Abstract
A two-year (2020-21) study was conducted to investigate the possibility of relying of ten-years old pear trees grown on sandy loam soil irrigated by drip on citric acid (CA), gibberellic acid (GA3) and humic acid (HA). The CA was applied at the concentrations of 500, 1000 and 1500 ppm, GA3 at 50, 100 and 150 ppm and HA at 3, 4 and 5%, whereas water spray was used as the control. The results of our study proved that CA, GA3 and HA improved the shoot length, shoot thickness, leaf area and leaf chlorophyll of pear as compared with the control. Moreover, they also positively increased the fruit set percentage and final yield of ‘Le Conte’ pear. The fruit weight, size and firmness were also improved under the influence of aforementioned treatments. The fruit soluble solids, total sugars, leaf nitrogen, leaf phosphorus and leaf potassium of pear were also enhanced as compared with the control. Additionally, spraying of GA3 at 150 ppm, as well as, HA at 5 and 4% were the superior treatments and showed the most significant impact on plant growth, yield, fruit quality and leaf mineral content of pear. This study provides a basis for the future elucidation of HA-, GA3- and CA-modulated molecular mechanisms in pear, which can make a significant contribution in the scientific community.
1. Introduction
Pear (Pyrus communis L.) belongs to family Rosaceae [1] and is cultivated on an area of 1,379,387 hectares in the world with the production of approximately 23,919,075 tons [2]. The exogenous application of plant bio-stimulants, such as citric acid (CA), gibberellic acid (GA3) and humic acid (HA), has emerged as an effective approach to improve plant growth and yield and thus sustain food production.
Research reported that the pear soil characteristics are preferably light acidic soil, which a pH of 6–7. However, soil outside of that range will not have an undesirable impact on growth. The ideal environmental conditions are of cold winter, which is required to stimulate the productivity of the fruit, and the majority of cultivars require approximately 400–800 h of temperatures under 45 F. Moreover, pear also requires a wet summer that supply the tree with the water needed for fruit production, although the high humidity can lead to incidence with diseases.
The excess and long application of chemical fertilizer in the agricultural sector may cause problems, such as salt gathering; soil degradation, deterioration and acidification; nitrogen leaching; loss of soil carbon; and imbalance of elements and may increase the concentration of heavy metals [3,4,5]. NPK chemical fertilizer impact on plant crop decreases with time, and it also lessens the bacterial variance [6,7,8]. Thus, organic agriculture is required to raise the soil microbes; enzyme activity; N, P and K uptake [9]; ameliorate the soil’s physical and chemical properties [10,11,12]; attract helpful bacteria to the rhizosphere [13,14]; lessen the concentration of soil heavy metals [4]; and reduce soil acidification and the undesirable impacts of synthetic fertilizers on human health and environment [3], and thus it can improve the soil productivity [15,16] and yield [17,18].
CA, a 6-carbon tricarboxylic acid synthesized by the citrate synthase (CS)-catalyzed condensation of oxaloacetate (OAA) and acetyl-CoA, is an intermediate of the mitochondrial tricarboxylic acid (TCA) cycle [19,20]. Recent studies emphasized on the significance of foliar-applied CA in terms of its vital effects on metabolic and physiological activities, such as cell elongation and division, leading to raises in the plant biomass and photosynthesis process in numerous plant species [21]. Orange trees sprayed with 1000 ppm CA exhibited improved leaf chlorophyll, leaf Fe, fruit soluble solids, vitamin C, fruit weight, fruit diameter and juice content, with minimized fruit titratable acidity, when compared to the control [22].
Maksoud et al. [23] reported that the CA in its effect is similar to the influence of auxin in increasing the evolution, flowering and fruiting of fruit trees. The exogenous application of CA improved the fruit quality of grapes [21], apples [24] and ‘Le-Cont’ pears [25]. Additionally, Guneri et al. [26] documented that CA at 0.05% increased the leaf width, fruit weight and vitamin C in orange and lemon plants. Mandour et al. [27] stated that the foliar spray with citric acid on strawberry cv. Festival at 2 g/L increased the yield components, plant height, number of leaves per plant, shoot dry weight, fruit physical and chemical quality standards and decreased the weight loss and decay percentage.
GA3 is a naturally occurring plant hormone that is of economic and industrial significance [28]. It has an effect on stem elongation, germination, dormancy elimination, flowering, sex expression, enzyme induction and the withering of leaves and fruits in plants [29]. The foliar application of GA3 significantly improved the vegetative growth attributes and fruit firmness in apple [30], peach [31] and pear [32]. Gibberellins can enhance cell elongation [33]; thus, it stimulated fruit growth and size in guava [34], citrus [35] and mango [36].
Through the regulation of photosynthesis, the carbohydrate metabolism and amino acid synthesis, GA3 can affect the growth of plants, root and stem prolongation, flowering encouragement, fruit numbers and, consequently, the obtained yield [37,38,39]. Ennab [40] documented that spraying plum with GA3, raised the ratio between TSS and acidity and lessened the fruit acidity percentage. Spraying mandarin cv. Kinnow with GA3 [41], pomegranate [42,43,44] and grape [45,46] notably raised the peel color, fruit weight, TSS, acidity, ascorbic acid, antioxidants, phenols, flavonoids and carotenoids, and they lessened cracking and sunburn. Askarieh et al. [47] noticed that treating sweet cherry with 100 ppm GA3 enhanced the flowering rate, fruit number and yielding, while it lowered the fruit drop.
HA is an organically charged bio-stimulant that has been shown to considerably boost plant growth and development while also increasing crop output [48,49]. HA induces plant growth by inducing the cell respiration, photo-synthesis, water uptake, enzyme activation, cation exchange capacity, availability and transportation of nutrients, soil structure and water reservation capability [50,51,52]. HA could promote the growth parameters, yield, fruit weight and size in apricot [53], ‘Le-Conte’ pear [54] and kiwifruit [55]. Spraying of pomegranate cvs. Manfalouty [56] with HA at 1%, 2% and 3% and Wonderful [57] with 0.2%, 0.3% and 0.4% significantly improved the parameters of vegetative traits, fruit set percentage, yielding and fruit physicochemical characteristics, such as sugars, anthocyanin and firmness. Foliar application of HA caused to improvement of nutrient efficiency and an increase in leaf Zn and Fe contents, which raised carbohydrate and protein production [58].
It was noticed that HA enhanced the weight, size, TSS, and V.C. in grape [59], apricots [60] and Guava paluma [56]. Moreover, mango performance via the vegetative yield and fruit chemical or physical quality were markedly improved as a result of the foliar spray of HA on mango cv. Zebda as mentioned by El-Hoseiny et al. [61]. Hence, considering the aforementioned beneficial aspects of CA, GA3 and HA on plant performance, this study was aimed to investigate their impacts on vegetative growth, yield and fruit quality of ‘Le Conte’ pear as a good nutritional alternative to lessen the reliance on chemical fertilizers in fruit nutrition. Although the current study provided ample information to signify the roles of CA, GA3 and HA in improving pear performance, the molecular mechanisms behind the improved yield and fruit quality remain to be explored.
2. Materials and Methods
2.1. Plant Material, Treatments, Experimental Site and Design
The current study was carried out during the 2020 and 2021 seasons to examine the impact of the exogenous applications of Citric acid (CA), Gibberellic acid (GA3) and Humic acid (HA) on the performance of ten-years-old ‘Le Conte’ pear trees budded on Pyrus communis rootstock. The trees were cultivated on the distances of 3.5 × 3.5 m in a private orchard in Burg EL-Arab, Alexandria governorate, Egypt, at 29.55 longitude and 30.90 latitude in sandy loam soil under drip irrigation.
The experiment was composed from ten treatments; control (water only); CA (Sunshine Biotech International Co., Ltd., Prachin Buri, Thailand) at 500, 1000 and 1500 ppm; GA3 (Shijiazhuang Awiner Biotech Co., Ltd. Shijiazhuang, China) at 50, 100 and 150 ppm; HA (X-humate 100% super powder potassium humate manufacturing—Humate (Tianjin) International Ltd., Tianjin, China) at 3, 4 and 5%. Each treatment had eight replicates (trees), which were arranged in a randomized complete block design (RCBD). The trees were sprayed three times; the first spray was in February (before starting of the bloom), the second was during full bloom (March), and the third spray was performed two weeks later than the second spray. The pear trees received the same horticultural treatment in the orchard. The analysis of the soil composition is illustrated in Table 1.

Table 1.
Physical and chemical analysis of the experimental soil.
2.2. Vegetative Growth Attributes
Six healthy branches on each tree were selected and tagged, and the average of their shoot length was measured in cm at the end of each vegetative season. The thickness of shoots was measured two times i.e., at the start of the vegetative season (March) and after harvesting (July) to measure the increase in shoot thickness in cm by using a Digital Vernier Caliper in both seasons.
The leaf total chlorophyll was measured using a digital chlorophyll meter (SPAD-502Plus, Konica Minolta, Inc., Tokyo, Japan). Thirty leaves were randomly picked from each tree in each treatment to determine the leaf area in cm2 using a CI-203 laser area meter (CID Inc., Washington, DC, USA). The average leaf area (cm2) was determined using the following equation as reported by Demirsoy [62].
LA = leaf area, L is the length, and W is the width of the leaves
2.3. Fruit Set Percentage, Fruit Drop and Yield
Fruit set (%) was determined by counting the number of flowers on each labelled branch. The fruit set percentage was calculated using the following formula:
Fruit drop (%) was measured by counting the number of dropped fruits after fruit set (mid of March) till the harvesting time (June), and then it was finally calculated as a percentage according the following equation:
Fruit yield was estimated in kg per replicate/tree at harvesting time (July).
2.4. Fruit Physical Characteristics
Fruit fresh weight, length (from maximum vertical point) and diameter (from maximum horizontal point) were calculated by taking the average of thirty fruits from each replication of same treatment. Fruit weight was measured with digital weighing balance (MJ-W176P, Panasonic, Kadoma, Japan), whereas the length and diameter were measured with digital Vernier callipers (DR-MV0100NG, Ningbo Dongrun Imp. & Exp. Co., Ltd., Ningbo, China). The length was divided by diameter of each fruit to calculate the length-to-width ratio (fruit shape index). Fruit firmness was calculated using a Magness and Taylor pressure tester with a 7/18-inch plunger (mod. FT 02) (0–2 Lb., Via Reale, 63, 48011 Alfonsine, Italy).
2.5. Fruit Biochemical Characteristics
The total soluble solids were determined with a hand-held digital refractometer (Atago, Hybrid PAL-BXIACID F5, Japan). The total titratable acids were determined with NaOH-based titrimetric method and expressed as percent citric acid. The sugar-acid ratio was calculated by dividing the total soluble solids to total titratable acids within the same sample. Sugars were determined as detailed earlier by Ali et al. [63]. Briefly, an aliquot of fruit pulp, treated with 25% lead acetate and 20% potassium oxalate, was hydrolysed with HCl and kept overnight to convert non-reducing sugars into reducing sugars. Then, the HCl-hydrolysed aliquot was neutralized with 0.1 N NaOH and titrated against Fehling solutions.
2.6. Leaf Minerals
In July, and after harvesting the fruit, thirty leaves were taken from the middle part of branches of the trees, and then they were washed, cleaned, well-crushed and digested with H2SO4 and H2O2 to assess their nitrogen, phosphorus and potassium contents. Nitrogen was assessed using the micro-Kjeldahl method [64], phosphorus was determined using the vanadomolybdate method [65], and potassium was determined using a flame photometer (SKZ International Co., Ltd., Jinan, China) [66]. Iron, zinc and manganese were determined using inductively coupled plasma–mass spectrometry (ICP-MS) [67,68,69].
2.7. Statistical Analysis
One-way analysis of variance (ANOVA) was utilized to analyze the obtained results statistically, and the least significant difference (LSD) at 0.05% was used to make a comparison between the means of treatments, and it was measured using the statistical software ‘Statistix v8.1′. Variables with a statistically significant impact of treatments (p ≤ 0.05) were further subjected to principal component analysis (PCA) using XLSTAT ver. 2018. Correlation coefficient values were determined with the Pearson (n) method. The clustering of variables with associated treatments was determined with their highest squared cosine values corresponding to factor, F1 or F2.
3. Results
3.1. Vegetative Growth Attributes
The exogenous application of CA, GA3 and HA greatly enhanced the vegetative performance of ‘Le Conte’ pear plants in terms of their shoot length, shoot thickness, leaf area and leaf total chlorophyll content in both growing seasons (Table 2). Among all treatments, the foliar application of HA at 5% or 4% and GA3 at 150 ppm had the most notable impact on the previously listed vegetative measurements. Furthermore, it was noticed that the application of GA3 at 100 ppm also highly increased the shoot length and thickness as well as the leaf area and chlorophyll content over untreated plants.

Table 2.
Influence of Citric acid, Gibberellic acid and Humic acid on the shoot length, shoot thickness, leaf area and leaf chlorophyll of ‘Le Conte’ pear during the 2020 and 2021 seasons.
3.2. Fruit Set Percentage, Fruit Drop and Yield
The foliar application of CA, GA3 and HA raised the percentage of fruit set and fruit yield per tree over control. On the other hand, the fruit drop percentages were lowered by these treatments (Figure 1). The maximum fruit set (19.22% “2020” and 21.51% “2021”) and minimum fruit drop (55.89% “2020”and 51.59% “2021”) was obtained by the foliar application of 5% HA. The most remarkable yield was resulted from the application of HA at 5% and 4% and GA3 at 150 or 100 ppm over untreated plants. Moreover, GA3 also improved the fruit set percentage and lessened the percentage of fruit drop, and thus HA and GA3 improved the productivity of the trees over the usage of CA during the studied seasons. It was clear from the data that the impact of HA, GA3 or CA was enhanced gradually in parallel to the increase of the applied concentration.
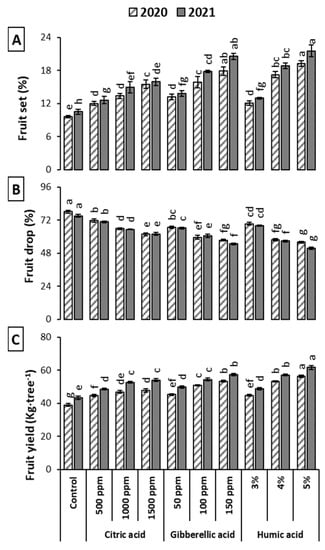
Figure 1.
Influence of Citric acid, Gibberellic acid and Humic acid on the fruit set (A), fruit drop (B) and fruit yield (C) of ‘Le Conte’ pear during the 2020 and 2021 seasons. Vertical bars indicate ± standard error (n = 8). Same letters indicate non-significant difference among treatments according to Fisher’s LSD method (p ≤ 0.05).
3.3. Fruit Physical Characteristics
The results shown in Table 3 clearly indicated that, in comparison to the control, the spraying of HA at 4% or 5%, as well as the application of GA3 at the rate of 150 ppm resulted in remarkable increments in fruit weight, size, length, diameter and fruit firmness of ‘Le Conte’ pear. Additionally, the application of 100 ppm GA3 and 1500 ppm CA also had a positive impact on boosting the same attributes compared to the control in both growing seasons. Moreover, the fruit shape index was significantly increased by the foliar application of CA at 1500 ppm and 100 or 150 ppm GA3 as well as HA at 4% or 5% compared with other applied treatments in both seasons.

Table 3.
Influence of Citric acid, Gibberellic acid and Humic acid on the fruit weight, size, length, diameter, firmness and fruit shape in ‘Le Conte’ pear trees during the 2020 and 2021 seasons.
3.4. Fruit Biochemical Characteristics
From the results demonstrated in Table 4, the spraying of CA, GA3 and HA had a beneficial impact on fruit biochemical characteristics as compared to the control. The exogenous application of 5% or 4% HA and 150 ppm GA3 markedly increased the soluble solids, total sugars and the ratio between TSS and acidity compared to the control. Additionally, the foliar application of HA at 5% and GA3 at 150 ppm remarkably improved the content of reducing and non-reducing sugars in pear fruits. Additionally, fruit acidity was noted higher in untreated pears as compared with CA-, GA3- and HA-treated pear plants. The clearest effect on decreasing the fruit acidity percentage was noticeably related to the application of HA at 4% and 5% and also GA3 at 150 and 100 ppm.

Table 4.
Influence of Citric acid, Gibberellic acid and Humic acid on the percentages of TSS, acidity, total, reduced and non- reduced sugars and TSS/acid ratio in ‘Le Conte’ pear trees during the 2020 and 2021 seasons.
3.5. Leaf Minerals
The foliar supplied CA, GA3 and HA considerably increased the leaf nitrogen, phosphorus, potassium, iron, zinc and manganese of pear plants as compared to the control (Table 5). Furthermore, during both growing seasons, the high content of macro and micronutrients was accompanied to the foliar sprays of HA at 5% or 4%, as well as GA3 at 150 ppm, rather than the other treatments. Additionally, it was noticed that the influence of CA, GA3 and HA on the measured parameters was increased in dose-dependent manner, where the highest applied concentration gave the most increase in the leaf mineral content and this reflected on improving the final obtained productivity and fruit quality.

Table 5.
Influence of Citric acid, Gibberellic acid and Humic acid on leaf content of N, P, K, Fe, Zn and Mn of ‘Le Conte’ pear trees during the 2020 and 2021 seasons.
4. Discussion
There were differences between CA, GA3 and HA treatments in terms of their impact on plant growth and fruit quality of ‘Le Conte’ pear. However, the results are in line with the findings of Mansour et al. [70], who reported that spraying CA on pear trees enhanced the fruit production, fruit weight, fruit firmness, as well as the percentages of total soluble solids and total sugars, while diminishing the fruit acidity. El-Badawy [71] reported that CA enhanced the shoot length, leaves/shoot ratio, leaf area index, TSS percent, vitamin C and fruit firmness in apricot cv. Canino. Ca raises the intake of elements’ rate and diminishes the adverse impacts of Pb under environmental stressors; thus, it can ameliorate photosynthetic rate [72,73], photosynthetic pigment production [74] and plant growth [75].
Osama et al. [76] noticed that spraying of CA at 400 and 800 ppm on ‘Keitt’ mango trees increased the percentages of fruit set and fruit retention, fruit number, fruit production, fruit physical attributes in terms of fruit and peel weight and pulp/fruit ratio in comparison with the control. Additionally, they added that CA also increased the fruit chemical features such as total soluble solids and sugars, while reduced the fruit acidity in comparison to the control.
Treating ‘Washington’ navel orange trees with CA at 1 g/L enhanced the total chlorophyll content in the leaves, number of shoots, shoot length and thickness, leaf number per shoot, leaf area and leaf mineral content of N, P, K, Ca, Mg, Fe, Mn and Zn [24]. Mohamed [77] reported that the foliar application of CA at 1000 ppm on grapevines increased yield, number of clusters per vine, cluster weight, length, width, berry weight, berry length, berry diameter, berry content from reducing sugars, anthocyanin and total soluble solids, while it reduced the acidity of berries in the two seasons compared to the control.
Iqbal et al. [78] documented that GA3 reduced flower drop, while increasing the flower retention and the yield and fruit quality in many fruit crops such as mango, citrus, apple and guava. In relative to the control treatment, treating apples with 20 mg/L GA3 enhanced the number of buds, fruit setting, fruit number, weight and yield, as well as the total sugar content, while it lessened the buds dropping before anthesis [79]. Nkansah et al. [80] found that the application of mango trees cv. Keitt with 25 mg/L GA3 at full bloom raised the fruit set, fruit retention, number of fruits, fruit weight and yield. Treating apricot trees with 50 or 100 mg/L from gibberellic acid in mid-May had a positive effect on improving the fruit weight, length and diameter [81].
Our results are also in line with the findings of Mosa et al. [82], who noticed that the application of 50 and 100 mg/L GA3 on apple trees (cv. Anna) obviously increased the shoot length, shoot thickness, leaf area, fruit set % and fruit yield, while it lessened the fruit drop percentage. Furthermore, it also increased the fruit weight, length, diameter and firmness, as well as TSS %, ascorbic acid and leaf nitrogen, phosphorus, potassium, calcium and magnesium compared to the untreated plants.
In the same trend, it was noticed by Beerappa et al. [83] that, when GA3 at the rate of 50 and 100 ppm was applied on pomegranate trees (cv. Bhagwa), it raised the fruit length, diameter, number of arils, total aril weight, fruit weight, peel weight and peel thickness. In addition, it also improved the fruit number and consequently the fruit yield, TSS, ascorbic acid content, total sugars, reducing sugars, non-reducing sugars, juice content and sugar/acid ratio. Similarly, the exogenous application of GA3 at 10, 20 and 30 mg/L on pear tree (cv. Le-Conte) increased shoot length and diameter, leaf area and fruit length, diameter and size. Additionally, they also boosted the number and weight of fruits, as well as the yield in comparison to untreated plants [84].
In case of HA application, our results are in corroboration with the earlier findings [85,86,87], when HA was applied to grapevines at full bloom, it enhanced the leaf chlorophyll content, berry weight, size, width, yield, sugars content and the sugar/acidity ratio while it lessened the fruit titratable acidity comparing with untreated vines. Moreover, the same authors explained the increase in the size of berries was as result of raising the mineral nutrient uptake, and thus HA’s effect seemed to be similar with the influence of auxin, gibberellin and cytokinin-like activity.
As HA includes multiple minerals, it could improve the soil fertility and raise the nutrient availability by adsorbing them on mineral surfaces, resulting in improving the plant development and yield of broad bean [88]. In grapes, HA increased the leaf chlorophyll content and photosynthesis rate; therefore, it led to increasing the growth [85,89]. Treating olive cultivars with HA 100 mg/L increased the fruit content from the oil, protein, total chlorophyll and phenol contents [90]. Under water stress, it was noted that HA increased the leaf water retention, photosynthetic rate, the roots density and absorbed elements; thus, it could improve the growth, yield and quality of fruit [91,92].
Application of HA encourages the growth of plants and crop production [93] as well as microbial activities [92,94,95], and it significantly affects the soil physicochemical characteristics, microbe number and activity; thus, it can raise the availability and absorption of macro and micro nutrients and the prolongation of roots [86,92,96,97,98]. The foliar spraying of HA ameliorated the shoot length, shoot diameter and leaf area, fruit set percentage and fruit yield and decreased the fruit drop percentage. Furthermore, it also enhanced the fruit weight, length, diameter, firmness, TSS and TSS-acid ratio, while decreasing the fruit acidity. In addition, it increased the concentration of sugars in the fruit and leaf contents of macro and micronutrients, such as N, P, K, Ca, Fe, Zn, Mn and B when compared with untreated plants in apple [82] and apricot [99].
The efficacy of CA, GA and HA to modulate plant physiology depends on its concentration, application method and plant [63,100]. The results from this study also showed that response of pear plant growth and fruit development to CA, GA3 and HA applications changed with change in their concentrations. Thus, principal component analysis was conducted to delineate treatment and concentration-dependent effects (Figure 2). Based on the highest squared cosine value corresponding to factors F1 or F2, the plant growth, yield and fruit quality attributes were clustered around CA, GA3 and HA treatments.
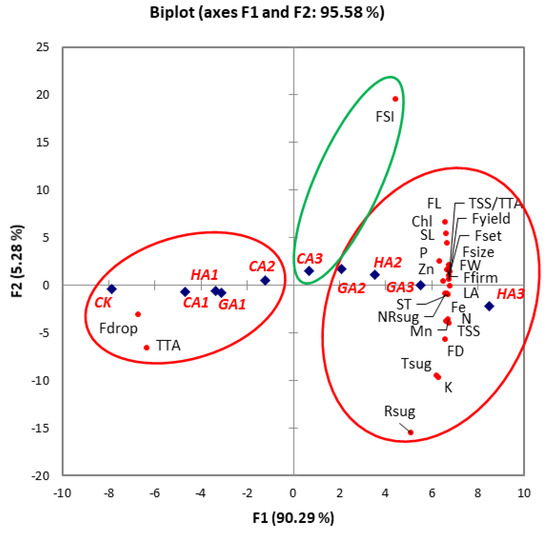
Figure 2.
Principal component analysis among CA, GA3 and HA treatments and various plant growth, yield and fruit quality attributes of ‘Le Conte’ pear. Clustering of observation (treatment) and variables (plant growth, yield or fruit quality attributes) into groups (coloured circles) is based on their highest squared cosine values corresponding to the factor, F1 (red) or F2 (green). Abbreviations: CK—control; CA1—500 ppm citric acid; CA2—1000 ppm citric acid; CA3—1500 ppm citric acid; GA1—50 ppm gibberellic acid; GA2—100 ppm gibberellic acid; GA3—150 ppm gibberellic acid; HA1—3% humic acid; HA2—4% humic acid; HA3—5% humic acid; SL—shoot length; ST—shoot thickness; LA—leaf area; Chl—leaf chlorophyll; Fset—fruit set (%); Fdrop—fruit drop (%); Fyield—fruit yield; FW—fruit weight; FL—fruit length; FD—fruit diameter; FSI—fruit shape index; Ffirm—fruit firmness; TSS—total soluble solids; TTA—total titratable acidity; TSS/TTA—Sugar acid ratio; Rsug—reducing sugars; NRsug—non-reducing sugars; Tsug—total sugars; N—leaf nitrogen; P—leaf phosphorus; K—leaf potassium; Fe—leaf iron; Zn—leaf zinc; and Mn—leaf manganese.
Factor F1, covering 90.29% variability in data (eigenvalue 22.573), showed clustering of the shoot thickness, shoot length, leaf area, leaf chlorophyll, fruit weight, fruit size, fruit firmness, fruit length, fruit diameter, leaf nitrogen, leaf phosphorus, leaf potassium, leaf iron, leaf zinc, leaf manganese, fruit TSS, fruit TTA, sugar-acid ratio, total sugars, reducing sugars, non-reducing sugars, fruit set (%) and fruit yield of pear with 100–150 ppm GA3 and 4–5% HA suggesting pa ositive influence of GA3 and HA applications on these parameters.
The fruit drop (%) and total titratable acidity showed a positive association with the control, 500–1000 ppm CA, 50 ppm GA3 and 3% HA applications. This cluster was located opposite to 100–150 ppm GA3 and 4–5% HA on the F1 axis depicting a strong negative correlation of 100–150 ppm GA3 and 4–5% HA applications on these parameters compared to the control. The second factor, covering 5.28% variability in data (eigenvalue 1.320), showed clustering of fruit shape index with 1500 ppm CA. Thus, principal component analysis helped to delineate the individual roles of CA, GA3 and HA treatments in regulating various aspects of plant growth, yield and fruit quality of pears.
5. Conclusions
This study suggests that foliar applications of CA, GA3 and HA can be used to increase the yield and nutritional quality of ‘Le Conte’ pear—two important factors contributing to economic profit and consumer health, respectively. Since foliar applications of CA, GA3 and HA at different levels differentially regulate distinct aspects of plant growth and development, specific concentrations of these bio-stimulants may help to achieve specific objectives of fruit production. Overall, 1500 ppm CA, 150 ppm GA3 and 4–5% HA may be used as an effective preharvest application strategy to improve plant health, increase yield and improve the quality of pears. Citric, gibberellic and humic acids are effective tools to improve fruit vegetative growth, yield and physiochemical characteristics under stress conditions; thus, they could be used as alternatives to the chemical fertilizers to alleviate undesirable impacts on the soil composition, microbe activity and nutritional value of the produced crops.
Author Contributions
Conceptualization, W.F.A.M. and N.A.A.E.-M.; methodology, W.F.A.M., N.A.A.E.-M. and H.S.A.; software, W.F.A.M., H.M.A., M.H.S. and M.M.A.; validation, N.A.A.E.-M., L.S.-P., H.M.A. and M.M.A.; formal analysis, W.F.A.M., M.M.A., H.M.A., H.S.A. and M.H.S.; investigation, W.F.A.M., N.A.A.E.-M. and H.S.A.; resources, W.F.A.M., N.A.A.E.-M., M.M.A. and L.S.-P.; data curation, W.F.A.M., M.M.A., M.H.S. and H.S.A.; writing—original draft preparation, W.F.A.M., H.M.A., N.A.A.E.-M. and L.S.-P.; writing—review and editing, W.F.A.M., H.M.A., M.H.S. and M.M.A.; and supervision, N.A.A.E.-M. and L.S.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Researchers Supporting Project number (RSP-2021/123) King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
This study does not involve humans or animals “Not applicable”.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project (RSP-2021/123) King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Álvarez-Fernández, A.; García-Laviña, P.; Fidalgo, C.; Abadía, J.; Abadía, A.J.P. Foliar fertilization to control iron chlorosis in pear (Pyrus communis L.) trees. Plant Soil 2004, 263, 5–15. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2019: Safeguarding against Economic Slowdowns and Downturns; FAO: Rome, Italy, 2019; Licence: CC BY-NC-SA 3.0 IGO. Rome; 2019. [Google Scholar]
- Sharma, A.; Chetani, R. A review on the effect of organic and chemical fertilizers on plants. Int. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 677–680. [Google Scholar] [CrossRef]
- Lin, W.; Lin, M.; Zhou, H.; Wu, H.; Li, Z.; Lin, W. The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE 2019, 14, e0217018. [Google Scholar] [CrossRef]
- Mosa, W.F.; Ali, H.M.; Abdelsalam, N.R. The utilization of tryptophan and glycine amino acids as safe alternatives to chemical fertilizers in apple orchards. Environ. Sci. Pollut. Res. 2021, 28, 1983–1991. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Horrigan, L.; Lawrence, R.S.; Walker, P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ. Health Perspect. 2002, 110, 445–456. [Google Scholar] [CrossRef]
- Wang, X.; Fan, J.; Xing, Y.; Xu, G.; Wang, H.; Deng, J.; Wang, Y.; Zhang, F.; Li, P.; Li, Z. The effects of mulch and nitrogen fertilizer on the soil environment of crop plants. Adv. Agron. 2019, 153, 121–173. [Google Scholar]
- Du, Z.-L.; Wu, W.-L.; Zhang, Q.-Z.; Guo, Y.-b.; Meng, F.-q. Long-term manure amendments enhance soil aggregation and carbon saturation of stable pools in North China plain. J. Integr. Agric. 2014, 13, 2276–2285. [Google Scholar] [CrossRef]
- Mäder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil fertility and biodiversity in organic farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Bairwa, H.L.; Kumar, U.; Javed, T.; Asad, M.; Lal, K.; Abdelsalam, N.R. Influence of organic manures on soil nutrient content, microbial population, yield and quality parameters of pomegranate (Punica granatum L.) cv. Bhagwa. PLoS ONE 2022, 17, e0266675. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xu, Y.; Xiang, L.; Wang, G.; Shen, X.; Chen, X. Effects of a mixture of bacterial manureand biochar on soil environment and physiological characteristics of Mals huupehens seedlings. Chin. Agric. Sci. Bull. 2017, 33, 52–59. [Google Scholar]
- Xu, H.; Xiao, R.; Xiang, Z.; Huang, Y.; Luo, W.; Qin, Z. Effects of different ecological manage-ment on the soil microbial biomass and microbial population of tea plantation in hilly red soil region. Chin. J. Soil Sci. 2010, 41, 1355–1359. [Google Scholar]
- He, L.-L.; Zhong, Z.-K.; Yang, H.-M. Effects on soil quality of biochar and straw amendment in conjunction with chemical fertilizers. J. Integr. Agric. 2017, 16, 704–712. [Google Scholar] [CrossRef]
- Kandil, E.E.; Abdelsalam, N.R.; EL Aziz, A.A.A.; Ali, H.M.; Siddiqui, M.H. Efficacy of nanofertilizer, fulvic acid and boron fertilizer on sugar beet (Beta vulgaris L.) yield and quality. Sugar Tech. 2020, 22, 782–791. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Z.W.; Lin, W.W.; Jiang, Y.H.; Weng, B.Q.; Lin, W.X. Effects of biochar and sheep manure on rhizospheric soil microbial community in continuous ratooning tea orchards. Ying Yong Sheng Tai Xue Bao 2018, 29, 1273–1282. [Google Scholar] [CrossRef]
- Kandil, E.E.; Abdelsalam, N.R.; Mansour, M.A.; Ali, H.M.; Siddiqui, M.H. Potentials of organic manure and potassium forms on maize (Zea mays L.) growth and production. Sci. Rep. 2020, 10, 8752. [Google Scholar] [CrossRef]
- Trejo-Tellez, L.; Gomez-Merino, F.; Schmitt, J.M.; Vargas, D.A.; Medina, J. Citric Acid: Biosynthesis, Properties and Applications on Higher Plants; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 43–70. [Google Scholar]
- Khatun, M.R.; Mukta, R.H.; Islam, M.A.; Nazmul Huda, A.K.M. Insight into citric acid-induced chromium detoxification in rice (Oryza sativa. L). Int. J. Phytoremediation 2019, 21, 1234–1240. [Google Scholar] [CrossRef]
- Fayed, T. Effect of some antioxidants on growth, yield and bunch characteristics of Thompson seedless grapevine. Am.-Eurasian J. Agric. Environ. Sci. 2010, 8, 322–328. [Google Scholar]
- Amri, E.; Shahsavar, A.R. Comparative efficacy of citric acid and Fe (II) sulfate in the prevention of chlorosis in orange trees (Citrus sinensis L. cv ‘Darabi’). J. Biol. Chem. Environ. Sci. 2009, 3, 61–65. [Google Scholar]
- Maksoud, M.; Saleh, M.A.; El-Shamma, M.; Fouad, A.A. The beneficial effect of biofertilizers and antioxidants on olive trees under calcareous soil conditions. World J. Agric. Res. 2009, 5, 350–352. [Google Scholar]
- El-Badawy, H.; El-Gioushy, S.; Baiea, M.; EL-Khwaga, A. Effect of some antioxidants and nutrients treatments on vegetative growth and nutritional status of Washington navel orange trees. Middle East J. Agric. Res. 2017, 6, 87–98. [Google Scholar]
- Fayek, M.; Fayed, T.; El-Fakhrani, E.; Sayed, S.N. Yield and fruit quality of” Le-conte” pear trees as affected by compost tea and some antioxidants applications. J. Hortic. Sci.Ornam. Plants 2014, 6, 1–8. [Google Scholar] [CrossRef]
- Guneri, M.; Misirli, A.; Yokas, I. Citric acid treatments on the vegetative, fruit properties and yield in Interdonat lemon and Valencia orange. Afr. J. Agric. Res. 2012, 7, 5525–5529. [Google Scholar] [CrossRef]
- Mandour, M.A.; Metwaly, H.A.; Ali, A.M. Effect of foliar spray with amino acids, citric acid, some calcium compounds and mono-potassium phosphate on productivity, storability and controlling gray mould of strawberry fruits under sandy soil conditions. Zagazig J. Agric. Res. 2019, 46, 985–997. [Google Scholar] [CrossRef]
- Javed, T.; Ali, M.M.; Shabbir, R.; Anwar, R.; Afzal, I.; Mauro, R.P.J.B. Alleviation of Copper-Induced Stress in Pea (Pisum sativum L.) through Foliar Application of Gibberellic Acid. Biology 2021, 10, 120. [Google Scholar] [CrossRef]
- Rodrigues, C.; Vandenberghe, L.P.d.S.; de Oliveira, J.; Soccol, C.R. New perspectives of gibberellic acid production: A review. Crit. Rev. Biotechnol. 2012, 32, 263–273. [Google Scholar] [CrossRef]
- Sharma, G.; Ananda, S. Effect of pre-bloom foliar application of plant bioregulators on growth, fruiting and quality of apple under warmer agroclimatic conditions. In Proceedings of the VII International Symposium on Temperate Zone Fruits in the Tropics and Subtropics 662, Nauni, Solan, India, 21–25 October 2003; pp. 353–357. [Google Scholar]
- El-Shazly, S.M.; Eisa, A.M.; Moảtamed, A.M.H.; Kotb, H.R.M. Effect of some agro-chemicals preharvest foliar application on yield and fruit quality of ”Swelling” peach trees. Alex. J. Agric. Res. 2013, 58, 219–229. [Google Scholar]
- Ouma, G. Use of gibberellins to improve fruit set in pears after frost damage. J. Biol. Sci. 2008, 8, 213–216. [Google Scholar] [CrossRef][Green Version]
- Davis, P. The plant hormones: Their nature, occurrence and functions. In Plant Hormones; Davis, P.J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 1–15. [Google Scholar]
- Pal, P.; Yadav, K.; Kumar, K.; Singh, N. Effect of gibberellic acid and potassium foliar sprays on productivity and physiological and biochemical parameters of parthenocarpic cucumber cv. ‘Seven Star F1‘. J. Hortic. Res. 2016, 24, 93–100. [Google Scholar] [CrossRef]
- El-Sese, A.M.A. Effect of gibberellic acid (GA3) on yield and fruit characteristics of Balady mandarin. Assiut J. Agric. Sci. 2005, 36, 23–35. [Google Scholar]
- Sarkar, S.; Ghosh, B. Effect of growth regulators on biochemical compostion of mango cv. Amrapali. Ecol. Environ. 2005, 23, 379–380. [Google Scholar]
- Arunadevi, A.; Kumar, S.; Rajangam, J.; Venkatesan, K. Effect of plant growth regulators on growth, yield and quality of acid lime (Citrus aurantifolia Swingle.) var. PKM. J. Pharmacogn. Phytochem. 2019, 8, 3438–3441. [Google Scholar]
- Bons, H.K.; Kaur, N.; Rattanpal, H.S. Quality and Quantity Improvement of Citrus: Role of Plant Growth Regulators. Int. J. Agric. Environ. Biotechnol. 2015, 8, 433–447. [Google Scholar] [CrossRef]
- Dilip, W.; Singh, D.; Moharana, D.; Rout, S.; Patra, S.S. Effect of gibberellic acid (GA) different concentrations at different time intervals on seed germination and seedling growth of Rangpur Lime. J. Agroeco. Nat. Resour. Manag. 2017, 4, 157–165. [Google Scholar]
- Ennab, H.A. Moshtohor. Effect of GA3 and Sitofex (CPPU) Spraying on Yield and Fruit Quality of “Kelsey” Plum Trees (Prunus salicina Lindl.). Ann. Agric. Sci. Moshtohor. 2019, 57, 993–1002. [Google Scholar] [CrossRef]
- Talat, H.; Shafqat, W.; Qureshi, M.A.; Sharif, N.; Raza, M.K.; ud Din, S.; Ikram, S.; Jaskani, M.J. Effect of gibberellic acid on fruit quality of Kinnow mandarin. J. Glob. Innov. Agric. Soc. Sci. 2020, 8, 59–63. [Google Scholar] [CrossRef]
- Hegazi, A.; Samra, N.; El-Baz, E.; Khalil, B.M.; Gawish, M.S. Improving fruit quality of manfaloty and wonderfull pomegranates by using bagging and some spray treatments with gibberellic acid, calcium chloride and kaolin. J. Plant Prod. Sci. 2014, 5, 779–792. [Google Scholar] [CrossRef]
- Harhash, M.M.; Ali, M.A.; El-Megeed, A.; Ben Hifaa, A.B. Effect of Some Growth Regulators, Nutrient Elements and Kaolin on Cracking and Fruit Quality of Pomegranate ‘Wonderful’ Cultivar. J. Adv. Agric. Res. 2019, 24, 280–297. [Google Scholar] [CrossRef]
- Merwad, M.; Eisa, R.; Merwad, A.M.M. Effect of GA3 and some nutrients on pomegranate under South Sinai Governorate conditions. Int. J. Chemtech Res. 2016, 9, 104–113. [Google Scholar]
- Khalil, H.A. Improved yield, fruit quality, and shelf life in ‘Flame Seedless’ grapevine with pre-harvest foliar applications of forchlorfenuron, gibberellic acid, and abscisic acid. J. Hortic. Res. 2020, 28, 77–86. [Google Scholar] [CrossRef]
- Xu, Y.; Hou, X.; Feng, J.; Khalil-Ur-Rehman, M.; Tao, J. Transcriptome sequencing analyses reveals mechanisms of eliminated russet by applying GA3 and CPPU on ‘Shine Muscat’ grape. Sci. Hortic. 2019, 250, 94–103. [Google Scholar] [CrossRef]
- Askarieh, A.; Suleiman, S.; Tawakalna, M. Sweet Cherry (Prunus avium L.) Fruit Drop Reduction by Plant Growth Regulators (Naphthalene Acetic Acid NAA and Gibberellic Acid GA3). Am. J. Plant Sci. 2021, 12, 1338–1346. [Google Scholar] [CrossRef]
- Mikkelsen, R.L. Humic materials for agriculture. Better Crops 2005, 89, 6–10. [Google Scholar]
- Bakry, M.A.; Soliman, Y.R.; Moussa, S.A.M. Importance of micronutrients, organic manure and biofertilizer for improving maize yield and its components grown in desert sandy soil. Res. J. Agric. Biol. Sci. 2009, 5, 16–23. [Google Scholar]
- Cavalcante, I.; Silva-Matos, R.; Albano, F.; Silva Junior, G.; Silva, A.; Soares da Costa, L. Foliar spray of humic substances on seedling production of yellow passion fruit. J. Food Agric. Environ. 2013, 11, 301–304. [Google Scholar]
- Paksoy, M.; Türkmen, Ö.; Dursun, A. Effects of potassium and humic acid on emergence, growth and nutrient contents of okra (Abelmoschus esculentus L.) seedling under saline soil conditions. Afr. J. Biotechnol. 2010, 9, 5343–5346. [Google Scholar]
- Nardi, S.; Tosoni, M.; Pizzeghello, D.; Provenzano, M.; Cilenti, A.; Sturaro, A.; Rella, R.; Vianello, A. Chemical characteristics and biological activity of organic substances extracted from soils by root exudates. Soil Sci. Soc. Am. J. 2005, 69, 2012–2019. [Google Scholar] [CrossRef]
- Tarantino, A.; Lops, F.; Disciglio, G.; Lopriore, G. Effects of plant biostimulants on fruit set, growth, yield and fruit quality attributes of ‘Orange rubis®’ apricot (Prunus armeniaca L.) cultivar in two consecutive years. Sci. Hortic. 2018, 239, 26–34. [Google Scholar] [CrossRef]
- Shalan, A.M. Effect of bio-stimulant and soil amendment on vegetative growth, yield and fruit quality of Pyrus communis cv.’Le Conte’Pear trees. J. Plant Prod. Sci. 2014, 5, 1973–1987. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Samavat, S.; Mostafavi, M.; Khalighi, A.; Cherati, A. The effects of proline and humic acid on quantitative properties of kiwi fruit. Int. Res. J. Appl. Basic Sci. 2013, 6, 1117–1119. [Google Scholar]
- Da Rocha, L.F.; Cavalcante, L.F.; Nunes, J.C.; de Luna Souto, A.G.; Cavalcante, A.C.P.; Cavalcante, Í.H.L.; Pereira, W.E. Fruit production and quality of guava ‘Paluma’as a function of humic substances and soil mulching. Afr. J. Biotechnol. 2016, 15, 1962–1969. [Google Scholar]
- Harhash, M.M.; Saad, R.M.; Mosa, W.F.A. Response of “Wonderful” pomegranate cultivar to the foliar application of some biostimulants. Plant Arch. 2021, 21, 474–487. [Google Scholar] [CrossRef]
- Sanjari, M.; Siroosmehr, A.; Fakheri, B. The effects of drought stress and humic acid on some physiological characteristics of roselle. J. Crop Improv. 2015, 17, 403–414. [Google Scholar] [CrossRef]
- Mohamadineia, G.; Farahi, M.H.; Dastyaran, M. Foliar and soil drench application of humic acid on yield and berry properties of ‘Askari’ grapevine. Agric. Commun. 2015, 3, 21–27. [Google Scholar]
- Shaaban, F.K.; Morsey, M.M.; Mahmoud Thanaa, S.M. Influence of spraying yeast extract and humic acid on fruit maturity stage of canino apricot fruits. Int. J. Chemtech. Res. 2015, 8, 530–543. [Google Scholar]
- El-Hoseiny, H.M.; Helaly, M.N.; Elsheery, N.I.; Alam-Eldein, S.M.J.H. Humic acid and boron to minimize the incidence of alternate bearing and improve the productivity and fruit quality of mango trees. HortScience 2020, 55, 1026–1037. [Google Scholar] [CrossRef]
- Demirsoy, H.J.F. Leaf area estimation in some species of fruit tree by using models as a non-destructive method. Fruits 2009, 64, 45–51. [Google Scholar] [CrossRef]
- Ali, M.M.; Anwar, R.; Malik, A.U.; Khan, A.S.; Ahmad, S.; Hussain, Z.; Hasan, M.U.; Nasir, M.; Chen, F. Plant growth and fruit quality response of strawberry is improved after exogenous application of 24-epibrassinolide. J. Plant Growth Regul. 2021, 41, 1786–1799. [Google Scholar] [CrossRef]
- Wang, H.; Pampati, N.; McCormick, W.M.; Bhattacharyya, L. Protein nitrogen determination by kjeldahl digestion and ion chromatography. J. Pharm. Sci. 2016, 105, 1851–1857. [Google Scholar] [CrossRef]
- Bowden, M.; Diamond, D.J.S.; Chemical, A.B. The determination of phosphorus in a microfluidic manifold demonstrating long-term reagent lifetime and chemical stability utilising a colorimetric method. Sens. Actuators B: Chem. 2003, 90, 170–174. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of soil, plant, and water analysis: A manual for the West Asia and North Africa region. Int. Cent. Agric. Res. Dry Areas (ICARDA) 2013, 134299328. [Google Scholar]
- Ali, M.M.; Li, B.; Zhi, C.; Yousef, A.F.; Chen, F. Foliar-Supplied Molybdenum Improves Phyto-Nutritional Composition of Leaves and Fruits of Loquat (Eriobotrya japonica Lindl.). Agronomy 2021, 11, 892. [Google Scholar] [CrossRef]
- Ali, M.M.; Anwar, R.; Shafique, M.W.; Yousef, A.F.; Chen, F.J.A. Exogenous Application of Mg, Zn and B Influences Phyto-Nutritional Composition of Leaves and Fruits of Loquat (Eriobotrya japonica Lindl.). Agronomy 2021, 11, 224. [Google Scholar] [CrossRef]
- Ali, M.M.; Rizwan, H.M.; Yousef, A.F.; Zhi, C.; Chen, F. Analysis of toxic elements in leaves and fruits of loquat by inductively coupled plasma-mass spectrometry (ICP-MS). Acta Sci. Pol. Hortoru. 2021, 20, 33–42. [Google Scholar] [CrossRef]
- Mansour, A.; Ahmed, F.; Shaaban, E.; Amera, A.F. The beneficial of using citric acid with some nutrients for improving productivity of Le-Conte pear trees. Res. J. Agric. Biol. Sci. 2008, 4, 245–250. [Google Scholar]
- El-Badawy, H. Effect of some antioxidants and micronutrients on growth, leaf mineral content, yield and fruit quality of Canino apricot trees. J. Appl. Sci. Res. 2013, 9, 1228–1237. [Google Scholar]
- Shakoor, M.B.; Ali, S.; Hameed, A.; Farid, M.; Hussain, S.; Yasmeen, T.; Najeeb, U.; Bharwana, S.A.; Abbasi, G.H. Citric acid improves lead (Pb) phytoextraction in Brassica napus L. by mitigating Pb-induced morphological and biochemical damages. Ecotoxicol. Environ. Saf. 2014, 109, 38–47. [Google Scholar] [CrossRef]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 11679–11689. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Abbas, F.; Bukhari, S.A.H.; Saeed, R.; Wu, L. Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotoxicol. Environ. Saf. 2017, 145, 90–102. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Gu, J.; Zhao, J.; Fu, J. Citric acid and EDTA on the growth, photosynthetic properties and heavy metal accumulation of Iris halophila Pall. cultivated in Pb mine tailings. Int. Biodeterior. Biodegrad. 2018, 128, 15–21. [Google Scholar] [CrossRef]
- Osama, H.; Amro, E.; Salama Saber, M.M.B. Effect of growth regulator, antioxidant and application date on fruiting and fruit quality of mango trees cv. Keitt. J. Agric. Vet. Sci. 2015, 8, 87–95. [Google Scholar] [CrossRef]
- Mohamed, H.M. Effect of spraying citric acid macro and micro nutrients on yield and berries quality of Red Globe Grapevines. J. Hortic. Sci. Ornam. Plants 2018, 10, 53–59. [Google Scholar] [CrossRef]
- Iqbal, M.; Khan, M.Q.; Rehman, K.; Munir, M. Effect of foliar application of NAA on fruit drop, yield and physico-chemical characteristics of guava (Psidium guajava L.) red flesh cultivar. J. Agric. Res. 2009, 47, 03681157. [Google Scholar]
- Moneruzzaman, K.M.; Hossain, A.; Normaniza, O.; Boyce, A.N. Growth, yield and quality responses to gibberellic acid (GA3) of Wax apple Syzygium samarangense var. Jambu air madu fruits grown under field conditions. Afr. J. Biotechnol. 2011, 10, 11911–11918. [Google Scholar] [CrossRef]
- Nkansah, G.; Ofosu-Anim, J.; Mawuli, A. Gibberellic acid and naphthalene acetic acid affect fruit retention, yield and quality of Keitt mangoes in the coastal savanna ecological zone of Ghana. Am. J. Plant Physiol. 2012, 7, 243–251. [Google Scholar] [CrossRef]
- Khajehyar, R.; Rahemi, M.; Fallahi, E. The impact of various rates and dates of gibberellic acid applications on fruit set in apricot. Inter. J. Fruit Sci. 2015, 15, 324–338. [Google Scholar] [CrossRef]
- Mosa, W.; Abd EL-Megeed, N.; SasPaszt, L. The effect of the foliar application of potassium, calcium, boron and humic acid on vegetative growth, fruit set, leaf mineral, yield and fruit quality of ’Anna’ apple trees. J. Exp. Agric. Int. 2015, 8, 224–234. [Google Scholar] [CrossRef]
- Beerappa, S.; Hipparagi, K.; Patil, D.; Suma, R.; Biradar, I. Effect of foliar application of gibberellic acid (GA3) and nutrients on yield and quality of pomegranate (Punica granatum L.) cv. Bhagwa. Int. J. Chem. Stud. 2019, 7, 2579–2584. [Google Scholar]
- Al-Doori, M.F.; Medan, R.A.; Hussein, S.A. Effect of Spray with some growth regulators and Zinc on growth and Yield of Pear Trees (Pyrus communis L.) CV. LE-CONTE. Int. J. Agricult. Stat. Sci. 2020, 16, 1181–1186. [Google Scholar]
- Ferrara, G.; Brunetti, G. Effects of the times of application of a soil humic acid on berry quality of table grape (Vitis vinifera L.) cv Italia. Span. J. Agric. Res. 2010, 8, 817–822. [Google Scholar] [CrossRef]
- De Castro, T.A.v.T.; Berbara, R.L.L.; Tavares, O.C.H.; da Graça Mello, D.F.; Pereira, E.G.; de Souza, C.d.C.B.; Espinosa, L.M.; García, A.C. Humic acids induce a eustress state via photosynthesis and nitrogen metabolism leading to a root growth improvement in rice plants. Plant Physiol. Biochem. 2021, 162, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Olaetxea, M.; Mora, V.; Baigorri, R.; Zamarreño, A.M.; García-Mina, J.M. The Singular molecular conformation of humic acids in solution influences their ability to enhance root hydraulic conductivity and plant growth. Molecules 2020, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Bulut, F.; Akinci, S. The effect of salinity on growth and nutrient composition in broad bean (Vicia faba L.) seedlings. Fresen. Environ. Bull. 2010, 19, 2901–2910. [Google Scholar]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A meta-analysis and review of plant-growth response to humic substances: Practical implications for agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar] [CrossRef]
- Nargesi, M.M.; Sedaghathoor, S.; Hashemabadi, D. Effect of foliar application of amino acid, humic acid and fulvic acid on the oil content and quality of olive. Saudi J. Biol. Sci. 2022, 29, 3473–3481. [Google Scholar] [CrossRef]
- Fahramand, M.; Moradi, H.; Noori, M.; Sobhkhizi, A.; Adibian, M.; Abdollahi, S.; Rigi, K. Influence of humic acid on increase yield of plants and soil properties. Int. J. Farming Allied Sci. 2014, 3, 339–341. [Google Scholar]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Zhou, L.; Mi, J.; McLaughlin, N.B.; Liu, J. Effect of synthetic and natural water absorbing soil amendments on soil microbiological parameters under potato production in a semi-arid region. J. Sci. Food Agric. 2016, 96, 1010–1017. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Alsamadany, H.; Hamooh, B.T.; Mujtaba, T.; Daur, I.; Al Zahrani, Y.; Alzahrani, H.A.; Ali, S. Humic substances: Determining potential molecular regulatory processes in plants. Front. Plant Sci. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Canellas, N.O.; Irineu, L.E.S.d.S.; Olivares, F.L.; Piccolo, A. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 2020, 7, 12. [Google Scholar] [CrossRef]
- Laskosky, J.D.; Mante, A.A.; Zvomuya, F.; Amarakoon, I.; Leskiw, L. Geosciences; Environment. A bioassay of long-term stockpiled salvaged soil amended with biochar, peat, and humalite. Agrosyst. Geosci. Environ. 2020, 3, e20068. [Google Scholar] [CrossRef]
- Sible, C.N.; Seebauer, J.R.; Below, F.E.J.A. Plant biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Taha, N.M.; El-Shahat, R. Influence of Azolla, Some Blue Green Algae Strains and Humic Acid on Soil, Growth, Productivity, Fruit Quality and Storability of “Canino” Apricot Cultivar Grown Under Clay Loamy Soil. J. Plant Prod. Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Trevisan, S.; Botton, A.; Vaccaro, S.; Vezzaro, A.; Quaggiotti, S.; Nardi, S. Humic substances affect Arabidopsis physiology by altering the expression of genes involved in primary metabolism, growth and development. Environ. Exp. Bot. 2011, 74, 45–55. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).