Antioxidant Properties of Tomato Fruit (Lycopersicon esculentum Mill.) as Affected by Cultivar and Processing Method
Abstract
:1. Introduction
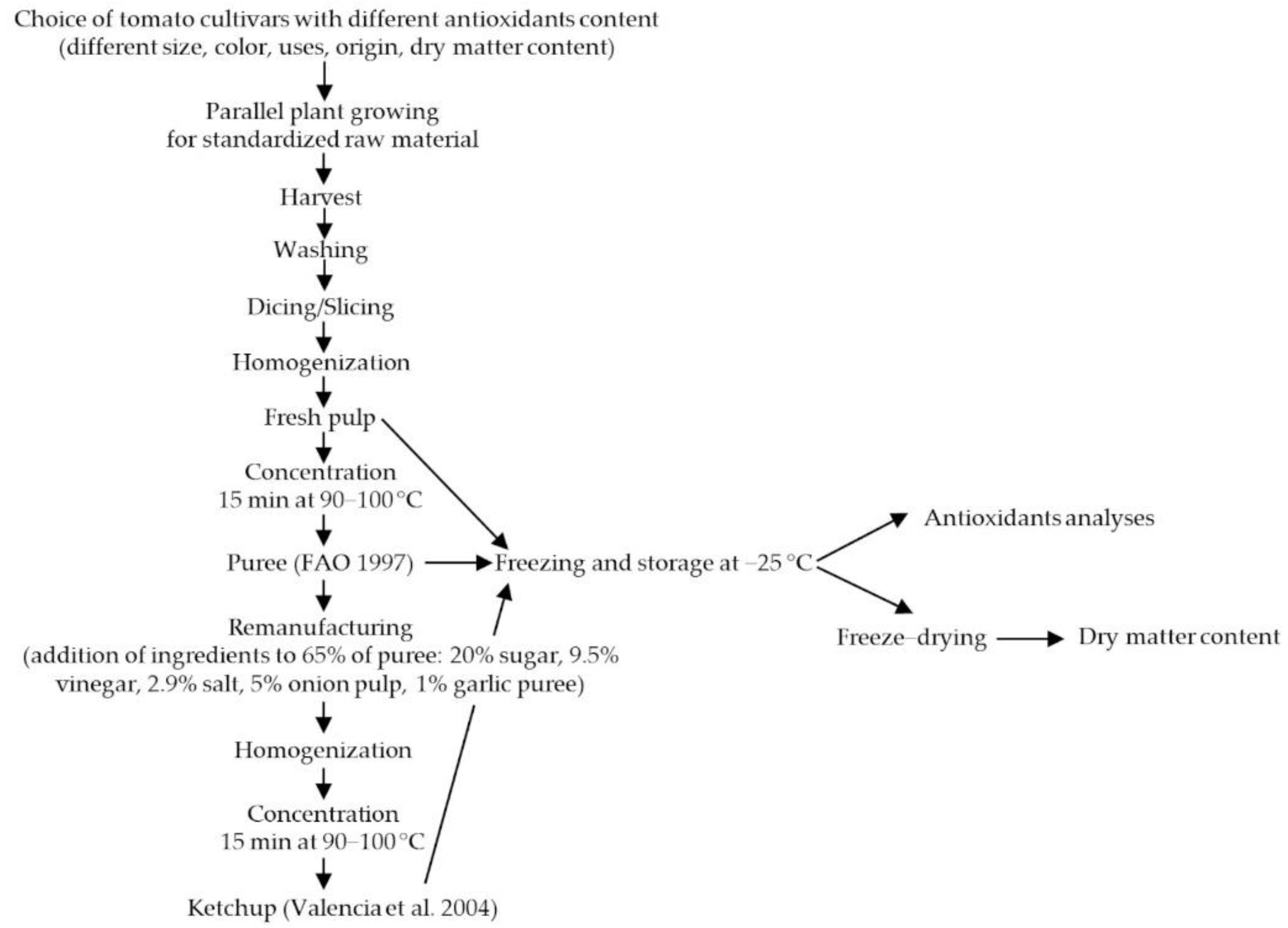
2. Materials and Methods
2.1. Plant Materials
2.2. Analyses of Fresh and Processed Tomato Fruits
2.3. Statistical Analyses
3. Results and Discussion
3.1. Dry-Matter Content
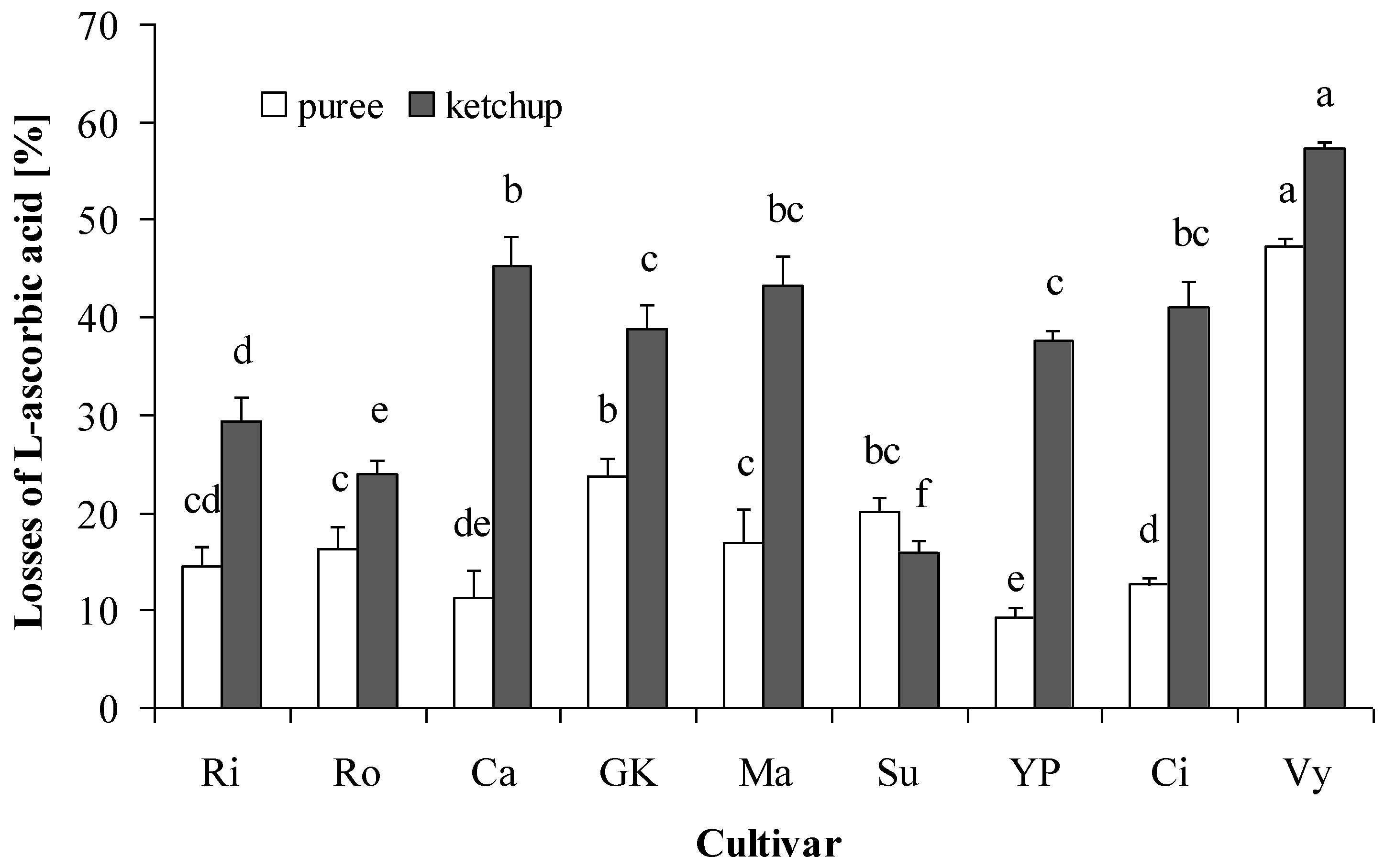
3.2. Ascorbic Acid
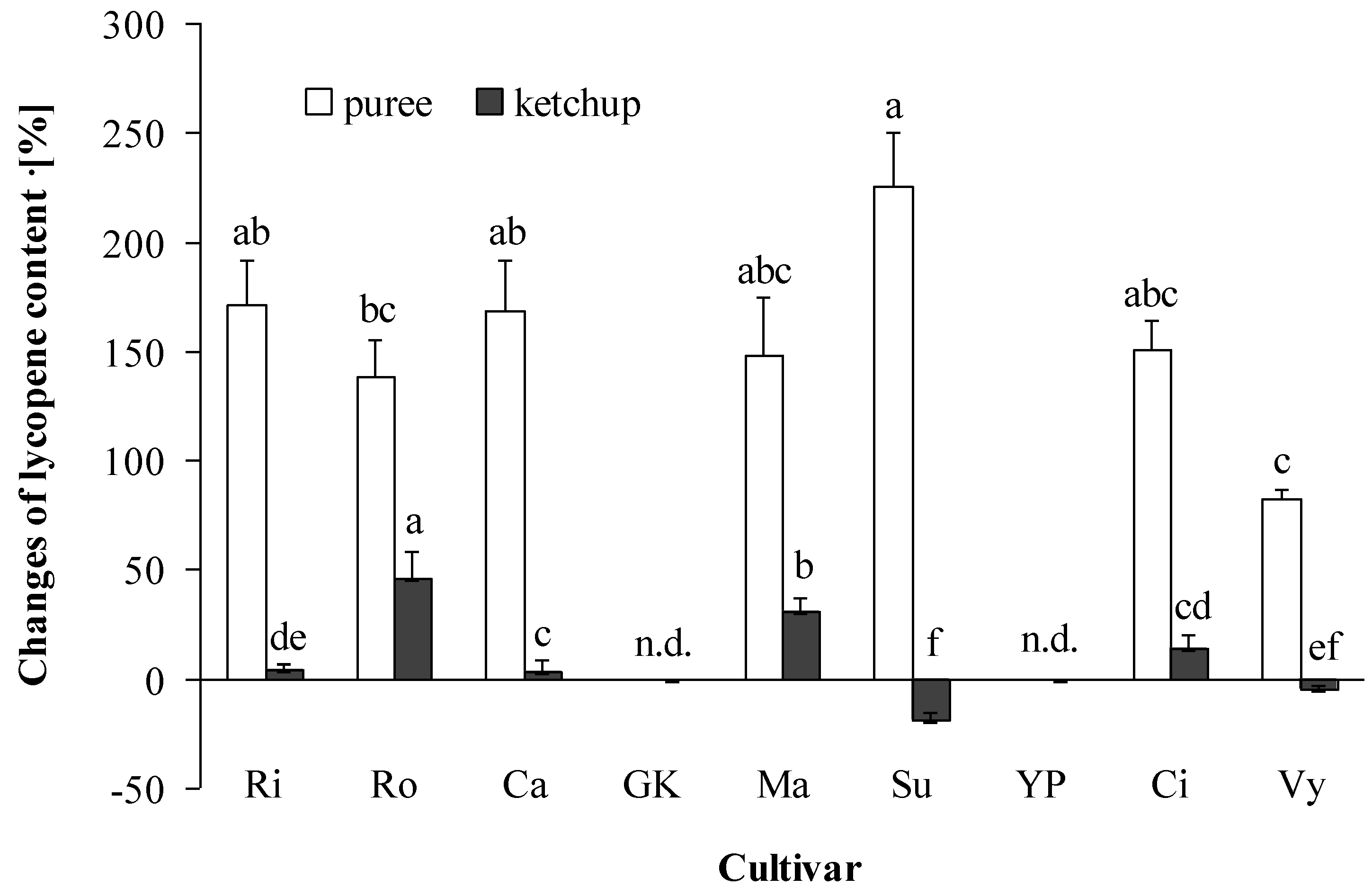
3.3. Lycopene
3.4. Total Phenolic Compounds
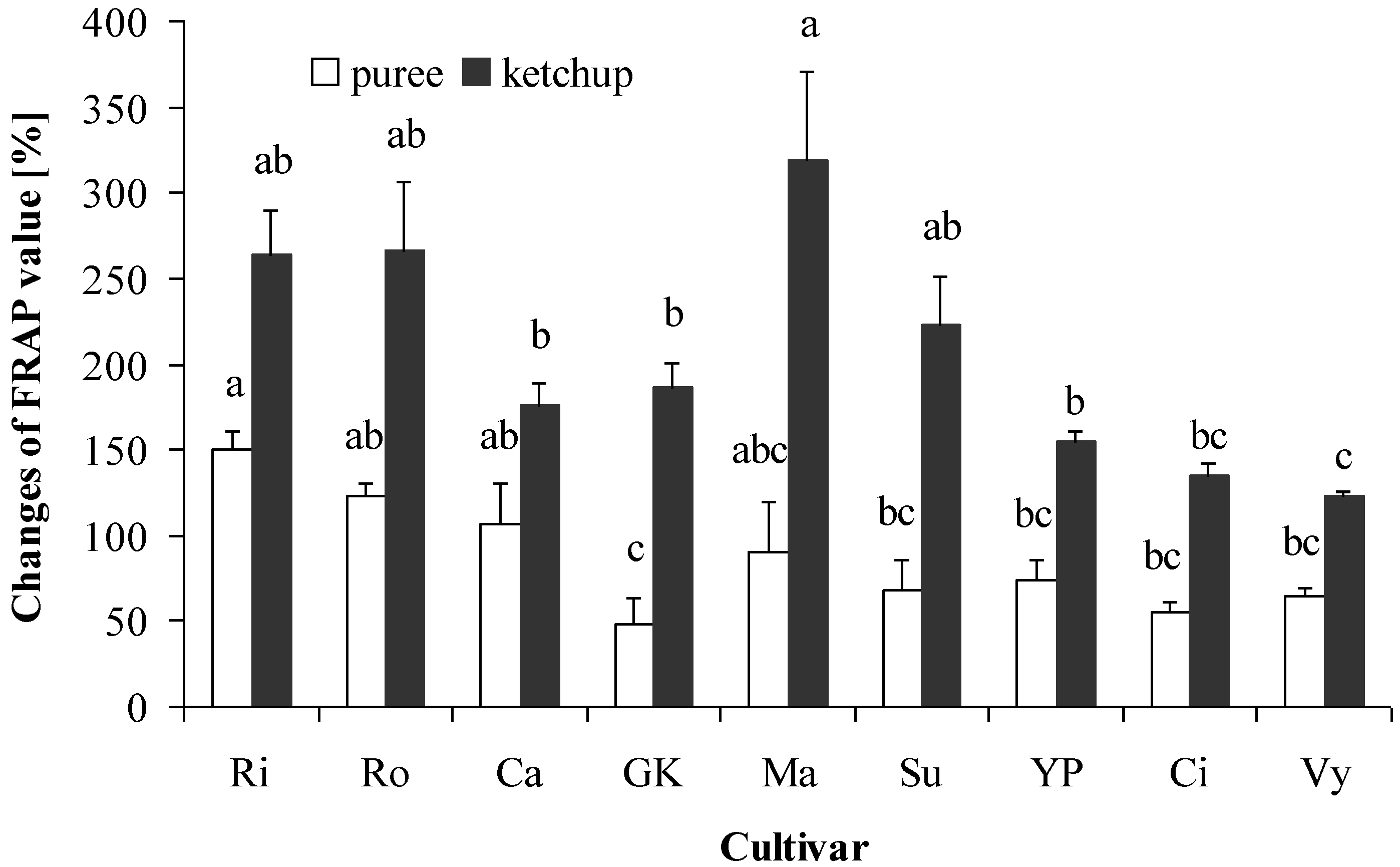
3.5. Total Antioxidant Capacity
3.6. Relationship between Antioxidant Capacity and Selected Bioactive Compounds
3.7. Contribution of Tomato Products to the Antioxidant Pool in Human Diet
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Crops and Livestock Products in 2020. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 April 2022).
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Tomato/Pomodoro/Domates Consumption in 2012–2013. Available online: https://www.reddit.com/r/mediterranea/comments/i28b7w/tomato_consumption_per_capita/ (accessed on 24 May 2022).
- Giuffrè, A.M.; Capocasale, M. Policosanol in Tomato (Solanum lycopersicum L.) Seed oil: The effect of cultivar. J. Oleo Sci. 2015, 64, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuffrè, A.M.; Capocasale, M. Sterol composition of tomato (Solanum lycopersicum L.) seed oil: The effect of cultivar. Int. Food Res. J. 2016, 23, 116–122. [Google Scholar]
- Akubor, P.I.; Owuse, A.U. Chemical Composition, functional and biscuit making properties of tomato peel flour. South Asian J. Food Technol. Environ. 2020, 6, 874–884. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M.; Zappia, C. Tomato seed oil for edible use: Cold break, hot break and harvest year effects. J. Food Process. Preserv. 2017, 41, e13309. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A Review. Foods 2021, 10, 45. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2020, 343, 128396. [Google Scholar] [CrossRef]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-apoptotic effects of carotenoids in neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef]
- Frankel, E.N.; Finley, J.W. How to standardize the multiplicity of methods to evaluate natural antioxidants. J. Agric. Food Chem. 2008, 56, 4901–4908. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Willcox, B.J.; Curb, J.D.; Rodriguez, B.L. Antioxidants in cardiovascular health and disease: Key lessons from epidemiologic studies. Am. J. Cardiol. 2008, 101, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Dillingham, B.L.; Rao, A.V. Biologically Active lycopene in human health. IntJNM 2009, 4, 23–27. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Reimers, K. Tomato consumption and health: Emerging benefits. Am. J. Lifestyle Med. 2011, 5, 182–191. [Google Scholar] [CrossRef]
- Tommonaro, G.; Gennaro Roberto Abbamondi, G.R.; Nicolaus, B.; Poli, A.; D’Angelo, C.; Iodice, C.; De Prisco, R. Productivity and nutritional trait improvements of different tomatoes cultivated with effective microorganisms technology. Agriculture 2021, 11, 112. [Google Scholar] [CrossRef]
- Pérez-Conesa, D.; Garcia-Alonso, J.; Garcia-Valverde, V.; Iniesta, M.-D.; Jacob, K.; Sánchez-Siles, L.M.; Ros, G.; Periago, M.J. Changes in bioactive compounds and antioxidant activity during homogenization and thermal processing of tomato puree. Innov. Food Sci. Emerg. Technol. 2009, 10, 179–188. [Google Scholar] [CrossRef]
- De Sio, F.; Rapacciuolo, M.; De Giorgi, A.; Trifirò, A.; Giuliano, B.; Vitobello, L.; Cuciniello, A.; Caruso, G. Yield quality and antioxidants of peeled tomato as affected by genotype and industrial processing in Southern Italy. Adv. Hort. Sci. 2018, 32, 379–387. [Google Scholar] [CrossRef]
- Tomas, M.; Beekwilder, J.; Hall, R.D.; Sagdic, O.; Boyacioglu, D.; Capanoglu, E. Industrial processing versus home processing of tomato sauce: Effects on phenolics, flavonoids and in vitro bioaccessibility of antioxidants. Food Chem. 2017, 220, 51–58. [Google Scholar] [CrossRef]
- Iswari, R.S.; Susanti, R. Antioxidant activity from various tomato processing. Biosaintifika J. Biol. Biol. Educ. 2016, 8, 129–134. [Google Scholar] [CrossRef]
- Ishida, B.K.; Roberts, J.S.; Chapman, M.H.; Burri, B.J. Processing tangerine tomatoes: Effects on lycopene-isomer concentrations and profile. J. Food Sci. 2007, 72, 307–312. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Abushita, A.; Daood, H.G.; Biacs, P.A. Changes in carotenoids and antioxidants vitamins in tomato as a function of varietal and technological factors. J. Agric. Food Chem. 2000, 48, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, E.; Savage, G.P.; Lister, C.E. Investigation on the antioxidant properties of tomatoes after processing. J. Food Compos. Anal. 2004, 17, 635–647. [Google Scholar] [CrossRef]
- Odrizola-Serrano, I.; Soliva-Fortuny, R.; Martin-Belloso, O. Effect of minimal processing of bioactive compounds and color attributes of fresh-cut tomatoes. LWT 2008, 41, 217–226. [Google Scholar] [CrossRef]
- Keutgen, A.; Pawelzik, E. Modification of strawberry fruit antioxidant pools and fruit quality under NaCl stress. J. Agric. Food Chem. 2007, 55, 4066–4072. [Google Scholar] [CrossRef]
- FAO Corporate Document Repository. Technical Manual on Small-Scale Processing of Fruits and Vegetables. 1997. Available online: http://www.fao.org/docrep/x0209e/x0209e00.HTM (accessed on 30 August 2021).
- Valencia, C.; Sanchez, M.C.; Ciruelos, A.; Gallegos, C. Influence of tomato paste processing on the linear viscoelasticity of tomato ketchup. Food Sci. Tech. Int. 2004, 10, 95–96. [Google Scholar] [CrossRef]
- Albrecht, J.A. Ascorbic acid and retention in lettuce. J. Food Quality. 1993, 16, 311–316. [Google Scholar] [CrossRef]
- Binoy, G.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersium esculentum) as a function genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Quality and nutritional value of strawberry fruit under long term salt stress. Food Chem. 2008, 107, 1413–1420. [Google Scholar] [CrossRef]
- Górecka, D.; Wawrzyniak, A.; Jędrusek-Golińska, A.; Dziedzic, K.; Hamułka, J.; Kowalczewski, P.Ł.; Walkowiak, J. Lycopene in tomatoes and tomato products. Open Chem. 2020, 18, 752–756. [Google Scholar] [CrossRef]
- Ochida, C.O.; Itodo, A.U.; Nwanganga, P.A. A review on postharvest storage, processing and preservation of tomatoes (Lycopersicon esculentum Mill). AFSJ 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Wu, X.; Yu, L.; Pehrsson, P.R. Are processed tomato products as nutritious as fresh tomatoes? Scoping review on the effects of industrial processing on nutrients and bioactive compounds in tomatoes. Adv. Nutr. 2022, 13, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Charoenprasert, S.; Mitchell, A.E. Effects of industrial tomato paste processing on ascorbic acid, flavonoids and carotenoids and their stability over one-year storage. J. Sci. Food Agric. 2012, 92, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lenucci, M.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant composition in cherry and high-pigment tomato cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Biotechnol. 2000, 20, 293–334. [Google Scholar] [CrossRef]
- Hadley, C.W.; Miller, E.C.; Schwartz, S.J.; Clinton, S.K. Tomatoes, lycopene, and prostate cancer: Progress and promise. Exp. Biol. Med. 2002, 227, 869–880. [Google Scholar] [CrossRef]
- Minoggio, M.; Bramati, L.; Simonetti, P.; Gardana, C.; Lemoli, L.; Santangelo, E.; Mauri, P.L.; Spigno, P.; Soressi, G.P.; Pietta, P.G. Polyphenol pattern and antioxidant activity of different tomato lines and cultivars. Ann. Nutr. Metab. 2003, 47, 64–69. [Google Scholar] [CrossRef]
- Chanforan, C.; Loonis, M.; Mora, N.; Caris-Veyrat, C.; Dufour, C. The impact of industrial processing on health-beneficial tomato microconstituents. Food Chem. 2012, 134, 1786–1795. [Google Scholar] [CrossRef]
- Shen, Y.C.; Chen, S.L.; Wang, C.K. Contribution of tomato phenolics to antioxidation and down-regulation of blood lipids. J. Agric. Food Chem. 2007, 55, 6475–6481. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Crozier, A.; Aruoma, O.I. Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J. Sci. Food. Agric. 2004, 84, 1553–1561. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.-B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. CMAJ 2000, 163, 739–744. Available online: https://www.cmaj.ca/content/163/6/739 (accessed on 28 April 2022). [PubMed]

| Cultivar | DM [%] | FRAP [mmol Fe2+] | LAA [mg] | Lycopene [mg] | Total Phenolics [mg GAE] |
|---|---|---|---|---|---|
| fresh fruits | |||||
| ‘Rilia’ | 6.06 ± 0.96 d | 25.3 ± 8.1 d | 192.3 ± 22.3 b | 111.7 ± 35.3 c | 234.5 ± 18.1 cd |
| ‘Roma‘ | 6.80 ± 1.62 d | 27.0 ± 10.8 d | 179.6 ± 10.8 c | 103.7 ± 42.0 cd | 211.7 ± 17.3 d |
| ‘Campbell-28‘ | 7.23 ± 0.07 cd | 23.1 ± 6.3 de | 165.6 ± 36.8 c | 117.5 ± 45.8 c | 217.5 ± 15.3 d |
| ‘Goldene Königin‘ | 7.81 ± 0.27 c | 33.7 ± 5.9 c | 226.2 ± 23.8 ab | - | 281.4 ± 18.0 c |
| ‘Marmande‘ | 5.93 ± 0.03 e | 21.9 ± 6.3 e | 139.4 ± 48.0 d | 96.0 ± 17.2 cd | 212.6 ± 15.3 d |
| ‘Suso’ | 8.21 ± 0.35 bc | 26.1 ± 2.8 d | 156.0 ± 10.7 cd | 89.7 ± 3.3 d | 106.9 ± 1.1 e |
| ‘Yellow Pearshaped’ | 8.81 ± 0.33 b | 24.8 ± 1.2 d | 185.2 ± 15.7 bc | - | 102.1 ± 8.3 e |
| ‘CIMA’ | 9.03 ± 0.21 ab | 43.6 ± 0.4 b | 204.7 ± 6.3 b | 144.8 ± 4.6 b | 483.7 ± 9.4 b |
| ‘Vyta’ | 9.53 ± 0.04 a | 47.9 ± 0.3 a | 255.7 ± 10.5 a | 202.5 ± 3.8 a | 567.6 ± 10.1 a |
| tomato puree | |||||
| ‘Rilia’ | 16.07 ± 0.05 b | 63.2 ± 17.7 b | 164.6 ± 20.0 a | 303.2 ± 57.9 ab | 758.7 ± 384.9 ab |
| ‘Roma‘ | 18.04 ± 0.03 a | 60.3 ± 23.7 b | 150.5 ± 36.7 ab | 246.9 ± 116.4 c | 765.4 ± 378.7 ab |
| ‘Campbell-28‘ | 14.15 ± 0.11 c | 47.8 ± 8.8 cd | 146.8 ± 15.9 ab | 315.0 ± 71.0 ab | 634.5 ± 308.5 b |
| ‘Goldene Königin‘ | 17.99 ± 0.38 ab | 50.0 ± 8.1 d | 172.6 ± 13.1 a | - | 681.5 ± 293.2 b |
| ‘Marmande‘ | 14.15 ± 0.15 c | 41.5 ± 20.5 c | 115.7 ± 16.4 c | 238.4 ± 20.3 c | 918.8 ± 442.6 ab |
| ‘Suso’ | 18.05 ± 0.42 a | 43.8 ± 1.0 cd | 124.5 ± 15.7 ab | 291.6 ± 9.9 b | 452.4 ± 47.4 c |
| ‘Yellow Pearshaped’ | 13.84 ± 0.17 c | 43.1 ± 1.1 cd | 168.1 ± 4.1 a | - | 409.6 ± 5.4 c |
| ‘CIMA’ | 16.13 ± 0.73 b | 67.9 ± 1.0 b | 178.5 ± 3.5 a | 363.4 ± 6.9 a | 1062.0 ± 29.2 b |
| ‘Vyta’ | 18.30 ± 0.03 a | 79.0 ± 0.7 a | 134.8 ± 4.9 b | 370.0 ± 2.7 a | 1168.3 ± 35.3 a |
| Ketchup | |||||
| ‘Rilia’ | 28.05 ± 0.27 b | 92.1 ± 13.0 bc | 136.0 ± 27.4 a | 116.9 ± 45.6 cd | 1389.2 ± 761.4 ab |
| ‘Roma‘ | 29.42 ± 0.43 a | 98.9 ± 14.2 bc | 136.7 ± 19.8 a | 151.5 ± 55.5 abc | 1481.3 ± 804.3 ab |
| ‘Campbell-28‘ | 28.95 ± 0.22 ab | 63.7 ± 13.9 d | 90.5 ± 21.9 de | 121.4 ± 12.8 cd | 1414.2 ± 762.0 ab |
| ‘Goldene Königin‘ | 26.71 ± 0.05 c | 130.0 ± 6.7 a | 138.2 ± 24.3 a | - | 1270.2 ± 779.5 c |
| ‘Marmande‘ | 26.19 ± 0.09 c | 91.7 ± 11.2 bc | 79.2 ± 15.2 e | 125.8 ± 23.8 cd | 1234.3 ± 618.2 c |
| ‘Suso’ | 25.73 ± 0.61 cd | 84.3 ± 7.0 c | 131.3 ± 14.5 a | 72.6 ± 3.6 de | 705.0 ± 28.2 c |
| ‘Yellow Pearshaped’ | 24.65 ± 0.11 d | 63.1 ± 1.9 d | 115.4 ± 2.5 c | - | 643.6 ± 14.7 c |
| ‘CIMA’ | 26.03 ± 0.41 bc | 102.6 ± 5.3 b | 120.8 ± 1.3 b | 164.7 ± 0.7 b | 2005.7 ± 24.2 b |
| ‘Vyta’ | 29.97 ± 0.62 a | 106.8 ± 2.3 b | 109.0 ± 1.7 d | 193.3 ± 1.1 a | 2268.0 ± 72.1 a |
| Cultivar | FRAP [mmol Fe2+] | LAA [mg] | Lycopene [mg] | Total Phenolics [mg GAE] |
|---|---|---|---|---|
| Fresh fruits (200 g) | ||||
| ‘Rilia’ | 5.1 ± 1.62 d | 38.5 ± 4.46 b | 22.3 ± 7.06 c | 46.9 ± 36.24 cd |
| ‘Roma’ | 5.4 ± 2.17 d | 35.9 ± 2.16 c | 20.7 ± 8.39 cd | 42.3 ± 34.65 d |
| ‘Campbell-28’ | 4.6 ± 1.27 de | 33.1 ± 7.37 c | 23.5 ± 9.17 c | 43.5 ± 30.53 d |
| ‘Goldene Königin’ | 6.7 ± 1.18 c | 45.2 ± 4.76 ab | - | 56.3 ± 36.05 c |
| ‘Marmande’ | 4.4 ± 1.27 e | 27.9 ± 9.61 d | 19.2 ± 3.43 cd | 42.5 ± 30.61 d |
| ‘Suso’ | 5.2 ± 0.55 d | 31.2 ± 2.14 cd | 17.9 ± 0.66 d | 21.4 ± 2.15 e |
| ‘Yellow Pearshaped’ | 5.0 ± 0.25 d | 37.0 ± 3.13 bc | - | 20.4 ± 1.66 e |
| ‘CIMA’ | 8.7 ± 0.08 b | 40.9 ± 1.25 b | 29.0 ± 0.92 b | 96.6 ± 1.88 b |
| ‘Vyta’ | 9.6 ± 0.05 a | 51.1 ± 2.10 a | 40.5 ± 0.75 a | 113.5 ± 2.01 a |
| Tomato puree | ||||
| ‘Rilia’ | 3.8 ± 1.06 b | 9.9 ± 1.20 a | 18.2 ± 3.47 ab | 45.5 ± 23.09 ab |
| ‘Roma’ | 3.6 ± 1.42 b | 9.0 ± 2.20 ab | 14.8 ± 6.98 c | 45.9 ± 22.72 ab |
| ‘Campbell-28’ | 2.9 ± 0.53 cd | 8.8 ± 0.95 ab | 18.9 ± 4.26 ab | 38.1 ± 18.51 b |
| ‘Goldene Königin’ | 3.0 ± 0.48 d | 10.4 ± 0.79 a | - | 40.9 ± 17.59 b |
| ‘Marmande’ | 2.5 ± 1.23 c | 6.9 ± 0.99 c | 14.3 ± 1.22 c | 55.1 ± 26.56 ab |
| ‘Suso’ | 2.6 ± 0.06 cd | 7.5 ± 0.94 ab | 17.5 ± 0.59 b | 27.1 ± 2.84 c |
| ‘Yellow Pearshaped’ | 2.6 ± 0.07 cd | 10.1 ± 0.25 a | - | 24.6 ± 0.32 c |
| ‘CIMA’ | 4.1 ± 0.06 b | 10.7 ± 0.21 a | 21.8 ± 0.42 a | 63.7 ± 1.75 b |
| ‘Vyta’ | 4.7 ± 0.04 a | 8.1 ± 0.29 b | 22.2 ± 0.16 a | 70.1 ± 2.12 a |
| Ketchup | ||||
| ‘Rilia’ | 1.4 ± 0.20 bc | 2.0 ± 0.41 a | 1.8 ± 0.68 cd | 20.8 ± 11.42 ab |
| ‘Roma’ | 1.5 ± 0.21 bc | 2.1 ± 0.30 a | 2.3 ± 0.83 abc | 22.2 ± 12.06 ab |
| ‘Campbell-28’ | 1.0 ± 0.21 d | 1.4 ± 0.33 de | 1.8 ± 0.19 cd | 21.2 ± 11.43 ab |
| ‘Goldene Königin’ | 2.0 ± 0.10 a | 2.1 ± 0.36 a | - | 19.1 ± 11.69 c |
| ‘Marmande’ | 1.4 ± 0.17 bc | 1.2 ± 0.23 e | 1.9 ± 0.36 cd | 18.5 ± 9.27 c |
| ‘Suso’ | 1.3 ± 0.11 c | 2.0 ± 0.22 a | 1.1 ± 0.05 de | 10.6 ± 0.42 c |
| ‘Yellow Pearshaped’ | 0.9 ± 0.03 d | 1.7 ± 0.04 c | - | 9.7 ± 0.22 c |
| ‘CIMA’ | 1.5 ± 0.08 b | 1.8 ± 0.02 b | 2.5 ± 0.01 b | 30.1 ± 0.36 b |
| ‘Vyta’ | 1.6 ± 0.03 b | 1.6 ± 0.03 d | 2.9 ± 0.02 a | 34.0 ± 1.08 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero, A.G.; Keutgen, A.J.; Pawelzik, E. Antioxidant Properties of Tomato Fruit (Lycopersicon esculentum Mill.) as Affected by Cultivar and Processing Method. Horticulturae 2022, 8, 547. https://doi.org/10.3390/horticulturae8060547
Rivero AG, Keutgen AJ, Pawelzik E. Antioxidant Properties of Tomato Fruit (Lycopersicon esculentum Mill.) as Affected by Cultivar and Processing Method. Horticulturae. 2022; 8(6):547. https://doi.org/10.3390/horticulturae8060547
Chicago/Turabian StyleRivero, Annia Gonzalez, Anna J. Keutgen, and Elke Pawelzik. 2022. "Antioxidant Properties of Tomato Fruit (Lycopersicon esculentum Mill.) as Affected by Cultivar and Processing Method" Horticulturae 8, no. 6: 547. https://doi.org/10.3390/horticulturae8060547
APA StyleRivero, A. G., Keutgen, A. J., & Pawelzik, E. (2022). Antioxidant Properties of Tomato Fruit (Lycopersicon esculentum Mill.) as Affected by Cultivar and Processing Method. Horticulturae, 8(6), 547. https://doi.org/10.3390/horticulturae8060547






