Abstract
In manufacturing food powders, foam-mat drying provides a cost-effective alternative to traditional drying methods. This study aimed to select the foaming conditions which support the subsequent drying of Magenta leaves extract. In the initial stage, concentrations of egg albumin (5 to 15%) as a foaming agent, xanthan gum as foam stabilizer (0.1 to 0.5%), and whipping time (2 to 8 min) were designed. Multiple regression analysis was applied to analyze the relationship between the dependent variables (Foam expansion volume and foam density) and three independent variables as an indicator of foaming capacity and foam stability. The multiple response optimization was applied to optimize the foam density and foam expansion. The optimum foam density (0.25 g/mL) and foam expansion volume (298.12%) were obtained at the optimum egg albumin and xanthan gum concentrations, and whipping time at 12.21%, 0.24%, and 5.8 min, respectively, indicating a stable foam structure. Experimental moisture loss data are fitted for five selected drying models. The mathematical models were compared according to three statistical parameters, such as coefficient of determination (R2), chi-square (χ2), and root mean square error (RMSE). Among the five mathematical models tested with experimental data, the Page model could be applied to describe the foam-mat drying process of magenta leaves extract. The highest value of R2 (99.54%), the lowest value of χ2 (0.0007), and RMSE (0.0253) were observed for a air drying temperature of 60 °C. The effect of temperature on diffusion is described by the Arrhenius equation with an activation energy of 100.21 kJ/mol. Effective moisture diffusion values ranged from 2.27 × 10−10 to 6.71 × 10−10 m2/s as the temperature increased. The effect of drying conditions on anthocyanin changes of magenta leaves powder was compared. The results showed that the highest quality of the sample was observed when the sample was dried at 60 °C.
1. Introduction
The magenta plant (Peristrophe roxburghiana) is a species of flowering plant in the family Acanthaceae, distributed mainly in Southeast Asia. Vietnam is also a place where the plants grow well all year round. Magenta leaves extract contains high levels of anthocyanins [1]. The purple magenta plant has a pinkish purple color and its leaves are ovate-shaped. The base is round, light green, relatively thin and contains little hairs. The area of white spots along the veins is large and the extract is purple [2]. Magenta leaves have a sweet taste and cool properties and have the effect of reducing cough and stopping bleeding. If combined with other drugs, they can treat bronchitis with lots of phlegm, bleeding, tendon injuries, and bruised muscles [2]. In Vietnam, people also use the leaves to color food or process food (sticky rice and cylindrical glutinous rice cake—Bánh tét) because the leaves are not toxic. However, the extract has a short shelf life due to its high water content. With large volumes of extracts, their preservation also becomes difficult. If the storage temperature is not controlled appropriately, the extract is very susceptible to spoilage due to various microbiological, physical, and chemical agents [1]. Therefore, it is necessary to reduce the water content to keep the color and quality of the extract.
Drying can be one of the main processes for preserving the bioactive compound in the extract because this process reduces the water content, the growth of enzymes and microorganisms, and ultimately increases the stability of the product [3,4]. Foam drying can be considered as a new and very suitable drying technique. Foam is formed by stirring the liquid in the presence of an edible foaming agent and foam stabilizer, in combination with air. The foam is then dried to a moisture content of about 2–2.5% [3]. The dehumidification rate of foam occurs rapidly due to the physical structure of the foam such as honeycomb structure. The increase in volume after stirring together with the increase of the drying surface area enables the drying speed to take place rapidly [3,5]. Therefore, this drying process also helps to preserve nutrients and heat-sensitive biological compounds [4]. It is difficult to dry unstable foam and remove moisture from the drying surface [5], which might lead to the poor quality of product [6]. In addition, the drying method and drying temperature also have an important influence. Many experimental studies and mathematical models on the drying qualities of various plants, such as papaya [7], carrot [8], tomato [9], and butterfly pea flowers [10], have been conducted.
The foaming ability was affected by foaming agents such as egg albumin, milk, etc. Foam stabilizers such as gelatin, carboxyl methyl cellulose, xanthan gum, etc., together with the whipping time, also affect the foam stability. It is important to optimize the process with appropriate concentrations of foaming agent, foam stabilizer, and foaming time [3]. The process of converting items from liquid to stable foam and then air drying them is known as foam-mat drying [11]. Foam-mat drying of papaya was performed by Kandasamy et al. [12], who used varying concentrations of egg albumin as a foaming ingredient (5 to 20% w/w) and whipped for varying periods to interact with air to generate a stable foam. The determination of foam properties including foam stability, foam density, and foam expansion was conducted. Sharada [13] investigated the sponge drying of tomato, guava, and banana using various quantities of egg albumin and soy protein isolate as foaming agents at drying temperatures ranging from 55 to 80 °C. Reis et al. [14] investigated the effects of hot air temperatures (40–65 °C), foam thickness (0.2 and 1 cm), and the use of egg white as one of the foaming agents on bioactive retention. Xanthan gum has been added as a stabilizer to maintain the stability of the foam to accelerate the drying of the foam mat in the packaging [15]. However, studies on foam drying from magenta leaves extract in Vietnam have not been researched. This study focuses on understanding the factors affecting foaming generation and foam stability, then optimizing the content of these agents along with the whipping time. This optimization method might assist in establishing the best conditions for a stable foam that does not collapse during the following stage of foam-mat drying of magenta leaves extract. Foam-mat drying kinetic modeling is also studied and evaluated.
2. Materials and Methods
2.1. Preparation of Magenta Extract
The materials used in the research are the Magenta plant, grown and harvested at the College of Agriculture, Can Tho University (Vietnam). Harvesting should be done in the cool hours of the day. Samples were cut to the length of both stems and leaves, choosing good leaves free from pests and diseases. When harvesting, care should be taken to avoid crushing the stems and leaves in order to protect the natural bioactive compounds available in the material. The stems and leaves were then washed, cut into pieces with a length of about 4 to 6 cm, and microwave-assisted extraction was performed, as described by Thuy, Han, Minh and Van Tai [1]. Briefly, the leaves were extracted of water with the ratio of 1:10.83 (w/v) by microwave. The power of microwave was a constant set up at 600 W, the extraction time was 4.39 min, and the obtained extract had an anthocyanin content of 30.97 mg/g db [1].
2.2. Investigate the Effects of Egg Albumin, Xanthan Gum and Whipping Time on Foam Properties
The obtained extract was mixed with the foaming ingredient as albumin, with the content varying from 5 to 15%, and xanthan gum was used as foam stabilizing agent, with the content varying from 0.1 to 0.5% (Table 1). The mixture was whipped and foamed with a mixer (Philips HR3705 300 W) at the highest speed, fixing the batch volume at 100 mL. During whipping, the foam was developed by incorporating air in it to increase the surface area, which expanded in volume. The whipping time was recorded in experiments ranging from 2 to 8 min. The multilevel factorial design was conducted (27 runs and three replicates), and multi-regression analysis was applied to observe the optimal conditions for forming foam. At the end of each treatment, the mixture was evaluated based on the foam properties, including foam expansion volume and foam density.

Table 1.
Factors level of this study.
2.2.1. Foam Expansion Volume Determination
Expansion of foam is used to represent the quantity of air integrated into the extract during foaming and is expressed as a percentage increase in extract volume (Equation (1)).
where V1 is initial volume (mL) and V2 is final volume (mL).
2.2.2. Foam Density Determination
Abd Karim and Wai [16] described a method for determining foam density (Equation (2)). In total, 100 mL of foam was weighed after being transferred to a 250 mL measuring cylinder. To avoid breaking the foam structure or trapping the air spaces filling the cylinder, the foam transferring was done with extreme caution.
2.2.3. Anthocyanin Content Determination
The pH differential method was used to determine the anthocyanin concentration. Anthocyanin content was expressed as cyanidin-3-glucoside equivalents and the calculation process was followed as described by Maran et al. [17].
2.2.4. Hygroscopicity Determination
Ten grams of magenta powder after drying was placed in an open glass container [NaCl saturated solution (75.29% RH)] at 25 °C. After one week, samples were weighed and hygroscopicity was expressed as g of adsorbed moisture per 100 g dry solids (g/100 g), following the method of Tonon et al. [18]. Hygroscopicity (%) was calculated using Equation (3).
where m (g) is the increase in weight of powder after equilibrium, M is the initial mass of powder, and Mi (% wb) is the free water content of the powder before exposure to the humid air environment.
2.2.5. Statistical Analysis
To fit the model of the observed data, a statistical analysis (STATGRAPHIC) was employed. For each response (Y), the proposed model (Equation (4)) was as follows:
where bo Y is the intercept (constant), bn is the regression coefficient for the linear effect of Xn on Y, bnn and bnm are the regression coefficients for the interaction and quadratic effect on Y and Xn, and Xm are the independent values
Multiple regression analysis was utilized to find the optimum condition for forming stable foam. The dependent variables were foam density and foam expansion volume, whereas the independent factors were egg albumin content, xanthan gum content, and whipping duration, which were expressed as X1, X2, and X3, respectively. Based on the R2 value obtained through multiple regression, the reference equation was chosen to match the data. When compared to the R2 value for the other reference interaction, the selected reference equation should have a higher R2 value.
2.3. Drying Procedure
The oven drier (Memmert, UN260, Germany) was set to four different temperatures (50, 60, 70, and 80 °C), with an air velocity of 1.0 m/s and a 4 mm thick drying layer parallel to the sample’s drying surface. During the drying process, a half-hourly weight change was measured using a digital balance (Ohaus, Parsippany, NJ, USA, d = 0.001). The sample was dried until the weight remained unchanged. For further analysis, the sample was ground and sieved through a 0.5 mm sieve (Test Sieve, Thailand).
2.3.1. Calculation of Drying Rate
Using the drying curves, the drying behavior of the magenta leaves extract was investigated, and the drying rate, DR (g water/g dry matter per min), was estimated using Equation (5) [19].
where Mt+dt and Mt are moisture contents (g water/g dry matter) at time (t + dt) and time t, respectively.
2.3.2. Mathematical Modeling
The experimental drying data was fitted into five mathematical models that are typically used to describe drying behavior (Table 2) [19,20,21].

Table 2.
Mathematical models used to predict the drying of magenta leaves extract.
The moisture content of different temperatures was transformed into the moisture ratio (MR) in order to determine the mathematical model. Because of the long drying time, the values of equilibrium moisture content are relatively little compared to those of Mt or Mo, and the error involved in the simplification is negligible. Therefore, Equation (6) was used to represent the simplest form of MR [22].
where Mt and Mo are the instantaneous and initial moisture content (kg water/kg dry matter) of the product.
The correlation coefficient (R2), chi-square (χ2), and root mean square error (RMSR) were used in the regression analysis to determine the best model for drying the magenta leaves extract at various temperatures. The best fit with experimental data and mathematical model is shown by the highest R2 and the lowest χ2 and RMSE [20,21].
2.3.3. Calculation of the Effective Moisture Diffusivity and Activation Energy
Fick’s diffusion equation accurately describes the drying characteristics of a variety of foods [23]. A variant of the equation given by Crank [24] can be derived during drying, assuming one-dimensional moisture movement, minor shrinkage, constant diffusivity, homogeneous initial moisture distribution, and negligible external resistance [25]. For extended drying times, only the first term in the series equation is significant, resulting in a linear solution. Then, a natural logarithm is used to produce Equation (7) [19].
where Deff is the effective diffusivity (m2/s), t is drying time (s), and L is the half thickness of the slab (m).
Because the plot produces a straight line with the same slope as Equation (8), the diffusion coefficients are obtained by graphing experimental drying data in terms of ln(MR) vs. drying time (t) [26].
The Arrhenius Equation (Equation (9)) is used to calculate the activation energy (Ea), which expresses the dependency of the effective diffusion coefficient on the air drying temperature [27].
where Do is the diffusion coefficient corresponding to infinite temperature (m2/s), Ea is the activation energy (kJ/mol), R is the universal gas constant (8.314 J/mol.K), and T is the absolute drying temperature (K).
2.3.4. Statistical Analysis
Each experiment was replicated three times, and the results were presented as mean ± standard deviation. Duncan’s multiple range test with a confidence level of 95% (α = 0.05) was used in STATGRAPHICS Centurion XV.I to assess significant variations between means.
3. Results and Discussion
3.1. Effect of Egg Albumin, Xanthan Gum, and Whipping Time on Foam Properties
3.1.1. Foam Density
The effect of the sponge property is expressed through foam density. Foam density decreases as more air is incorporated in the whipping process, and the more air, the higher the foaming potential. The relationship between foam density with the albumin, xanthan gum content, and whipping time was presented in the multiple regression equation (Equation (10)). Analysis of Variance (ANOVA) was used to assess the statistical significance of the equation (Table 3). Egg albumin concentration (X1) and whipping time (X3) were shown to have significant impacts on foam density (p < 0.05); however, xanthan gum concentration (X2) and interactions (X1X3, X2X3) were not affected (p > 0.05). Foam density was significantly impacted by the quadratic effects of X1 and X3, as well as the interaction effect of the remainder (p < 0.05). The non-essential terms have already been removed, and Equation (10) contains the significant parameters that enhanced the foam density response.

Table 3.
ANOVA for the response surface quadratic model for foam density.
The high values of R2 (93.47%) and adjusted R2 (92.94%) for response variables were observed. The Lack-of-fit is insignificant (p = 0.476 > 0.05) and the p-value of the model also was significantly different(p = 0.001 < 0.05), suggesting that the selected model is accurate enough to explain the behavior and predict foam density.
where X1 is egg albumin (%), X2 is xanthan gum (%), and X3 is whipping time (min).
Figure 1 shows the response surface plots for the effect of independent variables (X1, X2, and X3) on foam density.
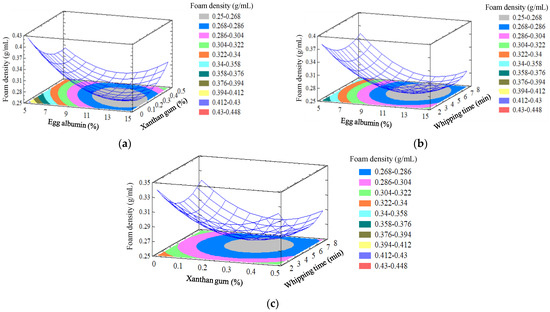
Figure 1.
Response surface of foam density as a function of egg albumin, xanthan gum and whipping time. (a) whipping time 5 min; (b) xanthan gum 0.5%; and (c) egg albumin 10%.
Response surface and contour plots showed that the whipping time and egg albumin content both have a quadratic effect on foam density. The lower the foam density, the more foam expands, and this helps in good heat transfer for the next drying process, where the foam density ranges from range from 0.253 to 0.423 g/mL. Keeping whipping time constant (Figure 1a), the foam density decreased with increasing concentrations of generated stable foam (egg albumin) and stabilizer (xanthan gum), increasing to the optimum point and then equalizing or decreasing slightly. With the same concentrations of xanthan gum (Figure 1b) and egg albumin (Figure 1c), the foam density decreased as the whipping time increased from 2 to 5.77 min, and as the time increased past the optimum point, the foam density increased. Foam density is minimized across the designated region by a combination of factor levels. It was found that the foam density reached the optimum value (0.25 g/mL) at optimum values of egg albumin, xanthan gum, and whipping time, at 12.21%, 0.24%, and 5.8 min, respectively. Some food items are typically foam-mat dried with egg albumin [28,29,30,31].
3.1.2. Foam Expansion Volume
The amount of air integrated into the magenta extract during foaming was measured using the expansion in volume of foam, which indicates the percentage increase in volume of the extract. Similarly, the results of ANOVA statistical analysis indicated that the models are established with the coefficients of linearity, interaction, and square of the factors; the content of egg albumin (X1), xanthan gum (X2), and the whipping time (X3) all influenced the foam expansion volume, with the exception of the double interaction (X1X3, and X2X3), which did not affect the foam expansion volume (Table 4). The linear factors (X1, X2, and X3), double interactions (X1X2, X1X3, and X2X3) and second order interactions (X12, X22, and X32) all show high reliability (p < 0.05). The significant parameters that improved the foam expansion volume response were given in Equation (11). The high coefficient of determination (R2 = 98.96%) and adjusted R2 (98.85%) for response variables were observed. The Lack-of-fit is insignificant (p = 0.188 > 0.05) and the p-value of model was lower than 0.05, which indicates the goodness fit of a model. The correlation between the experimental data and the predicted data from Equation (11) is found.
where X1 is egg albumin (%), X2 is xanthan gum (%), and X3 is whipping time (min).

Table 4.
ANOVA for the response surface quadratic model for foam expansion volume.
The response surface and contour plots of each pair of factors were presented in Figure 2. It was observed that xanthan gum and egg albumin concentrations have a similar effect on foam expansion compared with foam density because both reactions are related; greater foam expansion leads to lesser foam density. The combination of factor levels which maximizes foam expansion volume over the indicated region and the optimization is to be performed. It indicated that the foam expansion volume reached the maximum value (299.479%) at optimum values of egg albumin, xanthan gum, and whipping time at 11.67%, 0.29%, and 5.98 min, respectively.
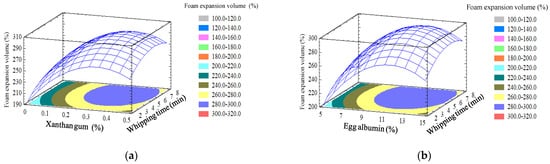
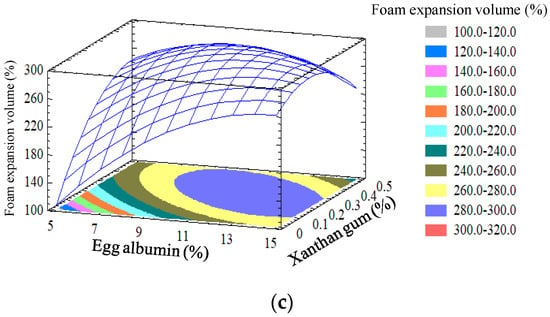
Figure 2.
Response surface of foam expansion volume as a function of egg albumin, xanthan gum, and whipping time. (a) Egg albumin 10%; (b) Xanthan gum 0.3%; and (c) Whipping time 5 min.
At the fixed egg albumin content of 10%, the foam expansion volume increased as the used concentration of gum xanthan and whipping time increased from 0.1 to 0.29% and 2 to 5.98 min, respectively (Figure 2a). However, when this optimal point is exceeded, the expansion volume of the foam tends to decrease. Similarly, when foaming time and xanthan gum were constant at 5 min and 0.3% (Figure 2b,c), respectively, the foam expansion volume increased as the concentration of egg albumin used increased from 5% to 11.67%. However, when the albumin content exceeds the optimal point, the foam expansion volume is equalized and then tends to decrease slightly. Muthukumaran et al. [32] investigated the effect of foam stabilizers on the stability of egg white foam. The authors discovered that xanthan gum had a substantial stabilizing effect and 0.125% xanthan gum was adequate to create excellent egg white foam.
3.1.3. Multiple Response Optimization
Simultaneous optimization of multiple response surfaces is a major concern for industrial applications [33], since process energy costs are significantly reduced when the parameters are simultaneously optimized [34]. In this study, dependent variables such as foam density (g/mL) and foam expansion volume (%) were individually optimized using the RSM. Therefore, the independent variables (egg albumin, xanthan gum, and whipping time) exhibited different optimal values. Multiple response optimization should be performed to create a combination of response surfaces to minimize foam density and maximize the foam expansion volume with the same optimal values of egg albumin, xanthan gum, and whipping time. To obtain optimal values of a foam density (the lowest value) of 0.25 g/mL and a foam expansion volume (the highest value) of 298.12%, the optimal values of egg albumin, xanthan gum, and whipping time were obtained: 12.21%, 0.24%, and 5.8 min. The contour plot shows the effect of egg albumin, xanthan gum, and whipping time on foam density (g/mL) and foam expansion volume (%), and is shown in Figure 3 with an asterisk (*) when generally optimized. At a fixed factor level, for example at whipping time 5 min (Figure 3a), xanthan gum 0.3% (Figure 3b) and egg albumin 10% (Figure 3c), foam density and foam expansion volume of magenta leaves extract were optimized. The optimal parameters obtained in this study are quite similar to some relevant scientific publications. Balasubramanian, Paridhi, Bosco, and Kadam [3] succeeded in using response surface method to optimize foaming conditions of tomato juice with egg albumin and CMC (instead of xanthan gum in our study), and the foaming time was found to be 11.45%, 0.33%, and 5.21 min, respectively. Ratti and Kudra [35] reported that foam-mat drying enables the processing of difficult-to-dry biomaterials such as heat sensitive compounds, as well as the creation of materials that rehydrate quickly while maintaining quality indicators including color, aroma, texture, and nutritional content. When compared to convective drying of non-foamed materials, these attributes are the result of a shorter exposure period to a generally lower temperature, which reduces thermal degradation of the dry products. After optimal conditions have been determined, predictive models can only be used after validating those conditions with the sample done at observed optimum conditions. The results are presented in Table 5. The expansion volume was found to be slightly lower than the predicted value (3.79%), and the foam density was slightly greater (2.6%), but both were within acceptable ranges (5%).
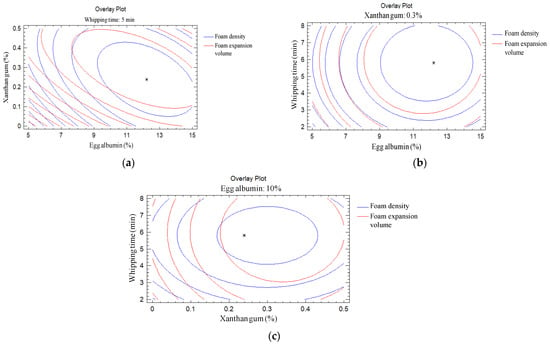
Figure 3.
The overlay plot of foam density and foam expansion volume of magenta leaves extract and optimum value * (different levels of input variables). (a) Whipping time 5 min; (b) Xanthan gum 0.3%; and (c) Egg albumin 10%.

Table 5.
Predicted and actual value of responses from optimal conditions.
3.2. Drying
After the magenta leaf extract is foamed, the stabilized foam is then dried. Drying of the stable foam structure will make an important contribution to the improvement of moisture transmission, as the surface area increases due to air incorporation with foaming [4]. The low temperature and short drying time of foam mat drying were chosen to maintain the available nutrients present in the extract [10,31].
3.2.1. Effect of Temperature on Moisture Ratio and Dehydration Rate
Figure 4 shows the moisture ratios achieved during the drying of foamed magenta leaves extract samples at different temperatures. The moisture ratio (MR) dropped steadily with drying time; however, the drying rate rose as drying temperature increased (Figure 5). The drying temperature has been found to have a substantial impact on the drying time. Drying time was reduced when the drying temperature was raised. After 5.5, 3.5, 2.75, and 2.25 h at drying temperatures of 50, 60, 70, and 80 °C, the final moisture content of the powder was 3.3 to 3.7% dry basis.
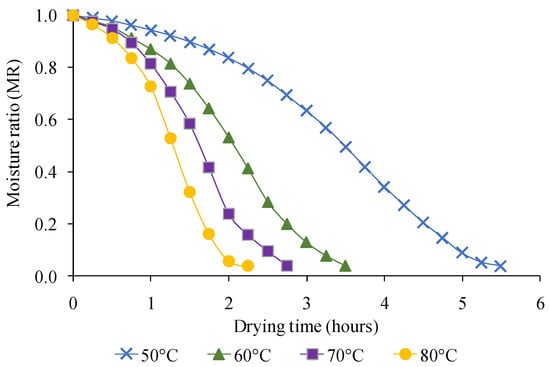
Figure 4.
The change in moisture ratio at various temperatures by foam-mat drying.
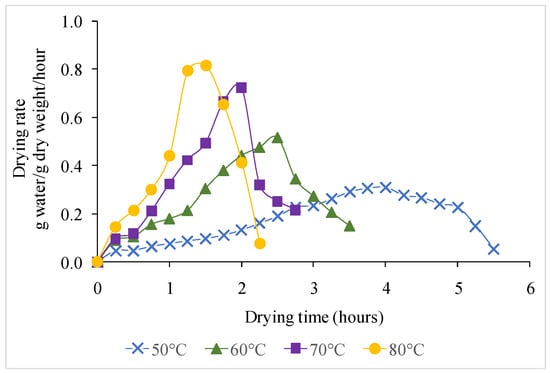
Figure 5.
Drying rate of foamed magenta leaves extract versus drying time at different temperatures.
During hot air drying, the drying rate was changed. The moisture loss rate was rapid during the first one and a half hour of the drying process, then gradually reduces until minimummoisturecontent is achieved. The continuously decreasing percentage of moisture indicates that moisture diffusion dominated the mass transfer within the foam. Because of the higher internal temperature of the product [36], the drying rate is usually judged to be significantly higher in the early stages of the drying process [37]. Furthermore, higher thermal energy promotes the movement of water molecules in the food, resulting in a reduction in drying time with increasing drying temperature [38]. These results are quite similar to previous studies reported on different plant food such as blanched pumpkin slices [39], butterfly pea flowers [10], and mango slices [40].
3.2.2. Drying Kinetics
The statistical analysis and correlation coefficients of foam-mat-dried magenta leaves extract samples dried at 50, 60, 70, and 80 °C forthe five drying models are presented in Table 6. The higher R2, lower chi square χ2, and RMSE values are the best criteria for selecting an appropriate drying model. Almost all the models tested had high correlations with the experimental data, and the R2 values were found between 77.93 to 99.69%, RSME 0.0206 to 0.184, and χ2 from 0.0005 to 0.564. The Page model, which had the highest R2 value and lower χ2 and RMSE, was the most successful in describing the drying behavior of magenta leaves extract. Table 6 also summarizes the constant values for all the models. The Page model may be assumed as the best-fitted model to exhibit the good drying behavior for most of the peach samples. Doymaz [41] indicated that the Page model fit the experimental drying data of persimmon slices better than other empirical models at 50, 60, and 70 °C. The drying process of butterfly pea flowers was well-described by the Page model, according to Thuy, Minh, Ha, and Tai [10]. The Page model was discovered to be suitable for drying red chiles [42]. Figure 6 shows a high correlation between experimental and calculated data, with a high R2 value (99.51%).

Table 6.
Regression parameters of tested mathematical models for foam-mat drying of magenta leaves extract at different temperatures.
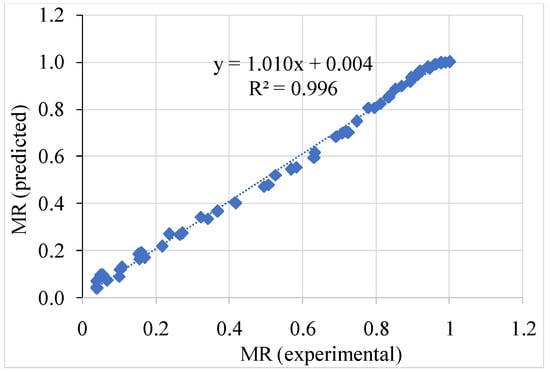
Figure 6.
Correlation between the experimentally determined MR values and the MR values predicted for magenta leaves powder using Page model.
3.2.3. The Moisture Diffusivity and Activation Energy
The Deff values showed an upward trend with increasing temperatures. The effective diffusivity values of foam-mat dried magenta leaves extract at 50 to 80 °C were in the range of 2.267 × 10−10 to 6.707 × 10−10 m2/s. As others reported, the resultant values are found to be greater. The estimated Deff values for the Vernonia amygdalina leaves [43] with the effective diffusivities ranged from 4.55 × 10−12 to 5.48 × 10−12 m2/s at 40 to 60 °C. The estimated Deff values of dried butterfly pea flowers varied in the range of 2.392 × 10−12 to 7.756 × 10−12 m2/s at 55 to 70 °C [10]. The values of ln(Deff) vs. 1/T (1/K) are plotted to determine the influence of temperature on effective diffusivity. Over the temperature range studied, the plot was found to be a straight line, demonstrating Arrhenius dependence. The activation energy (Ea) was determined to be 100.21 kJ/mol by calculating the slope of the straight line [26]. This value obtained from our investigation is found to be different with other dried products, such as mint leaf (82.93 kJ/mol), as reported by Jin Park et al. [44], and black tea 406.028 kJ/mol [45]. Lesser activation energy corresponds to lower sensitivity to air temperature [44].
3.2.4. Effect of Drying Temperature on Physicochemical Properties of Foam-Mat Dried Magenta Leaves Powder
Temperatures of 50, 60, 70, and 80 °C were relatively low, ranging from 3.35 to 3.96% and aw in the range of 0.205 to 0.293 (Table 7).

Table 7.
Physical properties of foam-mat dried magenta at different drying temperatures.
Magenta leaves powder maintains its inherent natural characteristic color at different drying temperatures and times (Figure 7 and Table 8). The hygroscopic ability (moisture absorption capacity) of the powdered leaves is from 19.69 to 24.29%, and the highest hygroscopicity is recorded when the powder is obtained when dried at 80 °C. This result is probably because the hygroscopicity value increases inversely with the powder’s moisture content; due to the moisture differential between the product and the air, the powder with the lower moisture content has a greater ability to absorb the surrounding moisture. Costa et al. [46] reported that finer particles have larger contact surfaces and thus more active sites, and most other fruit powders have particle sizes between 500 μm and 600 μm.

Figure 7.
Color of magenta powder at different foam-mat drying temperatures and times.

Table 8.
The color parameters of magenta powder under different drying temperatures.
The results also clearly showed that a high content of anthocyanin was maintained with a shorter drying time. However, the loss occurred more at higher drying temperature; the remaining anthocyanin content of 2.044 mg/g and 1.759 mg/g in dried samples at 60 °C and 80 °C, respectively, probably due to the thermal destruction of anthocyanins. Anthocyanins were degraded at temperatures above 65 °C as presented from some authors [14,47]. The foam-mat dried magenta leaves extract could be dried for 3.5 h at 60 °C, which produced the product with a high anthocyanin content and a bright color.
4. Conclusions
High quality powder produced from Magenta leaves extract can be obtained by selecting the proper amount of egg albumin (12.21%), xanthan gum (0.24%), and whipping time (5.8 min). The condition that can be applied is the optimal foaming process to reach the maximum value of the foam expansion volume and the minimum value of the foam density. The Page model is suitable for foam-mat drying of extracts in oven dryers with different temperatures. It also showed that the best fit with the highest correlation coefficient (R2), the smallest chi-square value (χ2), and RMSE were obtained. In addition, anthocyanins in plants that are very sensitive to high temperatures and difficult to dry could be easily dried using a foam-mat drying method (at 60 °C) with the highest anthocyanin content maintained. By using this technique, the final product can be produced in a short drying time (3.5 h) with minimal quality change.
Author Contributions
Conceptualization, N.M.T. and V.Q.M.; methodology, N.M.T., N.V.T. and V.Q.M.; software, V.Q.T. and N.V.T.; validation, N.M.T. and V.Q.M.; formal analysis, V.Q.T. and N.V.T.; investigation, V.Q.T. and N.V.T.; writing—original draft preparation, N.M.T., V.Q.T., N.V.T. and V.Q.M.; writing—review and editing, N.M.T., V.Q.M. and N.V.T.; supervision, N.M.T. and V.Q.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thuy, N.M.; Han, D.H.N.; Minh, V.Q.; Van Tai, N. Effect of extraction methods and temperature preservation on total anthocyanins compounds of Peristrophe bivalvis L. Merr leaf. J. Appl. Biol. Biotechnol. 2022, 10, 146–153. [Google Scholar] [CrossRef]
- Chi, V.V. Từ Điển Cây Thuốc Việt Nam (Dictionary of Vietnamese Medicinal Plants); Medical Publishinghouse: Hanoi, Vietnam, 1999. [Google Scholar]
- Balasubramanian, S.; Paridhi, G.; Bosco, J.D.; Kadam, D.M. Optimization of process conditions for the development of tomato foam by box-behnken design. Food Nutr. Sci. 2012, 3, 925–930. [Google Scholar] [CrossRef]
- Wilson, R.A.; Kadam, D.M.; Chadha, S.; Sharma, M. Foam mat drying characteristics of mango pulp. Int. J. Food Sci. Nutr. Eng. 2012, 2, 63–69. [Google Scholar] [CrossRef]
- Lee, J.; Ye, L.; Landen, W.O., Jr.; Eitenmiller, R.R. Optimization of an extraction procedure for the quantification of vitamin E in tomato and broccoli using response surface methodology. J. Food Compos. Anal. 2000, 13, 45–57. [Google Scholar] [CrossRef]
- Bag, S.K.; Srivastav, P.P.; Mishra, H.N. Optimization of process parameters for foaming of bael (Aegle marmelos L.) fruit pulp. Food Bioprocess Technol. 2011, 4, 1450–1458. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.A.; Zambra, C.E.; Vega-Gálvez, A.; Moraga, N.O. Coupled 3D heat and mass transfer model for numerical analysis of drying process in papaya slices. J. Food Eng. 2013, 116, 109–117. [Google Scholar] [CrossRef]
- Kaya, A.; Aydın, O.; Demirtaş, C. Experimental and theoretical analysis of drying carrots. Desalination 2009, 237, 285–295. [Google Scholar] [CrossRef]
- Das Purkayastha, M.; Nath, A.; Deka, B.C.; Mahanta, C.L. Thin layer drying of tomato slices. J. Food Sci. Technol. 2013, 50, 642–653. [Google Scholar] [CrossRef]
- Thuy, N.M.; Minh, V.Q.; Ha, H.T.N.; Tai, N.V. Impact of different thin layer drying temperatures on the drying time and quality of butterfly pea flowers. Food Res. 2021, 5, 197–203. [Google Scholar] [CrossRef]
- Sangamithra, A.; Sivakumar, V.; John, S.G.; Kannan, K. Foam Mat Drying of Food Materials: A Review. J. Food Process. Preserv. 2015, 39, 3165–3174. [Google Scholar] [CrossRef]
- Kandasamy, P.; Varadharaju, N.; Kalemullah, S. Foam-mat drying of papaya (Carica papaya L.) using glycerol monostearate as foaming agent. Food Sci. Qual. Manag. 2012, 9, 17–27. [Google Scholar]
- Sharada, S. Studies on effect of various operating parameters & foaming agents-Drying of fruits and vegetables. Int. J. Mod. Eng. Res. 2013, 3, 1512–1519. [Google Scholar]
- Reis, F.R.; de Moraes, A.C.S.; Masson, M.L. Impact of Foam-Mat Drying on Plant-Based Foods Bioactive Compounds: A Review. Plant Foods Hum. Nutr. 2021, 76, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Susanti, D.Y.; Sediawan, W.B.; Fahrurrozi, M.; Hidayat, M. Foam-mat drying in the encapsulation of red sorghum extract: Effects of xanthan gum addition on foam properties and drying kinetics. J. Saudi Soc. Agric. Sci. 2021, 20, 270–279. [Google Scholar] [CrossRef]
- Abd Karim, A.; Wai, C.C. Foam-mat drying of starfruit (Averrhoa carambola L.) puree. Stability and air drying characteristics. Food Chem. 1999, 64, 337–343. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Extraction of natural anthocyanin and colors from pulp of jamun fruit. J. Food Sci. Technol. 2015, 52, 3617–3626. [Google Scholar] [CrossRef][Green Version]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Akgun, N.A.; Doymaz, I. Modelling of olive cake thin-layer drying process. J. Food Eng. 2005, 68, 455–461. [Google Scholar] [CrossRef]
- Akpinar, E.; Midilli, A.; Bicer, Y. Single layer drying behaviour of potato slices in a convective cyclone dryer and mathematical modeling. Energy Convers. Manag. 2003, 44, 1689–1705. [Google Scholar] [CrossRef]
- Akpinar, E.K. Drying of mint leaves in a solar dryer and under open sun: Modelling, performance analyses. Energy Convers. Manag. 2010, 51, 2407–2418. [Google Scholar] [CrossRef]
- Toğrul, İ.T.; Pehlivan, D. Modelling of thin layer drying kinetics of some fruits under open-air sun drying process. J. Food Eng. 2004, 65, 413–425. [Google Scholar] [CrossRef]
- John, S.G.; Sangamithra, A.; Chandrasekar, V.; Sasikala, S.; Sanju, V.; Bhuvaneswari, S. Mathematical Modelling of the Thin Layer Drying of Banana Blossom. J. Nutr. Health Food Eng. 2014, 1, 42–49. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1979. [Google Scholar]
- Thorat, I.D.; Mohapatra, D.; Sutar, R.F.; Kapdi, S.S.; Jagtap, D.D. Mathematical Modeling and Experimental Study on Thin-Layer Vacuum Drying of Ginger (Zingiber officinale R.) Slices. Food Bioprocess Technol. 2012, 5, 1379–1383. [Google Scholar] [CrossRef]
- Zarein, M.; Samadi, S.H.; Ghobadian, B. Investigation of microwave dryer effect on energy efficiency during drying of apple slices. J. Saudi Soc. Agric. Sci. 2015, 14, 41–47. [Google Scholar] [CrossRef]
- Sanjuán, N.; Lozano, M.; García-Pascual, P.; Mulet, A. Dehydration kinetics of red pepper (Capsicum annuum L. var Jaranda). J. Sci. Food Agric. 2003, 83, 697–701. [Google Scholar] [CrossRef]
- Abbasi, E.; Azizpour, M. Evaluation of physicochemical properties of foam mat dried sour cherry powder. LWT Food Sci. Technol. 2016, 68, 105–110. [Google Scholar] [CrossRef]
- Franco, T.S.; Perussello, C.A.; Ellendersen, L.N.; Masson, M.L. Effects of foam mat drying on physicochemical and microstructural properties of yacon juice powder. LWT-Food Sci. Technol. 2016, 66, 503–513. [Google Scholar] [CrossRef]
- Dehghannya, J.; Pourahmad, M.; Ghanbarzadeh, B.; Ghaffari, H. Influence of foam thickness on production of lime juice powder during foam-mat drying: Experimental and numerical investigation. Powder Technol. 2018, 328, 470–484. [Google Scholar] [CrossRef]
- Ng, M.L.; Sulaiman, R. Development of beetroot (Beta vulgaris) powder using foam mat drying. LWT 2018, 88, 80–86. [Google Scholar] [CrossRef]
- Muthukumaran, A.; Ratti, C.; Raghavan, V.G.S. Foam-Mat Freeze Drying of Egg White—Mathematical Modeling Part II: Freeze Drying and Modeling. Dry. Technol. 2008, 26, 513–518. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Tong, L.-I.; Wang, C.-H. Optimization of multiple responses using data envelopment analysis and response surface methodology. J. Appl. Sci. Eng. 2010, 13, 197–203. [Google Scholar]
- Spigno, G.; De Faveri, D.M. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar] [CrossRef]
- Ratti, C.; Kudra, T. Drying of Foamed Biological Materials: Opportunities and Challenges. Dry. Technol. 2006, 24, 1101–1108. [Google Scholar] [CrossRef]
- Sadin, R.; Chegini, G.-R.; Sadin, H. The effect of temperature and slice thickness on drying kinetics tomato in the infrared dryer. Heat Mass Transf. 2014, 50, 501–507. [Google Scholar] [CrossRef]
- Workneh, T.S.; Oke, M.O. The influence of the combined microwave power and hot air ventilation on the drying kinetics and colour quality of tomato slices. Afr. J. Biotechnol. 2012, 11, 15353–15364. [Google Scholar]
- Maskan, A.; Kaya, S.; Maskan, M. Hot air and sun drying of grape leather (pestil). J. Food Eng. 2002, 54, 81–88. [Google Scholar] [CrossRef]
- Olurin, T.O.; Adelekan, A.O.; Olosunde, W.A. Mathematical modelling of drying characteristics of blanched field pumpkin (Cucurbita pepo L.) slices. Agric. Eng. Int. CIGR J. 2012, 14, 246–254. [Google Scholar]
- Mugodo, K.; Workneh, T.S. The kinetics of thin-layer drying and modelling for mango slices and the influence of differing hot-air drying methods on quality. Heliyon 2021, 7, e07182. [Google Scholar] [CrossRef]
- Doymaz, İ. Evaluation of some thin-layer drying models of persimmon slices (Diospyros kaki L.). Energy Convers. Manag. 2012, 56, 199–205. [Google Scholar] [CrossRef]
- Najla, M.; Bawatharani, R. Evaluation of Page Model on Drying Kinetics of Red Chillies. IRE J. 2019, 2, 6–10. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Olalere, O.A. Mathematical modelling and morphological properties of thin layer oven drying of Vernonia amygdalina leaves. J. Saudi Soc. Agric. Sci. 2019, 18, 309–315. [Google Scholar] [CrossRef]
- Jin Park, K.; Vohnikova, Z.; Pedro Reis Brod, F. Evaluation of drying parameters and desorption isotherms of garden mint leaves (Mentha crispa L.). J. Food Eng. 2002, 51, 193–199. [Google Scholar] [CrossRef]
- Panchariya, P.C.; Popovic, D.; Sharma, A.L. Thin-layer modelling of black tea drying process. J. Food Eng. 2002, 52, 349–357. [Google Scholar] [CrossRef]
- Costa, J.d.; Medeiros, M.d.; Mata, A. Isotermas de adsorção de pós de beterraba (Beta vulgaris L.), abóbora (Cucurbita moschata) e cenoura (Daucus carota) obtidos pelo processo de secagem em leito de jorro: Estudo comparativo. Rev. Ciência Agronômica 2003, 34, 5–9. [Google Scholar]
- Thuy, N.M.; Han, L.N.; Van Tai, N. Thermal stability of anthocyanin in mixed raspberry-pomegranate-banana nectar in the presence of ascorbic acid and citric acid. J. Appl. Biol. Biotechnol. 2022, 10, 189–195. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).