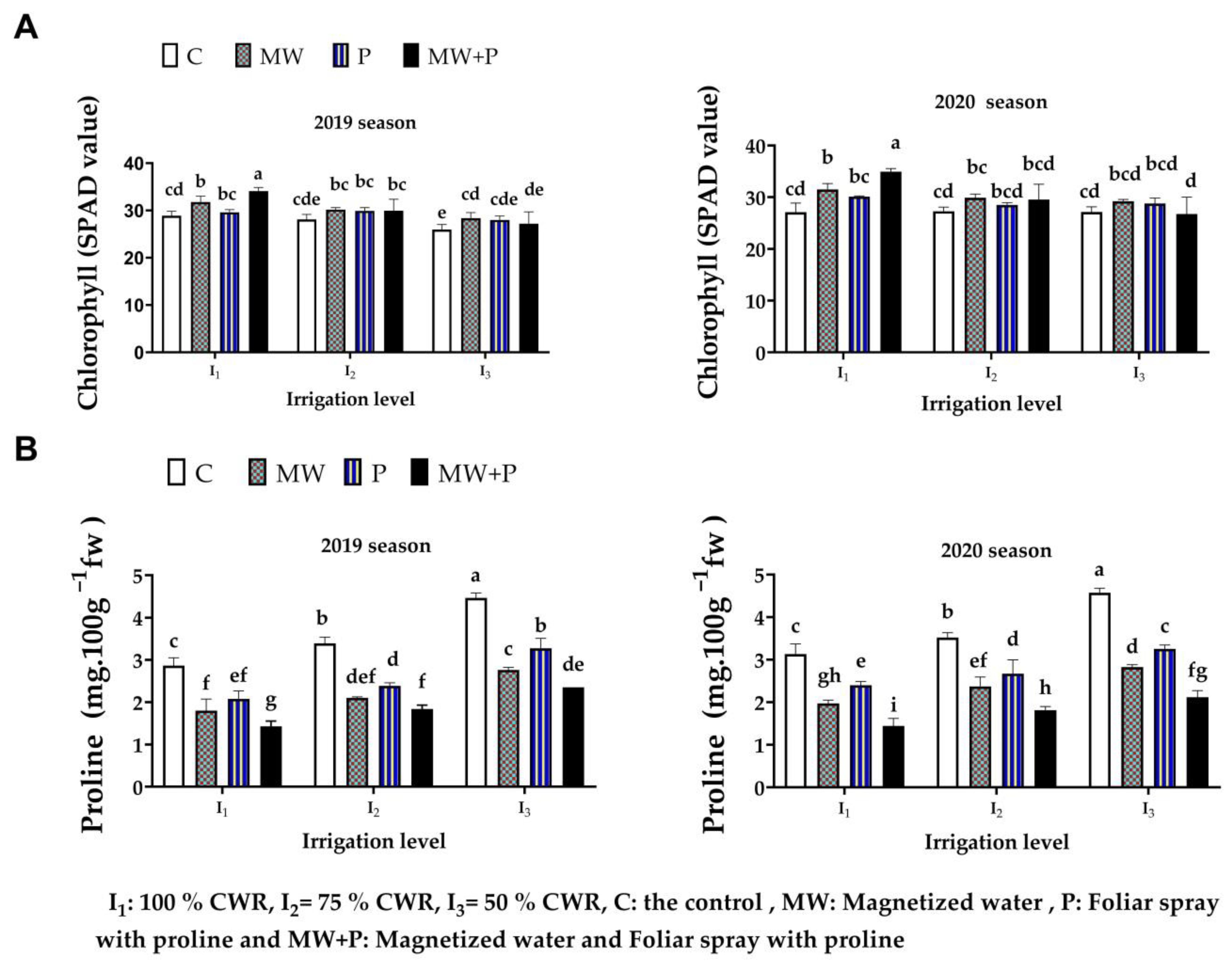
3.1. Leaf Chlorophyll and Proline Contents
3.2. Leaf Macro and Micronutrient Contents
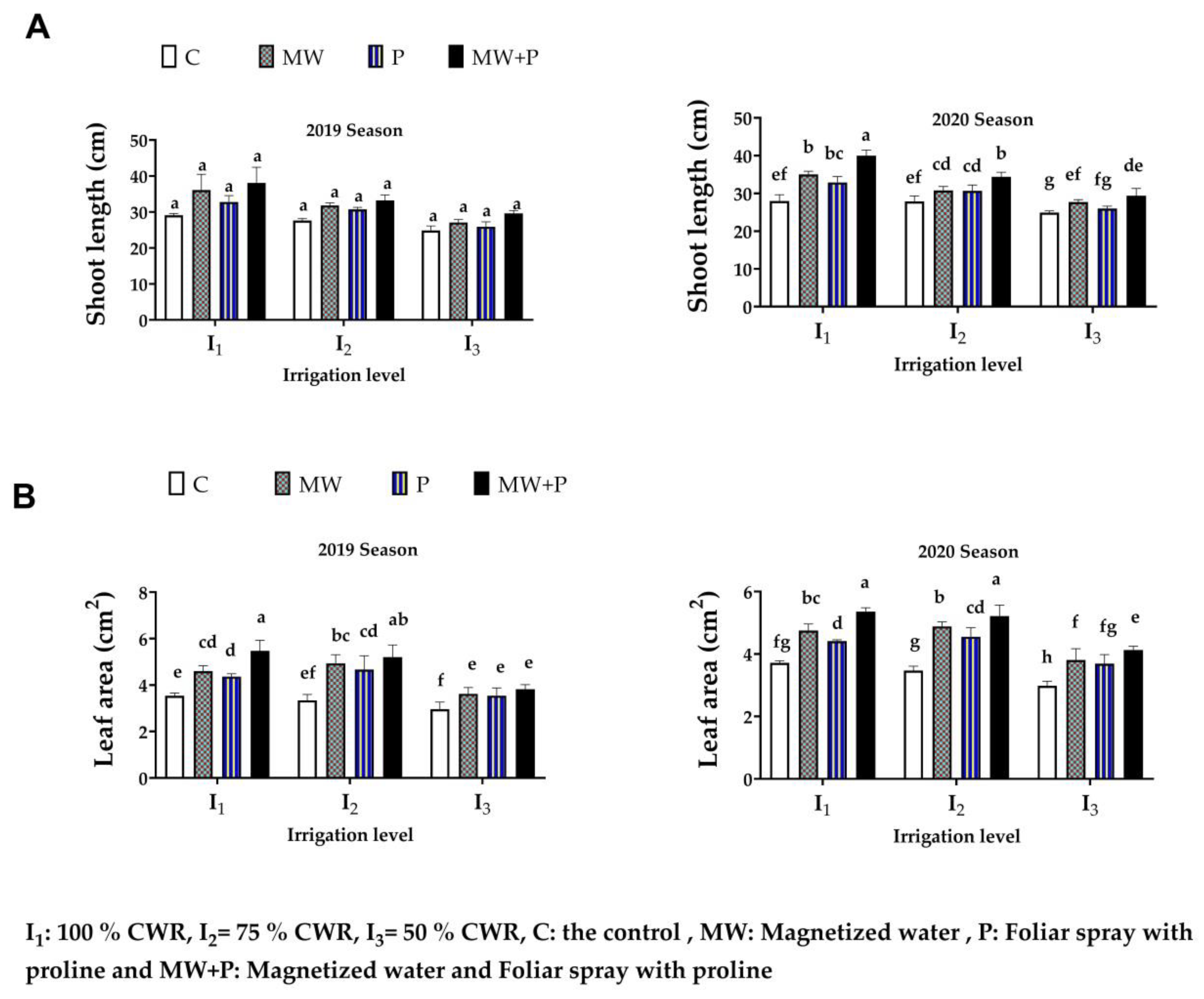
3.3. Shoot Length and Leaf Area
3.4. Plant/Water Relationships
3.5. Yield and Fruit Physiochemical Characteristics
3.6. Fruit Physiological Disorders
3.7. Soil Chemical Characteristics after the Experiment
3.8. Feasiability Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Still, D.W. Pomegranates: A botanical perspective. In Pomegranates: Ancient Roots to Modern Medicine; Seeram, N., Schulman, R., Heber, D., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 199–209. [Google Scholar]
- Kahramanoglu, I. Trends in pomegranate sector: Production, postharvesthandling and marketing. Int. J. Agric. For. Life Sci. 2019, 3, 239–246. [Google Scholar]
- Sheets, M.D.; Du Bois, M.L.; Williamson, J.G. The Pomegranate; University of Florida IFAS Extension Publication # HS44: Gainesville, FL, USA, 1994. [Google Scholar]
- Bhantana, P.; Lazarovitch, N. Evapotranspiration, crop coefficient and growth of two young pomegranate (Punica granatum L.) varieties under salt stress. Agric. Water Manag. 2010, 97, 715–722. [Google Scholar] [CrossRef]
- Tavousi, M.; Kaveh, F.; Alizadeh, A.; Babazadeh, H.; Tehranifar, A. Effects of drought and salinity on yield and water use efficiency in pomegranate tree. J. Mater. Environ. Sci. 2015, 6, 1975–1980. [Google Scholar]
- Pandey, P.; Ramegowda, V.; Senthil-Kumar, M. Shared and unique responses of plants to multiple individual stresses and stress combinations: Physiological and molecular mechanisms. Front. Plant Sci. 2015, 6, 723. [Google Scholar] [CrossRef]
- Saiki, S.T.; Ishida, A.; Yoshimura, K.; Yazaki, K. Physiological mechanisms of drought-induced tree die-off in relation to carbon, hydraulic and respiratory stress in a drought-tolerant woody plants. Sci. Rep. 2017, 7, 2995. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Blumwald, E. Developing salt-tolerant crop plants: Challenges and opportunities. Trends Plant Sci. 2005, 10, 615–620. [Google Scholar] [CrossRef]
- Li, J.; Pu, L.; Zhu, M.; Zhang, R. The present situation and hot issues in the salt-affected soil research. Acta Geogr. Sin. 2012, 67, 1233–1245. [Google Scholar]
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Plant Salt Tolerance. In ASCE Manual and Reports on Engineering Practice No. 71: Agricultural Salinity Assessment and Management, 2nd ed.; Wallender, W.W., Tanji, K.K., Eds.; American Society of Civil Engineers (ASCE) Library: Reston, VA, USA, 2012; Chapter 13; pp. 405–459. [Google Scholar]
- Zhu, J.K. Plant salt stress. In Encyclopedia of Life Science, 2nd ed.; O’Daly, A., Ed.; John and Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 1–3. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Sun, Y.; Niu, G.; Masabni, J.G.; Ganjegunte, G. Relative salt tolerance of 22 pomegranate (Punica granatum) cultivars. HortScience 2018, 53, 1513–1519. [Google Scholar] [CrossRef]
- Borochov-Neori, H.; Judeinstein, S.; Tripler, E.; Holland, D.; Lazarovitch, N. Salinity effects on colour and health traits in the pomegranate (Punica granatum L.) fruit peel. Int. J. Postharvest Technol. Innov. 2014, 4, 54–68. [Google Scholar] [CrossRef]
- Agricultural Statistics of Egypt, Ministry of Agriculture and Land Reclamation. Agricultural Economics Annual Report #781; Agricultural Statistics of Egypt, Ministry of Agriculture and Land Reclamation: Cairo, Egypt, 2020.
- El-Desouky, M.I.; Abd El-Hamied, S.A. Improving Growth and Productivity of Pomegranate Fruit Trees Planted on Sandy Slopes at Baloza District (N. Sinai) using Dfferent Methods of Drip Irrigation, Organic Fertilization and Soil Mulching. IOSR J. Agric. Vet. Sci. 2014, 7, 86–97. [Google Scholar] [CrossRef]
- Naser, T.A. Evergreen and Deciduus Fruits Production and Important Cultivars in the Arab World, 1st ed.; Dar Alma’arif Publishing Inc.: Cairo, Egypt, 1983. [Google Scholar]
- Al Shawish, F.; Hamed, F.; Al-Aisa, I. Evaluation of some qualitative and chemical characteristics for most important pomegranate (Punica granatum) accessions in Yemen. J. Agric Sci. Damascus Univ. Fac. Agric. Damascus Univ. 2006, 22, 117–241. [Google Scholar]
- Agricultural Statistics of Egypt. Water Scarcity in Egypt: The Urgent Need for Regional Cooperation among the Nile Basin Countries; Report of the Ministry of Water Resources and Irrigation; Government of Egypt: Cairo, Egypt, 2014; p. 5.
- Cosgrove, W.J.; Loucks, D.P. Water management: Current and future challenges and research directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuno, M.F.; Chaves, M.M. Deficit irrigation as a strategy to save water: Physiology and potential application to horticulture. J. Integr. Plant Biol. 2007, 49, 1421–1434. [Google Scholar] [CrossRef]
- Laribi, A.I.; Palou, L.; Intrigliolo, D.S.; Nortes, P.A.; Rojas-Argudo, C.; Taberner, V.; Bartual, J.; Perez-Gago, M.B. Effect of sustained and regulated deficit irrigation on fruit quality of pomegranate cv.‘Mollar de Elche’ at harvest and during cold storage. Agric. Water Manag. 2013, 125, 61–70. [Google Scholar] [CrossRef]
- Shafqat, W.; Mazrou, Y.S.A.; Nehela, Y.; Ikram, S.; Bibi, S.; Naqvi, S.A.; Hameed, M.; Jaskani, M.J. Effect of Three Water Regimes on the Physiological and Anatomical Structure of Stem and Leaves of Different Citrus Rootstocks with Distinct Degrees of Tolerance to Drought Stress. Horticulture 2021, 7, 554. [Google Scholar] [CrossRef]
- Tarantino, A.; Frabboni, L.; Disciglio, G. Water-yield relationship and vegetative growth of wonderful young pomegranate trees under deficit irrigation conditions in southeastern italy. Horticulturae 2021, 7, 79. [Google Scholar] [CrossRef]
- El-Hamied, A.; Sheren, A.; Ghieth, W.M. Use of magnetized water and compost tea to improve peach productivity under salinity stress of North Sinai conditions, Egypt. Egypt J. Desert Res. 2017, 67, 231–254. [Google Scholar] [CrossRef][Green Version]
- Van Oosten, M.J.; Pepe, O.; Pascale, S.D.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Ennab, H.; Alam-Eldein, S.M. Biostimulants Foliar Application to Improve Growth, Yield, and Fruit Quality of ‘Valencia’ Orange Trees under Deficit Irrigation Conditions. J. Am. Pomol. Soc. 2020, 74, 118–134. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Amiri, M.C.; Dadkhah, A.A. On reduction in the surface tension of water due to magnetic treatment. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 278, 252–255. [Google Scholar] [CrossRef]
- Holysz, L.; Szczes, A.; Chibowski, E. Effects of a static magnetic field on water and electrolyte solutions. J. Colloid Interface Sci. 2007, 316, 996–1002. [Google Scholar] [CrossRef]
- Han, H.B.; Guo, B.; Chai, F. Influence of Magnetic Field on Aqueous NaCl Solutions: A Foundational Research on the Desalination Method Based on the Rotating Electromagnetic Effect. Adv. Mat. Res. 2012, 591–593, 2607–2611. [Google Scholar] [CrossRef]
- Mostafazadeh-Fard, B.; Khoshravesh, M.; Mousavi, S.F.; Kiani, A.R. Effects of magnetized water on soil chemical components underneathtrickle irrigation. J. Irrig. Drain. Eng. 2012, 138, 1075–1081. [Google Scholar] [CrossRef]
- Hillal, M.H.; Hillal, M.M. Application of magnetic technologies in desert agriculture. 1. seed germination and seedling emergence of some crop in a saline calcareous soil. Egypt. J. Soil Sci. 2000, 40, 413–421. [Google Scholar]
- Coey, J.M.D.; Cass, S. Magnetic water treatment. J. Magn. Magn. Mater. 2000, 209, 71–74. [Google Scholar] [CrossRef]
- Tai, C.Y.; Wu, C.K.; Chang, M.C. Effects of magnetic field on the crystallization of CaCo3 using permanent magnets. J. Chem. Eng. Sci. 2008, 63, 5606–5612. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.M. The Effect of Magnetic Water on Soil Characteristics and Raphanus sativus L. Growth 2014, 9, 16–20. [Google Scholar]
- Aly, M.A.; Thanaa, M.E.; Osman, S.M.; Abdelhamed, A.A. Effect of magnetic irrigation water and some anti-salinity substances on the growth and production of Valencia orange. Middle East J. Agric. Res. 2015, 4, 88–98. [Google Scholar]
- Yu, X.; Liu, H.; Klejnot, J.; Lin, C. The Cryptochrome Blue Light Receptors. Arab. Book Am. Soc. Plant Biol. 2010, 8, 1–27. [Google Scholar] [CrossRef]
- Ghafoor, R.; Akram, N.A.; Rashid, M.; Ashraf, M.; Iqbal, M.; Lixin, Z. Exogenously applied proline induced changes in key anatomical features and physio-biochemical attributes in water stressed oat (Avena sativa L.) plants. Physiol. Mol. Biol. Plants 2019, 25, 1121–1135. [Google Scholar] [CrossRef]
- Meister, A. Biochemistry of the Amino Acids, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2012; ISBN 0323161472. [Google Scholar]
- El Sayed, O.M.; El Gammal, O.H.M.; Salama, A.S.M. Effect of proline and tryptophan amino acids on yield and fruit quality of Manfalouty pomegranate variety. Sci. Hortic. 2014, 169, 1–5. [Google Scholar] [CrossRef]
- Caronia, A.; Gugliuzza, G.; Inglese, P. Influence of L-proline on Citrus sinensis (L.) [’New Hall’and’Tarocco Scire’] fruit quality. In Proceedings of the XI International Symposium on Plant Bioregulators in Fruit Production. Acta Hortic. 2010, 884, 423–426. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc Oxide and Silicone Nanoparticles to Improve the Resistance Mechanism and Annual Productivity of Salt-Stressed Mango Trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Kahlaoui, B.; Hachicha, M.; Misle, E.; Fidalgo, F.; Teixeira, J. Physiological and biochemical responses to the exogenous application of proline of tomato plants irrigated with saline water. J. Saudi Soc. Agric. Sci. 2018, 17, 17–23. [Google Scholar] [CrossRef]
- Ezz, T.M.; Aly, M.A.M.; Nassem, M.G.; Abou Taleb, S.A.; Farag, M.E.H. Alleviation of salinity effect in irrigation water and soil on Manfalouty pomegranate trees using magnetic water, bio-fertilizer and some soil amendments. Egypt. J. Agric. Res. 2017, 95, 805–820. [Google Scholar] [CrossRef]
- Worldweatheronline. Kafr El-Sheikh, Egypt Historical Weather. 2020. Available online: https://www.worldweatheronline.com/kafr-ash-shaykh-weather-averages/kafr-ash-shaykh/eg.aspx (accessed on 3 June 2021).
- Chapman, H.D.; Pratt, F.P. Methods of Analysis for Soils, Plants and Waters, 1st ed.; University of California, Division of Agricultural Sciences: Davis, CA, USA, 1961; p. 309. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1990. [Google Scholar]
- Murquard, R.D.; Timpton, J.L. Relationship between extractable chlorophyll and the method to estimate leaf green. Hort. Sci. 1987, 22, 1327. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Evenhuis, B.; Dewaard, P.W. Nitrogen Determination; Department of Agriculture Research, Royal Tropical Institute: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Jones, J.B.; Wolf, B.; Mills, H.A. Plant Analysis Handbook. A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing, Inc.: Athens, GA, USA, 1991; p. 212. [Google Scholar]
- Tendon, H.L.S. Analysis of Soils, Plants, Waters and Fertilizers; Fertiliser Development and Consultation Organisation: New Delhi, India, 2005. [Google Scholar]
- Chang, K.L.; Bray, R.H. Determination of calcium and magnesium in soil and plant material. Soil Sci. 1951, 72, 449–458. [Google Scholar] [CrossRef]
- Rashid, A. Mapping Zinc Fertility of Soil Using Indicator Plant and Soil Analysis. Ph.D. Thesis, University of Hawaii, Manoa, HI, USA, 1986. [Google Scholar]
- Li-COR Inc. Li-3000A Portable Leaf Area Meter, Instruction Manual; Li- COR: Lincoln, NE, USA, 1987. [Google Scholar]
- Hansen, V.W.; Israelsen, D.W.; Stringharm, D.E. Irrigation Principle and Practices, 4th ed.; Johns Willey & Sons: New York, NY, USA, 1979. [Google Scholar]
- Ali, M.H.; Hoque, M.R.; Hassan, A.A.; Khair, A. Effects of deficit irrigation on yield, water productivity, and economic returns of wheat. Agric. Water Manag. 2007, 92, 151–161. [Google Scholar] [CrossRef]
- Al-Yahyai, R.; Al-Said, F.; Opara, L. Fruit growth characteristics of four pomegranate cultivars from northern Oman. Fruits 2009, 64, 335–341. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemist: Washington, DC, USA, 2000; pp. 16–20. [Google Scholar]
- Hsia, C.L.; Luh, B.S.; Chichester, C.O. Anthocyanin in Freestone Peaches. J. Food Sci. 1965, 30, 5–12. [Google Scholar] [CrossRef]
- Ikram, S.; Shafqat, W.; Qureshi, M.A.; ud Din, S.; ur-Rehman, S.; Mehmood, A.; Sajjad, Y.; Nafees, M. Causes and control of fruit cracking in pomegranate: A review. J. Glob. Imov. Agric. Soc. Sci. 2020, 8, 183–190. [Google Scholar] [CrossRef]
- Agehara, S.; Wang, W.; Sarkhosh, A. Guidelines for Pomegranate Nutrient Management in Florida; Horticultural Sciences Department, UF/IFAS Extension Publication # HS1347: Gainesville, FL, USA, 2019; p. 5. [Google Scholar]
- Grotjohann, I.; Jolley, C.; Fromme, P. Evolution of photosynthesis and oxygen evolution: Implications from the structural comparison of photosystem I and II. Phys. Chem. Chem. Phys. 2004, 6, 4743–4753. [Google Scholar] [CrossRef]
- Ratushnyak, A.A.; Andreeva, M.G.; Morozova, O.V.; Morozov, G.A.; Trushin, M.V. Effect of extremely high frequency Electromagnetic fields on the microbiological community in rhizosphere of plants. Int. Agrophysics 2008, 22, 71–74. [Google Scholar]
- Azharonok, V.V.; Goncharik, S.V.; Filatova, I.I.; Shik, A.S.; Antonyuk, A.S. The effect of the high frequency electromagnetic treatment of the sowing material for legumes on their sowing quality and productivity. Surf. Eng. Appl. Electrochem. 2009, 45, 318–328. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Vendruscolo, E.C.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinari, H.B.C.; Marur, C.J.; Vieira, L.G.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef]
- Abo-Ogiala, A. Managing crop production of pomegranate cv. Wonderful via foliar application of ascorbic acid, proline and glycinbetaine under environmental stresses. Int. J. Environ. 2018, 7, 95–103. [Google Scholar]
- Khattab, M.M.; Shaban, A.E.; El-shrief, A.H.; Mohamed, A.S.E. Growth and productivity of pomegranate trees under different irrigation levels. III: Leaf pigments, proline and mineral content. J. Hortic. Sci. Ornam. Plants 2011, 3, 265–269. [Google Scholar]
- Khoshravesh, M.; Mostafazadeh-Fard, B.; Mousavi, S.F.; Kiani, A.R. Effects of magnetized water on the distribution pattern of soil water with respect to time in trickle irrigation. Soil Use Manag. 2011, 27, 515–522. [Google Scholar] [CrossRef]
- Maheshwari, B.L.; Grewal, H.S. Magnetic treatment of irrigation water: Its effects on vegetable crop yield and water productivity. Agric. Water Manag. 2009, 96, 1229–1236. [Google Scholar] [CrossRef]
- De Souza, A.; García, D.; Sueiro, L.; Licea, L.; Porras, E. Pre-sowing magnetic treatment of tomato seeds: Effects on the growth and yield of plants cultivated late in the season. Span. J. Agric. Res. 2005, 3, 113–122. [Google Scholar] [CrossRef]
- Okba, S.K.; Mazrou, Y.; Elmenofy, H.M.; Ezzat, A.; Salama, A.-M. New Insights of Potassium Sources Impacts as Foliar Application on ‘Canino’Apricot Fruit Yield, Fruit Anatomy, Quality and Storability. Plants 2021, 10, 1163. [Google Scholar] [CrossRef]
- Mahmoud, T.A.; Youssef, E.A.; El-Harouny, S.B.; Abo Eid, M.A.M. Effect of irrigation with magnetic water on nitrogen fertilization efficiency of navel orange trees. Plant Arch. 2019, 19, 966–975. [Google Scholar]
- Kristl, J.; Slekovec, M.; Tojnko, S.; Unuk, T. Extractable antioxidants and non-extractable phenolics in the total antioxidant activity of selected plum cultivars (Prunus domestica L.): Evolution during on-tree ripening. Food Chem. 2011, 125, 29–34. [Google Scholar] [CrossRef]
- Alesiani, D.; Canini, A.; D’Abroca, B.; DellaGreca, M.; Fiorentino, A.; Mastellone, C.; Monaco, P.; Pacifico, S. Antioxidant and antiproliferative activities of phytochemicals from Quince (Cydonia vulgaris) peels. Food Chem. 2010, 118, 199–207. [Google Scholar] [CrossRef]
- Hassan, I.F.; Gaballah, M.S.; El-Hoseiny, H.M.; El-Sharnouby, M.E.; Alam-Eldein, S.M. Deficit irrigation to enhance fruit quality of the ’African Rose’ plum under the Egyptian semi-arid conditions. Agronoy 2021, 11, 1405. [Google Scholar] [CrossRef]
- Maatallah, S.; Guizani, M.; Hjlaoui, H.; Boughattas, N.E.H.; Lopez-Lauri, F.; Ennajeh, M. Improvement of fruit quality by moderate water deficit in three plum cultivars (Prunus salicina L.) cultivated in a semi-arid region. Fruits 2015, 70, 325–332. [Google Scholar] [CrossRef][Green Version]
- Hamayat, N.; Hafiz, I.A.; Ahmad, T.; Ali, I.; Qureshi, A.A. Biochemical and physiological responses of peach rootstocks against drought stress. J. Pure Appl. Agric. 2020, 5, 82–89. [Google Scholar]
- Zhao, Z.; Wang, W.; Wu, Y.; Xu, M.; Huang, X.; Ma, Y.; Ren, D. Leaf physiological responses of mature pear trees to regulated deficit irrigation in field conditions under desert climate. Sci. Hortic. 2015, 187, 122–130. [Google Scholar] [CrossRef]
- Blanco, V.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Influence of regulated deficit irrigation and environmental conditions on reproductive response of sweet cherry trees. Plants 2020, 9, 94. [Google Scholar] [CrossRef]
- Fernández-García, I.; Lecina, S.; Ruiz-Sánchez, M.C.; Vera, J.; Conejero, W.; Conesa, M.R.; Dominguez, A.; Pardo, J.J.; Léllis, B.C.; Montesinos, P. Trends and challenges in irrigation scheduling in the semi-arid area of Spain. Water 2020, 12, 785. [Google Scholar] [CrossRef]
- Wetzstein, H.Y.; Zhang, Z.; Ravid, N.; Wetzstein, M.E. Characterization of attributes related to fruit size in pomegranate. Hortscience 2011, 46, 908–912. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Gao, H.-F.; Wang, S.; Liu, X.-Y.; Hu, Q.-X.; Jian, Z.H.; Wan, R.; Song, J.-H.; Shi, J.-L. Comperhensive evaluation of 20 pomegranate (Punica granatum) cultivars in China. J. Integ. Agric. 2022, 21, 434–445. [Google Scholar] [CrossRef]
- Tehranifar, A.; Zarei, M.; Nemati, Z.; Esfandiyari, B.; Vazifeshenas, M.R. Investigation of physio-chemical properities and antioxidant activity of twenty Irania pomegranate (Punica granataum) cultivars. Sci. Hortic. 2010, 126, 180–185. [Google Scholar] [CrossRef]
- Hepaksoy, S.; Aksoy, U.; Can, H.Z.; Ui, M.A. Determination of relationship between fruit cracking and some physiological responses, leaf characteristics and nutritional status of some pomegranate varieties. Options Mediterr. 2000, 42, 87–92. [Google Scholar]
- Saei, H.; Sharifani, M.M.; Dehghani, A.; Seifi, E.; Akbarpour, V. Description of biochemical forces and physiological parameters of fruit cracking in pomegranate. Sci. Hortic. 2014, 178, 224–230. [Google Scholar] [CrossRef]
- Makeredza, B.; Schmeisser, M.; Lötze, E.; Steyn, W.J. Water stress increases sunburn in “Cripps” Pink’ apple. HortScience 2013, 48, 444–447. [Google Scholar] [CrossRef]
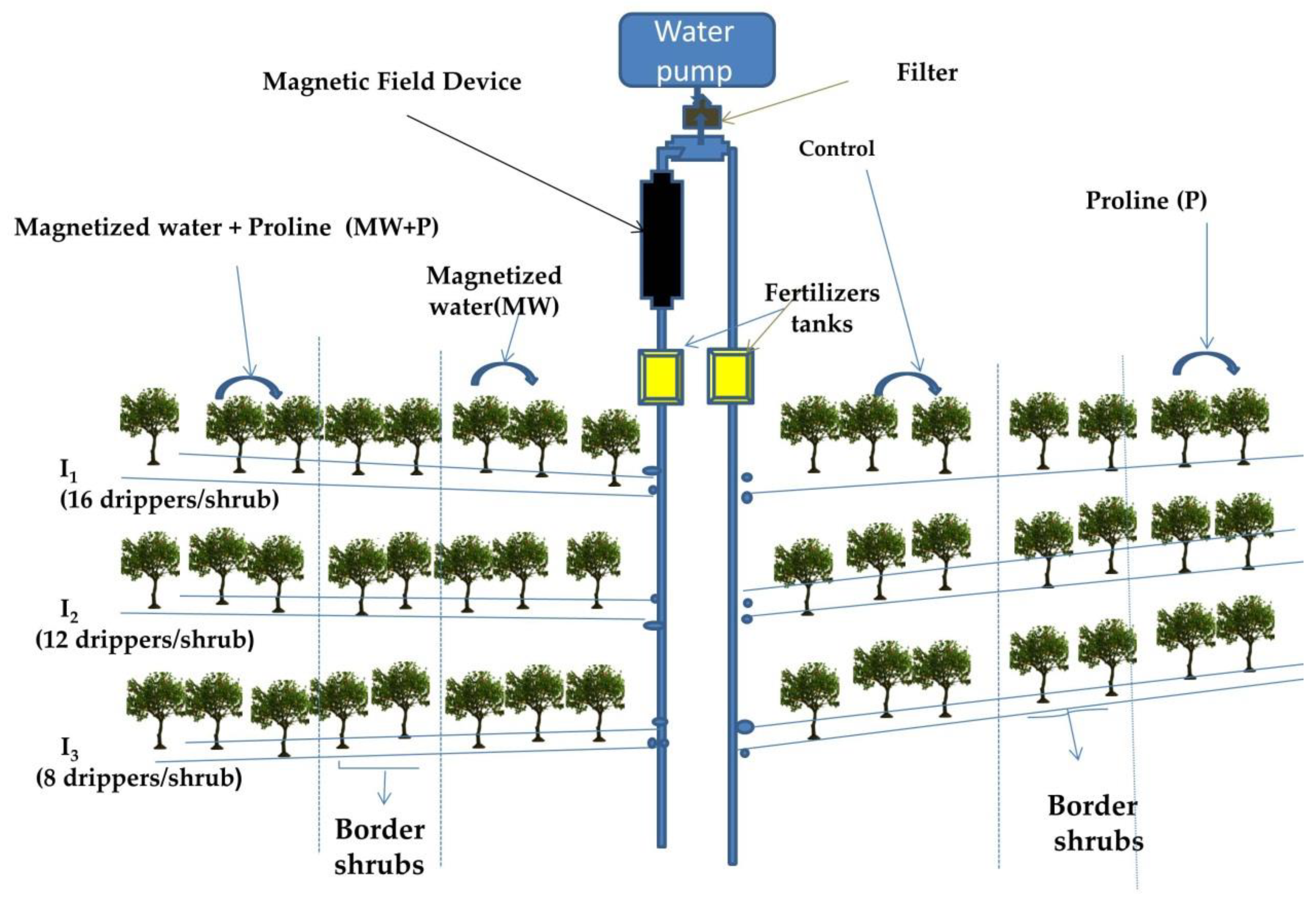


| Season | Temperature (°C) | Humidity (%) | Rainfall (mm·month−1) | Wind Speed (km·h−1) | Cloud (%) | Sun (days·month−1) | UV Index | |
|---|---|---|---|---|---|---|---|---|
| Winter | 2019 | 16.0 | 61.7 | 23.0 | 13.8 | 19.3 | 30.0 | 4.3 |
| 2020 | 16.0 | 68.0 | 26.8 | 13.2 | 31.7 | 28.7 | 4.0 | |
| Spring | 2019 | 23.0 | 57.3 | 15.7 | 14.0 | 13.3 | 30.0 | 6.0 |
| 2020 | 23.0 | 59.3 | 5.3 | 14.6 | 16.7 | 30.3 | 6.3 | |
| Summer | 2019 | 30.7 | 63.0 | 1.2 | 13.0 | 4.7 | 30.7 | 8.0 |
| 2020 | 30.7 | 62.7 | 0.07 | 13.9 | 5.0 | 30.7 | 7.7 | |
| Fall | 2019 | 26.7 | 63.7 | 6.03 | 11.9 | 10.0 | 30.0 | 6.3 |
| 2020 | 26.7 | 64.3 | 11.7 | 11.6 | 15.7 | 29.3 | 6.3 |
| Soil Analysis | Water Analysis | |||
|---|---|---|---|---|
| Depth (cm) | 0–30 | 30–60 | 60–90 | |
| Sand (%) | 25.2 | 22.7 | 25.5 | |
| Silt (%) | 27.1 | 28.1 | 39.3 | |
| Clay (%) | 47.7 | 49.2 | 35.1 | |
| Texture | Clay | Clay | Loamy clay | |
| Density (g·cm−3) | 1.29 | 1.36 | 1.42 | |
| Field capacity (%) | 42.2 | 39.7 | 38.9 | |
| Permineant wilting point (%) | 22.7 | 21.6 | 21.2 | |
| Available water (%) | 73.6 | 76.6 | 78.1 | |
| Depth (cm) | 0–60 | |||
| EC (ds·m−1) | 1.69 | |||
| pH | 8.2 | 7.3 | ||
| Total dissolved salts (ppm) | 400 | |||
| CaCO3 (%) | 8.54 | |||
| HCO3− (meq·L−1) | 0.90 | 2.1 | ||
| CO3− (meq·L−1) | 0.00 | |||
| So42− (meq·L−1) | 0.26 | 4.1 | ||
| Cl− (meq·L−1) | 0.50 | 2.3 | ||
| Na+ (meq·L−1) | 0.45 | 2.7 | ||
| K+ (meq·L−1) | 0.21 | 0.2 | ||
| Ca2+ (meq·L−1) | 0.80 | 3.1 | ||
| Mg2+ (meq·L−1) | 0.20 | 2.5 | ||
| Month | Irrigation Frequency Per Month | Irrigation Period (h) | Dripper Discharge Amount (L·h−1) | Irrigation Levels (L·Tree−1·Season−1) | ||
|---|---|---|---|---|---|---|
| I1 = 16 Drippers/Shrub (Control = 100% CWR *) | I2 = 12 Drippers/Shrub (25% Less = 75% CWR) | I3 = 8 Drippers/Shrub (50% Less = 50% CWR) | ||||
| January | 3 | 1 | 4 | 192 | 144 | 96 |
| Febreuary | 8 | 1 | 4 | 512 | 384 | 256 |
| March | 8 | 2 | 4 | 1024 | 768 | 512 |
| April | 14 | 2 | 4 | 1792 | 1344 | 896 |
| May | 14 | 2 | 4 | 1792 | 1344 | 896 |
| June | 15 | 2 | 4 | 1920 | 1440 | 960 |
| July | 15 | 3 | 4 | 2880 | 2160 | 1440 |
| August | 15 | 3 | 4 | 2880 | 2160 | 1440 |
| September | 10 | 2 | 4 | 1280 | 960 | 640 |
| October | 8 | 1.30 | 4 | 768 | 576 | 384 |
| November | 8 | 1 | 4 | 512 | 384 | 256 |
| December | 4 | 1 | 4 | 256 | 192 | 128 |
| Total water (m3.tree−1.season−1) | 15.81 | 11.86 | 7.90 | |||
| Treatment | N | P | K | Ca | Mg | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| Irrigation levels | ||||||||||
| I1: 100% CWR (control) | 1.86 | 1.84 | 0.23 | 0.23 | 0.98 | 0.95 | 0.13 | 0.13 | 1.25 | 1.29 |
| I2: 75% CWR | 1.82 | 1.81 | 0.21 | 0.21 | 0.94 | 0.94 | 0.13 | 0.13 | 1.26 | 1.22 |
| I3: 50% CWR | 1.74 | 1.72 | 0.19 | 0.19 | 0.82 | 0.82 | 0.13 | 0.13 | 0.82 | 0.87 |
| LSD (p ≤ 0.05) | 0.062 | 0.066 | 0.014 | 0.008 | 0.072 | 0.032 | ns | ns | 0.126 | 0.123 |
| Salinity-mitigating treatments | ||||||||||
| C: tap water (control) | 1.68 | 1.64 | 0.18 | 0.18 | 0.78 | 0.80 | 0.05 | 0.05 | 0.86 | 0.88 |
| MW: magnitized water | 1.83 | 1.83 | 0.22 | 0.22 | 0.95 | 0.96 | 0.14 | 0.15 | 1.20 | 1.21 |
| P: proline | 1.75 | 1.72 | 0.20 | 0.20 | 0.90 | 0.86 | 0.11 | 0.11 | 1.02 | 1.07 |
| MW + P | 1.97 | 1.96 | 0.23 | 0.23 | 1.02 | 1.00 | 0.17 | 0.18 | 1.36 | 1.34 |
| LSD (p ≤ 0.05) | 0.037 | 0.043 | 0.007 | 0.005 | 0.035 | 0.032 | 0.011 | 0.014 | 0.091 | 0.080 |
| Interaction | ||||||||||
| I1 + C (control) | 1.75 | 1.72 | 0.20 | 0.19 | 0.78 | 0.87 | 0.09 | 0.09 | 0.95 | 0.99 |
| I1 + MW | 1.86 | 1.94 | 0.23 | 0.24 | 1.04 | 1.01 | 0.14 | 0.14 | 1.32 | 1.41 |
| I1 + P | 1.82 | 1.75 | 0.22 | 0.22 | 1.00 | 0.86 | 0.10 | 0.11 | 1.19 | 1.22 |
| I1 + MW + P | 2.01 | 1.95 | 0.26 | 0.25 | 1.08 | 1.06 | 0.18 | 0.19 | 1.54 | 1.54 |
| I2 + C (75% CWR) | 1.72 | 1.64 | 0.18 | 0.18 | 0.85 | 0.81 | 0.08 | 0.08 | 0.95 | 0.91 |
| I2 + MW | 1.82 | 1.85 | 0.22 | 0.22 | 0.96 | 1.01 | 0.15 | 0.15 | 1.43 | 1.33 |
| I2 + P | 1.77 | 1.73 | 0.21 | 0.20 | 0.89 | 0.91 | 0.11 | 0.12 | 1.09 | 1.15 |
| I2 + MW + P | 1.98 | 2.01 | 0.24 | 0.24 | 1.04 | 1.02 | 0.18 | 0.18 | 1.57 | 1.48 |
| I3 + C (50% CWR) | 1.56 | 1.55 | 0.17 | 0.17 | 0.71 | 0.71 | 0.11 | 0.11 | 0.67 | 0.73 |
| I3 + MW | 1.82 | 1.72 | 0.20 | 0.20 | 0.84 | 0.84 | 0.13 | 0.16 | 0.84 | 0.90 |
| I3 + P | 1.67 | 1.68 | 0.18 | 0.18 | 0.80 | 0.81 | 0.13 | 0.11 | 0.77 | 0.84 |
| I3 + MW + P | 1.92 | 1.91 | 0.21 | 0.21 | 0.93 | 0.90 | 0.15 | 0.16 | 0.98 | 0.99 |
| LSD (p ≤ 0.05) | 0.064 | 0.075 | ns | 0.009 | 0.061 | 0.055 | 0.018 | 0.024 | 0.158 | 0.139 |
| Treatment | Fe | Zn | Mn | |||
|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| Irrigation levels | ||||||
| I1: 100% CWR (control) | 126.1 | 123.2 | 115.7 | 104.4 | 114.5 | 115.9 |
| I2: 75% CWR | 125.4 | 124.1 | 101.1 | 103.2 | 111.3 | 111.1 |
| I3: 50% CWR | 101.2 | 102.0 | 75.1 | 71.6 | 85.8 | 84.6 |
| LSD (p ≤ 0.05) | 9.75 | 12.87 | 9.65 | 19.11 | 5.79 | 9.02 |
| Salinity-mitigating treatements | ||||||
| C: tap water (control) | 99.0 | 99.1 | 74.6 | 74.4 | 80.5 | 83.9 |
| MW: magnitized water | 123.8 | 122.7 | 103.8 | 98.6 | 113.8 | 109.2 |
| P: proline | 110.6 | 110.8 | 97.7 | 8.3 | 96.2 | 98.4 |
| MW + P | 136.7 | 133.1 | 113.1 | 111.0 | 124.9 | 124.0 |
| LSD (p ≤ 0.05) | 8.63 | 5.25 | 6.43 | 8.27 | 8.00 | 6.45 |
| Interaction | ||||||
| I1 + C (control) | 102.0 | 104.2 | 89.5 | 89.6 | 91.4 | 92.8 |
| I1 + MW | 133.8 | 129.2 | 123.7 | 109.0 | 122.8 | 122.5 |
| I1 + P | 120.4 | 120.8 | 113.2 | 90.4 | 106.7 | 108.0 |
| I1 + MW + P | 148.1 | 138.7 | 136.4 | 128.7 | 136.9 | 140.5 |
| I2 + C (75% CWR) | 102.6 | 101.2 | 90.4 | 90.6 | 86.7 | 86.0 |
| I2 + MW | 132.3 | 131.8 | 102.5 | 106.6 | 124.0 | 120.4 |
| I2 + P | 115.5 | 116.4 | 98.6 | 100.1 | 101.3 | 103.6 |
| I2 + MW + P | 151.3 | 147.0 | 112.9 | 115.6 | 133.0 | 134.3 |
| I3 + C (50% CWR) | 92.5 | 91.9 | 43.9 | 43.1 | 63.4 | 72.8 |
| I3 + MW | 105.4 | 107.0 | 85.2 | 80.2 | 94.6 | 85.0 |
| I3 + P | 96.2 | 95.3 | 81.1 | 74.5 | 80.9 | 83.6 |
| I3 + MW + P | 110.8 | 113.7 | 90.0 | 88.6 | 104.7 | 97.1 |
| LSD (p ≤ 0.05) | ns | 9.09 | 11.14 | 14.33 | ns | 11.16 |
| Treatment | Yield (Kg·shrub−1) | Fruit Weight (g) | Fruit Volume (cm3) | Fruit Shape Index (Sphericity) (length·diameter−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| Irrigation levels | ||||||||
| I1: 100% CWR (control) | 71.2 | 68.1 | 416.7 | 416.6 | 454.6 | 447.1 | 0.88 | 0.91 |
| I2: 75% CWR | 73.8 | 76.6 | 408.2 | 406.7 | 417.4 | 427.2 | 0.86 | 0.88 |
| I3: 50% CWR | 52.1 | 54.3 | 266.5 | 266.05 | 245.4 | 239.5 | 0.86 | 0.91 |
| LSD (p ≤ 0.05) | 1.51 | 3.34 | 8.53 | 16.83 | 62.15 | 32.47 | ns | ns |
| Salinity-mitigating treatments | ||||||||
| C: tap water (control) | 52.3 | 52.9 | 316.7 | 317.8 | 288.7 | 292.3 | 0.80 | 0.94 |
| MW: magnitized water | 70.2 | 71.6 | 383.2 | 365.3 | 405.1 | 404.8 | 0.84 | 0.87 |
| P: proline | 62.4 | 63.6 | 346.9 | 357.0 | 344.7 | 339.2 | 0.90 | 0.93 |
| MW + P | 77.9 | 77.3 | 408.4 | 412.4 | 451.3 | 448.7 | 0.88 | 0.87 |
| LSD (p ≤ 0.05) | 5.27 | 7.10 | 14.90 | 17.53 | 29.07 | 30.54 | 0.020 | 0.030 |
| Interaction | ||||||||
| I1 + C (control) | 59.8 | 55.9 | 379.9 | 358.0 | 359.7 | 356.7 | 0.77 | 1.02 |
| I1 + MW | 75.3 | 75.8 | 434.4 | 433.8 | 523.3 | 484.3 | 0.83 | 0.80 |
| I1 + P | 68.9 | 67.9 | 393.3 | 398.0 | 398.0 | 401.0 | 0.86 | 0.98 |
| I1 + MW + P | 80.7 | 73.0 | 459.3 | 476.5 | 537.3 | 546.3 | 0.87 | 0.87 |
| I2 + C (75% CWR) | 53.9 | 56.3 | 340.9 | 366.2 | 325.3 | 327.7 | 0.78 | 0.91 |
| I2 + MW | 77.6 | 80.1 | 429.3 | 384.0 | 421.2 | 464.4 | 0.83 | 0.91 |
| I2 + P | 69.7 | 71.5 | 392.6 | 408.3 | 394.3 | 389.0 | 0.96 | 0.90 |
| I2 + MW + P | 94.7 | 98.4 | 470.2 | 468.3 | 528.9 | 527.7 | 0.89 | 0.81 |
| I3 + C (50% CWR) | 43.0 | 46.5 | 229.3 | 229.2 | 181.2 | 192.6 | 0.85 | 0.89 |
| I3 + MW | 57.8 | 58.9 | 285.8 | 278.0 | 270.7 | 265.7 | 0.85 | 0.90 |
| I3 + P | 49.1 | 51.3 | 254.8 | 264.7 | 241.8 | 227.7 | 0.89 | 0.90 |
| I3 + MW + P | 58.4 | 60.6 | 295.8 | 292.3 | 287.7 | 272.2 | 0.86 | 0.95 |
| LSD (p ≤ 0.05) | 9.14 | 12.29 | 25.81 | 30.36 | 50.35 | 52.89 | 0.042 | 0.051 |
| Treatment | TSS (%) | Acidity (%) | Tss/Acid Ratio | Vitamin C (mg·100 mL−1) | Anthocyanins (mg·100 g−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| Irrigation levels | ||||||||||
| I1: 100% CWR (control) | 12.5 | 12.2 | 1.3 | 2.4 | 9.3 | 5.2 | 8.0 | 8.1 | 14.2 | 13.7 |
| I2: 75% CWR | 13.1 | 12.9 | 1.5 | 1.9 | 8.9 | 6.8 | 9.8 | 8.7 | 15.1 | 14.5 |
| I3: 50% CWR | 13.5 | 13.4 | 1.5 | 2.1 | 9.2 | 6.5 | 7.7 | 9.4 | 14.5 | 14.9 |
| LSD (p ≤ 0.05) | 0.51 | 0.32 | ns | 0.32 | 0.31 | 0.70 | ns | ns | 0.53 | 0.86 |
| Salinity-mitigating treatments | ||||||||||
| C: tap water (control) | 11.9 | 11.4 | 1.5 | 3.2 | 7.9 | 3.6 | 5.9 | 5.4 | 11.9 | 11.4 |
| MW: magnitized water | 13.6 | 13.5 | 1.2 | 2.1 | 11.0 | 6.3 | 8.2 | 9.6 | 15.3 | 15.3 |
| P: proline | 12.9 | 12.9 | 2.0 | 1.9 | 6.4 | 6.8 | 8.8 | 9.4 | 14.9 | 14.7 |
| MW + P | 13.6 | 13.6 | 0.9 | 1.2 | 14.5 | 11.2 | 11.0 | 10.6 | 16.3 | 16.0 |
| LSD (p ≤ 0.05) | 0.31 | 0.30 | 0.50 | 0.21 | 1.79 | 0.67 | 0.76 | 0.94 | 0.68 | 0.76 |
| Interaction | ||||||||||
| I1 + C (control) | 11.3 | 10.3 | 2.3 | 3.1 | 4.8 | 3.4 | 4.1 | 3.5 | 11.0 | 8.9 |
| I1 + MW | 13.3 | 13.3 | 1.1 | 2.2 | 11.6 | 6.1 | 8.2 | 9.0 | 15.1 | 15.2 |
| I1 + P | 12.5 | 12.5 | 1.1 | 3.1 | 10.8 | 4.0 | 9.1 | 9.0 | 14.1 | 14.5 |
| I1 + MW + P | 12.9 | 12.7 | 0.7 | 1.0 | 16.8 | 12.2 | 10.5 | 11.0 | 16.6 | 16.2 |
| I2 + C (75% CWR) | 11.8 | 11.5 | 1.1 | 2.8 | 10.3 | 4.1 | 7.7 | 5.6 | 12.7 | 12.3 |
| I2 + MW | 13.5 | 13.30 | 1.9 | 2.4 | 7.0 | 5.4 | 9.0 | 9.4 | 16.0 | 15.2 |
| I2 + P | 12.8 | 12.7 | 1.9 | 1.2 | 6.7 | 11.0 | 9.7 | 9.0 | 15.4 | 14.9 |
| I2 + MW + P | 14.1 | 13.9 | 0.9 | 1.2 | 15.7 | 11.9 | 12.6 | 10.9 | 16.3 | 15.6 |
| I3 + C (50% CWR) | 12.6 | 12.5 | 1.0 | 3.7 | 12.3 | 3.4 | 5.9 | 7.1 | 11.9 | 13.1 |
| I3 + MW | 14.1 | 14.0 | 0.6 | 1.8 | 22.0 | 7.8 | 7.3 | 10.3 | 14.9 | 15.6 |
| I3 + P | 13.5 | 13.3 | 3.03 | 1.4 | 4.4 | 9.4 | 7.7 | 10.1 | 15.2 | 14.8 |
| I3 + MW + P | 13.8 | 14.0 | 1.15 | 1.4 | 12.0 | 9.9 | 9.8 | 10.0 | 16.0 | 16.3 |
| LSD (p ≤ 0.05) | ns | 0.52 | 0.87 | 0.36 | 3.10 | 1.16 | 1.32 | 1.62 | ns | 1.31 |
| Treatment | pH | EC (dS·m−1) | Soluble Cations (meq·L−1) | |||
|---|---|---|---|---|---|---|
| K+ | Mg2+ | Na+ | Ca2+ | |||
| Irrigation levels | ||||||
| I1: 100% CWR (control) | 8.16 | 1.59 | 0.33 | 0.17 | 0.33 | 0.52 |
| 12: 75% CWR | 8.17 | 1.60 | 0.27 | 0.32 | 0.36 | 0.60 |
| 13: 50% CWR | 8.39 | 1.67 | 0.27 | 0.39 | 0.43 | 0.66 |
| LSD (p ≤ 0.05) | ns | 0.039 | ns | 0.052 | 0.043 | 0.035 |
| Salinity-mitigating treatments | ||||||
| C: tap water (control) | 8.36 | 1.65 | 0.28 | 0.27 | 0.42 | 0.64 |
| MW: magnitized water | 8.18 | 1.61 | 0.30 | 0.28 | 0.34 | 0.54 |
| P: proline | 8.27 | 1.67 | 0.29 | 0.30 | 0.39 | 0.63 |
| MW + P | 8.15 | 1.58 | 0.29 | 0.32 | 0.35 | 0.56 |
| LSD (p ≤ 0.05) | 0.140 | 0.034 | ns | ns | 0.032 | 0.049 |
| Interaction | ||||||
| I1 + C (control) | 8.24 | 1.58 | 0.28 | 0.14 | 0.36 | 0.57 |
| I1 + MW | 8.13 | 1.60 | 0.34 | 0.16 | 0.32 | 0.46 |
| I1 + P | 8.16 | 1.65 | 0.35 | 0.19 | 0.40 | 0.58 |
| I1 + MW + P | 8.12 | 1.65 | 0.34 | 0.20 | 0.26 | 0.46 |
| I2 + C (75% CWR) | 8.26 | 1.65 | 0.33 | 0.28 | 0.39 | 0.59 |
| I2 + MW | 8.15 | 1.59 | 0.29 | 0.30 | 0.31 | 0.50 |
| I2 + P | 8.16 | 1.64 | 0.24 | 0.32 | 0.36 | 0.66 |
| I2 + MW + P | 8.12 | 1.52 | 0.23 | 0.38 | 0.39 | 0.63 |
| I3 + C (50% CWR) | 8.59 | 1.71 | 0.21 | 0.39 | 0.50 | 0.76 |
| I3 + MW | 8.26 | 1.63 | 0.28 | 0.39 | 0.40 | 0.66 |
| I3 + P | 8.50 | 1.71 | 0.28 | 0.38 | 0.41 | 0.65 |
| I3 + MW + P | 8.23 | 1.66 | 0.31 | 0.38 | 0.39 | 0.58 |
| LSD (p ≤ 0.05) | ns | ns | 0.060 | ns | 0.050 | 0.080 |
| Treatment | (1) TreatmentCost (USD·ha−1) | (2) Fixed Cost (USD·ha−1) | (3 = 1 + 2) Total Cost (USD·ha−1) | (4) Total Yield (t·ha−1) | (5 = 4 × ℗) Total Return (USD·ha−1) | (6 = 5 − 3) Net Profit (USD·ha−1) | |||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||||
| I1 (100% CWR) + C (tap water) [control] | - | 1786.12 | 1786.12 | 23.91 | 22.29 | 7476.22 | 6970.82 | 5690.10 | 5184.7 |
| I1 + MW (magnitized water) | 500.31 | 1786.12 | 2286.43 | 30.12 | 30.94 | 10,358.88 | 10,642.28 | 8072.45 | 8355.85 |
| I1 + P (proline) | 59.54 | 1786.12 | 1845.66 | 27.55 | 26.81 | 8876.33 | 8980.20 | 7030.67 | 7134.54 |
| I1 + MW + P | 559.85 | 1786.12 | 2345.97 | 32.29 | 29.43 | 11,105.75 | 10,123.81 | 8759.78 | 7777.84 |
| I2 (75% CWR) + C | - | 1786.12 | 1786.12 | 21.57 | 22.53 | 6745.08 | 7045.24 | 4958.96 | 5259.12 |
| I2 + MW | 500.31 | 1786.12 | 2286.43 | 31.04 | 32.53 | 10,675.58 | 10,170.94 | 8389.15 | 7884.51 |
| I2 + P | 59.54 | 1786.12 | 1845.66 | 27.65 | 28.56 | 9222.38 | 9253.08 | 7376.72 | 7407.42 |
| I2 + MW + P | 559.85 | 1786.12 | 2345.97 | 37.86 | 39.67 | 13,021.84 | 13,643.94 | 10,675.87 | 11,297.97 |
| I3 (50% CWR) + C | - | 1786.12 | 1786.12 | 17.21 | 18.56 | 4305.32 | 4643.90 | 2519.20 | 2857.78 |
| I3 + MW | 500.31 | 1786.12 | 2286.43 | 21.37 | 21.66 | 5346.90 | 5346.90 | 3060.47 | 3060.47 |
| I3 + P | 59.54 | 1786.12 | 1845.66 | 21.38 | 22.13 | 5348.91 | 5536.96 | 3503.25 | 3691.30 |
| I3 + MW + P | 559.85 | 1786.12 | 2345.97 | 23.35 | 24.2 | 6299.15 | 6299.15 | 3953.18 | 3953.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okba, S.K.; Mazrou, Y.; Mikhael, G.B.; Farag, M.E.H.; Alam-Eldein, S.M. Magnetized Water and Proline to Boost the Growth, Productivity and Fruit Quality of ‘Taifi’ Pomegranate Subjected to Deficit Irrigation in Saline Clay Soils of Semi-Arid Egypt. Horticulturae 2022, 8, 564. https://doi.org/10.3390/horticulturae8070564
Okba SK, Mazrou Y, Mikhael GB, Farag MEH, Alam-Eldein SM. Magnetized Water and Proline to Boost the Growth, Productivity and Fruit Quality of ‘Taifi’ Pomegranate Subjected to Deficit Irrigation in Saline Clay Soils of Semi-Arid Egypt. Horticulturae. 2022; 8(7):564. https://doi.org/10.3390/horticulturae8070564
Chicago/Turabian StyleOkba, Sameh K., Yasser Mazrou, Gehad B. Mikhael, Mohamed E. H. Farag, and Shamel M. Alam-Eldein. 2022. "Magnetized Water and Proline to Boost the Growth, Productivity and Fruit Quality of ‘Taifi’ Pomegranate Subjected to Deficit Irrigation in Saline Clay Soils of Semi-Arid Egypt" Horticulturae 8, no. 7: 564. https://doi.org/10.3390/horticulturae8070564
APA StyleOkba, S. K., Mazrou, Y., Mikhael, G. B., Farag, M. E. H., & Alam-Eldein, S. M. (2022). Magnetized Water and Proline to Boost the Growth, Productivity and Fruit Quality of ‘Taifi’ Pomegranate Subjected to Deficit Irrigation in Saline Clay Soils of Semi-Arid Egypt. Horticulturae, 8(7), 564. https://doi.org/10.3390/horticulturae8070564






