Surface Moisture Induces Microcracks and Increases Water Vapor Permeance of Fruit Skins of Mango cv. Apple
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Moisture Treatment
2.3. Transpiration Assays
2.4. Microcracking
2.5. Experiments
2.6. Histology
2.7. Data Analyses
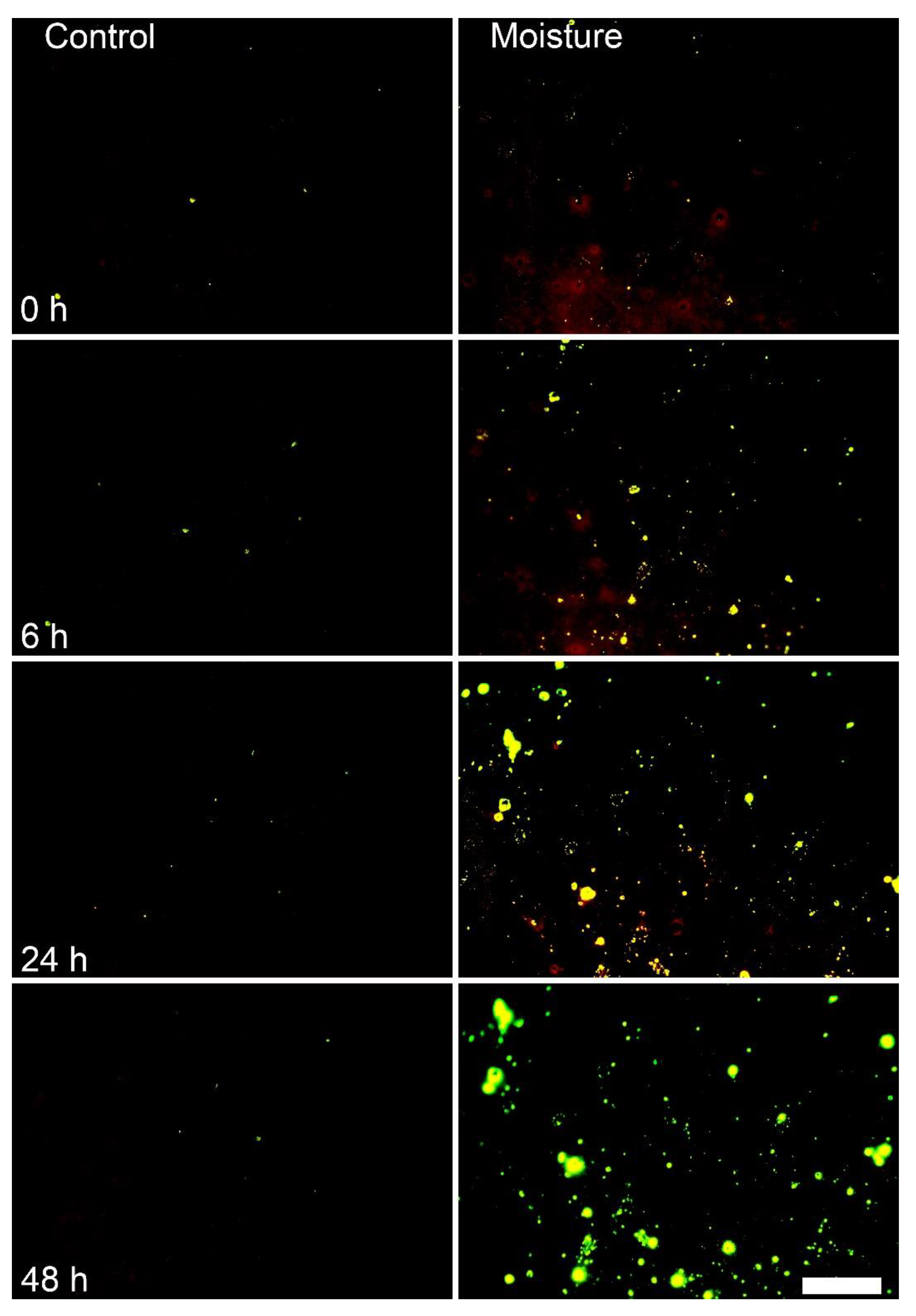
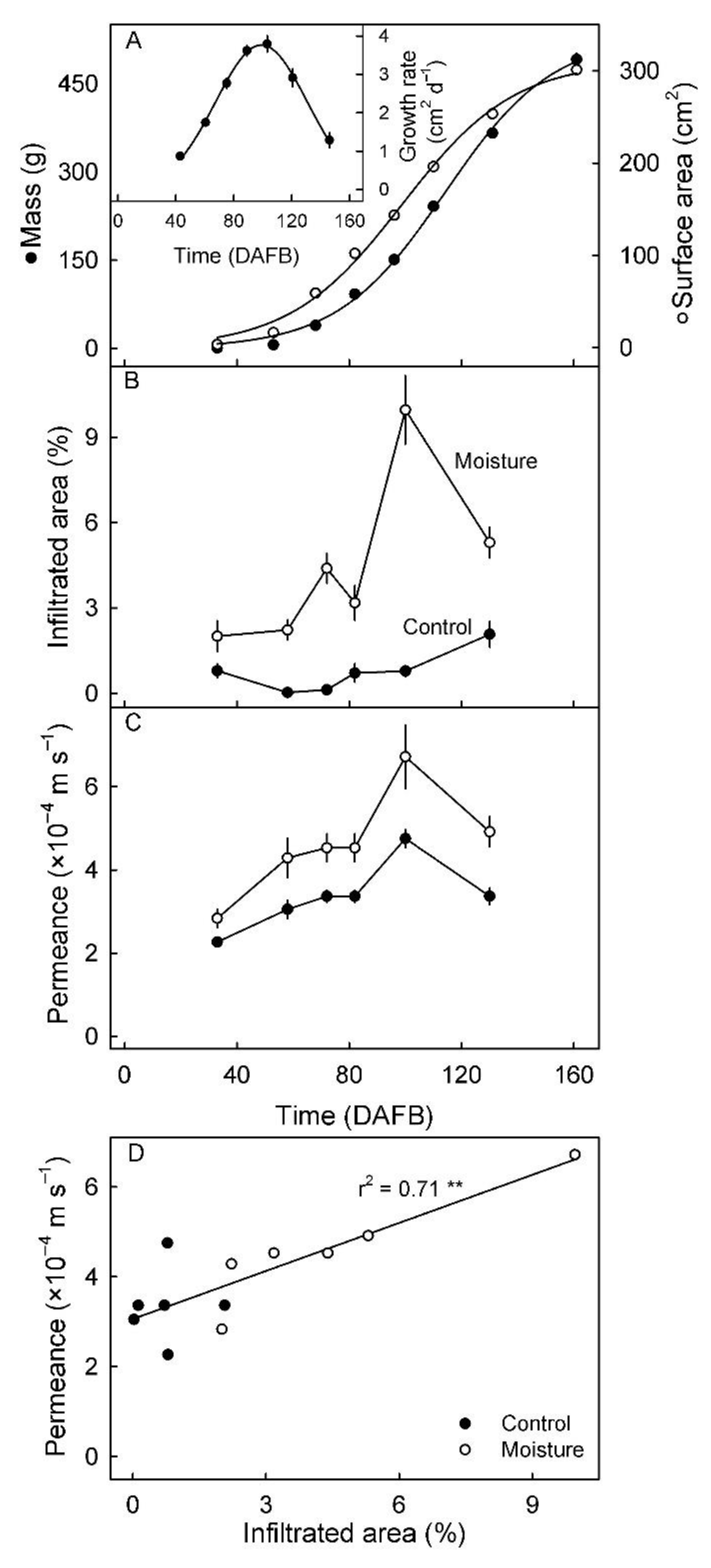
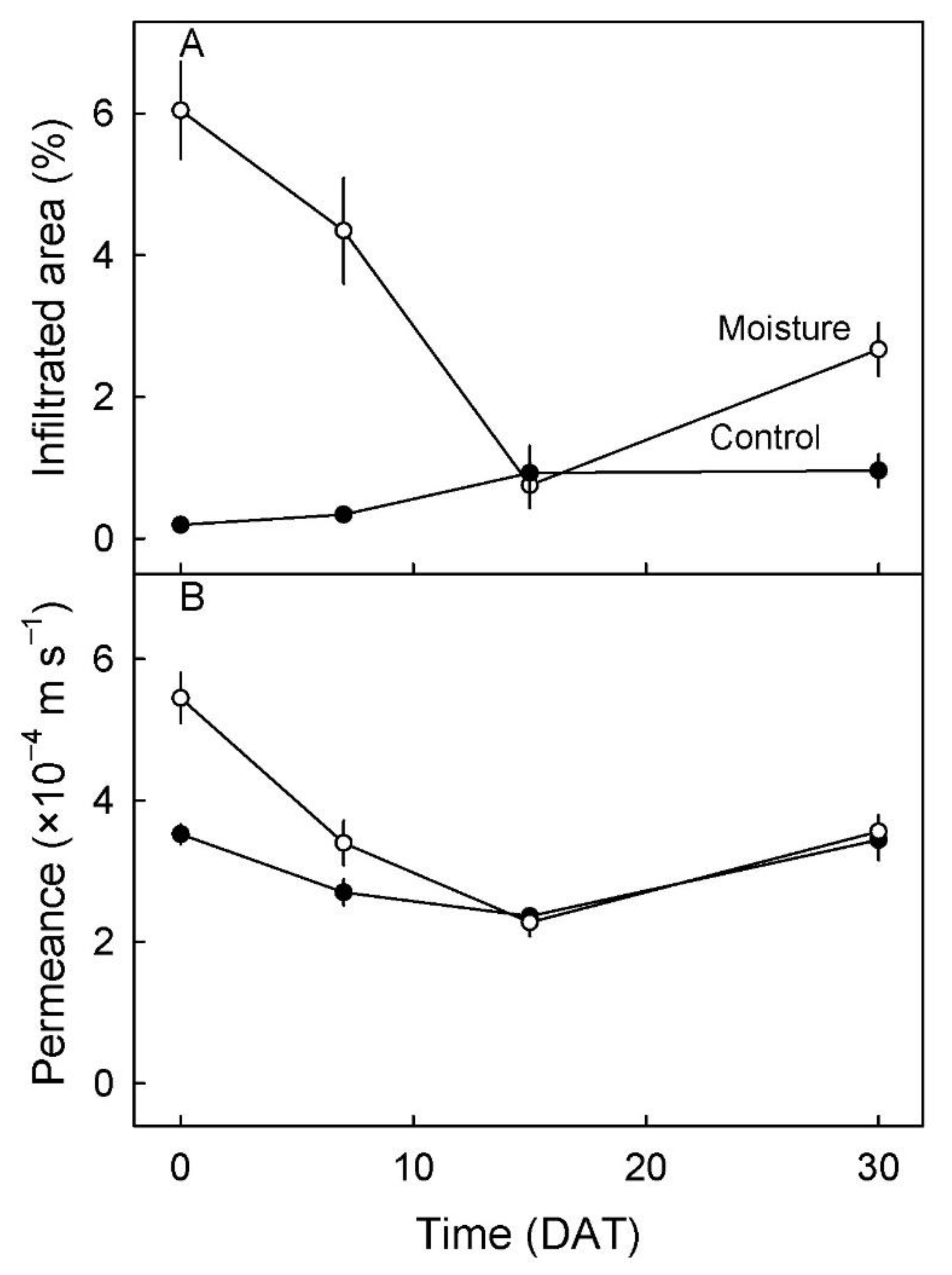
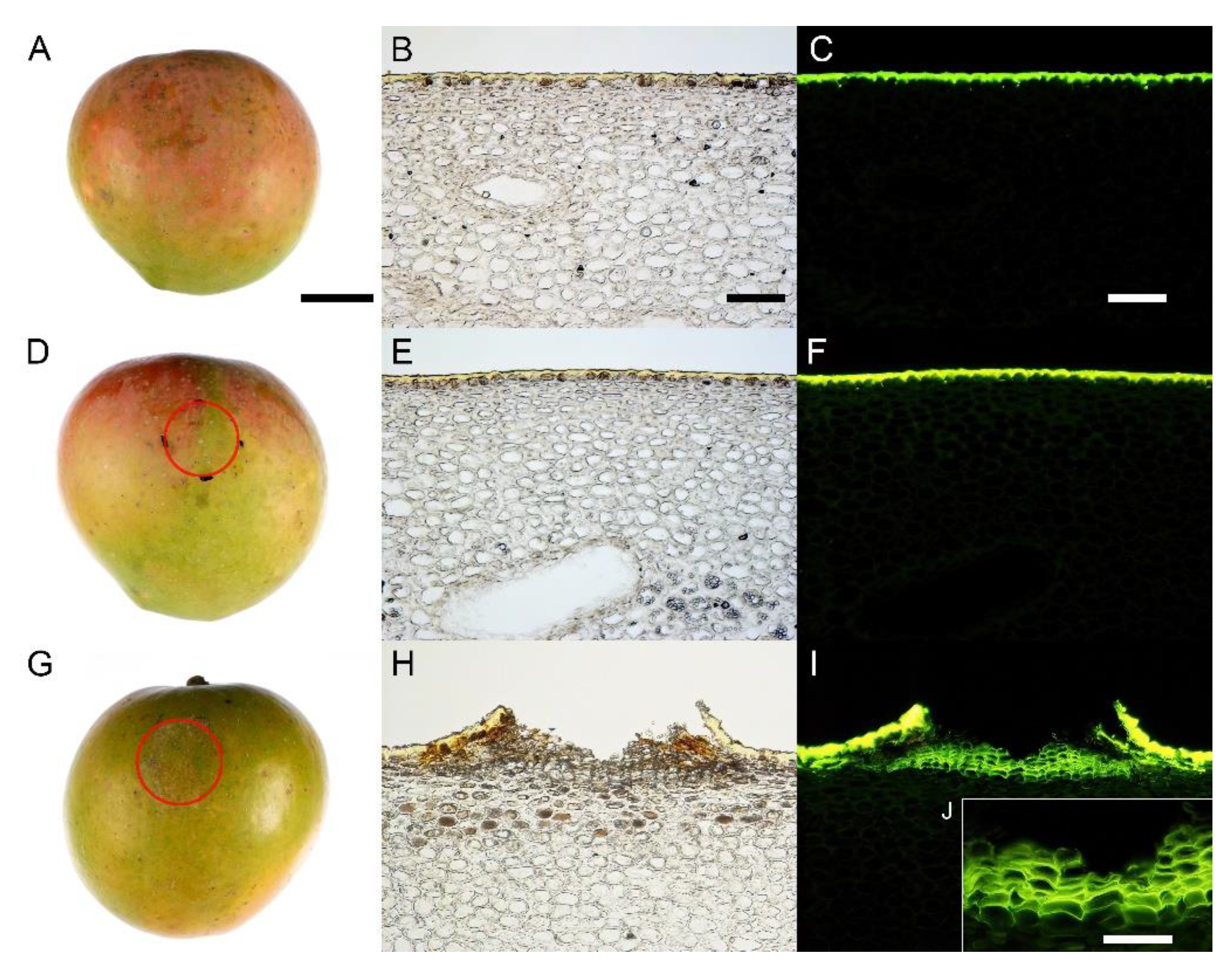
3. Results
4. Discussion
4.1. Surface Moisture Induces Microcracking and Russeting in Mango cv. Apple
4.2. Microcracking Increased Skin Permeance to Water Vapor
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Athoo, T.O.; Winkler, A.; Knoche, M. Russeting in ‘Apple’ mango: Triggers and mechanisms. Plants 2020, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Tukey, L.D. Observations on the russeting of apples growing in plastic bags. Proc. Amer. Soc, Hort. Sci. 1969, 74, 30–39. [Google Scholar]
- Skene, D.S. The development of russet, rough russet and cracks on the fruit of the apple Cox’s Orange Pippin during the course of the season. J. Hortic. Sci. 1982, 57, 165–174. [Google Scholar] [CrossRef]
- Bell, H.P. the origin of russeting in the Golden Russet apple. Can. J. Res. 1937, 15c, 560–566. [Google Scholar] [CrossRef]
- Scharwies, J.D.; Grimm, E.; Knoche, M. Russeting and relative growth rate are positively related in “Conference” and “Condo” pear. HortScience 2014, 49, 746–749. [Google Scholar] [CrossRef] [Green Version]
- Macnee, N.C.; Rebstock, R.; Hallett, I.C.; Schaffer, R.J.; Bulley, S.M. A review of current knowledge about the formation of native peridermal exocarp in fruit. Funct. Plant Biol. 2020, 47, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Michailides, T.J. Russeting and russet scab of prune, an environmentally induced fruit disorder: Symptomatology, induction, and control. Plant Dis. 1991, 75, 1114. [Google Scholar] [CrossRef]
- McCoy, C.W. Stylar feeding injury and control of eriophyoid mites in citrus. In World Crop Pests; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Achor, D.S.; Albrigo, L.G.; McCoy, C.W. Developmental anatomy of lesions on ‘Sunburst’ mandarin leaves initiated by citrus rust mite feeding. J. Am. Soc. Hortic. Sci. 1991, 116, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Avidan, B.; Klein, I. Physiological disorders in loquat (Eriobotrya japonica Lindl.). I. Russeting. Adv. Hortic. Sci. 1998, 12, 190–195. [Google Scholar]
- Bakker, J.C. Russeting (cuticle cracking) in glasshouse tomatoes in relation to fruit growth. J. Hortic. Sci. 1988, 63, 459–463. [Google Scholar] [CrossRef]
- Gerchikov, N.; Keren-Keiserman, A.; Perl-Treves, R.; Ginzberg, I. Wounding of melon fruits as a model system to study rind netting. Sci. Hortic. 2008, 117, 115–122. [Google Scholar] [CrossRef]
- Cohen, H.; Dong, Y.; Szymanski, J.; Lashbrooke, J.; Meir, S.; Almekias-Siegl, E.; Zeisler-Diehl, V.V.; Schreiber, L.; Aharoni, A. A multilevel study of melon fruit reticulation provides insight into skin ligno-suberization hallmarks. Plant Physiol. 2019, 179, 1486–1501. [Google Scholar] [CrossRef] [Green Version]
- Evert, R.F. Esau’s Plant Anatomy, 3rd ed.; Evert, R.F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 9780470047385. [Google Scholar]
- Knoche, M.; Lang, A. Ongoing growth challenges fruit skin integrity. CRC. Crit. Rev. Plant Sci. 2017, 36, 190–215. [Google Scholar] [CrossRef]
- Winkler, A.; Athoo, T.; Knoche, M. Russeting of fruits: Etiology and management. Horticulturae 2022, 8, 231. [Google Scholar] [CrossRef]
- Khanal, B.P.; Imoro, Y.; Chen, Y.H.; Straube, J.; Knoche, M. Surface moisture increases microcracking and water vapour permeance of apple fruit skin. Plant Biol. 2020, 23, 74–82. [Google Scholar] [CrossRef]
- Sugar, D.; Villardel, P.; Asin, L. Relationship of weather factors to russet incidence in “comice” and “bosc” pear fruit. Acta Hortic. 2015, 1094, 533–538. [Google Scholar] [CrossRef]
- Chen, Y.H.; Straube, J.; Khanal, B.P.; Knoche, M.; Debener, T. Russeting in apple is initiated after exposure to moisture ends—i. Histological evidence. Plants 2020, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Asín, L.; Torres, E.; Vilardell, P. Orchard cooling with overtree microsprinkler irrigation to increase fruit russet on ‘Conference’ pear. Acta Hortic. 2011, 909, 557–564. [Google Scholar] [CrossRef]
- Simons, R.K.; Chu, M.C. Periderm morphology of mature “Golden Delicious” apple with special reference to russeting. Sci. Hortic. 1978, 8, 333–340. [Google Scholar] [CrossRef]
- Joshi, M.; Schmilovitch, Z.; Ginzberg, I. Pomegranate fruit growth and skin characteristics in hot and dry climate. Front. Plant Sci. 2021, 12, 725479. [Google Scholar] [CrossRef]
- Noè, N.; Eccher, T. ‘Golden Delicious’ apple fruit shape and russeting are affected by light conditions. Sci. Hortic. 1996, 65, 209–213. [Google Scholar] [CrossRef]
- Brown, G.S.; Kitchener, A.E.; Barnes, S. Calcium hydroxide sprays for the control of black spot on apples—Treatment effects on fruit quality. Acta Hortic. 1998, 513, 47–52. [Google Scholar] [CrossRef]
- Palmer, J.W.; Davies, S.B.; Shaw, P.W.; Wünsche, J.N. Growth and fruit quality of ‘Braeburn’ apple (Malus domestica) trees as influenced by fungicide programmes suitable for organic production. New Zeal. J. Crop Hortic. Sci. 2003, 31, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.B.; King, J.R.; Mcbride, J.J. Zineb controls citrus rust mites. Proc. Florida State Hortic. Soc. 1957, 70, 38–47. [Google Scholar]
- Lindow, S.E.; Desurmont, C.; Elkins, R.; McGourty, G.; Clark, E.; Brandl, M.T. Occurrence of indole-3-acetic acid-producing bacteria on pear trees and their association with fruit russet. Phytopathology 1998, 88, 1149–1157. [Google Scholar] [CrossRef] [Green Version]
- Faust, M.; Shear, C.B. Russeting of apples, an interpretive review. HortScience 1972, 7, 233–235. [Google Scholar]
- Faust, M.; Shear, C.B. Fine structure of the fruit surface of three apple cultivars. J. Am. Soc. Hortic. Sci. 1972, 97, 351–355. [Google Scholar]
- Peschel, S.; Knoche, M. Characterization of microcracks in the cuticle of developing sweet cherry fruit. J. Am. Soc. Hortic. Sci. 2005, 130, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Borve, J.; Sekse, L.; Stensvand, A. Cuticular fractures promote postharvest fruit rot in sweet cherries. Plant Dis. 2000, 84, 1180–1184. [Google Scholar] [CrossRef] [Green Version]
- Nguyen-the, C. Structure of epidermis wall, cuticle and cuticular microcracks in nectarine fruit. Agronomie 1991, 11, 909–920. [Google Scholar] [CrossRef]
- Knoche, M.; Grimm, E.; Winkler, A.; Alkio, M.; Lorenz, J. Characterizing neck shrivel in European plum. J. Am. Soc. Hortic. Sci. 2019, 144, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Geyer, U.; Schönherr, J. In vitro test for effects of surfactants and formulations on permeability of plant cuticles. In Pesticide Formulations: Innovations and Developments; Cross, B., Scher, H.B., Eds.; American Chemical Society: Washington, DC, USA, 1988; pp. 22–33. [Google Scholar]
- Knoche, M.; Peschel, S.; Hinz, M.; Bukovac, M.J. Studies on water transport through the sweet cherry fruit surface: Characterizing conductance of the cuticular membrane using pericarp segments. Planta 2000, 212, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology, 5th ed.; Academic Press: San Diego, CA, USA, 2020; ISBN 978-0-12-819146-0. [Google Scholar]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137A–138A. [Google Scholar]
- Straube, J.; Chen, Y.-H.; Khanal, B.P.; Shumbusho, A.; Zeisler-Diehl, V.; Suresh, K.; Schreiber, L.; Knoche, M.; Debener, T. Russeting in apple is initiated after exposure to moisture ends: Molecular and biochemical evidence. Plants 2020, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Sugar, D.; Basile, S.R. Russet induction in “Beurr’e Bosc” and “Taylor’s Gold” pears. Acta Hortic. 2008, 800, 257–261. [Google Scholar] [CrossRef]
- Creasy, L.L. The correlation of weather parameters with russet of “Golden Delicious” apples under orchard conditions. J. Am. Soc. Hortic. Sci. 1980, 105, 735–738. [Google Scholar]
- Knoche, M.; Khanal, B.P.; Stopar, M. Russeting and microcracking of “Golden Delicious” apple fruit concomitantly decline due to gibberellin a4+7 application. J. Am. Soc. Hortic. Sci. 2011, 136, 159–164. [Google Scholar] [CrossRef]
- Shi, C.; Qi, B.; Wang, X.; Shen, L.; Luo, J.; Zhang, Y. Proteomic analysis of the key mechanism of exocarp russet pigmentation of semi-russet pear under rainwater condition. Sci. Hortic. 2019, 254, 178–186. [Google Scholar] [CrossRef]
- Yuan, G.; Bian, S.; Han, X.; He, S.; Liu, K.; Zhang, C.; Cong, P. An integrated transcriptome and proteome analysis reveals new insights into russeting of bagging and non-bagging “Golden Delicious” apple. Int. J. Mol. Sci. 2019, 20, 4462. [Google Scholar] [CrossRef] [Green Version]
- Mathooko, F.M.; Kahangi, E.M.; Runkua, J.M.; Onyango, C.A.; Owino, W.O. Preharvest mango (Mangifera indica L. ’Apple’) fruit bagging controls lenticel discolouration and improves postharvest quality. Acta Hortic. 2011, 906, 55–62. [Google Scholar] [CrossRef]
- Edelmann, H.G.; Neinhuis, C.; Bargel, H. Influence of hydration and temperature on the rheological properties of plant cuticles and their impact on plant organ integrity. J. Plant Growth Regul. 2005, 24, 116–126. [Google Scholar] [CrossRef]
- Khanal, B.P.; Knoche, M. Mechanical properties of cuticles and their primary determinants. J. Exp. Bot. 2017, 68, 5351–5367. [Google Scholar] [CrossRef] [PubMed]
- Knoche, M.; Peschel, S. Water on the surface aggravates microscopic cracking of the sweet cherry fruit cuticle. J. Am. Soc. Hortic. Sci. 2006, 131, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Brüggenwirth, M.; Knoche, M. Cell wall swelling, fracture mode, and the mechanical properties of cherry fruit skins are closely related. Planta 2017, 245, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Khanal, B.P.; Knoche, M. Mechanical properties of apple skin are determined by epidermis and hypodermis. J. Am. Soc. Hortic. Sci. 2014, 139, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Khanal, B.P.; Grimm, E.; Knoche, M. Russeting in apple and pear: A plastic periderm replaces a stiff cuticle. AoB Plants 2013, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Petracek, P.D.; Bukovac, M.J. Rheological properties of enzymatically isolated tomato fruit cuticle. Plant Physiol. 1995, 109, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Khanal, B.P.; Knoche, M.; Bußler, S.; Schlüter, O. Evidence for a radial strain gradient in apple fruit cuticles. Planta 2014, 240, 891–897. [Google Scholar] [CrossRef]
- Gibert, C.; Chadœuf, J.; Vercambre, G.; Génard, M.; Lescourret, F. Cuticular cracking on nectarine fruit surface: Spatial distribution and development in relation to irrigation and thinning. J. Am. Soc. Hortic. Sci. 2007, 132, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Knoche, M.; Grimm, E. Surface moisture induces microcracks in the cuticle of “Golden Delicious” apple. HortScience 2008, 43, 1929–1931. [Google Scholar] [CrossRef] [Green Version]
- Knoche, M.; Khanal, B.P.; Brüggenwirth, M.; Thapa, S. Patterns of microcracking in apple fruit skin reflect those of the cuticular ridges and of the epidermal cell walls. Planta 2018, 248, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Curry, E.; Arey, B. Apple cuticle: The perfect interface. Scanning Microsc. 2010, 7729, 77291P. [Google Scholar] [CrossRef]
- Curry, E.A. Growth-induced microcracking and repair mechanisms of fruit cuticles. In Proceedings of the SEM Annual Conference, Albuquerque, NM, USA, 1–4 June 2009. [Google Scholar]
- Lipton, W.J. Some effects of low-oxygen atmospheres on potato tubers. Am. Potato J. 1967, 44, 292–299. [Google Scholar] [CrossRef]
- Wei, X.; Mao, L.; Han, X.; Lu, W.; Xie, D.; Ren, X.; Zhao, Y. High oxygen facilitates wound induction of suberin polyphenolics in kiwifruit. J. Sci. Food Agric. 2018, 98, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, S.J. Fruit russeting in apple as affected by various gibberellins. J. Hortic. Sci. 1982, 57, 283–288. [Google Scholar] [CrossRef]

| Treatment | Area Russeted (%) |
|---|---|
| Untreated fruit | 1.3 ± 0.2 a |
| Open tube, no moisture | 1.3 ± 0.2 a |
| Closed tube, moisture | 20.2 ± 3.9 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athoo, T.O.; Winkler, A.; Owino, W.O.; Knoche, M. Surface Moisture Induces Microcracks and Increases Water Vapor Permeance of Fruit Skins of Mango cv. Apple. Horticulturae 2022, 8, 545. https://doi.org/10.3390/horticulturae8060545
Athoo TO, Winkler A, Owino WO, Knoche M. Surface Moisture Induces Microcracks and Increases Water Vapor Permeance of Fruit Skins of Mango cv. Apple. Horticulturae. 2022; 8(6):545. https://doi.org/10.3390/horticulturae8060545
Chicago/Turabian StyleAthoo, Thomas O., Andreas Winkler, Willis O. Owino, and Moritz Knoche. 2022. "Surface Moisture Induces Microcracks and Increases Water Vapor Permeance of Fruit Skins of Mango cv. Apple" Horticulturae 8, no. 6: 545. https://doi.org/10.3390/horticulturae8060545
APA StyleAthoo, T. O., Winkler, A., Owino, W. O., & Knoche, M. (2022). Surface Moisture Induces Microcracks and Increases Water Vapor Permeance of Fruit Skins of Mango cv. Apple. Horticulturae, 8(6), 545. https://doi.org/10.3390/horticulturae8060545






