Rhamnogalacturonan Endolyase Family 4 Enzymes: An Update on Their Importance in the Fruit Ripening Process
Abstract
:1. Introduction
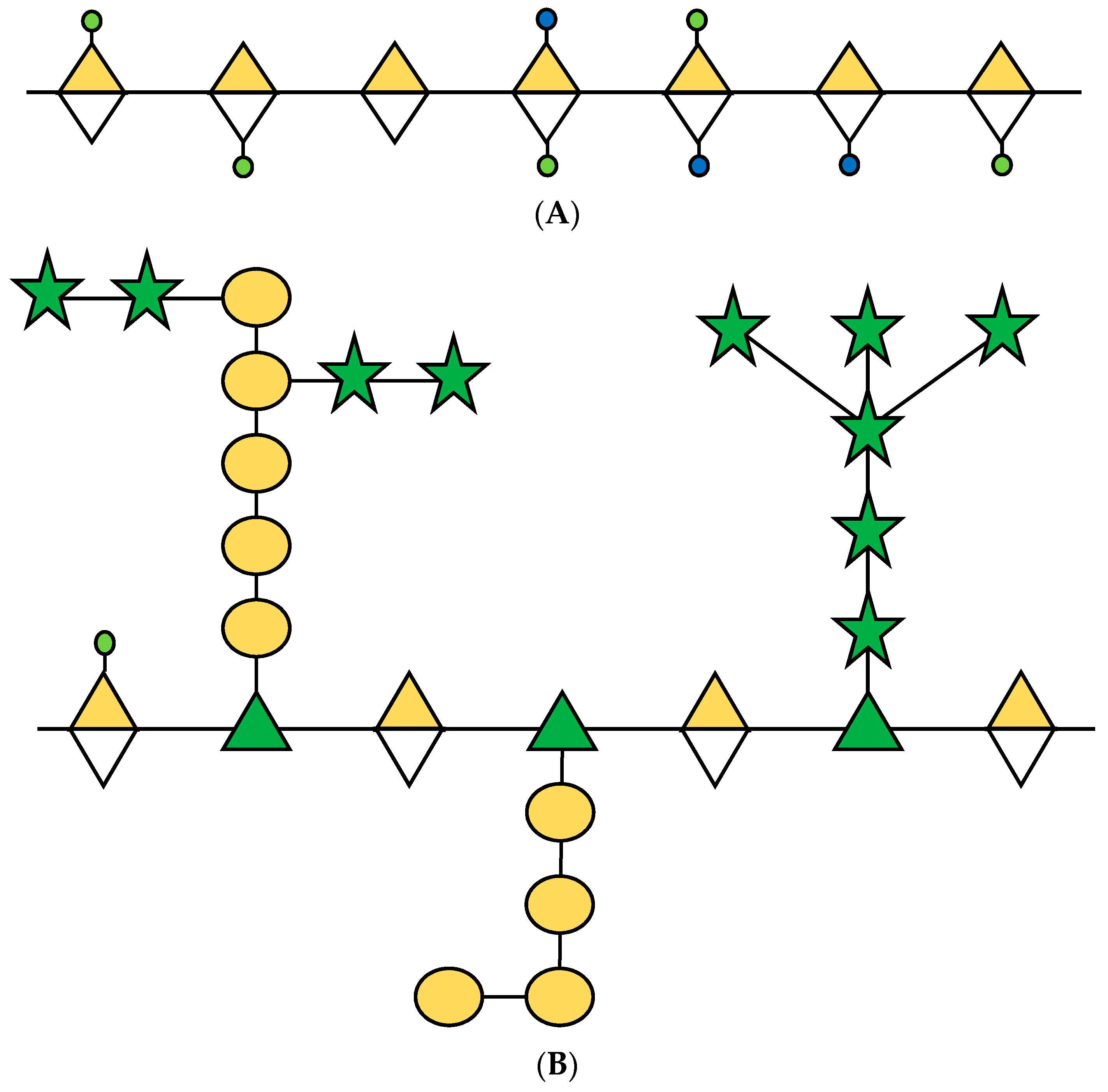
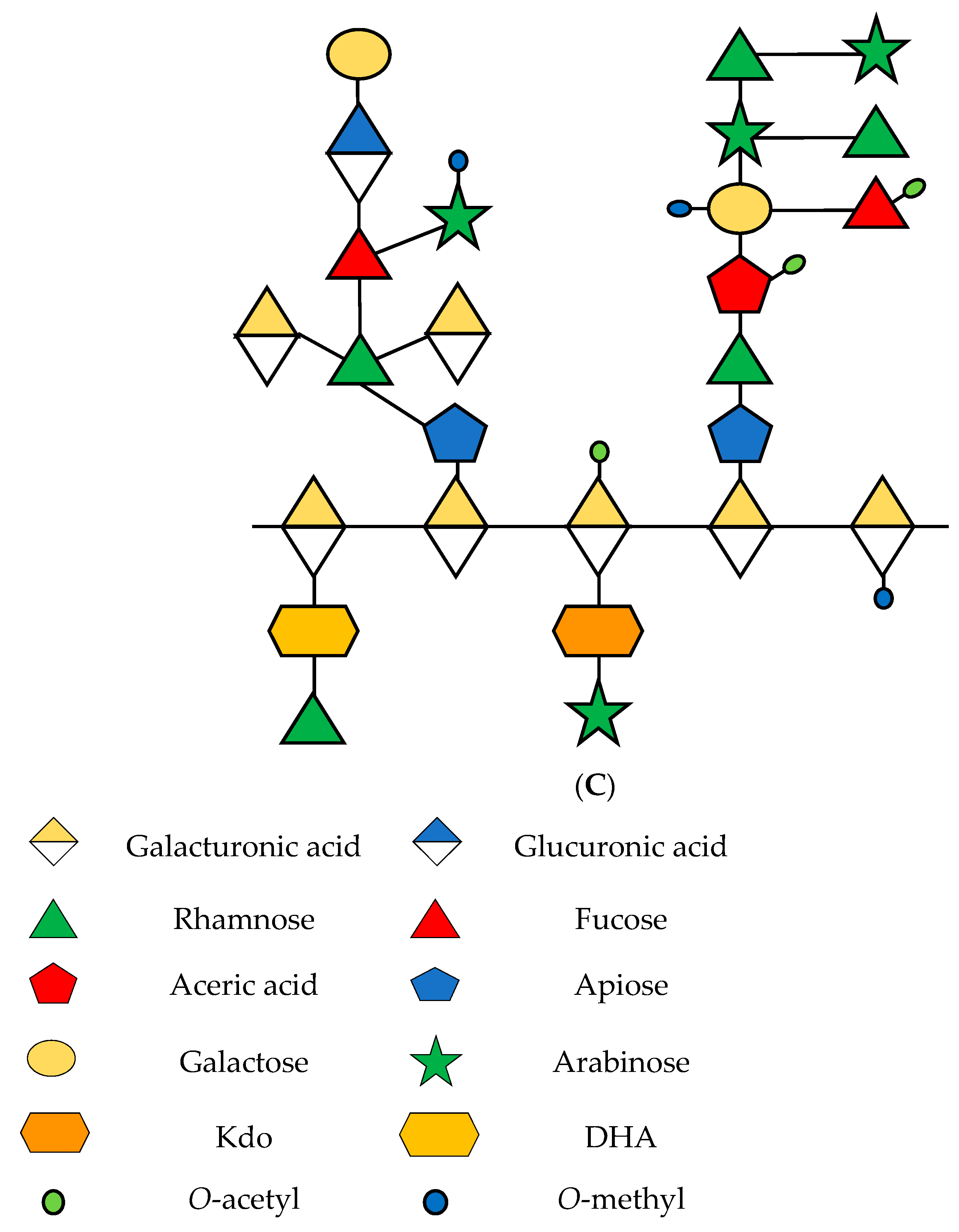
2. Rhamnogalacturonan-I in the Fruit Ripening
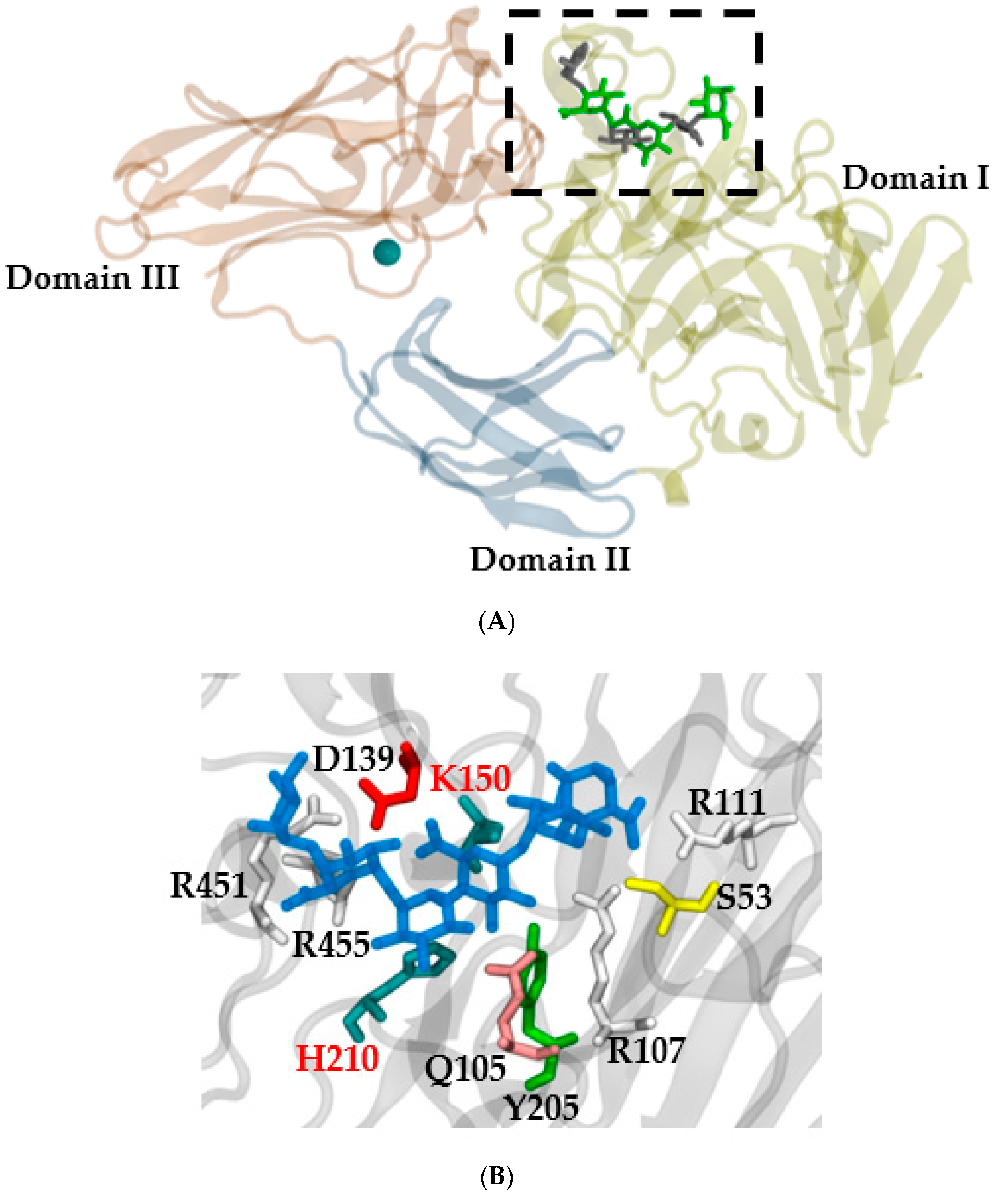
3. Structural and Phylogenetic Classification of the Rhamnogalacturonan Endolyase Enzyme
4. Rhamnogalacturonan Endolyase in Fruit Ripening
| Specie | Results | Reference |
|---|---|---|
| F. x ananassa | Changes in the spatio-temporal expression of FaRGlyase1 across the fruit ripening. Transient silencing of FaRGlyase1 induces a high conservation of cell wall. FaRGlyase1 expression could be induced by abscisic acid. FaRGlyase1 gene is located in a QTL linked to firmness. | [14] |
| F. chiloensis | Structural characterization and biochemical parameters of RGL enzyme. Transcripts levels increase during fruit ripening. Phylogenetic tree with same results according to Mokshina et al. (2018) [37]. Calcium dependence on enzymatic activity. | [15] |
| F. chiloensis | Elements of response to phytohormones: ABA, methyl jasmonate, gibberellins, salicylic acid, and auxin; response elements to biotic and abiotic stress such as defense, low temperatures, and drought. | [46] |
| S. lycopersicum L. | GUS under the control of an RGL promoter induces expression in the red ripe stage of fruit ripening. Increased expression levels of GUS 40 days after anthesis. | [49] |
| S. lycopersicum L. | Constitutive expression of RGL promoter to produce tomatoes with greater firmness and a longer shelf life. Overexpression under CaMV35S of Solyc11g011300 promoter reduces viability and pollen germination and decreases the number of seeds and fruits. Delay in fruit ripening of one week. | [12] |
| S. lycopersicum L. and A. thaliana | RGL genes respond to abscisic acid, dehydration, and light. | [48] |
| S. lycopersicum L. | Solyc11g011300 gene increases activity in fruit ripening, correlated with ethylene production increasing. Solyc04g076630 and Solyc04g076660 could participate in re-engineering on RG-I pectin at the beginning of fruit ripening. | [13] |
5. New Perspectives and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, S170–S180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, P.; Powell, A.L.T.; Orlando, R.; Bergmann, C.; Gutiérrez-Sánchez, G. A proteomic analysis of ripening tomato fruit infected by Botrytis cinerea. J. Proteome Res. 2012, 11, 2178–2192. [Google Scholar] [CrossRef] [Green Version]
- Alkan, N.; Fortes, A.M. Insights into molecular and metabolic events associated with fruit response to post-harvest fungal pathogens. Front. Plant Sci. 2015, 6, 889. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, L.; Domon, J.; Klimek, J.F.; Fournet, F.; Sellier, H.; Gillet, F.; Pelloux, J.; Lejeune-Hénaut, I.; Carpita, N.C.; Rayon, C. Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 2014, 104, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Amos, R.A.; Mohnen, D. Critical review of plant cell wall matrix polysaccharide glycosyltransferase activities verified by heterologous protein expression. Front. Plant Sci. 2019, 10, 915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacete, L.; Hamann, T. The role of mechanoperception in plant cell wall integrity maintenance. Plants 2020, 9, 574. [Google Scholar] [CrossRef]
- Mwaniki, M.W.; Mathooko, F.M.; Hiwasa, K.; Tateishi, A.; Yokotani, N.; Ushijima, K.; Nakano, R.; Inaba, A. β-galactosidase and α-L-arabinofuranosidase activities and gene expression in European and Chinese pear fruit during ripening. J. Jpn. Soc. Hortic. Sci. 2007, 76, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, J.P.; Su, J.; Li, X.P.; Chen, W.X. Changes in alpha-L-arabinofuranosidase activity in peel and pulp of banana (Musa sp.) fruits during ripening and softening. J. Plant Physiol. Mol. Biol. 2007, 33, 131–136. [Google Scholar]
- Rosli, H.G.; Civello, P.M.; Martínez, G.A. Alpha-L-arabinofuranosidase from strawberry fruit: Cloning of three cDNAs, characterization of their expression and analysis of enzymatic activity in cultivars with contrasting firmness. Plant Physiol. Biochem. 2009, 47, 272–281. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Dang, M.; Zhang, B.; Li, H.; Meng, R.; Qu, D.; Yang, Y.; Zhao, Z. Analysis of β-galactosidase during fruit development and ripening in two different texture types of apple cultivars. Front. Plant Sci. 2018, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Jiménez, V.; Berumen-Varela, G.; Burgara-Estrella, A.; Orozco-Avitia, J.; Ojeda-Contreras, A.; Trillo-Hernández, E.; Rivera-Domínguez, M.; Troncoso-Rojas, R.; Báez-Sañudo, R.; Datsenka, T.; et al. Functional analysis of tomato rhamnogalacturonan lyase gene Solyc11g011300 during fruit ripening development and ripening. J. Plant Physiol. 2018, 231, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Trillo-Hernández, E.A.; Orozco-Avitia, J.A.; Ojeda-Contreras, A.J.; Berumen-Varela, G.; Ochoa-Jiménez, V.A.; Troncoso-Rojas, R.; Rivera-Domínguez, M.; Baez-Flores, M.E.; Hernández-Oñate, M.A.; Tiznado-Hernández, M.E. Análisis de expresión de genes codificantes de isoenzimas de ramnogalacturonano liasa durante el desarrollo y la madurez del fruto de tomate. Rev. Fitotec. Mex. 2021, 44, 529–536. [Google Scholar] [CrossRef]
- Molina-Hidalgo, F.J.; Franco, A.R.; Villatoro, C.; Medina-Puche, L.; Mercado, J.A.; Hidalgo, M.A.; Monfort, A.; Caballero, J.L.; Muñoz-Blanco, J.; Blanco-Portales, R. The strawberry (Fragaria x ananassa) fruit-specific rhamnogalacturonate lyase 1 (FaRGLyase1) gene encodes an enzyme involved in the degradation of cell wall middle lamellae. J. Exp. Bot. 2013, 64, 1471–1483. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Yáñez, A.; González, M.; Carrasco-Orellana, C.; Herrera, R.; Moya-León, M.A. Isolation of a rhamnogalacturonan lyase from the Chilean strawberry fruit and its biochemical characterization. Plant Physiol. Biochem. 2020, 146, 411–419. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarska, A.; Pieczywek, P.M.; Cybulska, J.; Zdunek, A. Structure and functionality of Rhamnogalacturonan I in the cell wall and in solution: A review. Carbohydr. Polym. 2021, 278, 118909. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef] [Green Version]
- Nepogodiev, S.; Fais, M.; Hughes, D.; Field, R.A. Synthesis of apiose-containing oligosaccharide fragments of the plant cell wall: Fragments of rhamnogalacturonan-II side chains A and B, and apiogalacturonan. Org. Biomol. Chem. 2011, 9, 6670. [Google Scholar] [CrossRef] [PubMed]
- Yapo, B. Rhamnogalacturonan-I: A structurally puzzling and functionally versatile polysaccharide from plant cell walls and mucilages. Polym. Rev. 2011, 52, 391–413. [Google Scholar] [CrossRef]
- Van Audenhove, J.; Bernaerts, T.; De Smet, V.; Delbaere, S.; Van Loey, A.M.; Hendrickx, M.E. The structure and composition of extracted pectin and residual cell wall material from processing tomato: The role of a stepwise approach versus high-pressure homogenization-facilitated acid extraction. Foods 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.; Yin, X.; Zhang, A.; Wang, M.; Zhu, Q.; Liu, X.; Xie, X.; Chen, K.; Grierson, D. Ethylene and fruit softening. Food Qual. Saf. 2017, 1, 253–267. [Google Scholar] [CrossRef]
- Peña, M.J.; Carpita, N.C. Loss of highly branched arabinans and debranching of rhamnogalacturonan I accompany loss of firm texture and cell separation during prolonged storage of apple. Plant Physiol. 2004, 135, 1305–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, A.; Rodríguez, R.; Fernández-Caro, I.; Guillén, R.; Fernández-Bolaños, J.; Heredia, A. Olive fruit cell wall: Degradation of pectic polysaccharides during ripening. J. Agric. Food Chem. 2001, 49, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.A.; Cardoso, S.M.; Lopes-da-Silva, J.A. Olive pomace, a source for valuable arabinan-rich pectic polysaccharides. Top. Curr. Chem. 2010, 294, 129–141. [Google Scholar]
- Missang, C.E.; Renard, C.; Baron, A.; Drilleau, J. Changes in the pectic fraction of bush butter (Dacryodes edulis (G Don) HJ Lam) fruit pulp during ripening. J. Sci. Food Agric. 2001, 81, 781–789. [Google Scholar] [CrossRef]
- Laatu, M.; Condemine, G. Rhamnogalacturonate lyase RhiE is secreted by the out system in Erwinia chrysanthemi. J. Bacteriol. 2003, 185, 1642–1649. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, A.; Itoh, T.; Kawamata, A.; Hashimoto, W.; Murata, K. Plant cell wall degradation by saprophytic Bacillus subtilis strains: Gene clusters responsible for rhamnogalacturonan depolymerization. J. Appl. Environ. Microbiol. 2007, 73, 3803–3813. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.H.; Otten, H.; Christensen, U.; Borchert, T.V.; Christensen, L.L.H.; Larsen, S.; Lo Leggio, L. Structural and biochemical studies elucidate the mechanism of rhamnogalacturonan lyase from Aspergillus aculeatus. J. Mol. Biol. Mol. 2010, 404, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.R.; Larsen, D.M.; Meyer, A.S.; Mikkelsen, J.D. Identification, expression and characterization of a novel bacterial RGI Lyase enzyme for the production of biofunctional fibers. Enzym. Microb. Technol. 2011, 49, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.G. Polysaccharide degradation systems of the saprophytic bacterium Cellvibrio japonicus. World J. Microbiol. Biotechnol. 2016, 32, 121. [Google Scholar] [CrossRef]
- Silva, I.R.; Jers, C.; Meyer, A.S.; Mikkelsen, J.D. Rhamnogalacturonan I modifying enzymes: An update. New Biotechnol. 2016, 33, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Bernard, T.; Rancurel, C.; Brumer, H.; Coutinho, P.; Henrissat, B. A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem. J. 2010, 432, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, Y.; Yi, H.; Zhang, G.; Zhang, L.; Mayo, K.H.; Yuan, Y.; Zhou, Y. Biochemical characterization of two rhamnogalacturonan lyases from Bacteroides ovatus ATCC 8483 with preference for RG-I substrates. Front. Microbiol. 2022, 12, 799875. [Google Scholar] [CrossRef] [PubMed]
- Garron, M.; Cygler, M. Structural and mechanistic classification of uronic acid-containing polysaccharide lyases. Glycobiology 2010, 20, 1547–1573. [Google Scholar] [CrossRef] [Green Version]
- Charnock, S.J.; Brown, I.E.; Turkenburg, J.P.; Black, G.W.; Davies, G.J. Convergent evolution sheds light on the anti- β-elimination mechanism common to family 1 and 10 polysaccharide lyases. Proc. Natl. Acad. Sci. USA 2002, 99, 12067–12072. [Google Scholar] [CrossRef] [Green Version]
- McDonough, M.A.; Kadirvelraj, R.; Harris, P.; Poulsen, J.; Larsen, S. Rhamnogalacturonan lyase reveals a unique three-domain modular structure for polysaccharide lyase family 4. FEBS Lett. 2004, 565, 188–194. [Google Scholar] [CrossRef]
- Azadi, P.; O’Neill, M.; Bergmann, C.; Darvill, A.G.; Albersheim, P. The backbone of the pectic polysaccharide rhamnogalacturonan I is cleaved by an endohydrolase and an endolyase. Glycobiology 1995, 5, 783–789. [Google Scholar] [CrossRef]
- Mokshina, N.; Makshanova, O.; Nazipova, A.; Gorshkov, O.; Gorshkova, T. Flax rhamnogalacturonan lyases: Phylogeny, differential expression and modeling of protein structure. Physiol. Plant. 2018, 167, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Humprey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Posé, S.; Paniagua, C.; Matas, A.J.; Gunning, P.; Morris, V.J.; Quesada, M.A.; Mercado, J.A. A nanostructural view of the cell wall disassembly process during fruit ripening and postharvest process during fruit ripening and postharvest storage by atomic force microscopy. Trends Food Sci. Technol. 2019, 87, 47–58. [Google Scholar] [CrossRef]
- Wang, D.; Yeasts, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Ajayi, O.O.; Held, M.A.; Showalter, A.M. Two β-glucuronosyltransferases involved in the biosynthesis of type II arabinogalactans function in mucilage polysaccharide matrix organization in Arabidopsis thaliana. BMC Plant Biol. 2021, 21, 245. [Google Scholar] [CrossRef]
- Méndez-Yañez, A. Aislamiento y caracterización de la enzima ramnogalacturonano endoliasa inducida durante la maduración de frutos de Fragaria chiloensis (L.) Mill. In Tesis Doctorado en Ciencias, Mención Ingeniería Genética Vegetal; Universidad de Talca: Región del Maule, Chile, 2019. [Google Scholar]
- Symons, G.M.; Chua, Y.J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal changes during non-climateric ripening in strawberry. J. Exp. Bot. 2012, 63, 4741–4750. [Google Scholar] [CrossRef] [Green Version]
- Berumen-Varela, G.; Rivera-Domínguez, M.; Troncoso-Rojas, R.; Báez-Sañudo, R.; Tiznado-Hernández, M. Physiological function of rhamnogalacturonan lyase genes based in the analysis of cis-acting elements located in the promoter region. Res. J. Biotechnol. 2017, 12, 77–108. [Google Scholar]
- Berumen-Varela, G.; Ochoa-Jiménez, V.; Burgara-Estrella, A.; Trillo-Hernández, E.; Ojeda-Contreras, A.; Orozco-Avitia, A.; Rivera-Domínguez, M.; Troncoso-Rojas, R.; Báez-Sañudo, R.; Datsenka, T.; et al. Functional analysis of a tomato (Solanum lycopersicum L.) rhamnogalacturonan lyase promoter. J. Plant Physiol. 2018, 229, 175–184. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Quintana, L.; Ramos, P.; Méndez-Yáñez, A. Rhamnogalacturonan Endolyase Family 4 Enzymes: An Update on Their Importance in the Fruit Ripening Process. Horticulturae 2022, 8, 465. https://doi.org/10.3390/horticulturae8050465
Morales-Quintana L, Ramos P, Méndez-Yáñez A. Rhamnogalacturonan Endolyase Family 4 Enzymes: An Update on Their Importance in the Fruit Ripening Process. Horticulturae. 2022; 8(5):465. https://doi.org/10.3390/horticulturae8050465
Chicago/Turabian StyleMorales-Quintana, Luis, Patricio Ramos, and Angela Méndez-Yáñez. 2022. "Rhamnogalacturonan Endolyase Family 4 Enzymes: An Update on Their Importance in the Fruit Ripening Process" Horticulturae 8, no. 5: 465. https://doi.org/10.3390/horticulturae8050465
APA StyleMorales-Quintana, L., Ramos, P., & Méndez-Yáñez, A. (2022). Rhamnogalacturonan Endolyase Family 4 Enzymes: An Update on Their Importance in the Fruit Ripening Process. Horticulturae, 8(5), 465. https://doi.org/10.3390/horticulturae8050465








