Growth, Physicochemical, Nutritional, and Postharvest Qualities of Leaf Lettuce (Lactuca sativa L.) as Affected by Cultivar and Amount of Applied Nutrient Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growing Condition, and Measurement of Growth Parameters
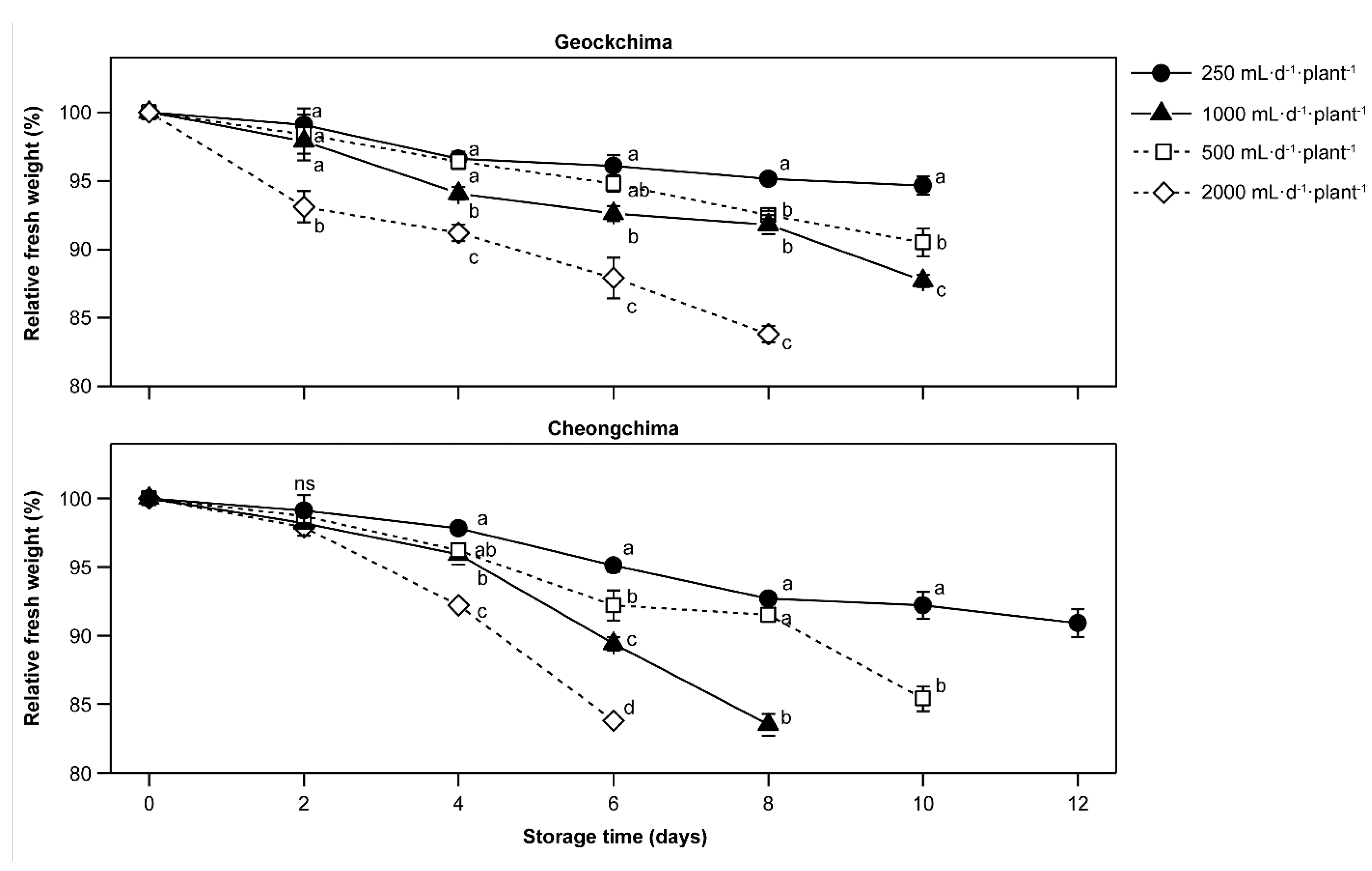
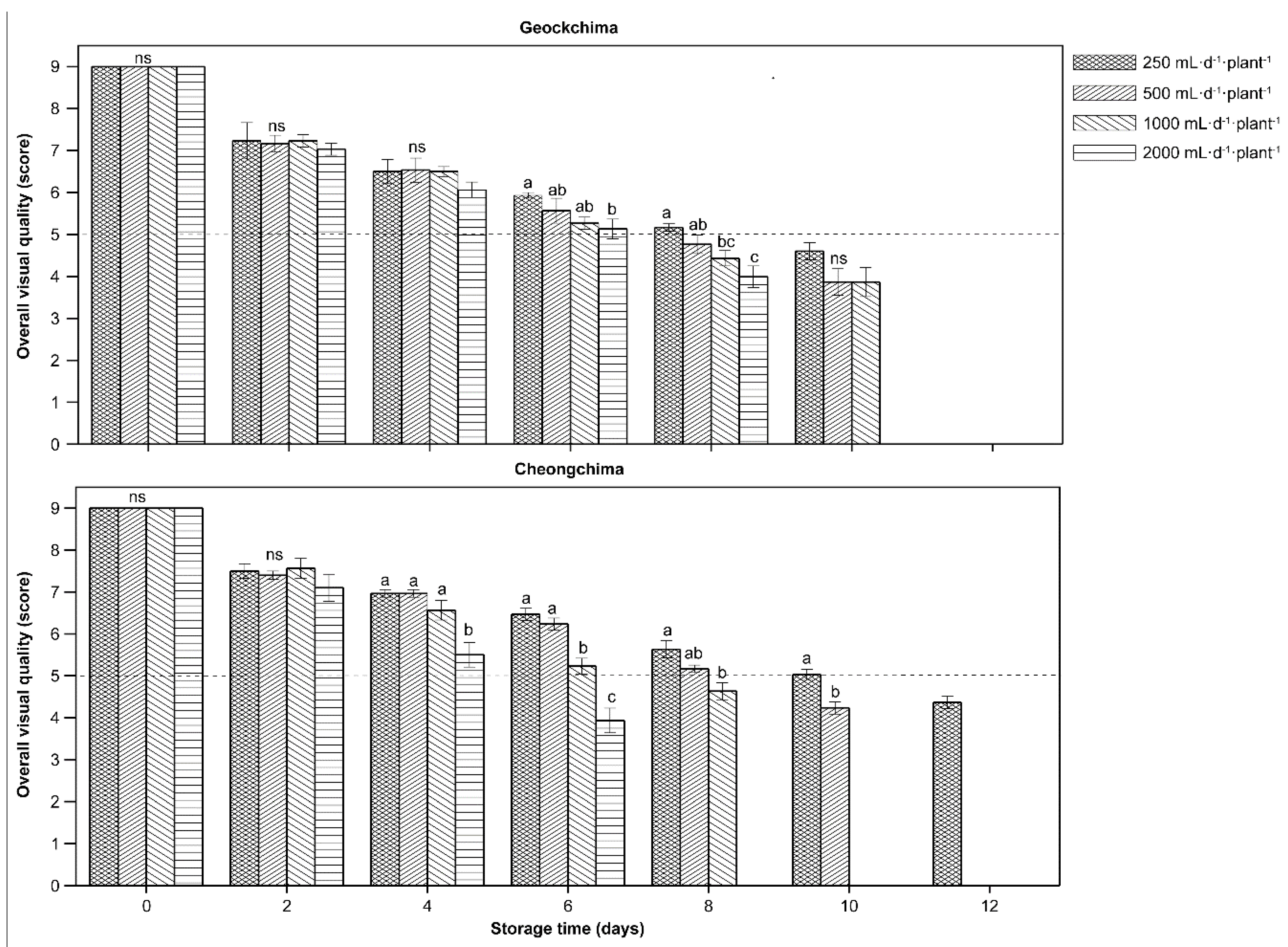
2.2. Harvest Practice, Storage Condition, and Postharvest Quality Evaluation
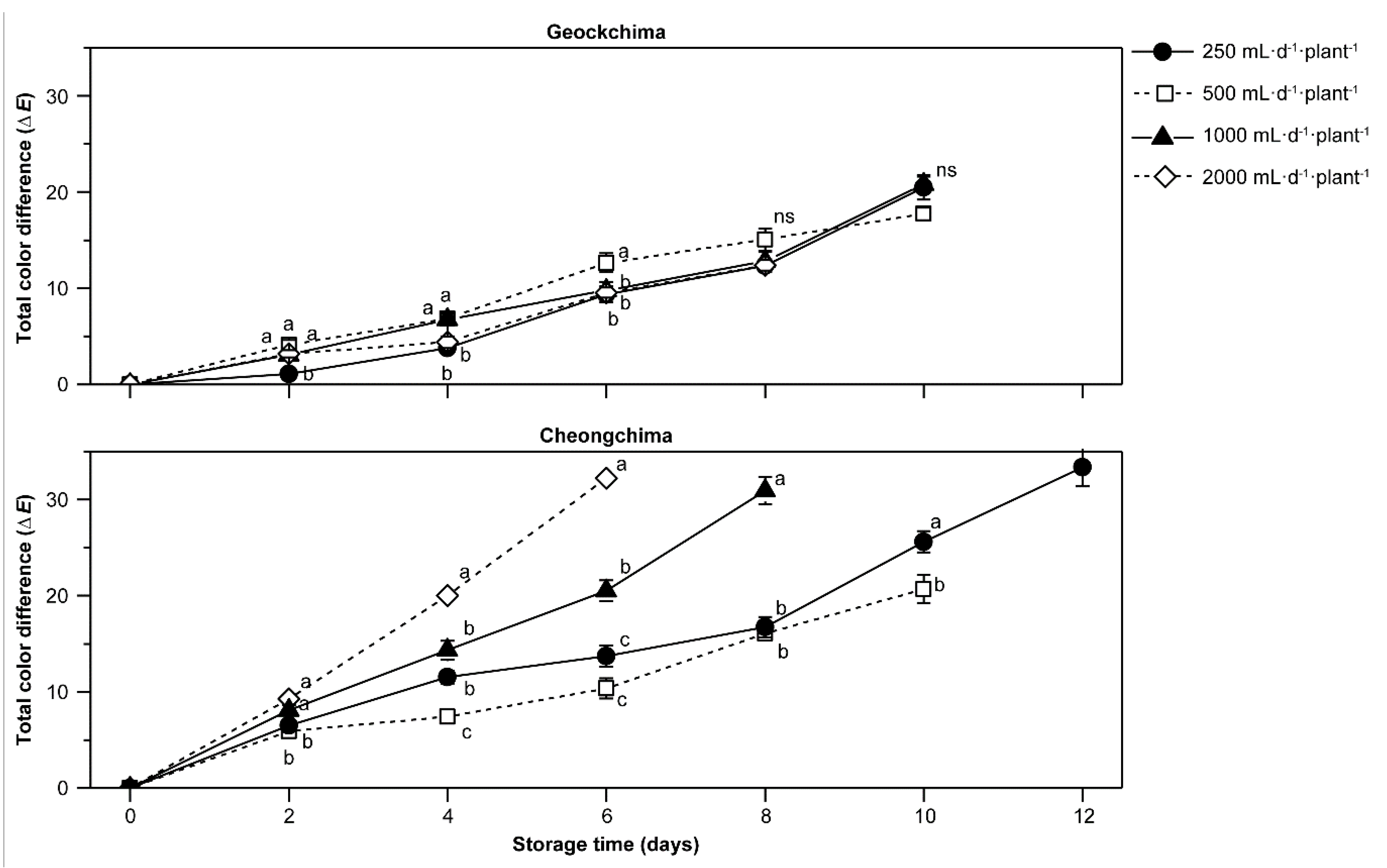
2.3. Color Measurement
2.4. Measurement of Dry Matter, Texture, Crude Fiber, Osmolality, and Ascorbic Acid Content
2.5. Microscopic Observation of Lettuce Tissue
2.6. Determination of Total Chlorophyll and Anthocyanin
2.7. Mineral Analysis
2.8. Statistical Analyses
3. Results and Discussion
3.1. Growth of Lettuce as Affected by Cultivar and Amount of Applied Nutrient Solution
3.2. Dry Matter, Crude Fiber, Texture, Osmolality, and Ascorbic Acid Content as Affected by Cultivar and Amount of Applied Nutrient Solution
3.3. Color Values, Chlorophyll, and Anthocyanin Contents as Affected by Cultivar and Amount of Applied Nutrient Solution
3.4. Mineral Contents as Affected by Cultivars and Amount of Applied Nutrient Solution
3.5. Postharvest Qualities as Affected by Cultivars and Amount of Applied Nutrient Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicola, S.; Hoeberechts, J.; Fontana, E. Comparison between traditional and soilless culture systems to produce rocket (Eruca sativa) with low nitrate content. Acta Hortic. 2005, 697, 549–555. [Google Scholar] [CrossRef]
- Selma, M.V.; Luna, M.C.; Martínez-Sánchez, A.; Tudela, J.A.; Beltrán, D.; Baixauli, C.; Gil, M.I. Sensory quality, bioactive constituents and microbiological quality of green and red fresh-cut lettuces (Lactuca sativa L.) are influenced by soil and soilless agricultural production systems. Postharvest Biol. Technol. 2012, 63, 16–24. [Google Scholar] [CrossRef]
- Blanch, M.; Alvarez, M.D.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Water relations, short-chain oligosaccharides and rheological properties in lettuces subjected to limited water supply and low temperature stress. Sci. Hortic. 2017, 225, 726–735. [Google Scholar] [CrossRef]
- Siomos, A.S.; Beis, G.; Papadopoulou, P.P.; Nasi, N.; Kaberidou, I.; Barbayiannis, N. Quality and composition of lettuce (cv. “Plenty”) grown in soil and soilless culture. Acta Hortic. 2001, 548, 445–450. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations FAO. Good Agricultural Practices for Greenhouse Vegetable Crops; FAO Plant Production and Protection Paper 217; FAO: Rome, Italy, 2013. [Google Scholar]
- Ministry of Agriculture, Food and Ministry of Agricultural Food and Rural Affairs (MAFRA). 2020 Greenhouse Status and Vegetable Production Performance. Available online: https://www.mafra.go.kr/bbs/mafra/71/251301/download.do (accessed on 28 March 2022).
- Ministry of Agricultural Food and Rural Affairs (MAFRA). 2017 Greenhouse Status and Vegetable Production Performance. Available online: https://www.mafra.go.kr/bbs/mafra/131/322442/artclView.do (accessed on 28 March 2022).
- Xu, H.L.; Gauthier, L.; Gosselin, A. Effects of fertigation management on growth and photosynthesis of tomato plants grown in peat, rockwool and NFT. Sci. Hortic. 1995, 6, 11–20. [Google Scholar] [CrossRef]
- Choi, E.Y.; Lee, H.J.; Lee, Y.B. (Mineral elements control of the nutrient solution in perlite culture of cucumber. J. Korean Soc. Hortic. Sci. Technol. 2001, 42, 497–500. [Google Scholar]
- Kang, N.J.; Cho, M.W.; Kweon, J.K.; Rhee, H.C.; Choi, Y.H. Effects of deficit irrigation on the total soluble solids and fruit yields of fresh tomato. Korean J. Bio-Environ. Control 2006, 15, 335–339. [Google Scholar]
- Yun, H.K.; Seo, T.C.; Zhang, C.H.; Huang, H.Z. Effect of selenium application on growth and quality of Chinese cabbage grown hydroponically in perlite media. J. Korean Soc. Hortic. Sci. 2005, 23, 363–366. [Google Scholar]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Cresswell, G.C.; Haigh, A.M. Comparison of sub-irrigation and overhead irrigation of tomato and lettuce seedlings. J. Hortic. Sci. Biotechnol. 2000, 75, 350–354. [Google Scholar] [CrossRef]
- Luna, M.C.; Tudela, J.A.; Martínez-Sánchez, A.; Allende, A.; Marín, A.; Gil, M.I. Long term deficit and excess of irrigation influences quality and browning related enzymes and phenolic metabolism of fresh-cut iceberg lettuce (Lactuca sativa L.). Postharvest Biol. Technol. 2012, 73, 37–45. [Google Scholar] [CrossRef]
- Vickers, L.H.; Grove, I.G.; Monaghan, J.M. Irrigation affects postharvest discolouration and yield in iceberg lettuce. Acta Hortic. 2015, 1091, 253–258. [Google Scholar] [CrossRef]
- Eichholz, I.; Forster, N.; Ulrichs, C.; Schreiner, M.; Huyskens-Keil, S. Survey of bioactive metabolites in selected cultivars and varieties of Lactuca sativa L. under water stress. J. Appl. Bot. Food Qual. 2014, 87, 265–273. [Google Scholar]
- Baslam, M.; Goicoechea, N. Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza 2012, 22, 347–359. [Google Scholar] [CrossRef]
- Stefanelli, D.; Winkler, S.; Jones, R. Reduced nitrogen availability during growth improves quality in red oak lettuce leaves by minimizing nitrate content, and increasing antioxidant capacity and leaf mineral content. Agric. Sci. 2011, 2, 477–486. [Google Scholar] [CrossRef]
- Fallovo, C.; Rouphael, Y.; Cardarelli, M.; Rea, E.; Battistelli, A.; Colla, G. Yield and quality of leafy lettuce in response to nutrient solution composition and growing season. J. Food Agric. Environ. 2009, 7, 456–462. [Google Scholar]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar]
- Kotsiras, A.; Vlachodimitropoulou, A.; Gerakaris, A.; Bakas, N.; Darras, A.I. Innovative harvest practices of Butterhead, Lollorosso and Bataviagreen lettuce (Lactuca sativa L.) types grown in floating hydroponic system to maintain the quality and improve storability. Sci. Hortic. 2016, 201, 1–9. [Google Scholar] [CrossRef]
- Lee, J.S.; Chang, M.S. Effect of nutrient solution concentration in the second half of growing period on the growth and postharvest quality of leaf lettuce (Lactuca sativa L.) in a deep flow technique system. Hortic. Sci. Technol. 2017, 35, 456–464. [Google Scholar]
- Chandra, D.; Matsui, T.; Suzuki, H.; Kosugi, Y.; Fujimura, K.; Bhowmik, P.K. Textural and compositional changes of stored iceberg lettuce in relation to harvest season and storage condition. Int. J. Veg. Sci. 2010, 16, 44–59. [Google Scholar] [CrossRef]
- Tian, W.; Lv, Y.; Cao, J.; Jiang, W. Retention of iceberg lettuce quality by low temperature storage and postharvest application of 1-methylcyclopropene or gibberellic acid. J. Food Sci. Technol. 2014, 51, 943–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirnak, H.; Tas, I.; Gokalp, Z.; Karaman, S. Effects of different irrigation levels on yield of lettuce grown in an unheated greenhouse. Curr. Trends Nat. Sci. 2016, 5, 145–151. [Google Scholar]
- Perez-Lopez, U.; Miranda-Apodaca, J.; Lacuesta, M.; Mena-Petite, A.; Munoz-Rueda, A. Growth and nutritional quality improvement in two differently pigmented lettuce cultivars grown under elevated CO2 and/or salinity. Sci. Hortic. 2015, 195, 56–66. [Google Scholar] [CrossRef]
- Fu, Y.; Lia, H.Y.; Yu, J.; Liu, H.; Cao, Z.Y.; Manukovsky, N.S.; Liu, H. Interaction effects of light intensity and nitrogen concentration on growth, photosynthetic characteristics and quality of lettuce (Lactucasativa L. Var. youmaicai). Sci. Hortic. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Rural Development Administration. Standardized Methods of Analysis for Agricultural Science and Technology; RDA: Suwon, Korea, 2012; Available online: http://lib.rda.go.kr/newlib/upload/prbook/%EC%97%B0%EA%B5%AC%EC%A1%B0%EC%82%AC%EB%B6%84%EC%84%9D%EA%B8%B0%EC%A4%80.pdf (accessed on 28 March 2022).
- Aguero, M.V.; Barg, M.V.; Yommi, A.; Camelo, A.; Roura, S.I. Postharvest changes in water status and chlorophyl content of lettuce (Lactuca sativa L.) and their relationship with overall visual quality. J. Food Sci. 2008, 73, S47–S55. [Google Scholar] [CrossRef]
- Association of Official Agricultural Cemists. Official Methods of Analysis, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Clarkson, G.J.J.; O’Byrne, E.E.; Rothwell, S.D.; Taylor, G. Identifying traits to improve postharvest processability in baby leaf salad. Postharvest Biol. Technol. 2003, 30, 287–298. [Google Scholar] [CrossRef]
- Jagota, S.K.; Dani, H.M. A new calorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Luna, M.C.; Tudela, J.A.; Martínez-Sánchez, A.; Allende, A.; Gil, M.I. Optimizing water management to control respiration rate and reduce browning and microbial load of fresh-cut romaine lettuce. Postharvest Biol. Technol. 2013, 80, 9–17. [Google Scholar] [CrossRef]
- Ferrante, A.; Maggiore, T. Chlorophyl a fluorescence measurements to evaluate storage time and temperature of Valeriana leafy vegetables. Postharvest Biol. Technol. 2007, 45, 73–80. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by uv-visible spectroscopy. Curr. Prot. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. (Eds.) Comparison between treatments means. In Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1983; pp. 187–240. [Google Scholar]
- Yazgan, S.; Ayas, S.; Demirtas, C.; Buyukcangaz, H.; Candogan, B.N. Deficit irrigation effects on lettuce(Lactuca sativa var. Olenka) yield in unheated greenhouse condition. J. Food Agric. Environ. 2008, 6, 357–361. [Google Scholar]
- Reference Chunli, L.; Nicki, J.E. Comparison of growth characteristics, functional qualities, and texture of hydroponically grown and soil-grown lettuce. LWT 2021, 150, 111931. [Google Scholar] [CrossRef]
- Seo, T.C.; Kim, Y.C.; Kim, K.Y.; Lee, J.W.; Yun, H.K.; Lee, S.G. Optimal supply amount and strength of nutrient solution for ripe-harvesting tomatoes grown under perlite culture system of semi-forcing cropping. J. Korean Soc. Hortic. Sci. 2003, 21, 79–85. [Google Scholar]
- Rajabbeigi, E.; Eichholz, I.; Beesk, N.; Ulrichs, C.; Kroh, L.W.; Rohn, S.; Huyskens-Keil, S. Interaction of drought stress and UV-B radiation–impact on biomass production andflavonoid metabolism in lettuce (Lactuca sativa L.). J. Appl. Bot. Food Qual. 2013, 86, 190–197. [Google Scholar]
- Zeipina, S.; Alsina, I.; Lepse, L.; Duma, M. The effect of irrigation on the biochemical content of leafy vegetables. In Proceedings of the Soil and Water Management, NJF 25th Congress of Nordic View to Sustainable Rural Development, Liga, Latvia, 16–18 June 2015; pp. 209–213. [Google Scholar]
- Ilker, R.; Szczesniak, A.S. Structural and chemical bases for texture of plant food stuffs. J. Tex. Stud. 1990, 21, 1–36. [Google Scholar] [CrossRef]
- Shinohara, Y.; Suzuki, Y. Quality improvement of hydroponically grown leaf vegetables. Acta Hortic. 1988, 230, 279–286. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Llorach, R.; Martinez-Sanchez, A.; Tomas-Barberan, F.A. Characterization of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, A108, 1028–1038. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Do anthocyanins function as osmoregulators in leaf tissues? Adv. Bot. Res. 2002, 37, 103–106. [Google Scholar]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Bryant, J.P.; Chapin, F.S.; Klein, D.R. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Nunes, C.N.; Emond, J.P. Relationship between weight loss and visual quality of fruits and vegetables. Proc. Fla. State Hortic. Soc. 2007, 120, 235–245. [Google Scholar]
- Hoque, M.M.; Ajwa, H.; Othman, M.; Smith, R.; Cahn, M. Yield and postharvest quality of lettuce in response to nitrogen, phosphorus, and potassium fertilizers. Hortic. Sci. 2010, 45, 1539–1544. [Google Scholar] [CrossRef] [Green Version]
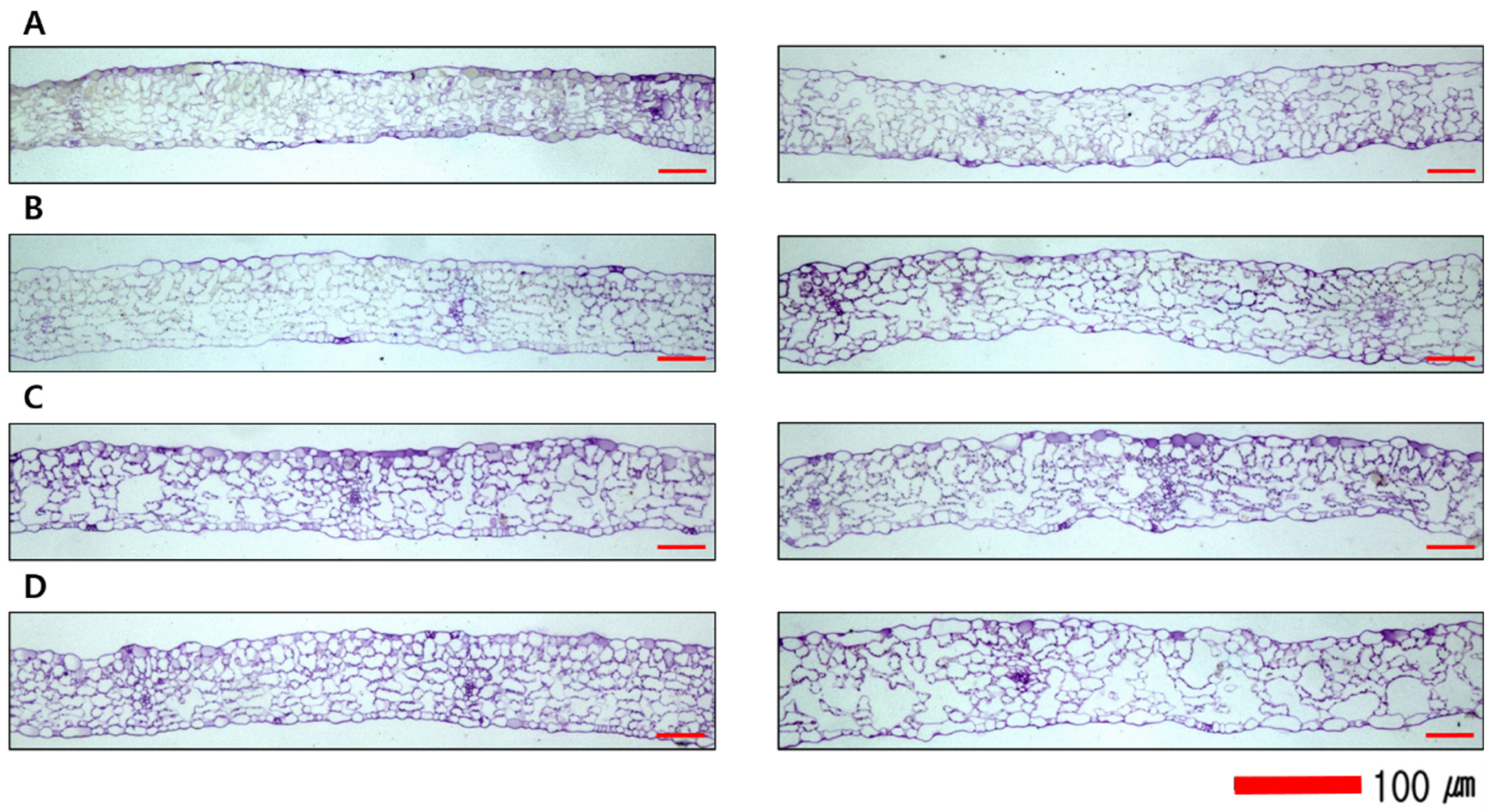
| Cultivar | Amount of Applied Nutrient Solution (mL·d−1·plant−1) | Plant Height (cm) | No. of Leaves x | Fresh Weight of Shoot (g·plant−1) | Dry Matter (%) | Crude Fiber (%) |
|---|---|---|---|---|---|---|
| Geockchima | 250 | 31.50 ± 1.58 b z | 24.73 ± 1.84 b | 76.75 ± 9.50 c | 5.60 ± 0.62 a | 1.29 ± 0.06 a |
| 500 | 32.18 ± 1.87 b | 27.43 ± 5.54 b | 119.35 ± 47.90 b | 4.50 ± 0.55 b | 1.43 ± 0.07 a | |
| 1000 | 36.80 ± 1.76 a | 34.33 ± 3.16 a | 253.43 ± 65.51 a | 3.25 ± 0.19 c | 0.91 ± 0.03 b | |
| 2000 | 35.65 ± 1.91 a | 33.75 ± 3.50 a | 249.05 ± 53.51 a | 3.35 ± 0.51 c | 0.84 ± 0.01 b | |
| Cheongchima | 250 | 25.70 ± 2.18 c | 31.93 ± 1.58 c | 78.05 ± 11.59 d | 6.20 ± 0.51 a | 1.31 ± 0.06 a |
| 500 | 29.93 ± 2.57 b | 35.28 ± 2.60 bc | 127.25 ± 26.01 c | 4.75 ± 0.62 b | 1.23 ± 0.06 a | |
| 1000 | 33.65 ± 0.97 a | 40.58 ± 2.68 a | 284.28 ± 12.62 a | 3.72 ± 0.36 c | 0.60 ± 0.06 b | |
| 2000 | 33.90 ± 1.50 a | 38.68 ± 3.91 ab | 228.05 ± 43.76 b | 3.65 ± 0.48 c | 0.61 ± 0.03 b | |
| Significance | Cultivar (A) | * | * | NS | NS | * |
| Irrigation amount (B) | ** | ** | ** | ** | ** | |
| Interaction (A × B) | NS | NS | NS | NS | ** |
| Cultivar | Amount of Nutrient Solution Applied (mL·d−1·plant−1) | |||
|---|---|---|---|---|
| 250 | 500 | 1000 | 2000 | |
| Geockchima |  |  |  |  |
| Cheongchima |  |  |  |  |
| Cultivar | Amount of Applied Nutrient Solution (mL·d−1·plant−1) | Texture/Puncture Force (N·m−2) | Osmolality (mmol·kg−1) | Ascorbic Acid Content (mg·100 g−1 FW) | Color Value of Leaf | Total Chlorophyll Content (mg·g−1 FW) | Total Anthocyanin Content (mg·100 g−1 FW) | ||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||||
| Geokchima | 250 | 1.8 a | 492.0 a | 10.28 c | 39.4 | −0.8 a z | 10.5 b | 1.71 a | 4.07 a |
| 500 | 1.7 a | 442.4 b | 20.01 b | 41.7 | −3.6 b | 11.7 b | 1.58 a | 3.10 b | |
| 1000 | 1.2 b | 415.6 c | 26.03 a | 42.9 | −7.3 c | 20.1 a | 0.87 b | 2.10 c | |
| 2000 | 1.0 b | 346.7 d | 24.43 a | 46.2 | −10.0 d | 21.2 a | 0.98 b | 1.49 c | |
| Cheongchima | 250 | 1.8 a | 447.3 a | 16.81 b | 45.4 | −12.7 a | 16.4 b | 2.03 a | - |
| 500 | 1.5 ab | 439.0 a | 12.52 c | 47.4 | −13.9 ab | 19.0 a | 1.89 a | - | |
| 1000 | 1.2 bc | 372.3 b | 21.14 a | 46.5 | −14.8 b | 20.2 a | 1.32 b | - | |
| 2000 | 0.9 c | 321.8 c | 21.52 a | 47.4 | −14.1 ab | 19.8 a | 1.42 b | - | |
| Significance | Cultivar (A) | NS | NS | NS | NS | ** | * | * | - |
| Irrigation amount (B) | ** | ** | ** | NS | ** | * | ** | ** | |
| Interaction (A × B) | NS | * | * | NS | ** | NS | NS | - | |
| Cultivar | Amount of Applied Nutrient Solution (mL·d−1·plant−1) | Mineral Content (%, DW) | C/N Ratio | ||||
|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | |||
| Geockchima | 250 | 4.68 ab z | 0.22 | 3.31 a | 1.16 a | 0.24 | 7.94 a |
| 500 | 4.40 b | 0.20 | 3.24 a | 1.04 ab | 0.20 | 8.13 a | |
| 1000 | 4.91 a | 0.25 | 3.51 a | 1.01 ab | 0.22 | 6.93 b | |
| 2000 | 4.87 a | 0.19 | 2.60 b | 0.91 b | 0.14 | 7.22 b | |
| Cheongchima | 250 | 4.55 b | 0.27 | 1.90 c | 0.83 a | 0.18 | 9.09 a |
| 500 | 4.88 b | 0.24 | 2.22 c | 0.74 ab | 0.17 | 8.53 a | |
| 1000 | 5.40 a | 0.31 | 3.98 b | 0.62 b | 0.17 | 6.63 b | |
| 2000 | 5.48 a | 0.27 | 5.80 a | 0.61 b | 0.16 | 6.49 b | |
| Significance | Cultivar (A) | ** | NS | NS | ** | NS | NS |
| Irrigation amount (B) | ** | NS | ** | * | NS | ** | |
| Interaction (A × B) | ** | NS | * | NS | NS | ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-S.; Chandra, D.; Son, J. Growth, Physicochemical, Nutritional, and Postharvest Qualities of Leaf Lettuce (Lactuca sativa L.) as Affected by Cultivar and Amount of Applied Nutrient Solution. Horticulturae 2022, 8, 436. https://doi.org/10.3390/horticulturae8050436
Lee J-S, Chandra D, Son J. Growth, Physicochemical, Nutritional, and Postharvest Qualities of Leaf Lettuce (Lactuca sativa L.) as Affected by Cultivar and Amount of Applied Nutrient Solution. Horticulturae. 2022; 8(5):436. https://doi.org/10.3390/horticulturae8050436
Chicago/Turabian StyleLee, Jung-Soo, Dulal Chandra, and Jinkwan Son. 2022. "Growth, Physicochemical, Nutritional, and Postharvest Qualities of Leaf Lettuce (Lactuca sativa L.) as Affected by Cultivar and Amount of Applied Nutrient Solution" Horticulturae 8, no. 5: 436. https://doi.org/10.3390/horticulturae8050436
APA StyleLee, J.-S., Chandra, D., & Son, J. (2022). Growth, Physicochemical, Nutritional, and Postharvest Qualities of Leaf Lettuce (Lactuca sativa L.) as Affected by Cultivar and Amount of Applied Nutrient Solution. Horticulturae, 8(5), 436. https://doi.org/10.3390/horticulturae8050436






