Calcium in Photosynthetic Restoration and Growth of Annona emarginata after Mechanical Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.1.1. Chlorophyll A Fluorescence, Gas Exchange, Biomass and Growth Index
2.1.2. Antioxidant Enzyme Activity, Hydrogen Peroxide Quantification and Lipid Peroxidation
2.1.3. Calcium Concentration in Plant Tissue
2.1.4. Statistical Analysis
3. Results
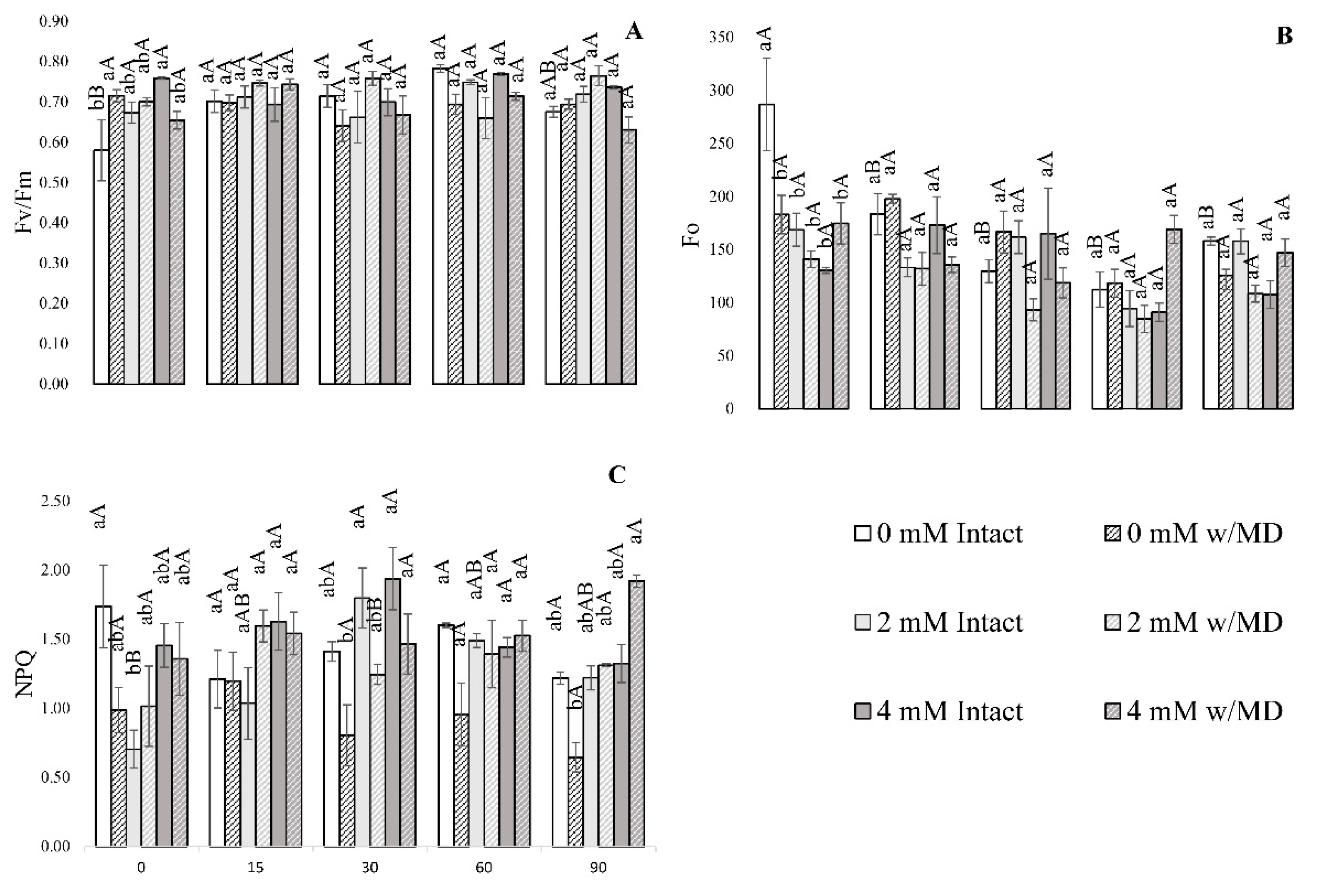
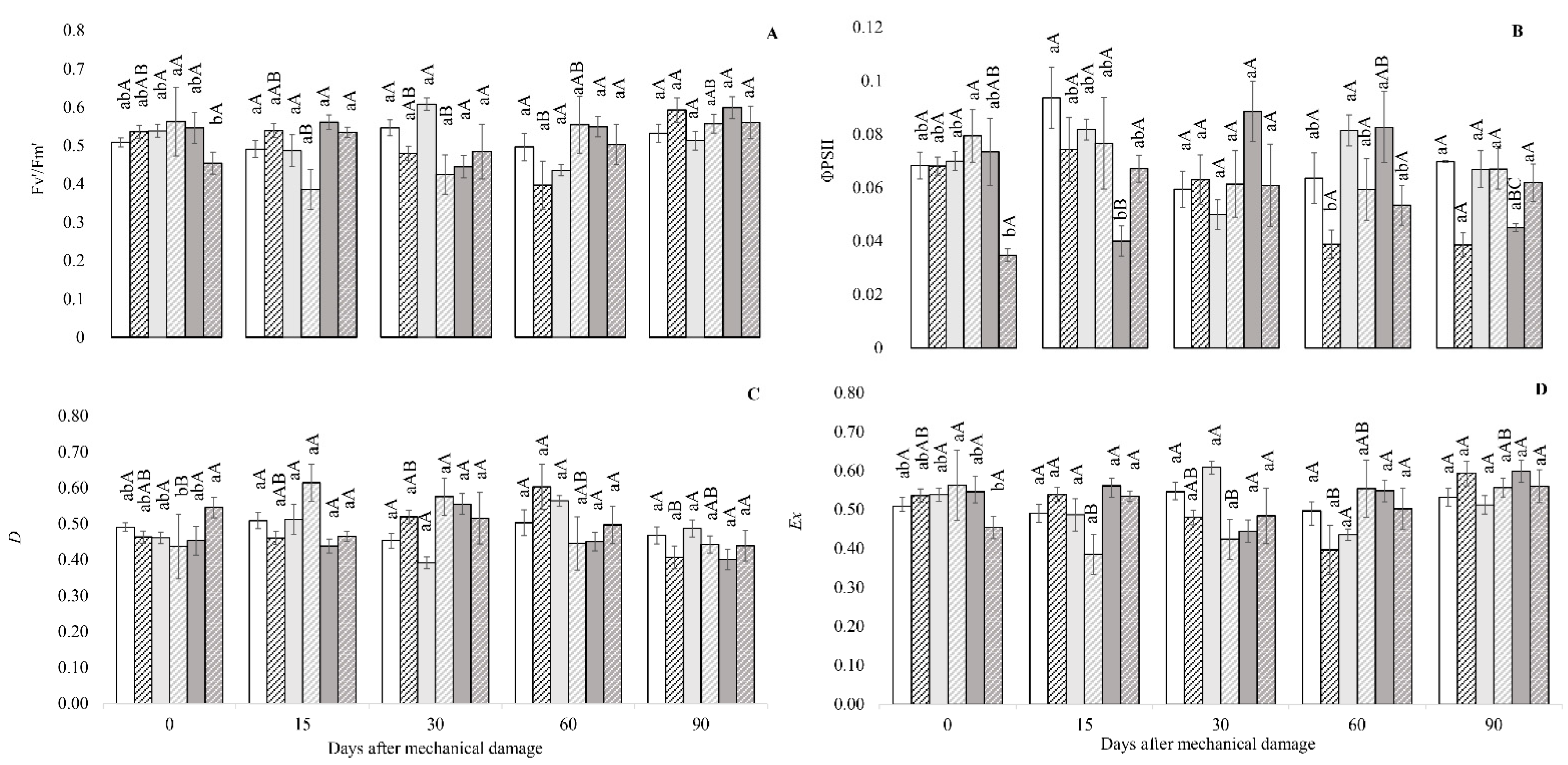
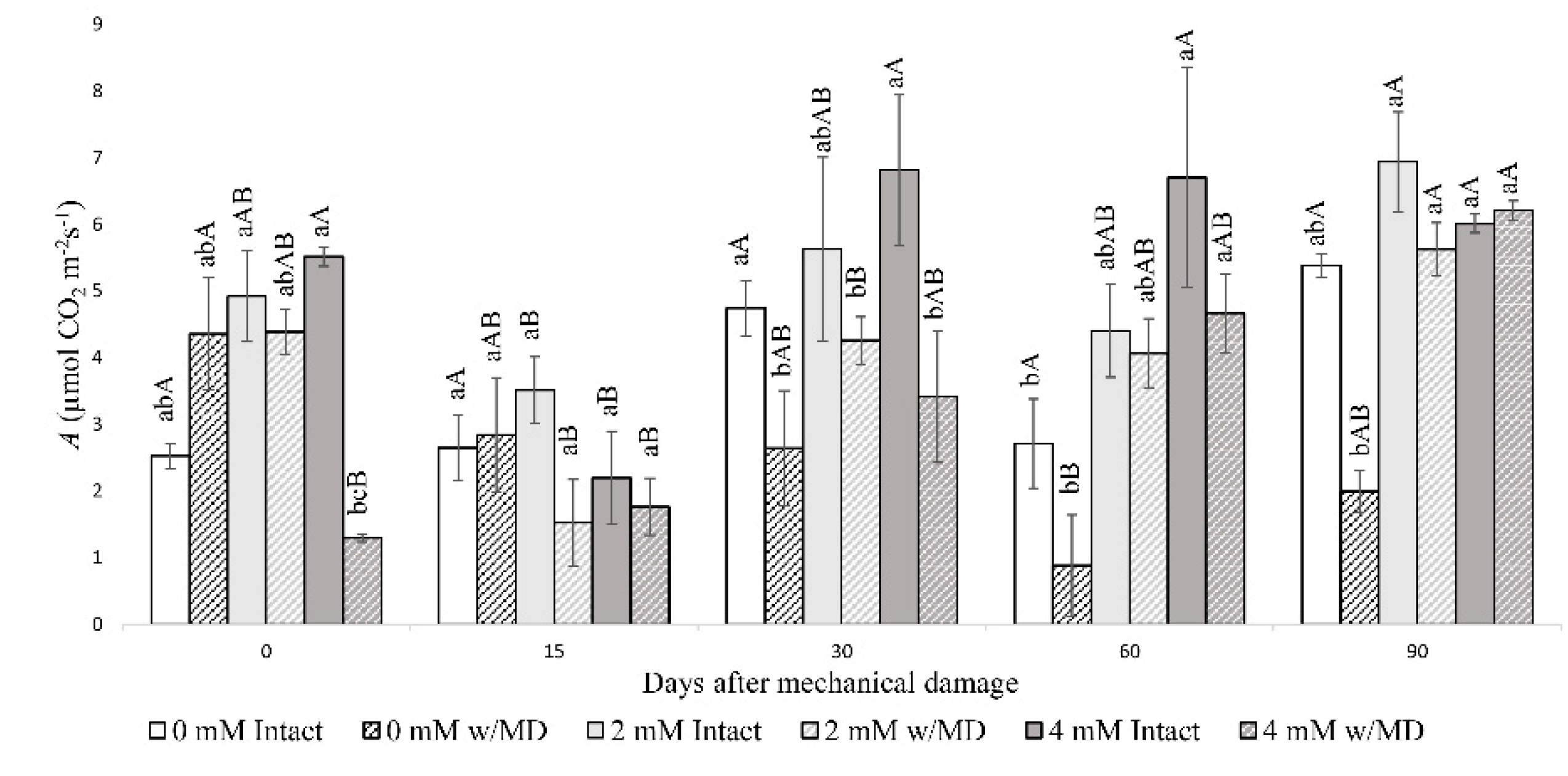
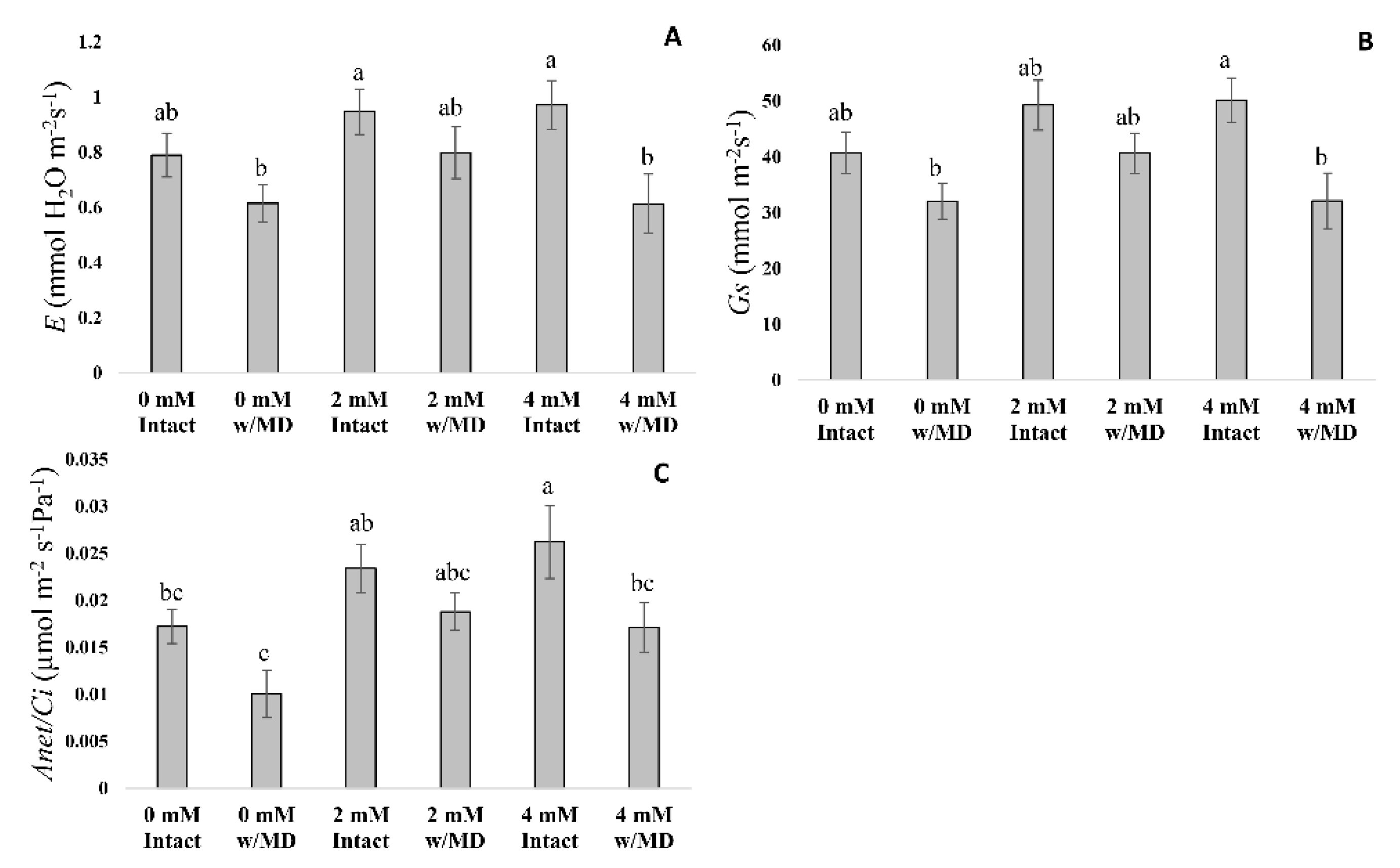
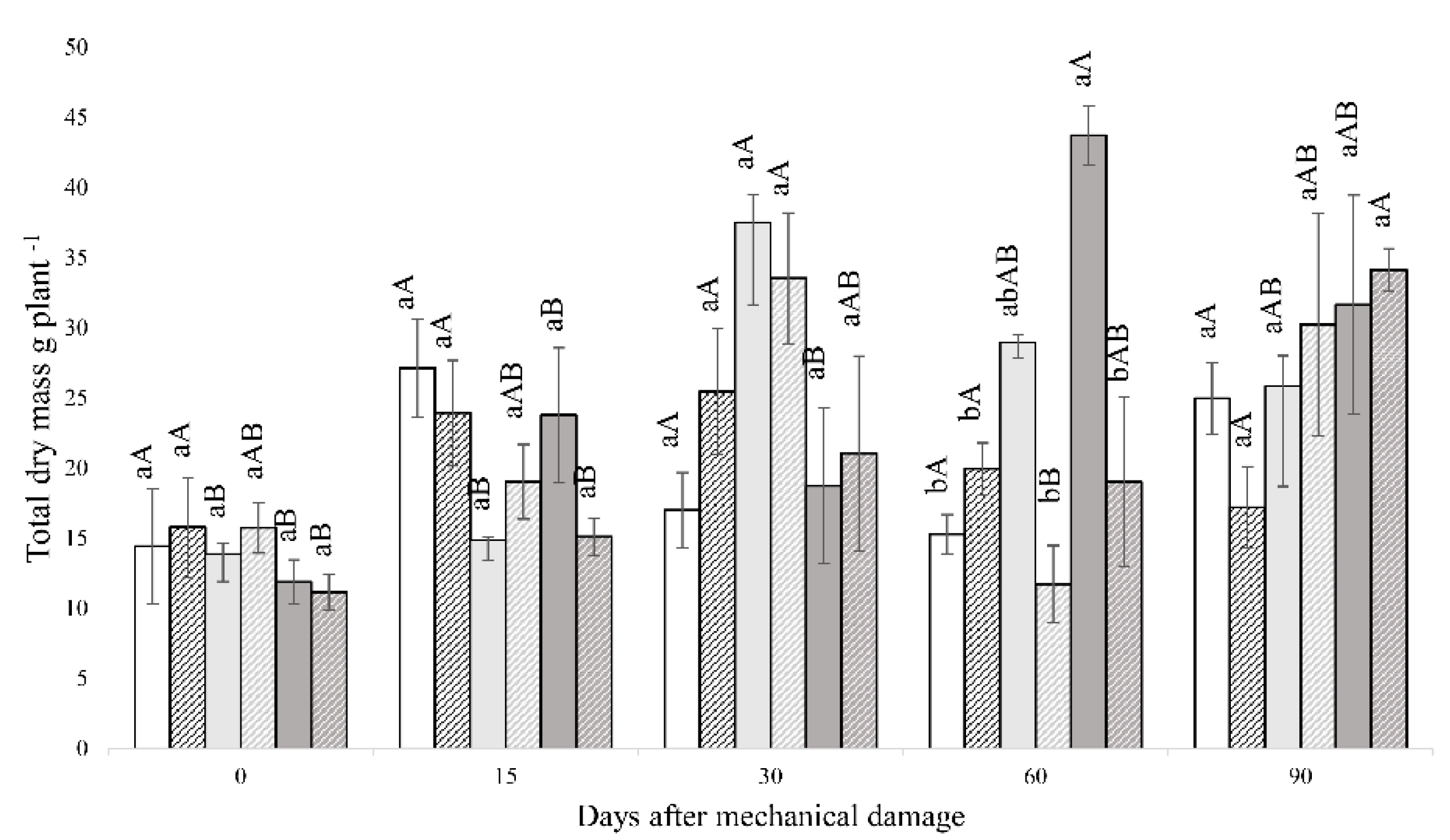
3.1. Fluorescence Analysis of Chlorophyll A, Gas Exchange and Biomass
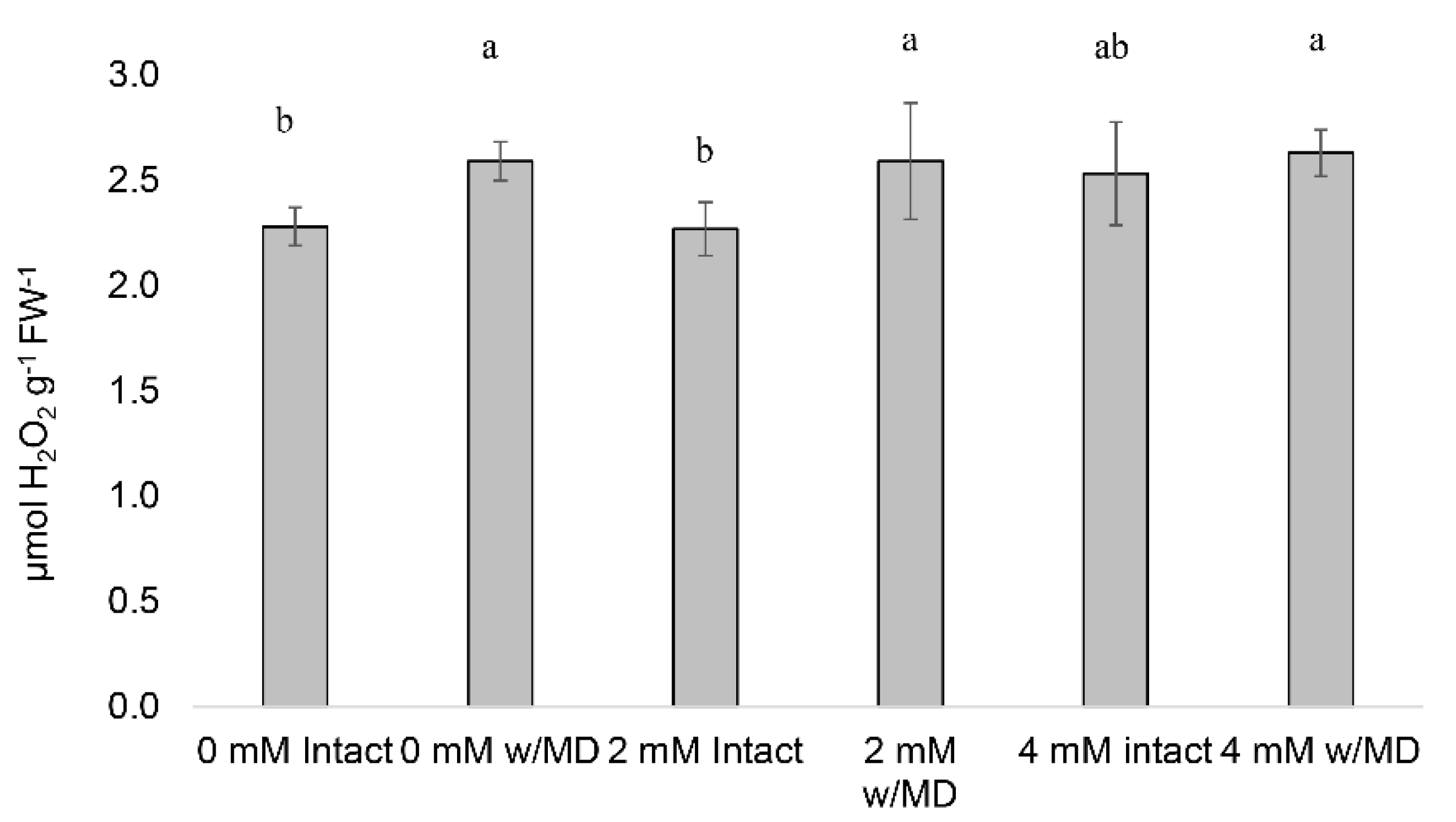
3.2. Hydrogen Peroxide Concentration (H2O2)
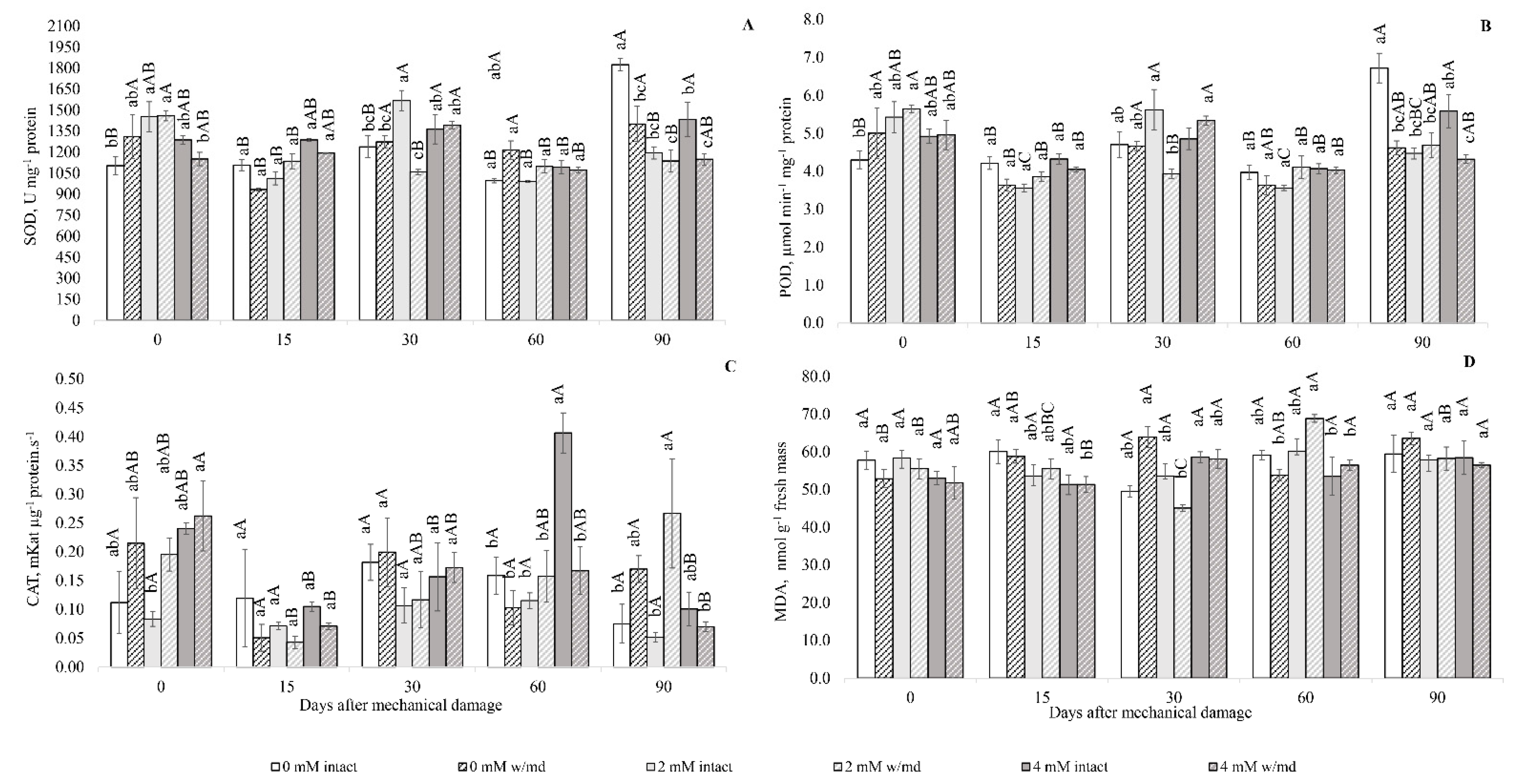
3.3. Antioxidant Enzyme Activity
3.4. Lipid Peroxidation Quantification
3.5. Calcium Concentration in Plant Tissue
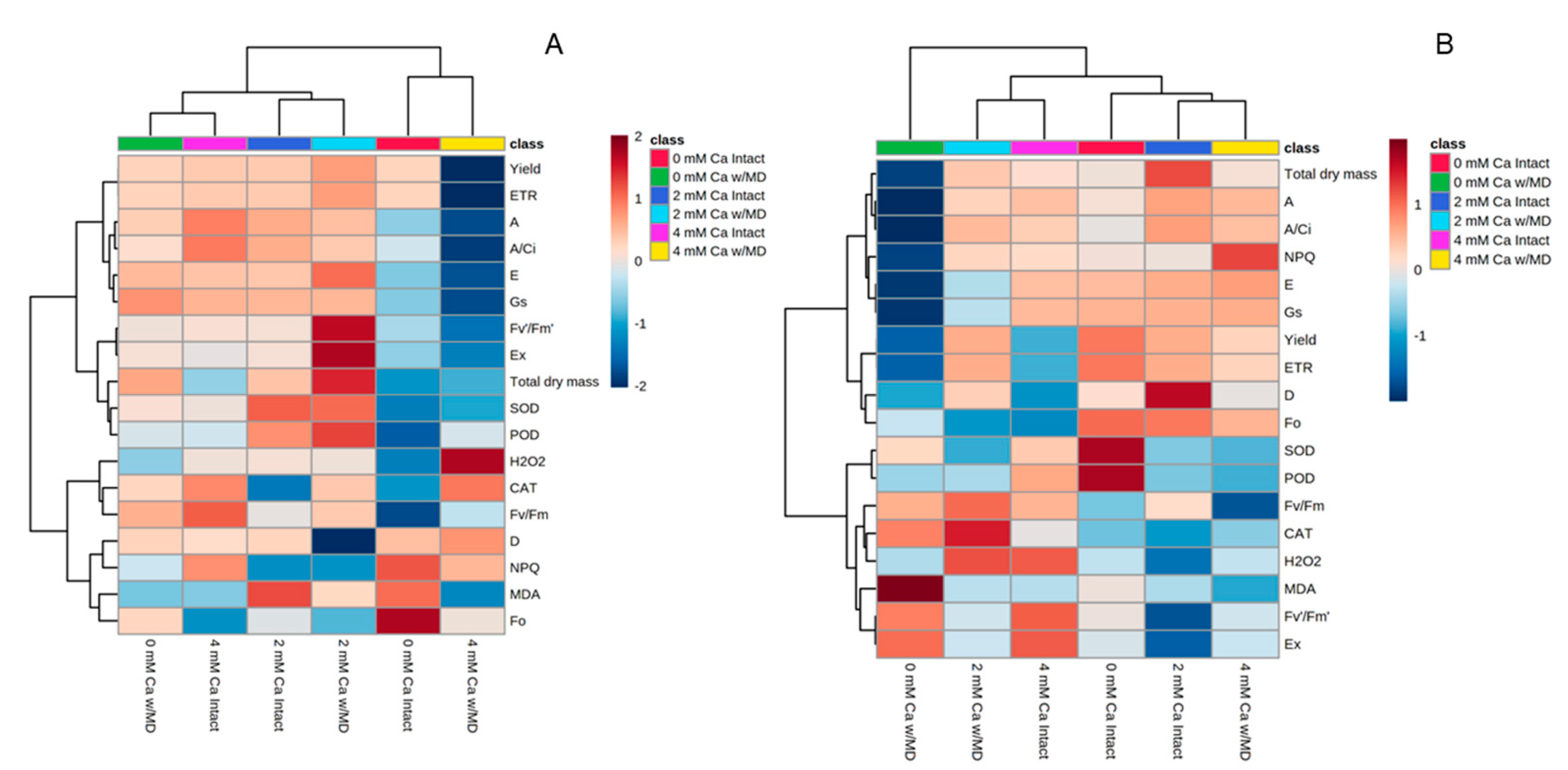
3.6. Heat Map
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denness, L.; McKenna, J.F.; Segonzac, C.; Wormit, A.; Madhou, P.; Bennett, M.; Mansfield, J.; Zipfel, C.; Hamann, T. Cell Wall Damage-Induced Lignin Biosynthesis Is Regulated by a Reactive Oxygen Species- and Jasmonic Acid-Dependent Process in Arabidopsis. Plant Physiol. 2011, 156, 1364–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Nagawa, S.; Wang, G.; Yang, Z. Cell Polarity Signaling: Focus on Polar Auxin Transport. Mol. Plant 2008, 1, 899–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-L.; Lan, S.-S.; Deng, F.-F.; Gong, M. Effects of Calcium and Calmodulin Antagonists on Chilling Stress-Induced Proline Accumulation in Jatropha curcas L. J. Plant Growth Regul. 2016, 35, 815–826. [Google Scholar] [CrossRef]
- Takahashi, F.; Mizoguchi, T.; Yoshida, R.; Ichimura, K.; Shinozaki, K. Calmodulin-Dependent Activation of MAP Kinase for ROS Homeostasis in Arabidopsis. Mol. Cell 2011, 41, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Steinhorst, L.; Kudla, J. Signaling in Cells and Organisms—Calcium Holds the Line. Curr. Opin. Plant Biol. 2014, 22, 14–21. [Google Scholar] [CrossRef]
- Białasek, M.; Górecka, M.; Mittler, R.; Karpiski, S. Evidence for the Involvement of Electrical, Calcium and ROS Signaling in the Systemic Regulation of Non-Photochemical Quenching and Photosynthesis. Plant Cell Physiol. 2016, 58, 207–215. [Google Scholar] [CrossRef]
- Mantoan, L.P.B.; Rolim de Almeida, L.F.; Macedo, A.C.; Ferreira, G.; Boaro, C.S.F. Photosynthetic Adjustment after Rehydration in Annona Emarginata. Acta Physiol. Plant. 2016, 38, 157. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.; De-La-Cruz-Chacón, I.; Boaro, C.S.F.; Baron, D.; Lemos, E.E.P. de Propagation of Annonaceous Plants. Rev. Bras. De Frutic. 2019, 41, 1–14. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Calif. Agric. Exp. Stn. 1950, 397, 32. [Google Scholar]
- Baron, D.; Ferreira, G.; Rodrigues, J.D.; Boaro, C.S.F.; Macedo, A.C. Gas Exchange, Phisiological Indexes and Ionic Accumulation in Annona Emarginata (Schltdl.) H. Rainer Seedlings in Nutrients Solution. Rev. Bras. De Frutic. 2013, 35, 361–376. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Adams III, W.W.; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using Chlorophyll Fluorescence to Assess the Fraction of Absorbed Light Allocated to Thermal Dissipation of Excess Excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Ma, K.; Chen, L. Temperature-Dependent Gas Exchange and Stomatal/Non-Stomatal Limitation to CO2 Assimilation of Quercus Liaotungensis under Midday High Irradiance. Photosynth. Res. 2001, 39, 383–388. [Google Scholar] [CrossRef]
- De Portes, T.A.; De Castro, L.G.J. Analise de Crescimento de Plantas: Um Programa Computacional Auxiliar. Rev. Bras. De Fisiol. Veg. 1991, 3, 53–56. [Google Scholar]
- Kar, M.; Mishra, D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Misra, N.; Gupta, A.K. Effect of Salinity and Different Nitrogen Sources on the Activity of Antioxidant Enzymes and Indole Alkaloid Content in Catharanthus Roseus Seedlings. J. Plant Physiol. 2006, 163, 11–18. [Google Scholar] [CrossRef]
- Teisseire, H.; Guy, V. Copper-Induced Changes in Antioxidant Enzymes Activities in Fronds of Duckweed (Lemna Minor). Plant Sci. 2000, 153, 65–72. [Google Scholar] [CrossRef]
- Peixoto, H.P.P.; Cambraia, J.; Sant, A.R.; Mosquim, R.; Moreira, M.A. Aluminum Effects on Lipid Peroxidation and on the Activities of Enzymes of Oxidative Metabolism in Sorghum 1. Rev. Bras. De Fisiol. Veg. 1999, 11, 137–143. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The Effect of Drought and Ultraviolet Radiation on Growth and Stress Markers in Pea and Wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Devi, S.R.; Prasad, M.N. V Copper Toxicity in Ceratophyllum Demersum L. (Coontail), a Free Floating Macrophyte: Response of Antioxidant Enzymes and Antioxidants. Plant Sci. 1998, 138, 157–165. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; de Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd ed.; Revista e Atualizada; POTAFOS: Piracicaba, Brazil, 1997. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Tooulakou, G.; Giannopoulos, A.; Nikolopoulos, D.; Bresta, P.; Dotsika, E.; Orkoula, M.G.; Kontoyannis, C.G.; Fasseas, C.; Liakopoulos, G.; Klapa, M.I.; et al. Alarm Photosynthesis: Calcium Oxalate Crystals as an Internal CO2 Source in Plants. Plant Physiol. 2016, 171, 2577–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Chen, Z.; Yu, J.-Q. RBOH1-Dependent H2O2 Production and Subsequent Activation of MPK1/2 Play an Important Role in Acclimation-Induced Cross-Tolerance in Tomato. J. Exp. Bot. 2014, 65, 595–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Minibayeva, F.; Beckett, R.P.; Kranner, I. Roles of Apoplastic Peroxidases in Plant Response to Wounding. Phytochemistry 2015, 112, 122–129. [Google Scholar] [CrossRef]
- Hochmal, A.K.; Schulze, S.; Trompelt, K.; Hippler, M. Calcium-Dependent Regulation of Photosynthesis. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 993–1003. [Google Scholar] [CrossRef] [Green Version]
- Selmar, D.; Kleinwächter, M. Influencing the Product Quality by Deliberately Applying Drought Stress during the Cultivation of Medicinal Plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Wilhelm, C.; Selmar, D. Energy Dissipation Is an Essential Mechanism to Sustain the Viability of Plants: The Physiological Limits of Improved Photosynthesis. J. Plant Physiol. 2011, 168, 79–87. [Google Scholar] [CrossRef]
- Neill, S.; Barros, R.; Bright, J.; Desikan, R.; Hancock, J.; Harrison, J.; Morris, P.; Ribeiro, D.; Wilson, I. Nitric Oxide, Stomatal Closure, and Abiotic Stress. J. Exp. Bot. 2008, 59, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Schippers, J.H.M. ROS-Mediated Redox Signaling during Cell Differentiation in Plants. Biochim. Biophys Acta 2015, 1850, 1497–1508. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as Key Players in Plant Stress Signalling. J. Exp. Bot. 2013, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic Stress: Interplay between ROS, Hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Santos, M.G.; Oliveira, R.F. Seasonal and Diurnal Changes in Photosynthetic Limitation of Young Sweet Orange Trees. Environ. Exp. Bot. 2009, 66, 203–211. [Google Scholar] [CrossRef]
- Silva, E.N.; Silveira, J.A.G.; Ribeiro, R.V.; Vieira, S.A. Photoprotective Function of Energy Dissipation by Thermal Processes and Photorespiratory Mechanisms in Jatropha curcas Plants during Different Intensities of Drought and after Recovery. Environ. Exp. Bot. 2015, 110, 36–45. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Abd-Allah, E.F.; Hashem, A.; Sarwat, M.; Anjum, N.A.; Gucel, S. Calcium and Potassium Supplementation Enhanced Growth, Osmolyte Secondary Metabolite Production, and Enzymatic Antioxidant Machinery in Cadmium-Exposed Chickpea (Cicer Arietinum L.). Front. Plant Sci. 2016, 7, 513. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Wang, F.; Guo, F.; Meng, J.J.; Li, X.G.; Wan, S.B. Calcium Contributes to Photoprotection and Repair of Photosystem II in Peanut Leaves during Heat and High Irradiance. J. Integr. Plant Biol. 2014, 57, 486–495. [Google Scholar] [CrossRef] [Green Version]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, Calcium, and Electric Signals: Key Mediators of Rapid Systemic Signaling in Plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef]
- Duarte, M.R.; Oliveira, R.B.; Souza, D.C. Anatomia Foliar e Caulinar de Duas Espécies de Rollinia (Annonaceae): R. Rugulosa E R. Mucosa. Visão Acadêmica 2015, 6, 4–21. [Google Scholar] [CrossRef] [Green Version]


| Ca2+ Leaves, g kg−1 | |||
|---|---|---|---|
| 2 mM Ca2+ | 11.438 ± 1.1805 ns | Intact | 13.37 ± 1.2609 a 1 |
| 0 Ca2+ | 11.91 ± 1.2525 | W/MD | 9.978 ± 0.4938 b |
| Ca2+ Stems, g kg −1 | |||
| 2 mM Ca2+ | 4.993 ± 0.1866 b | Intact | 5.743 ± 0.4553 ns |
| 0 Ca2+ | 6.182 ± 0.3756 a | W/MD | 5.432 ± 0.3168 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, F.G.; Barzotto, G.R.; Pagassini, J.A.V.; Sousa, M.C.; Ferreira, G.; Boaro, C.S.F. Calcium in Photosynthetic Restoration and Growth of Annona emarginata after Mechanical Damage. Horticulturae 2022, 8, 495. https://doi.org/10.3390/horticulturae8060495
Campos FG, Barzotto GR, Pagassini JAV, Sousa MC, Ferreira G, Boaro CSF. Calcium in Photosynthetic Restoration and Growth of Annona emarginata after Mechanical Damage. Horticulturae. 2022; 8(6):495. https://doi.org/10.3390/horticulturae8060495
Chicago/Turabian StyleCampos, Felipe Girotto, Gustavo Ribeiro Barzotto, Jonas Akenaton Venturineli Pagassini, Marilia Caixeta Sousa, Gisela Ferreira, and Carmen Sílvia Fernandes Boaro. 2022. "Calcium in Photosynthetic Restoration and Growth of Annona emarginata after Mechanical Damage" Horticulturae 8, no. 6: 495. https://doi.org/10.3390/horticulturae8060495
APA StyleCampos, F. G., Barzotto, G. R., Pagassini, J. A. V., Sousa, M. C., Ferreira, G., & Boaro, C. S. F. (2022). Calcium in Photosynthetic Restoration and Growth of Annona emarginata after Mechanical Damage. Horticulturae, 8(6), 495. https://doi.org/10.3390/horticulturae8060495






