Floral Nectary and Trichome Structure of Hoya cagayanensis, Hoya lacunosa, and Hoya coriacea (Apocynaceae, Marsdenieae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Light Microscopy (LM)
2.3. Scanning Electron Microscopy (SEM)
2.4. Histochemical Tests
3. Results
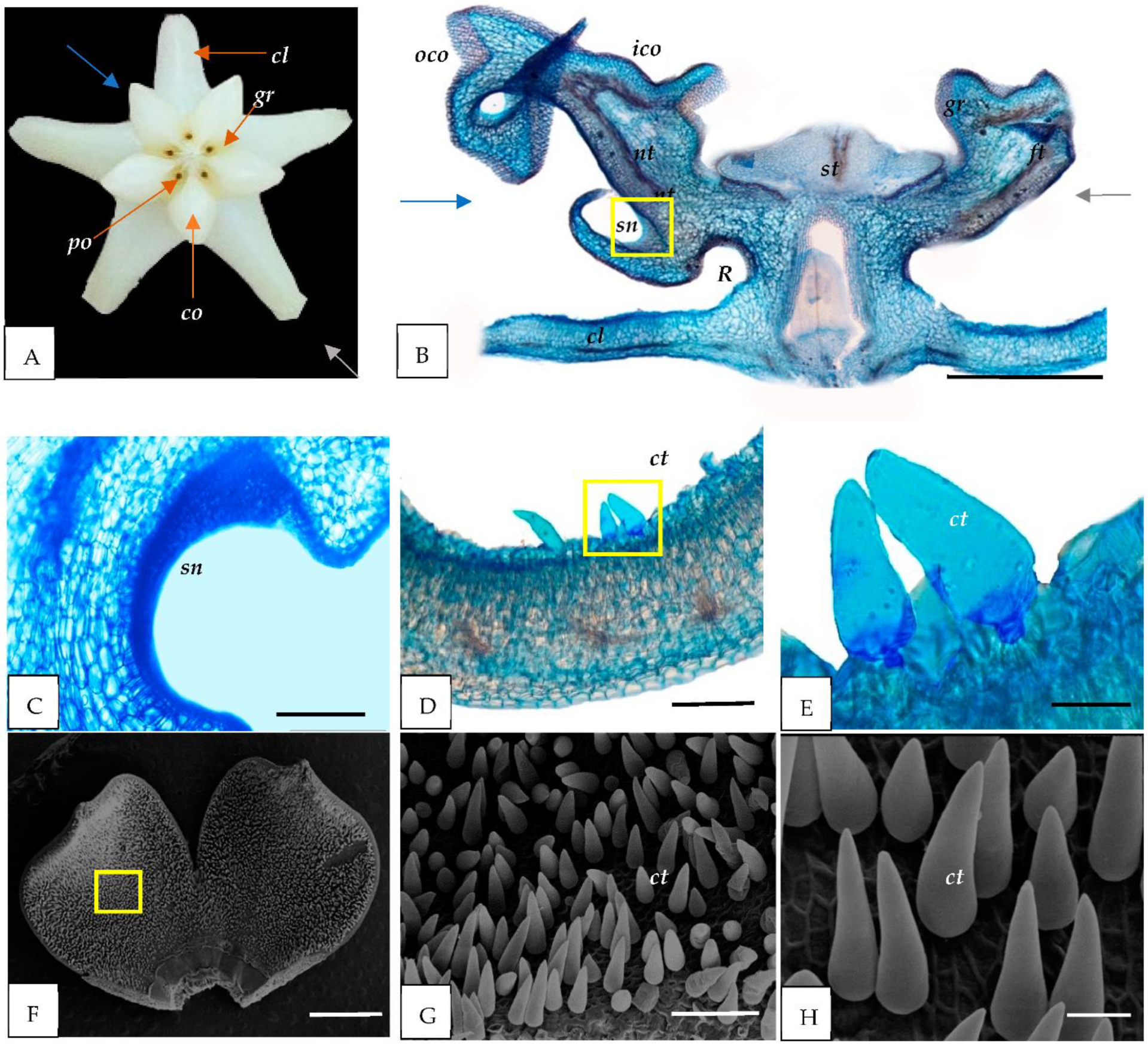
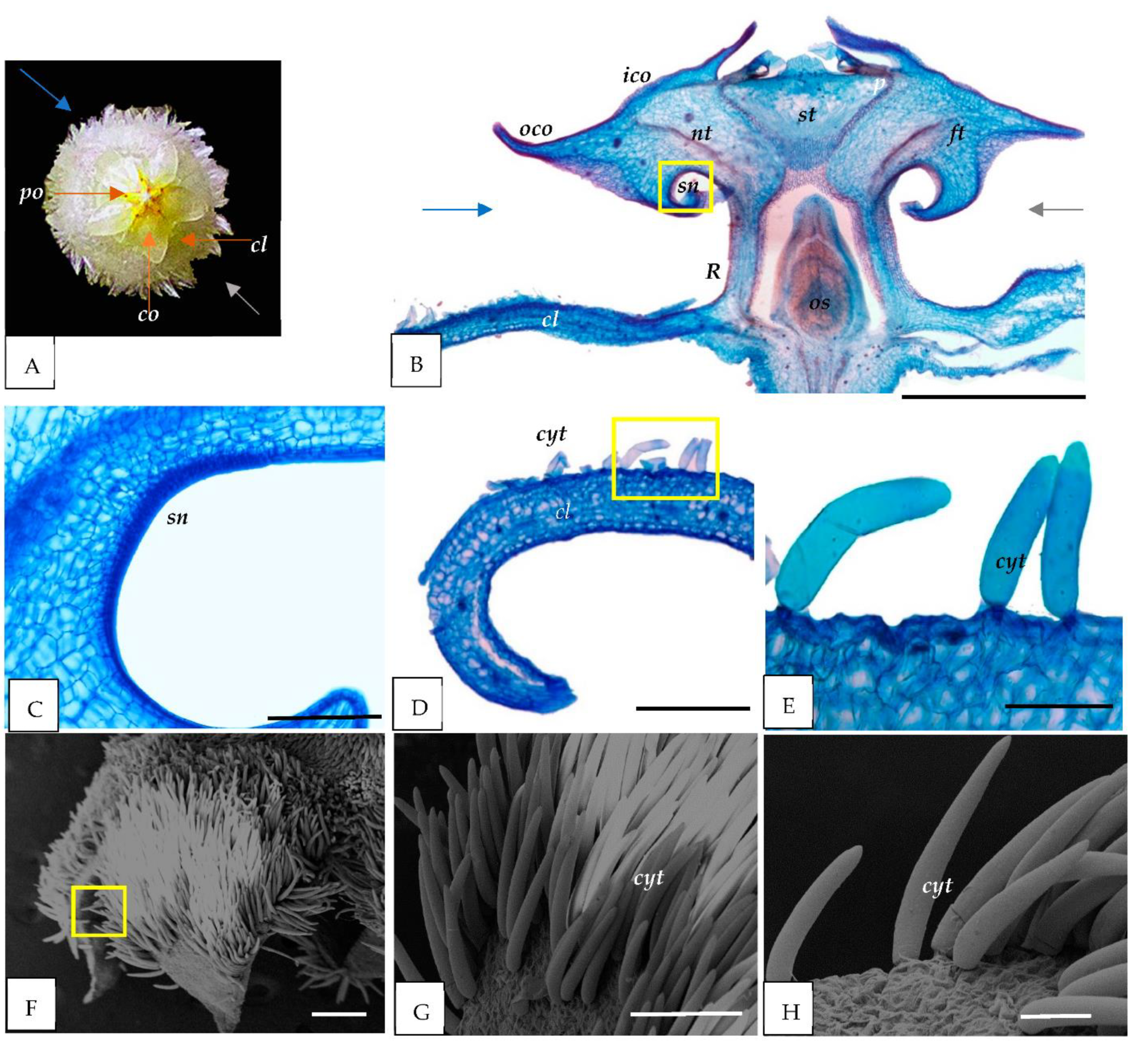
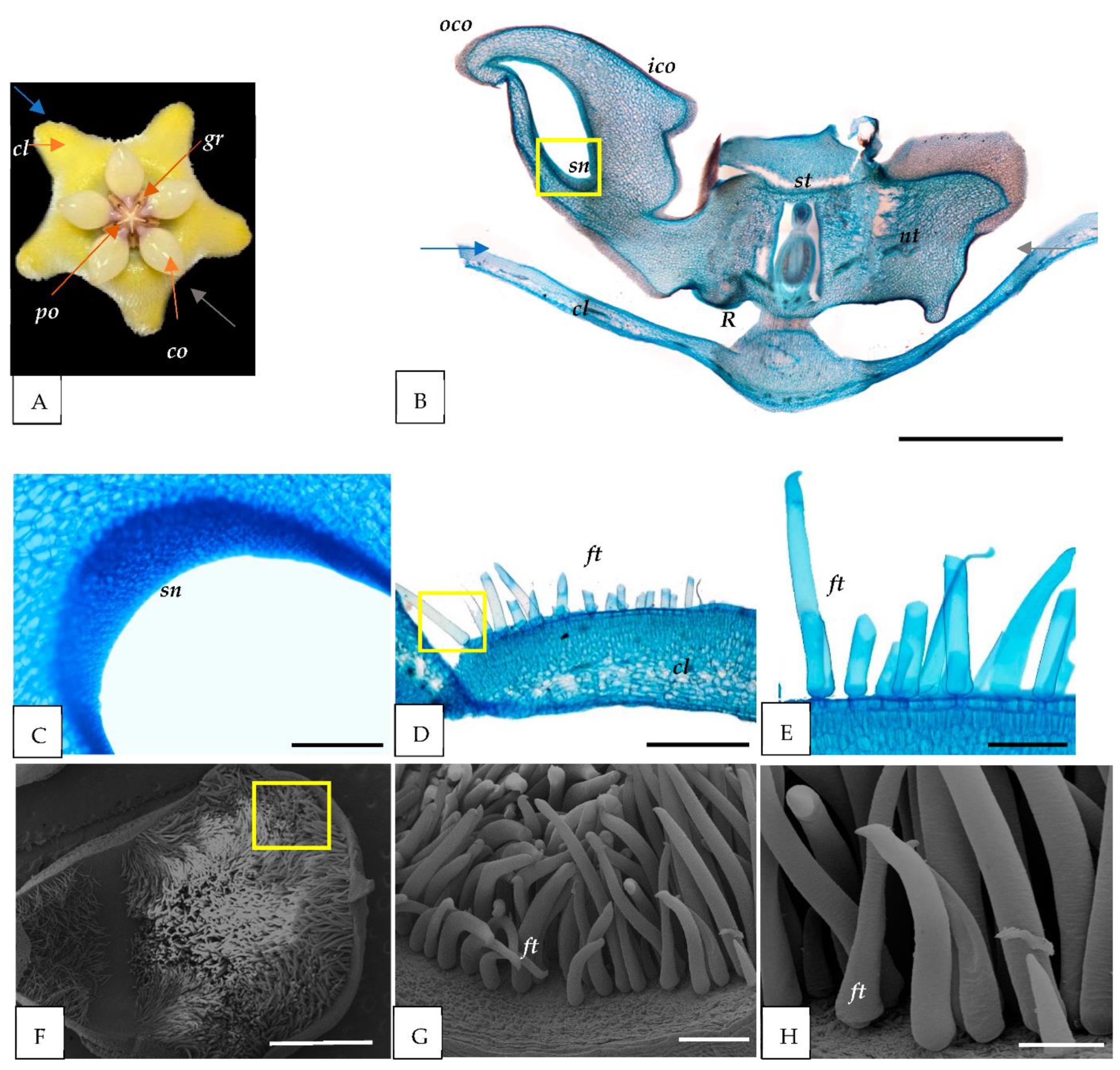
3.1. Morphological Study
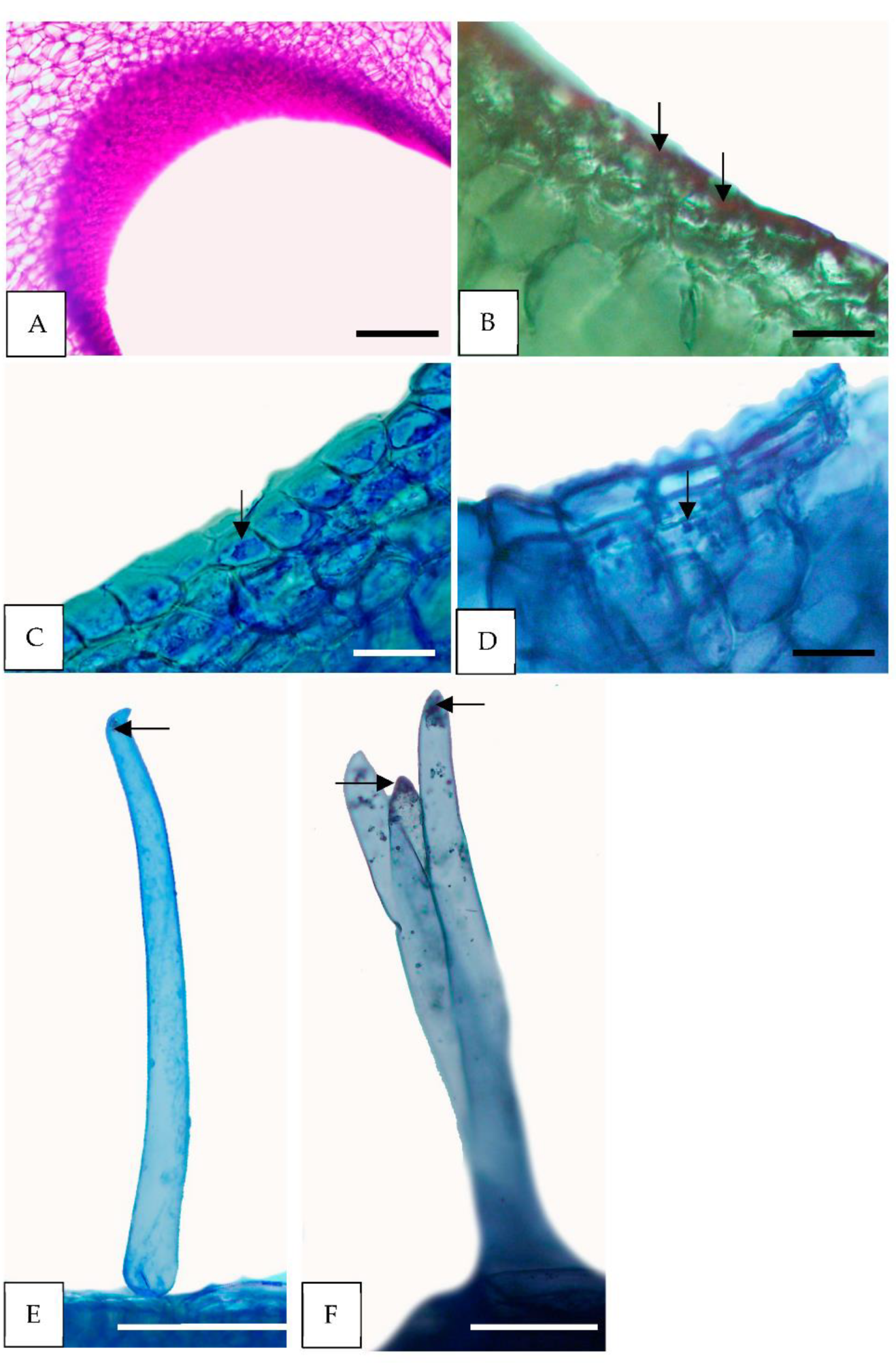
3.2. Light and Scanning Electron Microscopy
3.3. Histochemical Tests
4. Discussion
4.1. Nectary
4.2. Trichomes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodda, M.; Simonsson, N. Hoya medinillifolia (Apocynaceae Asclepiadoideae), A New Species from Lowland Forests of Sarawak, Borneo. Webbia 2011, 66, 149–154. [Google Scholar] [CrossRef]
- Rodda, M.; Ercole, E. Hoya papaschonii (Apocynaceae: Asclepiadoideae), A New Species from Southern Thailand with a Peculiar Corona. Phytotaxa 2014, 175, 97–106. [Google Scholar] [CrossRef][Green Version]
- Rodda, M.; Omlor, R. The Taxonomy of Oreosparte (Apocynaceae: Asclepiadoideae). Webbia 2014, 68, 91–95. [Google Scholar] [CrossRef]
- Lamb, A.; Rodda, M.; Gokulsing, L.; Bosuang, S.; Rahayu, S. A Guide to Hoyas of Borneo; Natural History Publications: Kota Kinabalu, Malaysia, 2016. [Google Scholar]
- Rahayu. Hoya as a Medical Plant. Warta Kebun Raya 2011, 11, 15–21. [Google Scholar]
- Rabbani, A.; Rahmad, Z.; Rosazlina, R.; Akomolafe, G.; Edzham, S.M.; Azmi, F. The Diversity of Hoya (Apocynaceae: Asclepiadoideae) in Some Parts of Kedah and Perak, Peninsular Malaysia. Trop. Agric. Sci. 2021, 44, 193–203. [Google Scholar] [CrossRef]
- Rahmad, Z.; Rabbani, A.; Hamzah, S.M.E.S.; Hamzah, Z.; Faizal, M. Notes on the Hoya (Apocynaceae, Asclepiadoideae) from Pergau Forest Reserve, Kelantan, Peninsular Malaysia. Conf. Ser. Earth Environ. Sci. 2020, 549, 012031. [Google Scholar]
- Rodda, M.; Juhonewe, N.S.; Middleton, D.J. The Taxonomic Status of the Presumed Extinct Singaporean Hoya wallichii (Apocynaceae: Asclepiadoideae). Gard. Bull. Singap. 2016, 68, 75–187. [Google Scholar] [CrossRef]
- Rodda, M.; Rahmad, Z. Hoya peninsularis (Apocynaceae, Asclepiadoideae), a New Species from Peninsular Malaysia, and Notes on Hoya maingayi and Gongronema wrayi. Nord. J. Bot. 2020, 38, 42–52. [Google Scholar] [CrossRef]
- Salim, J.M.; Nikong, D. Notes on Hoya of Terengganu, Peninsular Malaysia. Malay. Nat. J. 2020, 72, 43–51. [Google Scholar]
- Lakshmi, S.R.; Benjamin, J.F.; Kumar, T.S.; Murthy, G.V.S.; Rao, M.V. In Vitro Propagation of Hoya wightii ssp. Palniensis KT Mathew, A Highly Vulnerable and Endemic Species of Western Ghats of Tamil Nadu, India. Afr. J. Biotechnol. 2009, 9, 84–92. [Google Scholar]
- Das, A.; Lee, S.H.; Hyun, T.K.; Kim, S.W.; Kim, J.Y. Plant Volatiles as Method of Communication. Plant Biotechnol. Rep. 2013, 7, 9–26. [Google Scholar] [CrossRef]
- Ramya, H.G.; Palanimuthu, V.; Rachna, S. An Introduction to Patchouli (Pogostemon cablin Benth.). A Medicinal and Aromatic Plant: It’s Importance to Mankind. Agric. Eng. Int. CIGR J. 2013, 15, 243–250. [Google Scholar]
- Effmert, U.; Grobe, J.; Rose, U.S.R.; Ehrig, F.; Kagi, R.; Piechulla, B. Volatile Composition, Emission Pattern, and Localization of Floral Scent Emission in Mirabilis jalapa (nyctaginaceae). Am. J. Bot. 2005, 92, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Pansarin, L.M.; Castro, M.D.M.; Sazima, M. Osmophore and Elaiophores of Grobya amherstiae (Catasetinae, Orchidaceae) and Their Relation to Pollination. Bot. J. Linn. Soc. 2009, 159, 408–415. [Google Scholar] [CrossRef]
- Wiemer, A.P.; More, M.; Benitez-Vieyra, S.; Cocucci, A.A.; Raguso, R.A.; Sérsic, A.N. A Simple Floral Fragrance and Unusual Osmophore Structure in Cyclopogon elatus (Orchidaceae). Plant Biol. 2009, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Stpiczyńska, M.; Davies, K.L.; Świątek, P.; de Miranda, V.F.O. Floral Ultrastructure of Two Brazilian Aquatic–Epiphytic Bladderworts: Utricularia cornigera Studnička and U. nelumbifolia Gardner (Lentibulariaceae). Protoplasma 2017, 254, 353–366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demarco, D. Floral Glands in Asclepiads: Structure, Diversity and Evolution. Acta Bot. Bras. 2017, 31, 477–502. [Google Scholar] [CrossRef]
- Rao, V.S.; Ganguli, A. The Floral Anatomy of Some Asclepiadaceae. Proc. Indian Acad. Sci. 1963, 57, 15–44. [Google Scholar] [CrossRef]
- Kunze, H. Structure and Function in Asclepiad Pollination. Plant Syst. Evol. 1991, 176, 227–253. [Google Scholar] [CrossRef]
- Kunze, H. Corona and Nectar System in Asclepiadinae (Asclepiadaceae). Flora 1997, 192, 175–183. [Google Scholar] [CrossRef]
- Valente, M.C. Matelea maritima subsp. ganglinosa (Vell.) Font.—Anatomia e Vascularização Floral (Asclepiadaceae). Arq. Jard. Botânico Rio De Jan. 1995, 33, 75–98. [Google Scholar]
- Valente, M.C.; Costa, C.G. Estudo Anatômico da Flor de Marsdenia loniceroides E. Fournier (Asclepiadoideae—Apocynaceae). Rodriguésia 2005, 56, 51–66. [Google Scholar] [CrossRef]
- Castro, M.D.; Demarco, D. Phenolic Compounds Produced by Secretory Structures in Plants: A Brief Review. Nat. Prod. Commun. 2008, 3, 1273–1284. [Google Scholar]
- Wiemer, A.P.; Sérsic, A.N.; Marino, S.; Simões, A.O.; Cocucci, A.A. Functional Morphology and Wasp Pollination of Two South American Asclepiads (Asclepiadoideae—Apocynaceae). Ann. Bot. 2012, 109, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Demarco, D. Secretory Tissues, and the Morphogenesis and Histochemistry of Pollinarium in Flowers of Asclepiadeae (Apocynaceae). Int. J. Plant Sci. 2014, 175, 1042–1053. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Biology of Floral Scent; CRC Press: London, UK, 2006. [Google Scholar]
- Poinar, G.; Poinar, G. The Antiquity of Floral Secretory Tissues That Provide Today’s Fragrances. Hist. Biol. 2020, 32, 494–499. [Google Scholar] [CrossRef]
- Fahn, A. Secretory Tissues in Plants; Academic Press: London, UK, 1979. [Google Scholar]
- Chwil, M.; Kostryco, M.; Matraszek–Gawron, R. Comparative Studies on Structure of the Floral Nectaries and the Abundance of Nectar Produc-Tion of Prunus laurocerasus L. Protoplasma 2019, 256, 1705–1726. [Google Scholar] [CrossRef]
- Konarska, A.; Masierowska, M. Structure of Floral Nectaries and Female–Biased Nectar Production in Protandrous Species Geranium macrorrhizum and Geranium phaeum. Protoplasma 2020, 257, 501–523. [Google Scholar] [CrossRef]
- Faegri, K.; van der Pijl, L. The Principles of Pollination Ecology; Pergamon Press: New York, NY, USA, 1979. [Google Scholar]
- Galil, J.; Zeroni, M. Nectar System of Asclepias curassavica. Bot. Gaz. 1965, 126, 144–148. [Google Scholar] [CrossRef]
- Christ, P.; Schnepf, E. The Nectaries of Cynanchum vincetoxicum (Asclepiadaceae). Isr. J. Plant Sci. 1985, 34, 79–90. [Google Scholar]
- Kunze, H. Floral Morphology of some Gonolobeae (Asclepiadaceae). Bot. Jahrb. 1995, 117, 211–238. [Google Scholar]
- Kunze, H.; Liede, S. Observations on Pollination in Sarcostemma (Asclepiadaceae). Plant Syst. Evol. 1991, 178, 95–105. [Google Scholar] [CrossRef]
- Kunze, H. Pollination Ecology in Two Species of Gonolobus (Asclepiadaceae). Flora 1999, 194, 309–316. [Google Scholar] [CrossRef]
- Endress, M.E.; Bruyns, P.V. A Revised Classification of the Apocynaceae sl. Bot. Rev. 2000, 66, 1–56. [Google Scholar] [CrossRef]
- Vieira, M.F.; Shepherd, G.J. Oxypetalum banksii subsp. Banksii: A Taxon of Asclepiadaceae with an Extragynoecial Compitum. Plant Syst. Evol. 2002, 233, 199–206. [Google Scholar] [CrossRef]
- Valente, M.D.C.; Silva, N.M.F.D. Anatomia Floral de Barjonia erecta (Vell.) Schum. (Asclepiadaceae). Rodriguésia 1984, 36, 95–106. [Google Scholar] [CrossRef]
- Bruyns, P.V. A Revision of Hoodia and Lavrania (Asclepiadaceae-Stapelieae). Master’s Thesis, University of Cape Town, Cape Town, South Africa, 1994. [Google Scholar]
- Monteiro, M.M.; Demarco, D. Corona Development and Floral Nectaries of Asclepiadeae (Asclepiadoideae, Apocynaceae). Acta Bot. Bras. 2017, 31, 420–432. [Google Scholar] [CrossRef]
- Spence, J. Plant Histology. In Plant Cell Biology; Hawes, C., Satiat-Jeunemaitre, B., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 189–206. [Google Scholar]
- Zhao, Q.; Chen, X.Y. Development: A New Function of Plant Trichomes. Nat. Plants 2016, 2, 16096. [Google Scholar] [CrossRef]
- Kundan, M.; Gani, U.; Nautiyal, A.K.; Misra, P. Molecular Biology of Glandular Trichomes and Their Functions in Environmental Stresses. In Molecular Approaches in Plant Biology and Environmental Challenge; Singh, S.P., Upadhyay, S.K., Pandey, A., Kumar, S., Eds.; Springer: Singapore, 2019; pp. 365–393. [Google Scholar]
- Gershenzon, J.; Dudareva, N. The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Basir, S.; Akbar, M.A.; Talip, N.; Baharum, S.N.; Bunawan, H. An Integrative Volatile Terpenoid Profiling and Transcriptomics Analysis in Hoya cagayanensis, Hoya lacunosa and Hoya coriacea (Apocynaceae, Marsdenieae). Horticulturae 2022, 8, 224. [Google Scholar] [CrossRef]
- Puchtler, H.; Sweat Waldrop, F.; Conner, H.M.; Terry, M.S. Carnoy Fixation: Practical and Theoretical Considerations. Histochemie 1968, 16, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Noraini, M.A.A.; Ruzi, M. Plant Anatomy and Microscopy; Penerbit Universiti Kebangsaan Malaysia: Bangi, Malaysia, 2019. [Google Scholar]
- Kowalkowska, A.K.; Kozieradzka–Kiszkurno, M.; Turzynski, S. Morphological, Histological and Ultrastructural Features of Osmophores and Nectary of Bulbophyllum wendlandianum (Kraenzl.) Dammer (B. section Cirrhopetalum Lindl., Bulbophyllinae Schltr., Orchidaceae). Plant Syst. Evol. 2015, 301, 609–622. [Google Scholar] [CrossRef]
- Stern, W.L.; Curry, K.J.; Whitten, W.M. Staining Fragrance Glands in Orchid Flowers. Bull. Torrey Bot. Club 1986, 113, 288–297. [Google Scholar] [CrossRef]
- Vogel, S. The Role of Scent Glands Pollination; The National Science Foundation: Washington, DC, USA, 1990. [Google Scholar]
- Nepi, M. Nectary Structure and Ultrastructure. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 129–166. [Google Scholar]
- Pacini, E.; Nepi, M. Nectar production and presentation. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 167–214. [Google Scholar]
- Galetto, L.; Bernardello, G.; Sosa, C.A. The Relationship between Floral Nectar Composition and Visitors in Lycium (Solanaceae) from Argentina and Chile: What Does It Reflect? Flora 1998, 193, 303–314. [Google Scholar] [CrossRef]
- Kunze, H.; Wanntorp, L. The Gynostegium of Hoya spartioides (Apocynaceae–Asclepiadoideae): A Striking Case of Incongruence between Molecular and Phenotypic Evolution. Org. Divers. Evol. 2008, 8, 346–357. [Google Scholar] [CrossRef][Green Version]
- Wanntorp, L.; Kunze, H. Identifying Synapomorphies in the Flowers of Hoya and Dischidia, toward Phylogenetic Understanding. Int. J. Plant Sci. 2009, 170, 331–342. [Google Scholar] [CrossRef]
- Eisikowitch, D. Morpho–Ecological Aspects on the Pollination of Calotropis procera (Asclepiadaceae) in Israel. Plant Syst. Evol. 1986, 152, 185–194. [Google Scholar] [CrossRef]
- Lumer, C.; Yost, S.E. The Reproductive Biology of Vincetoxicum nigrum (L.) Moench (Asclepiadaceae), a Mediterranean Weed in New York State. Bull. Torrey Bot. Club 1995, 122, 15–23. [Google Scholar] [CrossRef]
- Vieira, M.F.; Shepherd, G.J. Pollinators of Oxypetalum (Asclepiadaceae) in Southeastern Brazil. Rev. Bras. Biol. 1999, 59, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, K.; Furukawa, S.; Kawakita, A. Pollinia Transfer on Moth Legs in Hoya carnosa (Apocynaceae). Am. J. Bot. 2017, 104, 953–960. [Google Scholar] [CrossRef]
- Medina, M.C.; Sousa-Baena, M.S.; Capelli, N.D.V.; Koch, R.; Demarco, D. Stinging Trichomes in Apocynaceae and Their Evolution in Angiosperms. Plants 2021, 10, 2324. [Google Scholar] [CrossRef] [PubMed]
- Ramayya, N. Classification, and Phylogeny of the Trichomes of Angiosperms. In Research Trends in Plant Anatomy; Ghouse, A.K.M., Yunus, M., Eds.; Tata McGraw-Hill Publishing Company Ltd.: New Delhi, India, 1972. [Google Scholar]
- Werker, E. Trichome Diversity and Development. In Advances in Botanical Research. Plant Trichomes; Hallahan, D.L., Gray, J.C., Eds.; Academic Press: New York, NY, USA, 2000; pp. 1–35. [Google Scholar]
- Bickford, C.P. Ecophysiology of Leaf Trichomes. Funct. Plant Biol. 2016, 43, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Dalin, P.; Ågren, J.; Björkman, C.; Huttunen, P.; Kärkkäinen, K. Leaf Trichome Formation and Plant Resistance to Herbivory. In Induced Plant Resistance to Herbivory; Springer: Dordrecht, The Netherlands, 2008; pp. 89–105. [Google Scholar]
- Moraes, T.M.S.; Guilherme, R.R.; Camilla, R.A.; Sebastião, J.S.N.; Maura, D.C. Comparative Leaf Anatomy and Micromorphology of Psychotria Species (Rubiaceae) from the Atlantic Rainforest. Acta Bot. Bras. 2011, 25, 178–190. [Google Scholar] [CrossRef]
- Spring, O. Chemotaxonomy Based on Metabolites from Glandular Trichomes. Adv. Bot. Res. 2000, 31, 153–174. [Google Scholar]


| Species | Voucher ID | Location | Coordinates |
|---|---|---|---|
| H. cagayanensis | ID031/2020 | Baling, Kedah | 5°30′06.8″ N 100°46′19.1″ E |
| H. lacunosa | ID033/2020 | Baling, Kedah | 5°34′02.7″ N 100°52′05.8″ E |
| H. coriacea | ID032/2020 | Sik, Kedah | 5°51′42.2″ N 100°50′11.1″ E |
| Tests | Target Constituents | Colour Observed | H. cagayanensis | H. lacunosa | H. coriacea | |||
|---|---|---|---|---|---|---|---|---|
| t | sn | t | sn | t | sn | |||
| PAS reagent | Polysaccharides | Pink | − | + | − | + | − | + |
| Lugol’s solution | Starch grains | Dark blue to black | − | + | − | + | − | + |
| Coomassie Blue | Protein | Blue | + | + | + | + | + | + |
| Sudan Black B | Lipids | Dark blue to black | + | + | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basir, S.; Saad, M.F.M.; Rahman, M.R.A.; Talip, N.; Baharum, S.N.; Bunawan, H. Floral Nectary and Trichome Structure of Hoya cagayanensis, Hoya lacunosa, and Hoya coriacea (Apocynaceae, Marsdenieae). Horticulturae 2022, 8, 420. https://doi.org/10.3390/horticulturae8050420
Basir S, Saad MFM, Rahman MRA, Talip N, Baharum SN, Bunawan H. Floral Nectary and Trichome Structure of Hoya cagayanensis, Hoya lacunosa, and Hoya coriacea (Apocynaceae, Marsdenieae). Horticulturae. 2022; 8(5):420. https://doi.org/10.3390/horticulturae8050420
Chicago/Turabian StyleBasir, Syazwani, Mohd Faiz Mat Saad, Mohamad Ruzi Abdul Rahman, Noraini Talip, Syarul Nataqain Baharum, and Hamidun Bunawan. 2022. "Floral Nectary and Trichome Structure of Hoya cagayanensis, Hoya lacunosa, and Hoya coriacea (Apocynaceae, Marsdenieae)" Horticulturae 8, no. 5: 420. https://doi.org/10.3390/horticulturae8050420
APA StyleBasir, S., Saad, M. F. M., Rahman, M. R. A., Talip, N., Baharum, S. N., & Bunawan, H. (2022). Floral Nectary and Trichome Structure of Hoya cagayanensis, Hoya lacunosa, and Hoya coriacea (Apocynaceae, Marsdenieae). Horticulturae, 8(5), 420. https://doi.org/10.3390/horticulturae8050420







