Impact of Cold Stress on Leaf Structure, Photosynthesis, and Metabolites in Camellia weiningensis and C. oleifera Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
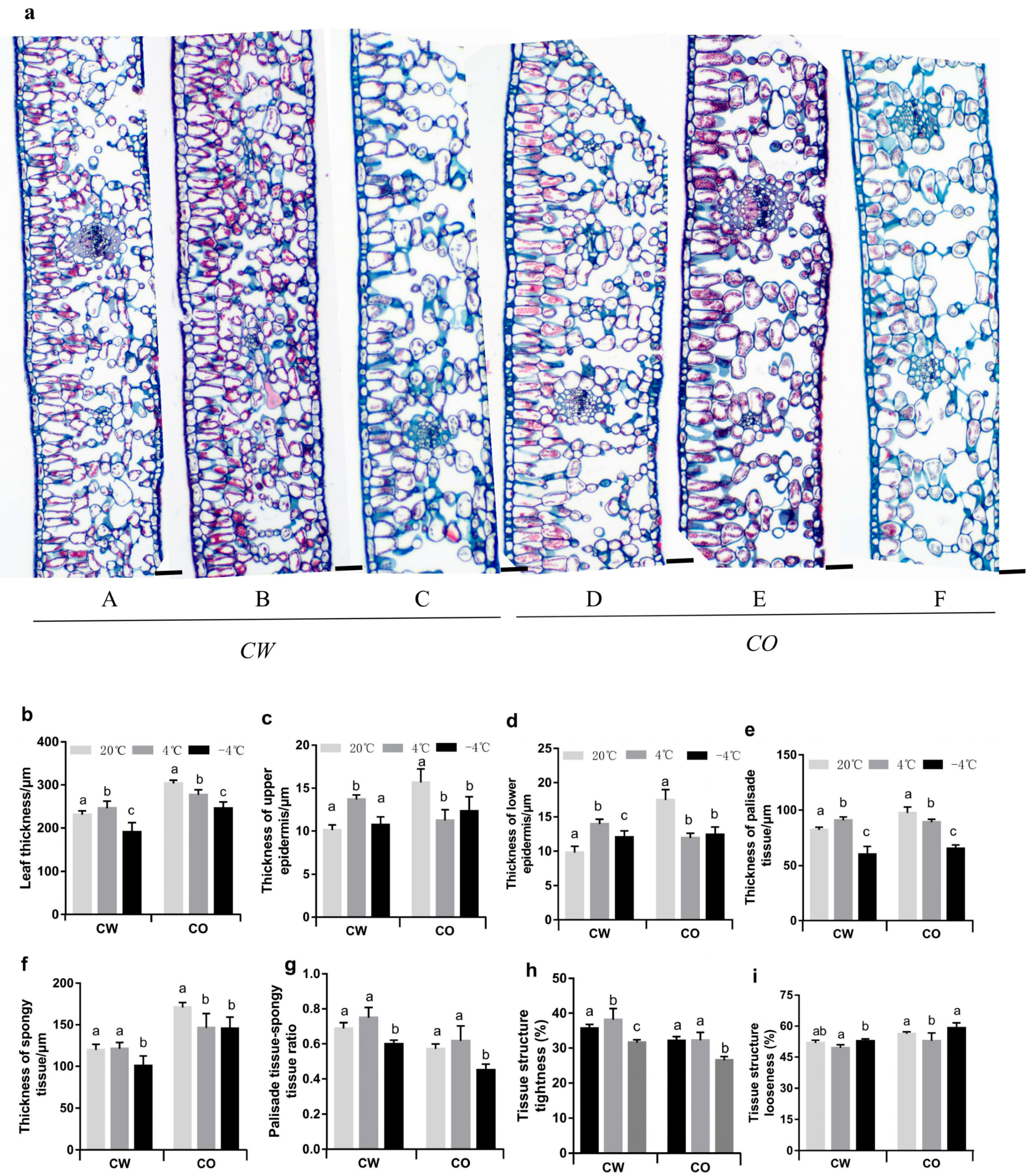
2.2. Studies on the Anatomical Structure of Leaves
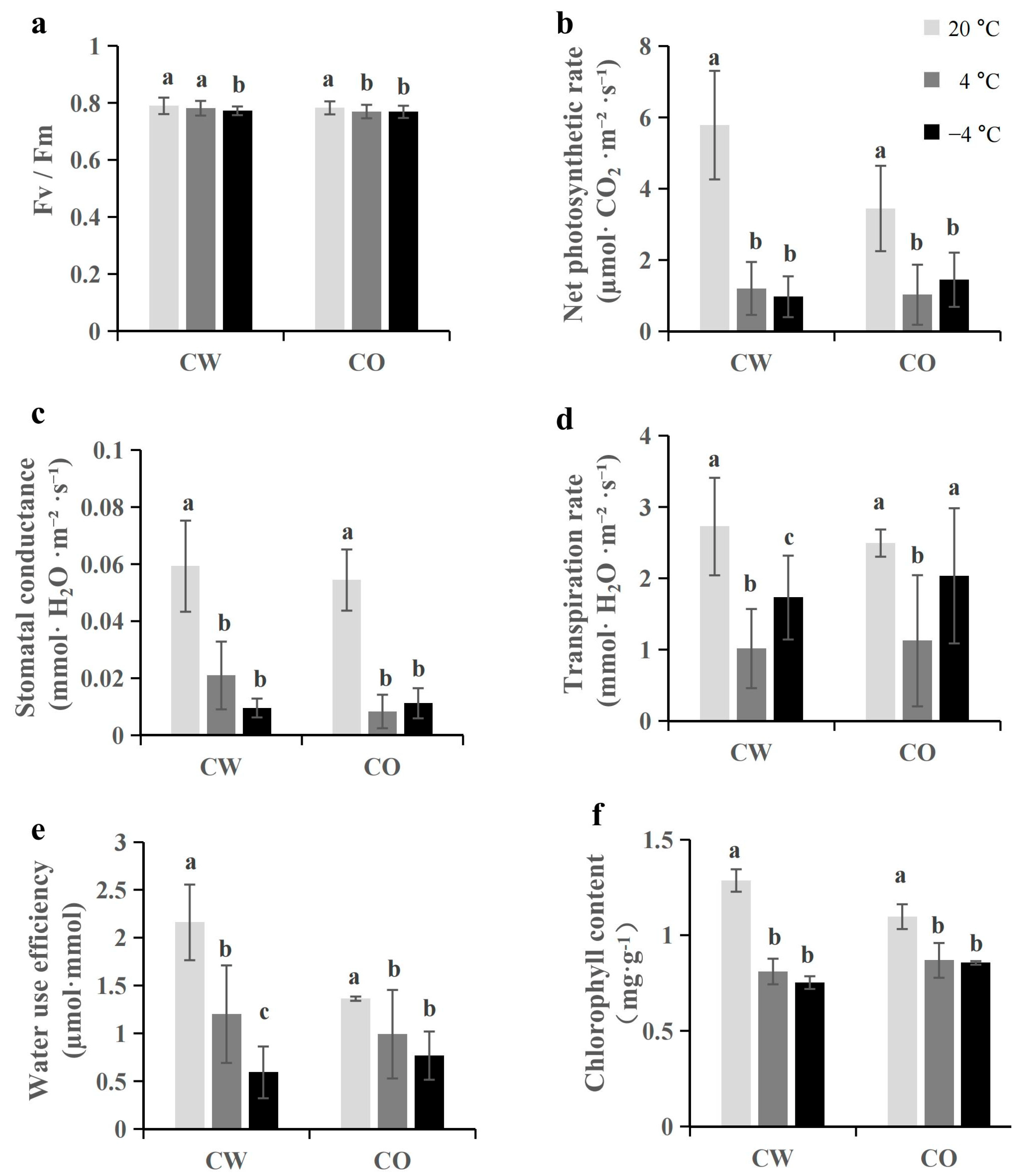
2.3. Measurement of Photosynthetic Parameters
2.4. Measurement of Chlorophyll Fluorescence and Chlorophyll Content
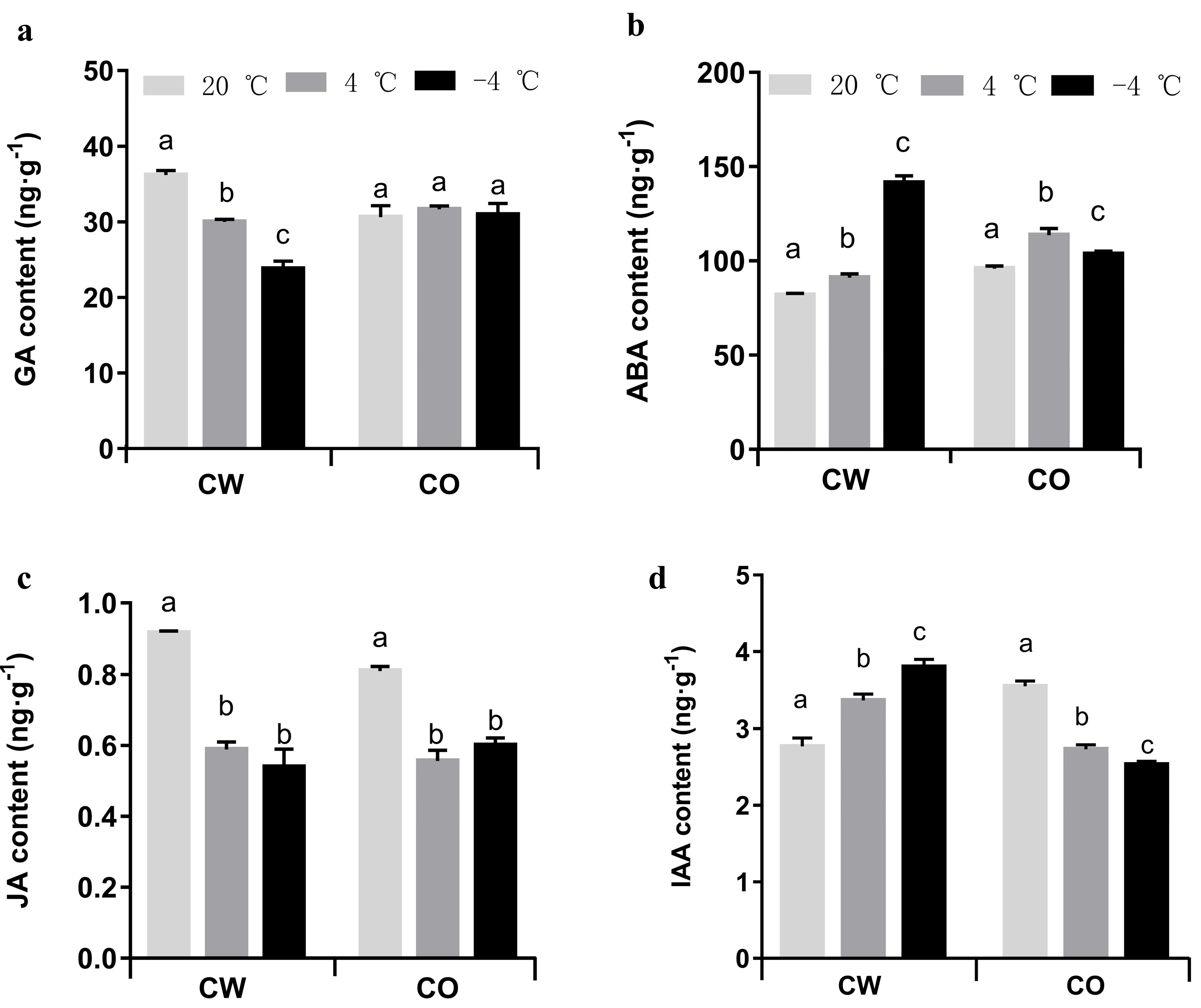
2.5. Quantitation of Plant Hormones
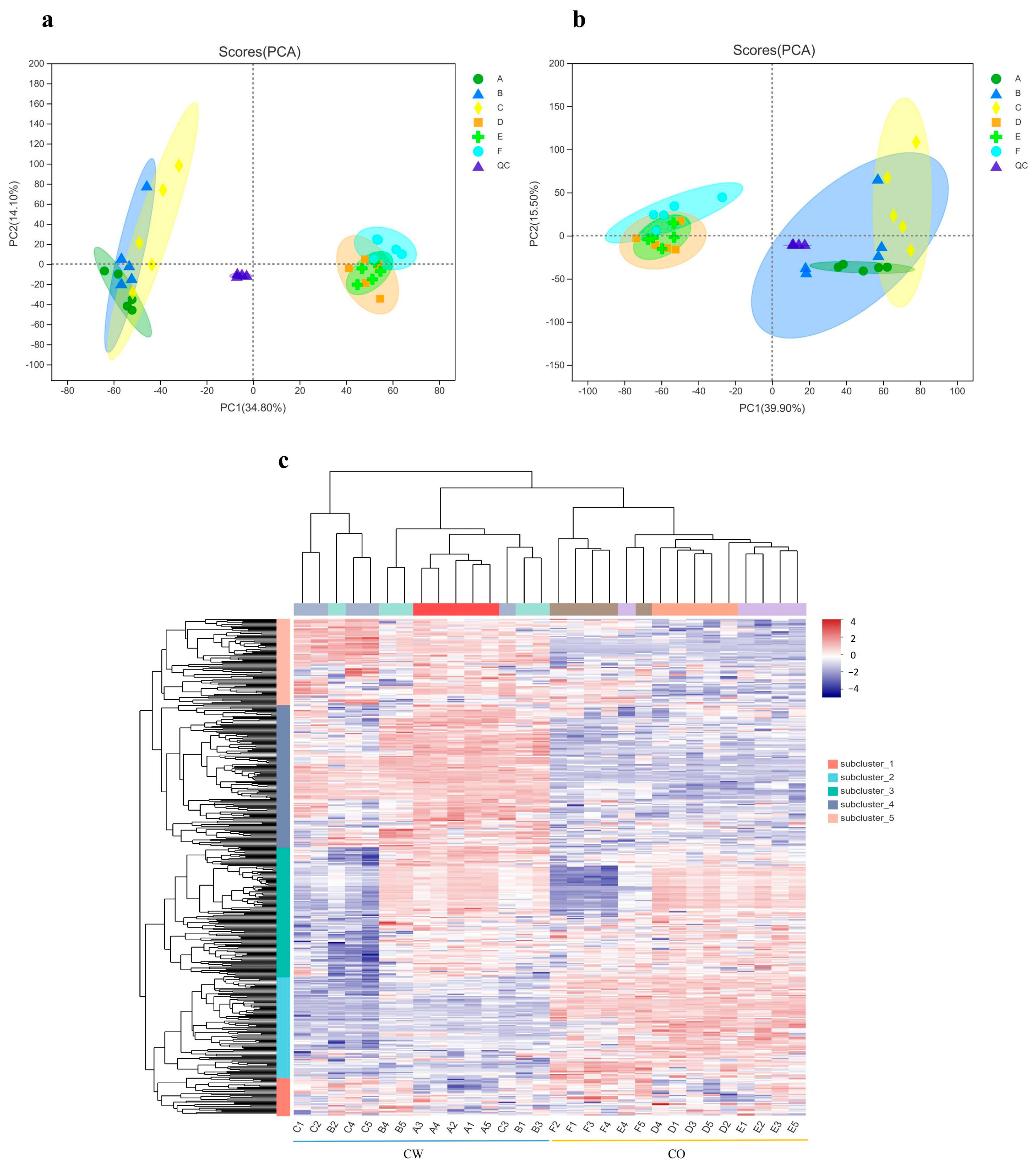
2.6. Untargeted Metabolomic Analysis
2.7. Separation of Metabolites and Data Analysis
2.8. Statistical Analysis
3. Results
3.1. Changes in Anatomical Features of Leaves in the Two Camellia Species under Cold Stress
3.2. Cold Stress Reduces Fv/Fm Value and Photosynthesis
3.3. Quantitation of Plant Hormones
3.4. Overview of Metabolite Profiling
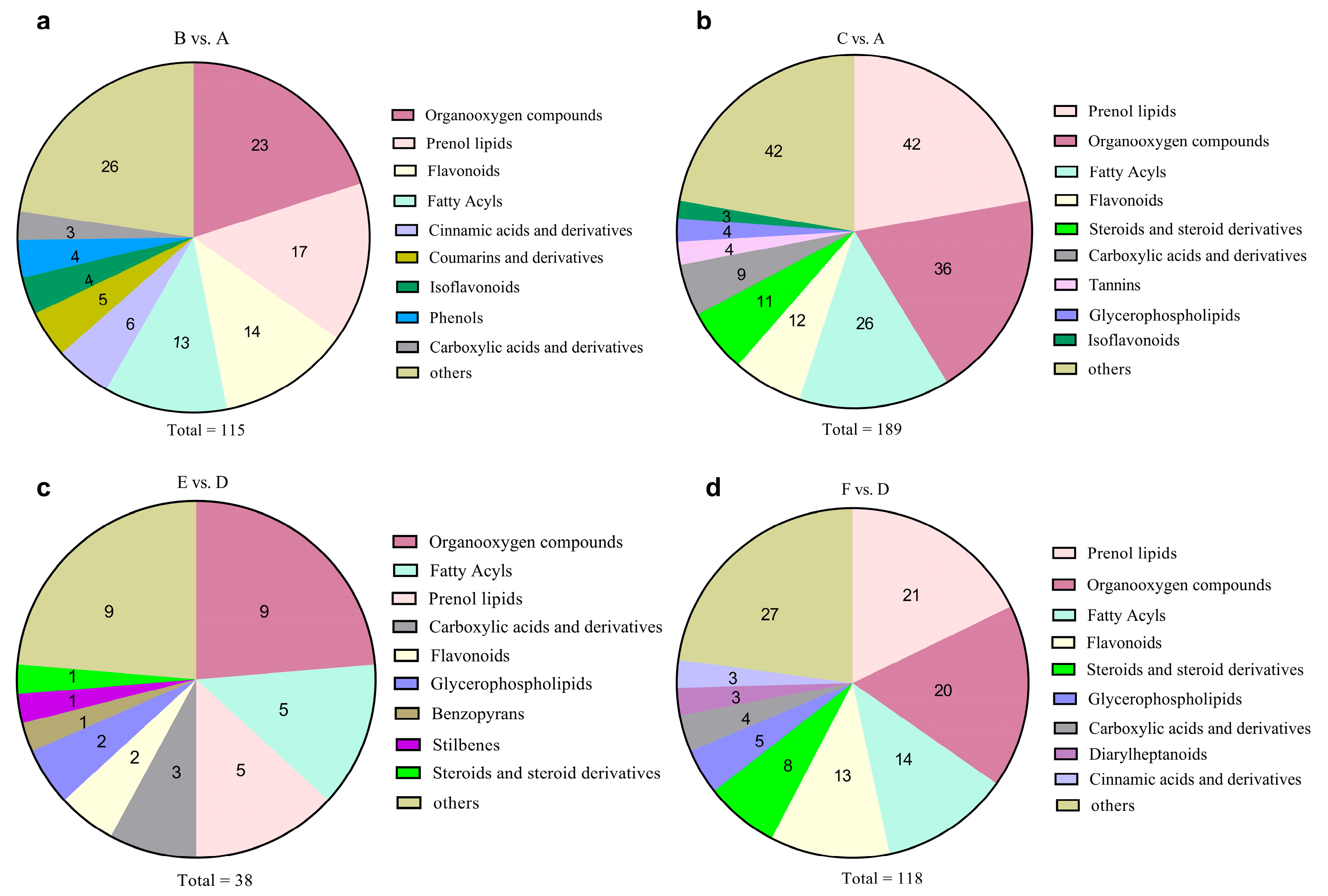
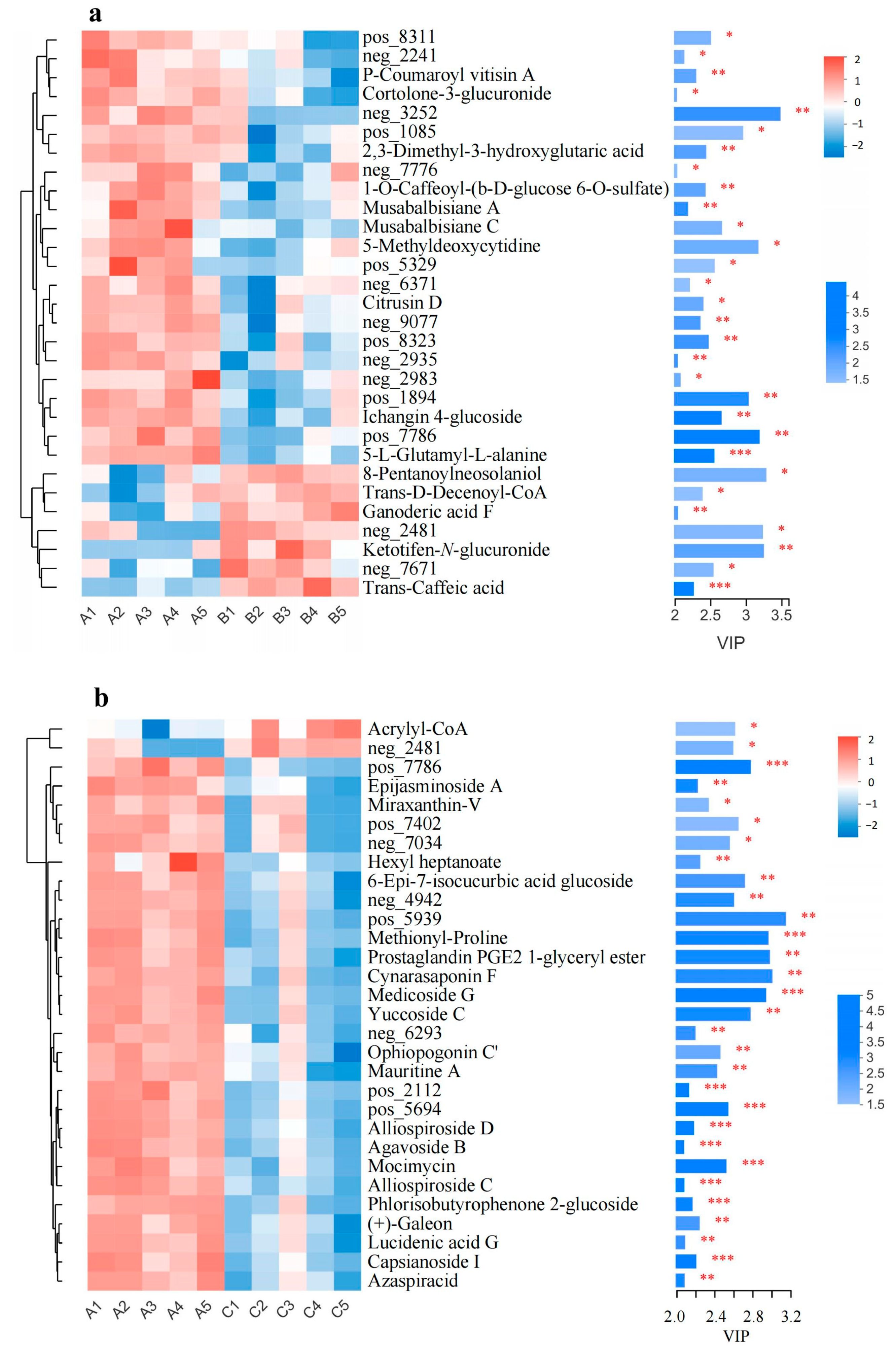
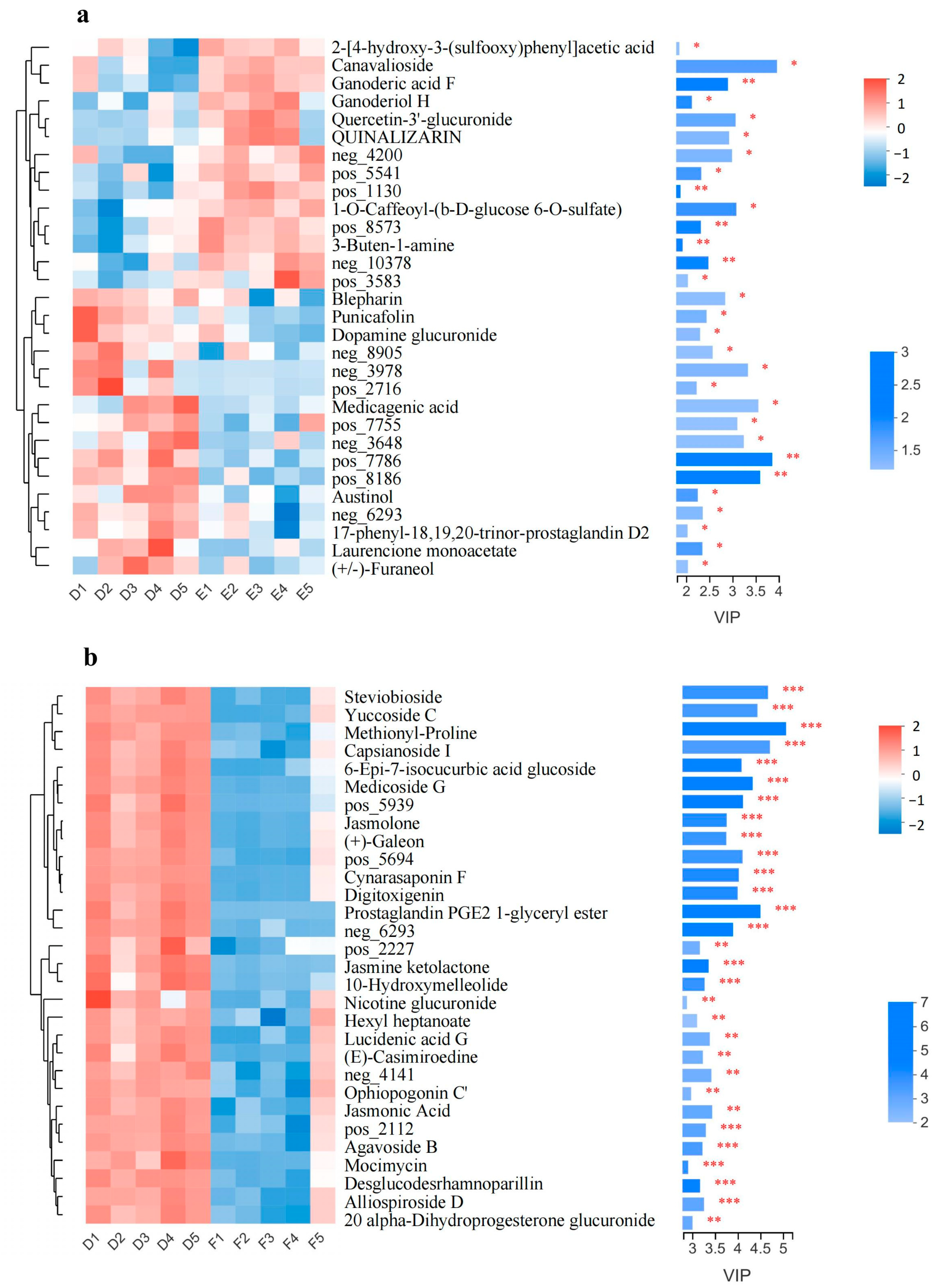
3.5. Significantly Changed Metabolites under Cold Stress
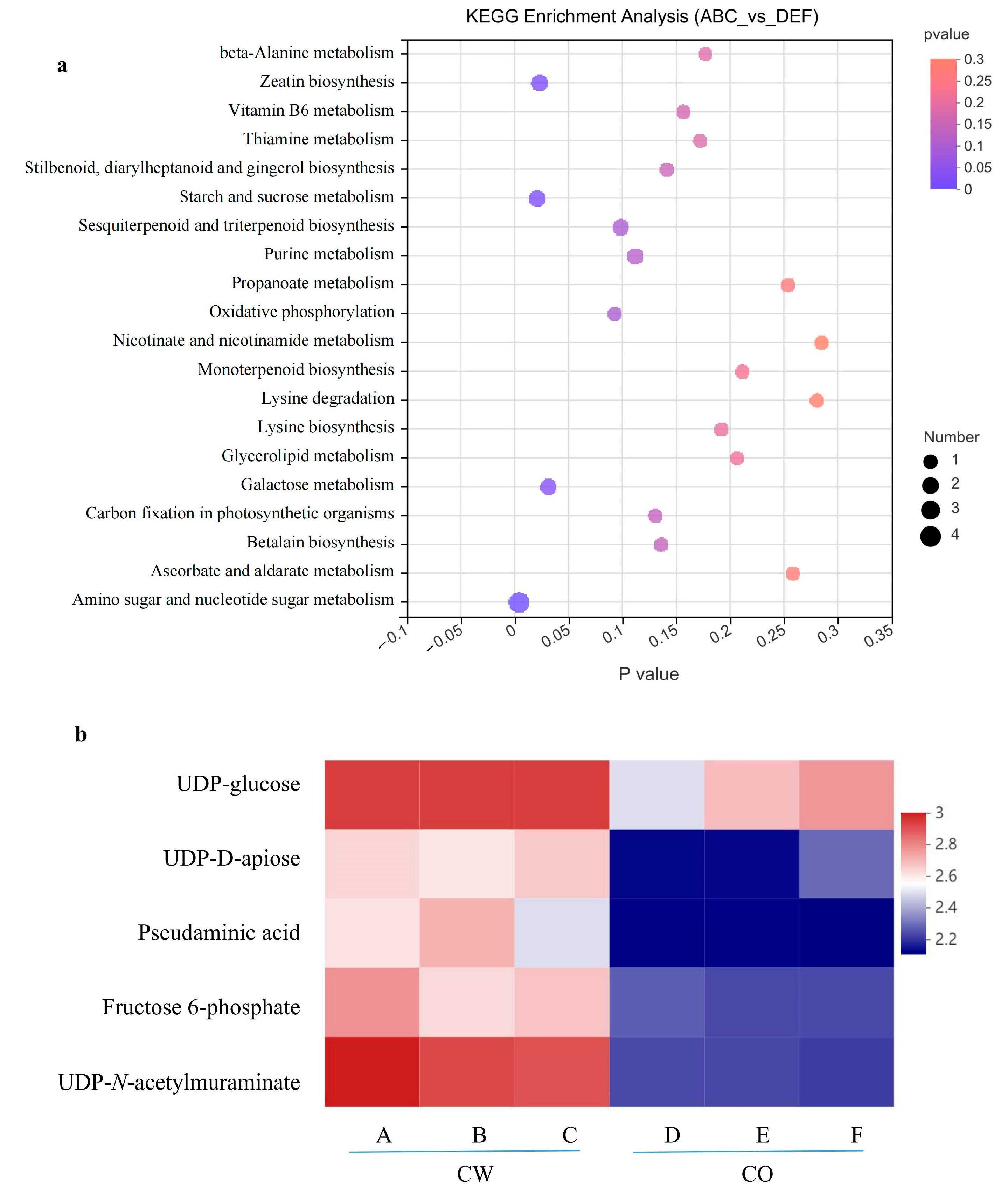
3.6. Metabolic Pathway Analysis of Metabolites in CW and CO Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, Q.; Li, J.; Gao, C.; Cheng, J.; Deng, X.; Jiang, D.; Li, L.; Yan, P. New perspective for evaluating the main Camellia oleifera cultivars in China. Sci. Rep. 2020, 10, 20676. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wu, G.; Jin, Q.; Wang, X. Camellia oil authentication: A comparative analysis and recent analytical techniques developed for its assessment. A review. Trends Food Sci. Technol. 2020, 97, 88–99. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Li, Z.; Zhang, F.; Tan, X. Transcriptomic analyses of Camellia oleifera ‘Huaxin’ leaf reveal candidate genes related to long-term cold stress. Int. J. Mol. Sci. 2020, 21, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, J.B.; Xu, J.; Wang, J.; Qiu, J.S. Resource Survey on Camellia weiningensis. Y. K. Li. sP. nov in Weining County, Guizhou Province and Utilization Status. J. Anhui. Agric. Sci. 2019, 47, 128–131. (In Chinese) [Google Scholar]
- Cao, H.X.; Huang, H.J.; Lei, X.T.; Zhang, D.P.; Zhang, R.L.; Sun, C.X. The effect of different low temperature treatment on the anatomical structure of oil palm leaves. Chin. J. Trop Crop. 2014, 35, 454–459. [Google Scholar]
- Kaur, R.; Kaur, N.; Singh, H. Pericarp and pedicel anatomy in relation to fruit cracking in lemon (Citrus limon, L. Burm.). Sci. Hortic. 2019, 246, 462–468. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, K.; Gu, Y.; Zhang, L.; Li, W.; Li, Z. Effects of Low-Temperature Stress and Brassinolide Application on the Photosynthesis and Leaf Structure of Tung Tree Seedlings. Front. Plant Sci. 2020, 10, 1767. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Deng, Q.; Li, J.; CHeng, J.; Deng, X.; Jiang, D. Relationship between leaf anatomic structure and cold tolerance in six Camellia oleifera cultivars. Non-Wood For. Res. 2020, 38, 117–124. (In Chinese) [Google Scholar]
- Wu, L.; Li, J.; Gu, Y.; Zhang, F.; Gu, L.; Tan, X.; Shi, M. Effect of chilling temperature on chlorophyll florescence, leaf anatomical structure, and physiological and biochemical characteristics of two Camellia oleifera cultivars. Int. J. Agric. Biol. 2020, 23, 777–785. [Google Scholar]
- Ploschuk, E.L.; Bado, L.A.; Salinas, M.; Wassner, D.F.; Windauer, L.B.; Insausti, P. Photosynthesis and fluorescence responses of jatropha curcas to chilling and freezing stress during early vegetative stages. Environ. Exp. Bot. 2014, 102, 18–26. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Q.; Chen, Y.; Sun, Y.; Zhang, L. Effects of Sub-chilling Stress in Spring on Photosynthetic Physiological Characteristics of Camellia Oleifera ‘Huashuo’ Leaves. J. Southwest For. Univ. 2021, 41, 1–8. (In Chinese) [Google Scholar]
- Wu, L.; Li, J.; Wang, N.; Gu, Y.; Zhang, F.; Tan, X. The effects of low temperature stress on the flowering, fruiting and physiological characteristics of two Camellia oleifera cultivars. Plant. Physiol. J. 2020, 56, 681–692. (In Chinese) [Google Scholar]
- Xu, H.Y.; Yu, C. Transcriptomic analysis reveals crucial biological pathways associated with cold response in Camellia weiningensis in Guizhou Province, China. Sci. Hortic. 2022, 295, 110883. [Google Scholar] [CrossRef]
- Qu, X.; Wang, H.; Chen, M.; Liao, J.; Yuan, J.; Niu, G. Drought Stress–induced Physiological and Metabolic Changes in Leaves of Two Oil Tea Cultivars. J. Am. Soc. Hort. Sci. 2019, 144, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Qu, D.; Chen, X.; Zeng, H.; Li, X.; Hu, C.Y. Metabolomics Reveals 5-Aminolevulinic Acid Improved the Ability of Tea Leaves (Camellia sinensis L.) against Cold Stress. Metabolites 2022, 12, 392. [Google Scholar] [CrossRef]
- Janmohammadi, M. Metabolomic analysis of low temperature responses in plants. Curr. Opin. Agric. 2012, 1, 1–6. [Google Scholar]
- Ye, H.; Folz, J.; Li, C.; Zhang, Y.; Hou, Z.; Zhang, L.; Su, S. Response of metabolic and lipid synthesis gene expression changes in Camellia oleifera to mulched ecological mat under drought conditions. Sci. Total Environ. 2021, 795, 148856. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Hart, R.; Abd Allah, E.F.; Alqarawi, A.A.; Alsubeie, M.S.; Calixto, E.S.; Ijaz, F.; Ali, N.; et al. Response of plant physiological attributes to altitudinal gradient: Plant adaptation to temperature variation in the Himalayan region. Sci. Total Environ. 2020, 706, 135714. [Google Scholar] [CrossRef]
- Tsegay, G.; Redi-Abshiro, M.; Chandravanshi, B.S.; Estifanos Ele, E.; Mohammed, A.M.; Mamo, H. Effect of altitude of coffee plants on the composition of fatty acids of green coffee beans. BMC Chem. 2020, 14, 36. [Google Scholar] [CrossRef]
- Shahandashti, S.S.K.; Amiri, R.M.; Zeinali, H.; Ramezanpour, S.S. Change in membrane fatty acid compositions and cold-induced responses in chickpea. Mol. Biol. Rep. 2013, 40, 893–903. [Google Scholar] [CrossRef]
- Ruzin, B.S.E. Plant microtechnique and microscopy. New Phytol. 2000, 148, 57–58. [Google Scholar]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Et. Biophys. Acta. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A. Determination of total carotenoids and chlorophylls a and b of leaf in extracts in different solvents. Biochem. Soc. Trans. 1983, 603, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhao, Y.; Yang, C.; Shi, H. The combination of transcriptome and metabolome reveals the molecular mechanism by which topping and salicylic acid treatment affect the synthesis of alkaloids in Nicotiana tabacum L. All Life 2022, 15, 147–159. [Google Scholar] [CrossRef]
- Wang, P.J.; Chen, S.; Gu, M.Y.; Chen, X.M.; Chen, X.J.; Yang, J.F.; Zhao, F.; Ye, N.X. Exploration of the Effects of Different Blue LED Light Intensities on Flavonoid and Lipid Metabolism in Tea Plants via Transcriptomics and Metabolomics. Int. J. Mol. Sci. 2020, 21, 4606. [Google Scholar] [CrossRef]
- Bach, K.A. Do Elevation and Aspect Predict Functional Trait Values in Alpine Plant Populations? California Polytechnic State University: San Luis Obispo, CA, USA, 2019. [Google Scholar]
- Zhang, W.J.; Huang, Z.L.; Wang, Q.; Guan, Y.N. Effects of low temperature on leaf anatomy and photosynthetic performance in different genotypes of wheat following a rice crop. Int. J. Agric. Biol. 2015, 17, 1165–1171. [Google Scholar]
- Yadav, S.K. Cold stress tolerance mechanisms in plants. Agron. Sustain. Dev. 2010, 303, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Mishra, K.B.; Höermiller, I.I.; Heyer, A.G.; Nedbal, L. Chlorophyll fluorescence emission as a reporter on cold tolerance in Arabidopsis thaliana accessions. Plant. Signal. Behav. 2011, 6, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Hajihashemi, S.; Noedoost, F.; Geuns, J.M.C.; Djalovic, I.; Siddique, K.H.M. Effect of Cold Stress on Photosynthetic Traits, Carbohydrates, Morphology, and Anatomy in Nine Cultivars of Stevia Rebaudiana. Front. Plant. Sci. 2018, 9, 1430. [Google Scholar] [CrossRef] [Green Version]
- Bayro-Kaiser, V.; Nelson, N. Temperature-sensitive PSII: A novel approach for sustained photosynthetic hydrogen production. Photosynth. Res. 2016, 130, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Zhang, X.; Fang, Z.; Wu, Y.; Tao, J. Physiological and Transcriptomic Analysis of Tree Peony (Paeonia section Moutan DC.) in Response to Drought Stress. Forests 2019, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ahammed, G.J.; Li, Z.X.; Zhang, L.; Wei, J.P.; Yan, P.; Zhang, L.P.; Han, W.Y. Freezing stress deteriorates tea quality of new flush by inducing photosynthetic inhibition and oxidative stress in mature leaves. Sci. Hortic. 2017, 230, 155–160. [Google Scholar] [CrossRef]
- Reinsberg, D.; Ottmann, K.; Booth, P.J.; Paulsen, H. Effects of chlorophyll a, chlorophyll b, and xanthophylls on the in vitro assembly kinetics of the major light-harvesting chlorophyll a/b complex, LHCIIb. J. Mol. Biol. 2001, 308, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, X.; Huangfu, L.; Shao, S.; Yi, C.D. Comparative analysis morphology, anatomical structure and transcriptional regulatory network of chlorophyll biosynthesis in Oryza longistaminata, O. sativa and their F 1 generation. Peer J. 2021, 9, e12099. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef]
- He, X.; Xu, L.; Pan, C.; Gong, C.; Wang, Y.; Liu, X.; Yu, Y. Drought resistance of Camellia oleifera under drought stress: Changes in physiology and growth characteristics. PLoS ONE 2020, 15, e0235795. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant. Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Oliver, S.N.; Dennis, E.S.; Dolferus, R. ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant. Cell Physiol. 2007, 48, 1319–1333. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Zhong, Q.; Yuan, T.; Cao, L.; Yan, C.; Yuan, Y. Effects of endogenous hormones on Camellia oleifera leaves and fruit growth under drought stress. For. Res. 2016, 29, 933–939. (In Chinese) [Google Scholar]
- Garbero, M.; Andrade, A.; Reinoso, H.; Fernández, B.; Cuesta, C.; Granda, V.; Escudero, C.; Abdala, G.; Pedranzani, H. Differential effect of short-term cold stress on growth, anatomy, and hormone levels in cold-sensitive versus -resistant cultivars of Digitaria eriantha. Acta Physiol. Plant. 2012, 34, 2079–2091. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Han, X.; Wang, H.; Pan, J.; Yu, D. Jasmonate regulates leaf senescence and tolerance to cold stress: Crosstalk with other phytohormones. J. Exp. Bot. 2017, 68, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Liu, Z.; Xing, M.; Yang, Y.; Wu, X.; Liu, H.; Liang, W. Heat Stress Suppresses Brassica napus Seed Oil Accumulation by Inhibition of Photosynthesis and BnWRI1 Pathway. Plant. Cell Physiol. 2019, 60, 1457–1470. [Google Scholar] [CrossRef]
- Wei, H.; Gao, C.; Qiu, J.; Long, L.; Wang, B.; Yang, L.; Hu, Y. Flowering Biological Characteristics of Camellia weiningensis Y.K. Li. Hortscience 2021, 56, 1331–1339. [Google Scholar] [CrossRef]
- Gutkowska, M.; Bienkowski, T.; Hung, V.S.; Wanke, M.; Hertel, J. Proteins are polyisoprenylated in Arabidopsis thaliana. Biochem. Biophys. Res. Comun. 2004, 322, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, Y.; Wang, M.; Hu, S.; Wu, J.; Yu, Z. Differences in lipid homeostasis and membrane lipid unsaturation confer differential tolerance to low temperatures in two Cycas species. BMC Plant Biol. 2020, 20, 15. [Google Scholar] [CrossRef]
- Bajda, A.; Chojnicki, T.; Hertel, J.; Swiezewska, E.; Wójcik, J.; Kaczkowska, A.; Marczewski, A.; Bojarczuk, T.; Karolewski, P.; Oleksyn, J. Light conditions alter accumulation of long chain polyprenols in leaves of trees and shrubs throughout the vegetation season. Acta Biochim. Pol. 2005, 52, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Basyuni, M.; Sagami, H.; Baba, S.; Putri, L.A.; Wati, R.; Oku, H. Salinity alters the polyisoprenoid alcohol content and composition of both salt-secreting and non-salt-secreting Mangrove seedlings. HAYATI J. Biosci. 2017, 24, 206–214. [Google Scholar] [CrossRef]
- Baczewska-Dąbrowska, A.H.; Dmuchowski, W.; Gozdowski, D.; Gworek, B.; Jozwiak, A.; Swiezewska, E.; Dąbrowski, P.; Suwara, I. The importance of prenol lipids in mitigating salt stress in the leaves of Tilia × euchlora. Trees 2022, 36, 393–404. [Google Scholar] [CrossRef]
- Xiang, L.; Zhu, L.; Huang, Y.; Cai, Z. Application of Derivatization in Fatty Acids and Fatty Acyls Detection: Mass Spectrometry-Based Targeted Lipidomics. Small Methods 2020, 4, 2000160. [Google Scholar] [CrossRef]
- Matteucci, M.; D’Angeli, S.; Errico, S.; Lamanna, R.; Perrotta, G.; Altamura, M.M. Cold affects the transcription of fatty acid desaturases and oil quality in the fruit of Olea europaea L. genotypes with different cold hardiness. J. Exp. Bot. 2011, 62, 3403–3420. [Google Scholar] [CrossRef] [Green Version]
- D’Angel, S.; Altamura, M.M. Unsaturated Lipids Change in Olive Tree Drupe and Seed during Fruit Development and in Response to Cold-Stress and Acclimation. Int. J. Mol. Sci. 2016, 17, 1889. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.F.; Morillon, R.; Anjum, M.A.; Ejaz, S.; Rao, M.J.; Ahmad, S.; Hussain, S. Volkamer Lemon Tetraploid Rootstock Transmits the Salt Tolerance When Grafted with Diploid Kinnow Mandarin by Strong Antioxidant Defense Mechanism and Efficient Osmotic Adjustment. J. Plant. Growth Regul. 2022, 41, 1125–1137. [Google Scholar] [CrossRef]
- Khalid, M.F.; Hussain, S.; Anjum, M.A.; Morillon, R.; Ahmad, S.; Ejaz, S.; Hussain, M.; Jaafar, H.Z.E.; Alrashood, S.T.; Ormenisan, A.N. Physiological and biochemical responses of Kinnow mandarin grafted on diploid and tetraploid Volkamer lemon rootstocks under different water-deficit regimes. PLoS ONE 2021, 16, e0247558. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Li, X.; Niu, L.; Jameson, P.E.; Zhou, W.B. Transcription-associated metabolomic adjustments in maize occur during combined drought and cold stress. Plant. Physiol. 2021, 186, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Wang, T.J.; Yu, X.H.; Yang, Y.; Wang, C.F.; Yang, Q.H.; Wang, X.H. Enhanced sugar accumulation and regulated plant hormone signalling genes contribute to cold tolerance in hypoploid Saccharum spontaneum. BMC Genom. 2020, 21, 507. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Huang, C.; Jiang, X.; Zhu, J.; Gao, X.; Yu, C. Impact of Cold Stress on Leaf Structure, Photosynthesis, and Metabolites in Camellia weiningensis and C. oleifera Seedlings. Horticulturae 2022, 8, 494. https://doi.org/10.3390/horticulturae8060494
Xu H, Huang C, Jiang X, Zhu J, Gao X, Yu C. Impact of Cold Stress on Leaf Structure, Photosynthesis, and Metabolites in Camellia weiningensis and C. oleifera Seedlings. Horticulturae. 2022; 8(6):494. https://doi.org/10.3390/horticulturae8060494
Chicago/Turabian StyleXu, Hongyun, Chengling Huang, Xian Jiang, Jing Zhu, Xiaoye Gao, and Cun Yu. 2022. "Impact of Cold Stress on Leaf Structure, Photosynthesis, and Metabolites in Camellia weiningensis and C. oleifera Seedlings" Horticulturae 8, no. 6: 494. https://doi.org/10.3390/horticulturae8060494
APA StyleXu, H., Huang, C., Jiang, X., Zhu, J., Gao, X., & Yu, C. (2022). Impact of Cold Stress on Leaf Structure, Photosynthesis, and Metabolites in Camellia weiningensis and C. oleifera Seedlings. Horticulturae, 8(6), 494. https://doi.org/10.3390/horticulturae8060494




