Effect of Silver Nitrate (AgNO3) and Nano-Silver (Ag-NPs) on Physiological Characteristics of Grapes and Quality during Storage Period
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Storage Conditions
2.2. Silver Nitrate (AgNO3) and Silver Nanoparticles (Ag-NPs) Preparation
2.3. Treatment of Research Work
2.4. Quality Assessments
2.4.1. Weight Loss Measurement
2.4.2. Firmness
2.4.3. Soluble Solid Content (SSC)
2.4.4. Titratable Acidity (TA) Measurement
- M = molarity of NaOH
- T = titre of NaOH required (mL)
- V = volume of sample used (mL)
2.5. Determination of Malondialdehyde (MDA) Content
2.6. Determination of Polyphenol Oxidase (PPO)
2.7. Determination of Pyrogallol Peroxidase (POD)
2.8. Determination of Pectin Methyl Esterase Activity (PME)
2.9. Storage Life
2.10. Statistical Analysis
3. Results
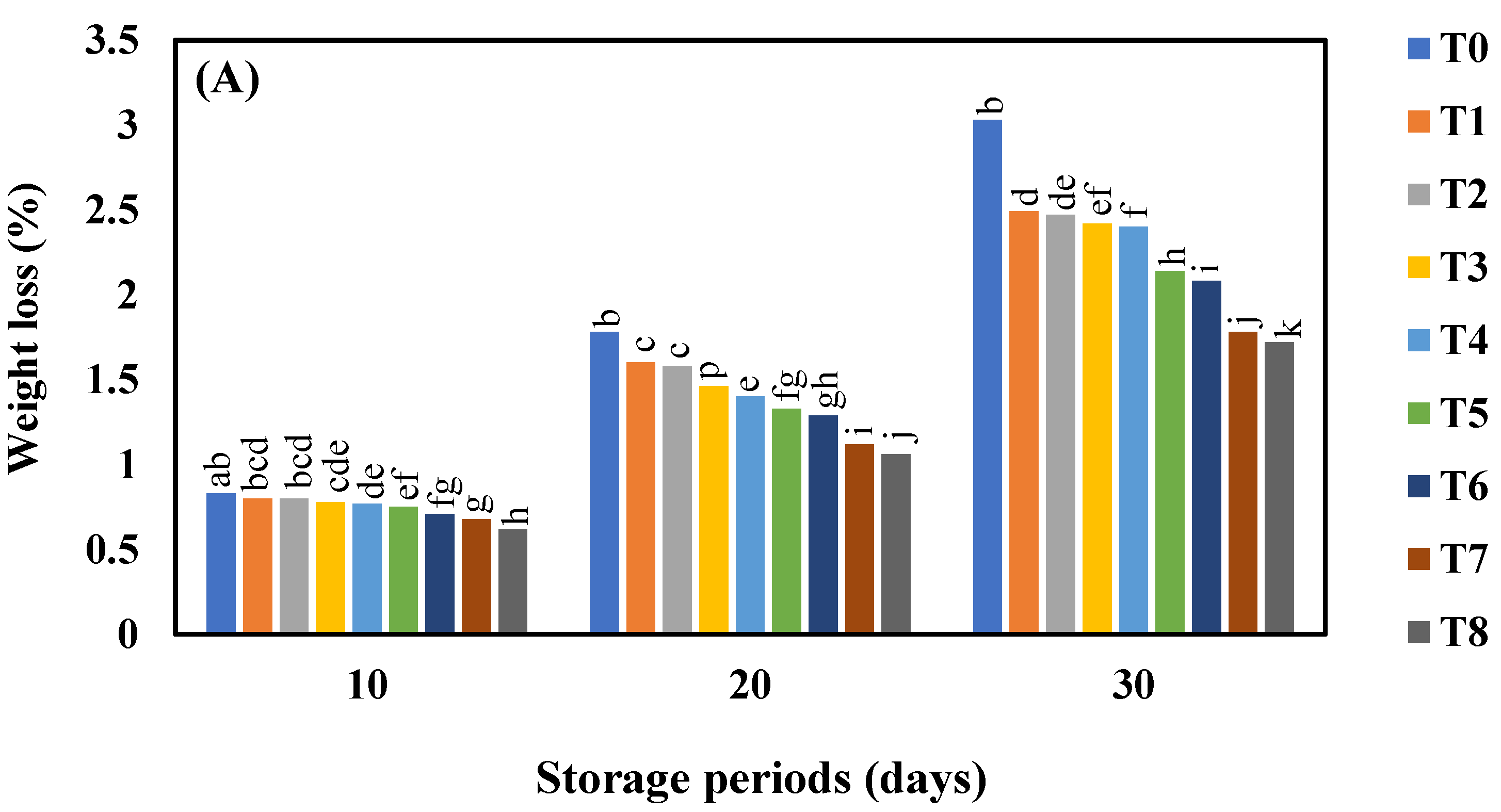
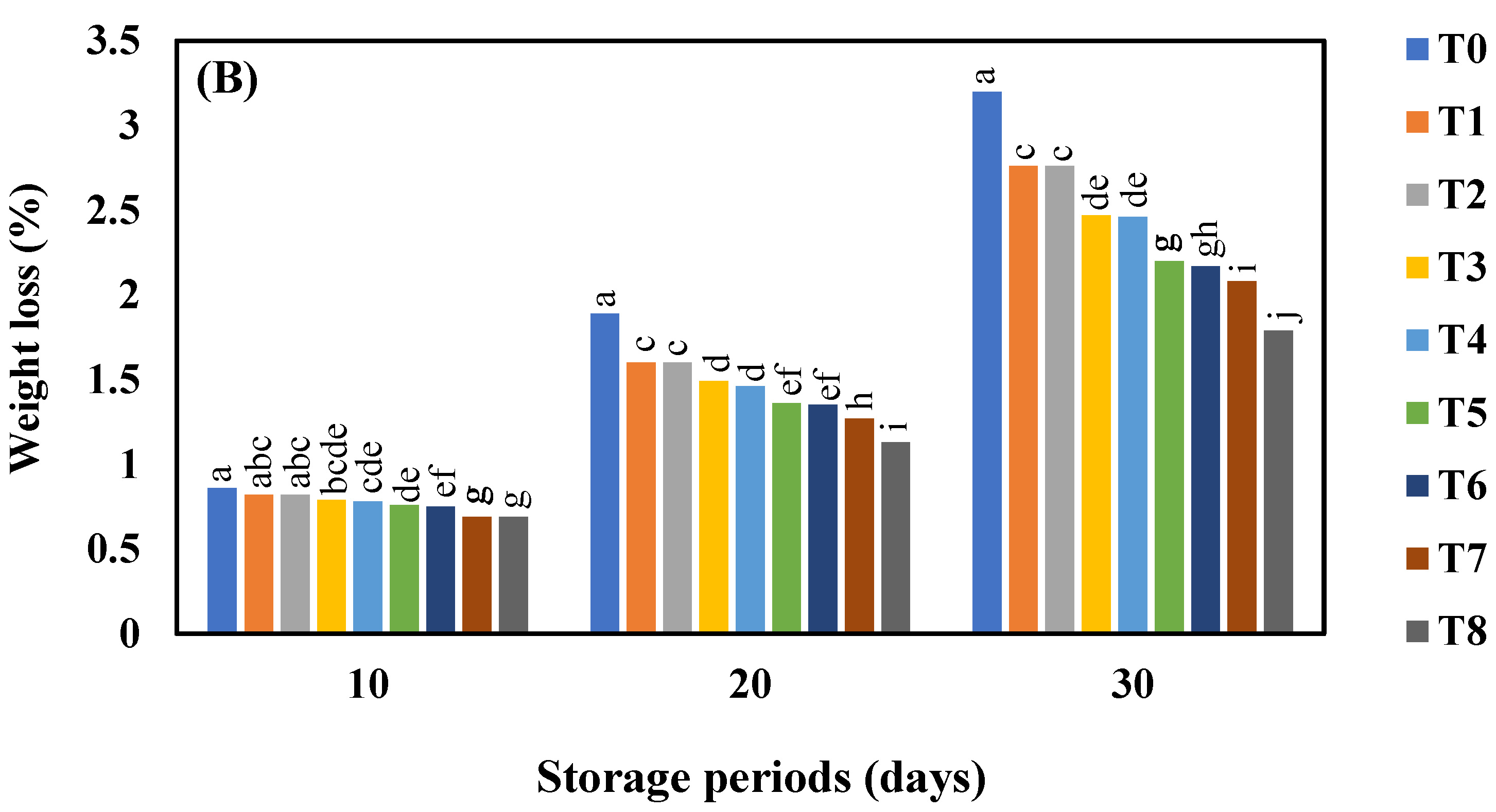
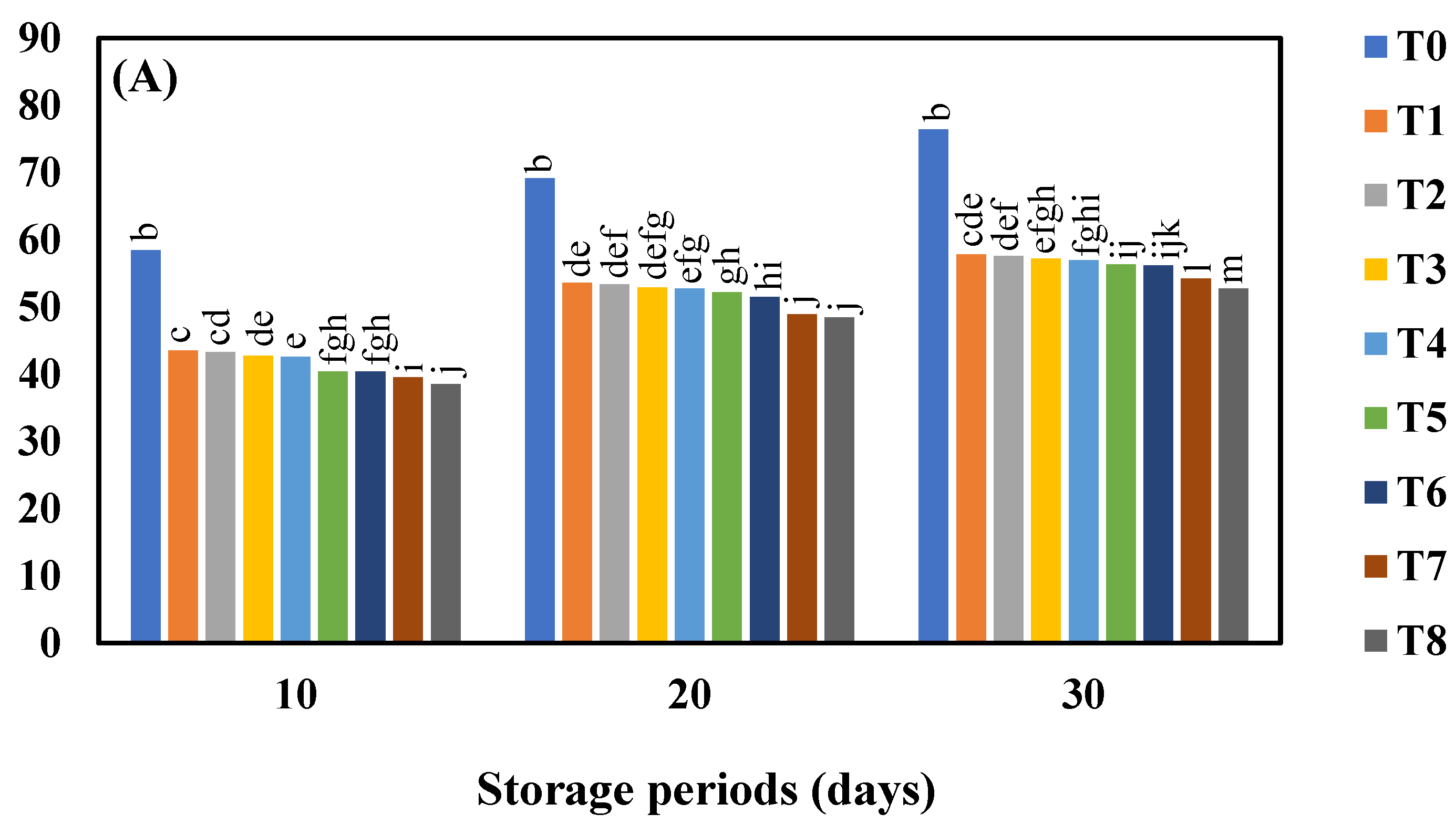
3.1. Effect of AgNO3 and Ag-NPs on the Weight Loss (%) in Shine Muscat (A) and Kyoho (B) Grape Varieties during 30 Days Cold Storage
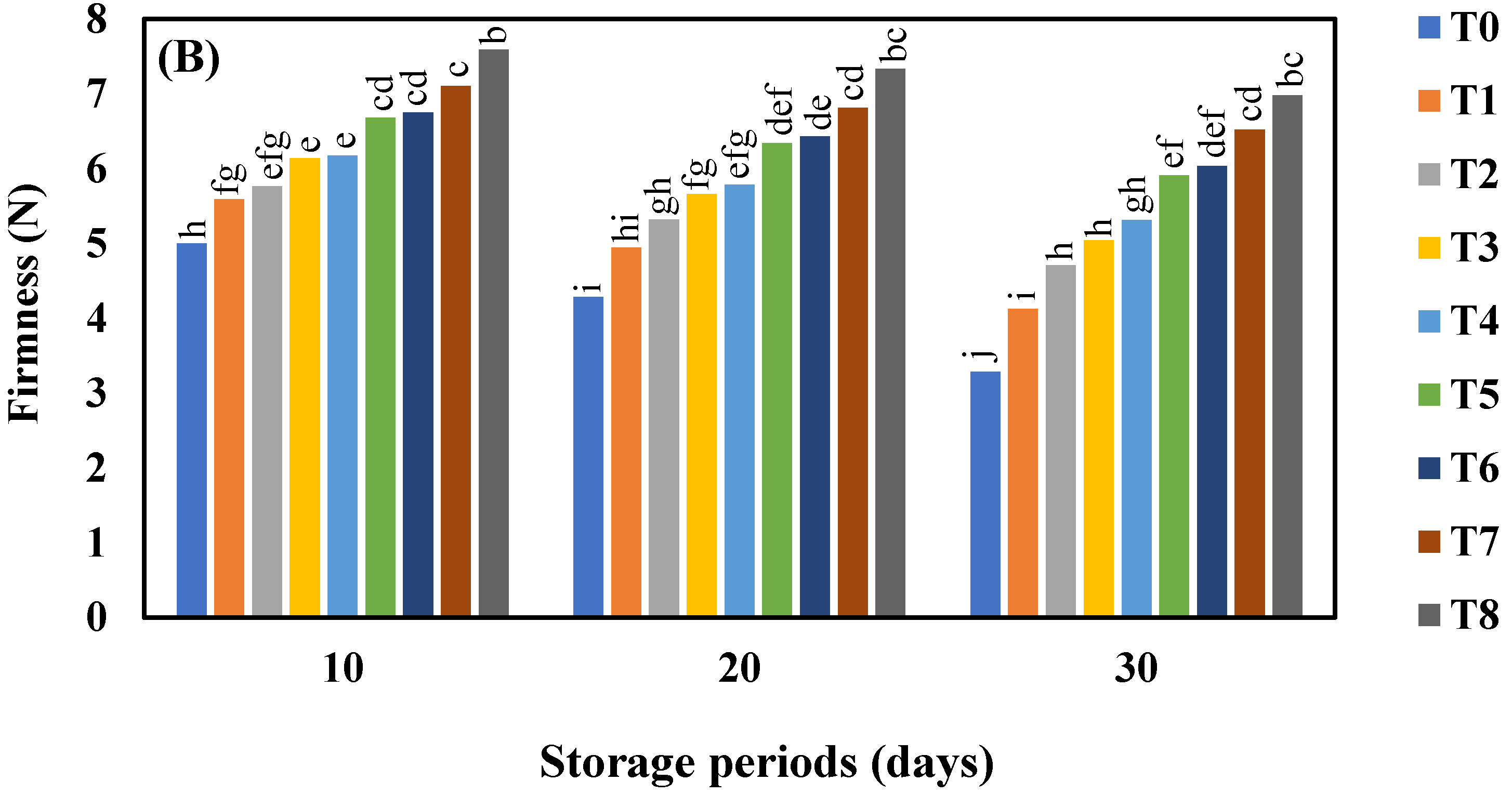
3.2. Effect of AgNO3 and Ag-NPs on Firmness Force (N) in Shine Muscat and Kyoho Grape Varieties during 30 Days Cold Storage
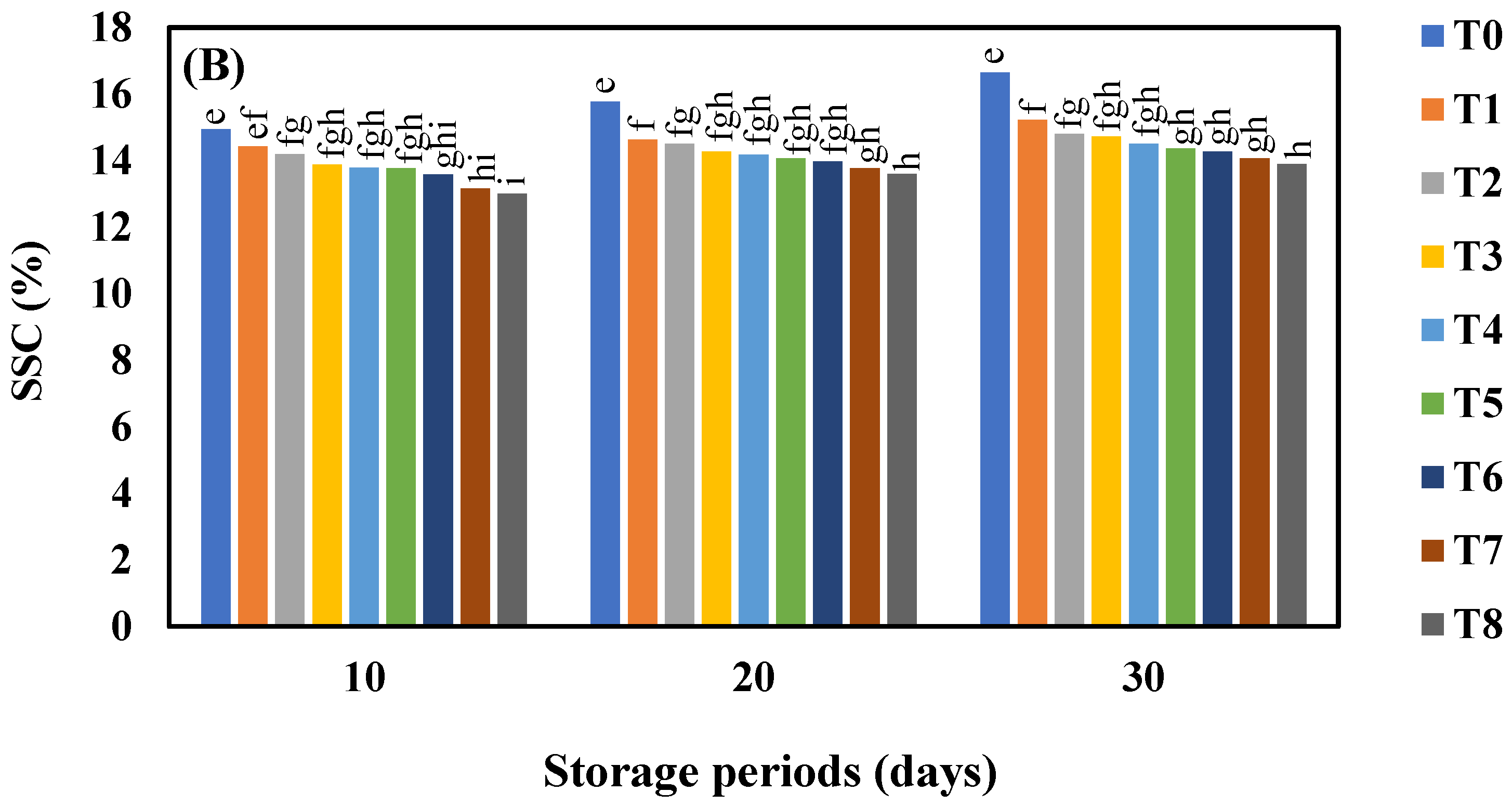
3.3. Effect of AgNO3 and Ag-NPs on Soluble Solids Content (%) in Shine Muscat and Kyoho Grape Varieties during 30 Days Cold Storage
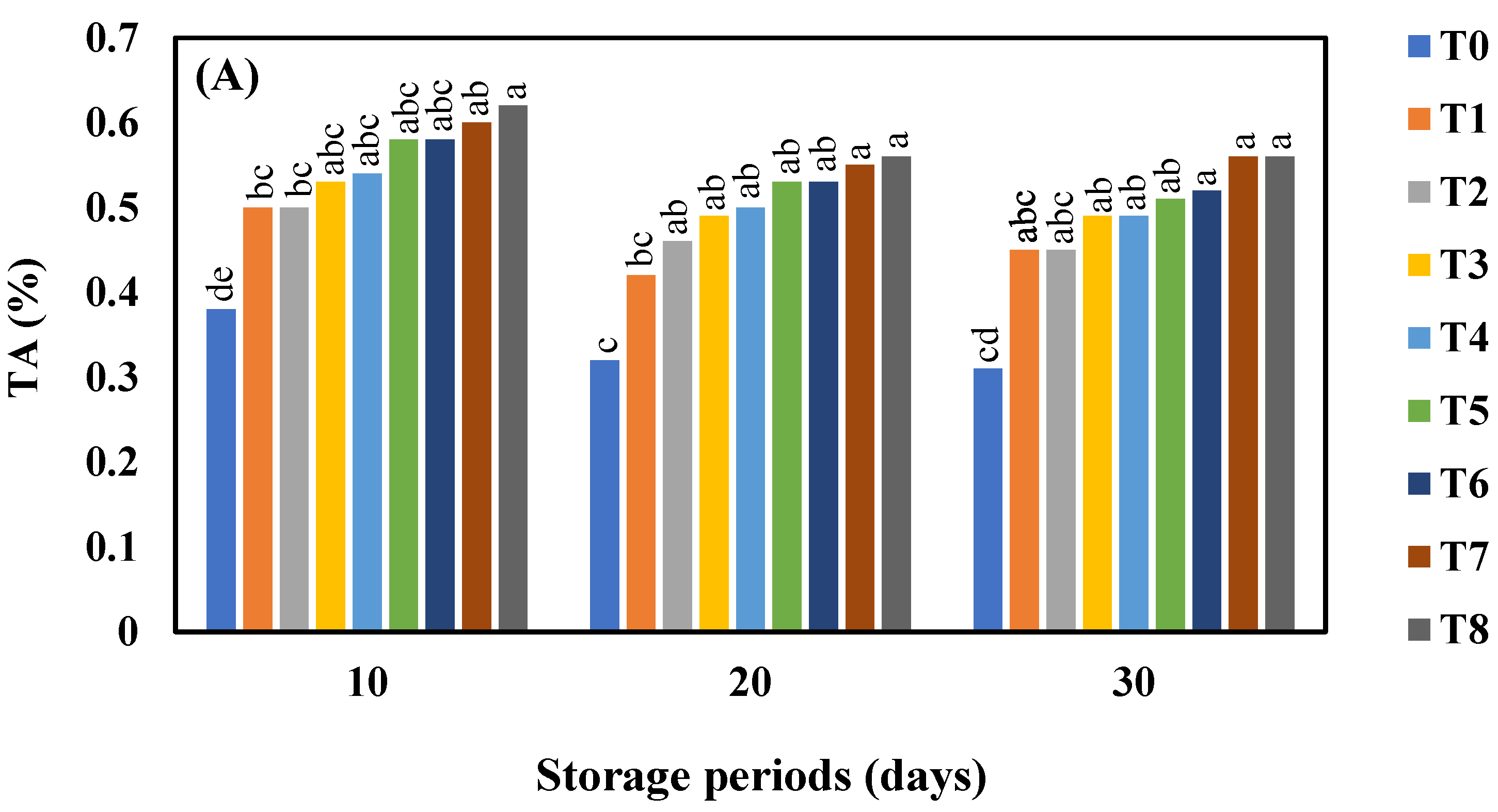
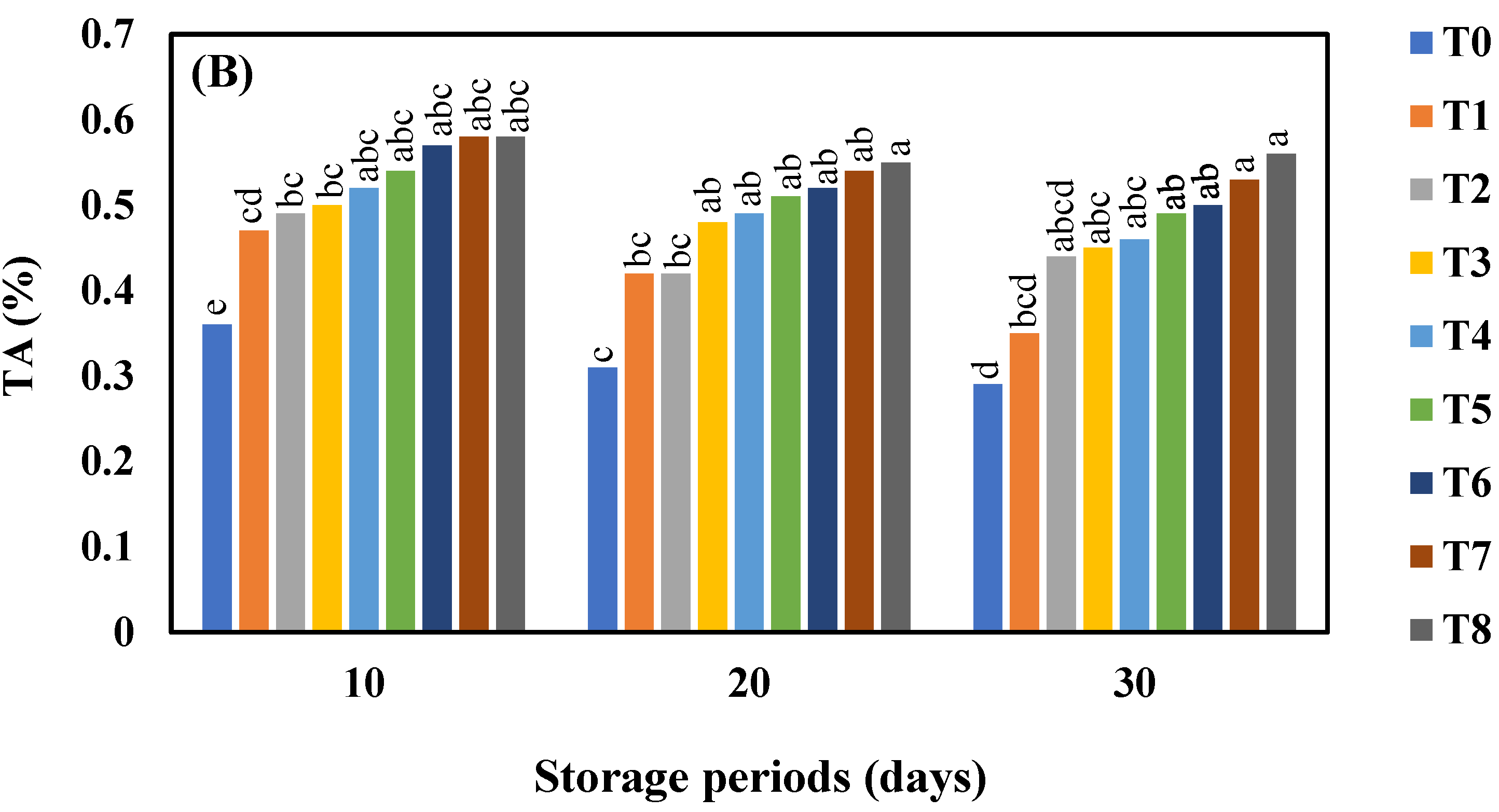
3.4. Effect of AgNO3 and Ag-NPs on Titratable Acidity (%) in Shine Muscat and Kyoho Grape Varieties during 30 Days Cold Storage
3.5. Effect of AgNO3 and Ag-NPs on Malondialdehyde (MDA) content in Shine Muscat and Kyoho Grape Varieties during 30 Days Cold Storage
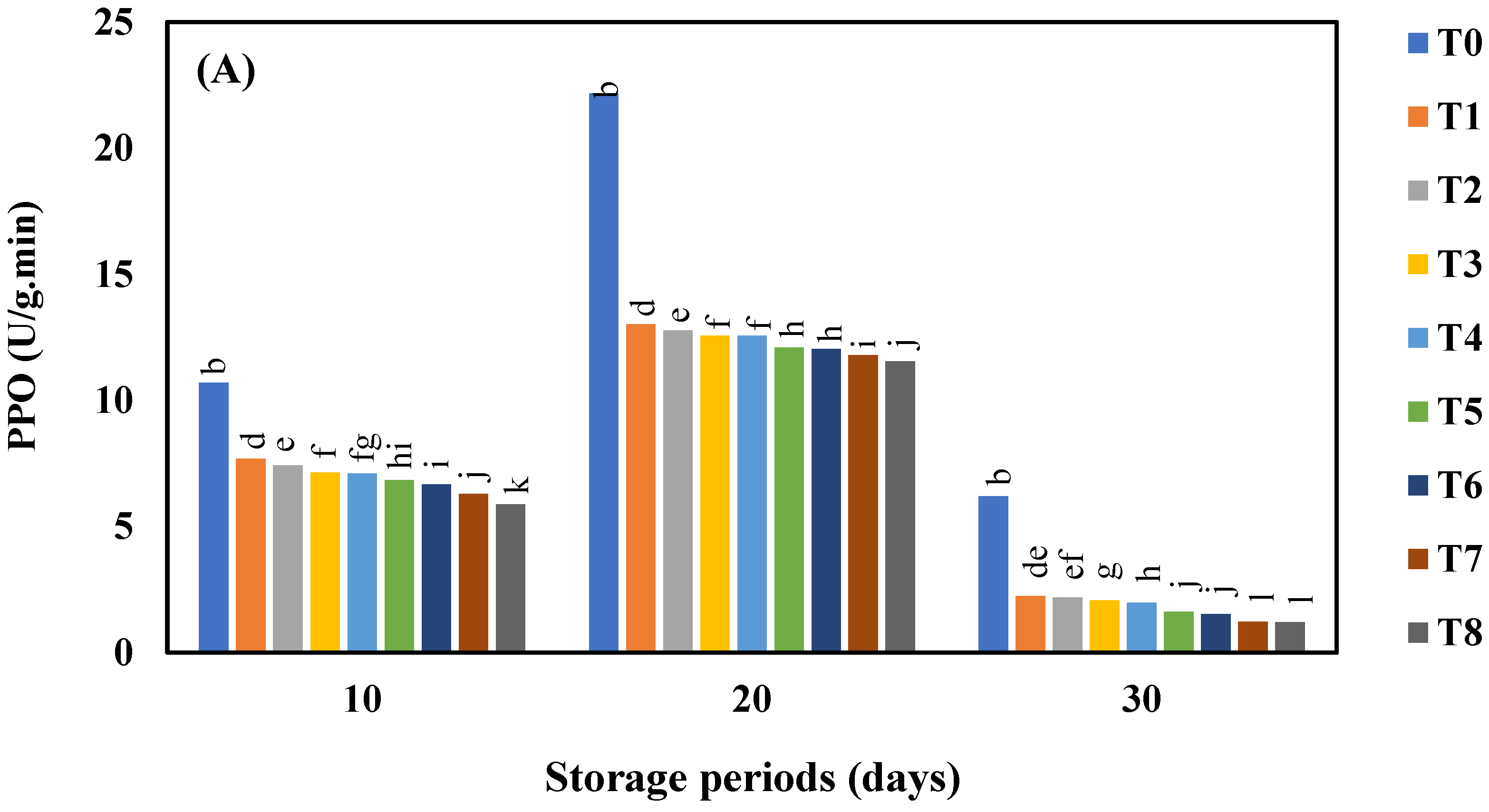
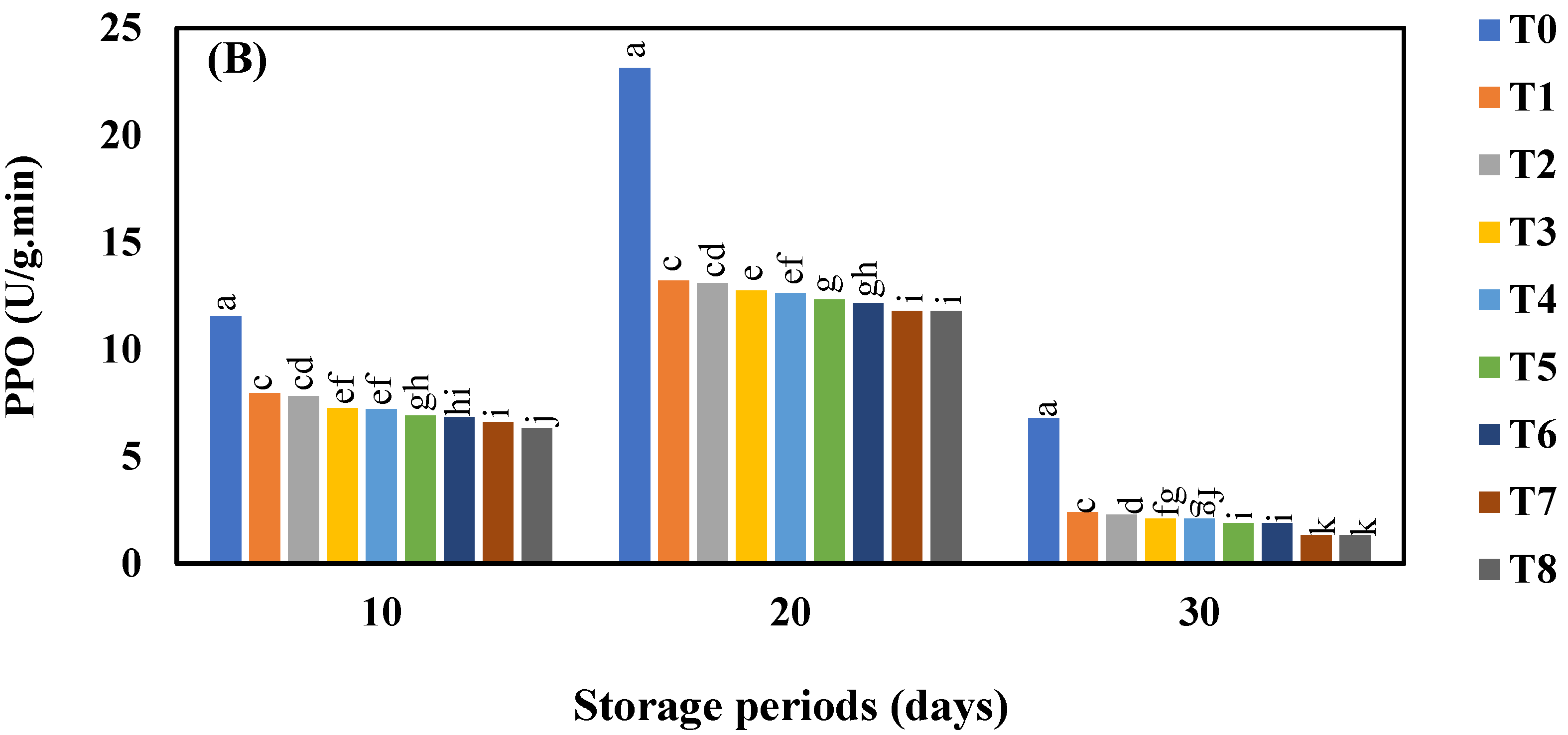
3.6. Effect of AgNO3 and Ag-NPs on Polyphenol Oxidase (PPO) Activity in Shine Muscat and Kyoho Grape Varieties during 30 Days Cold Storage
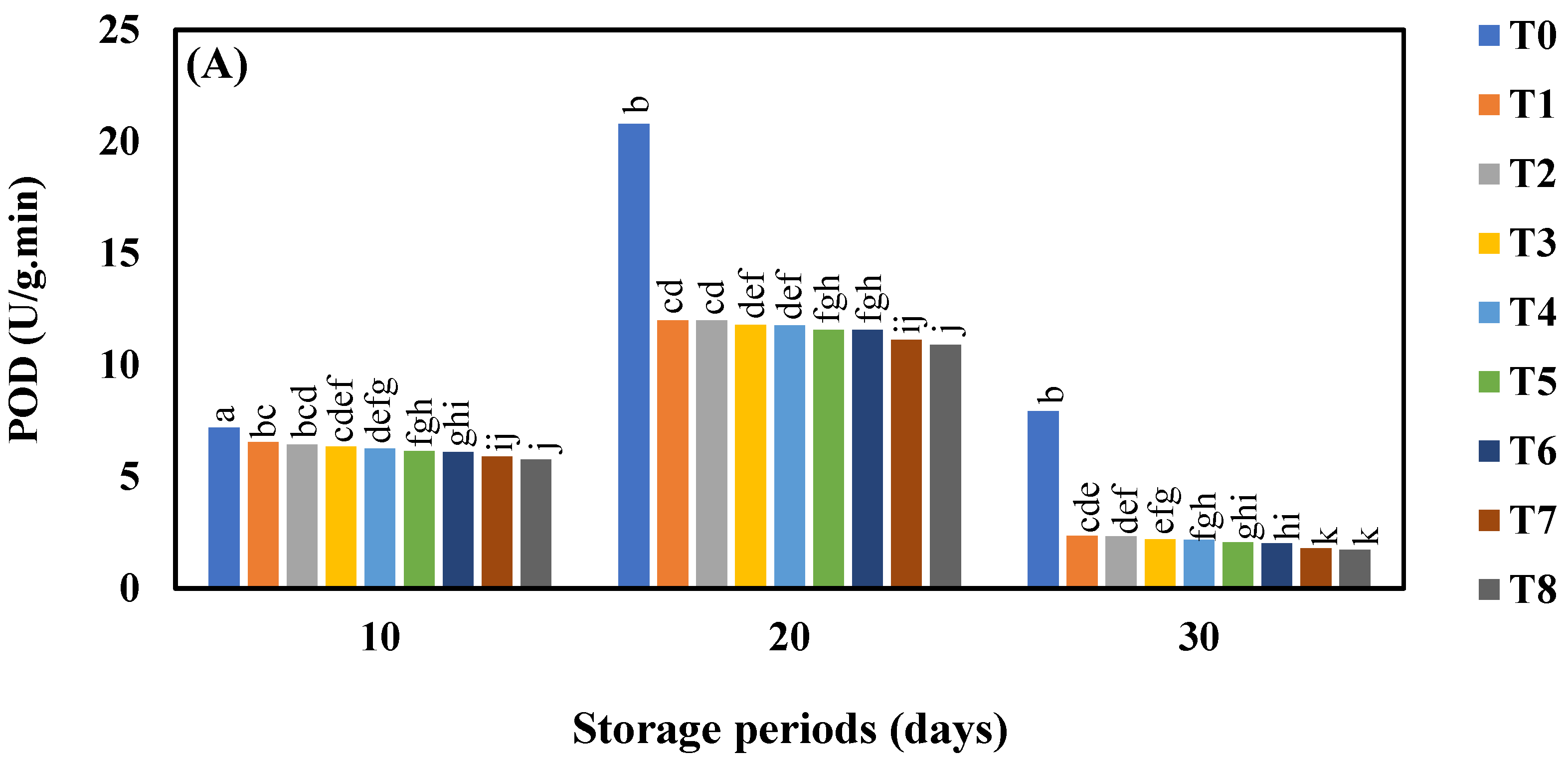
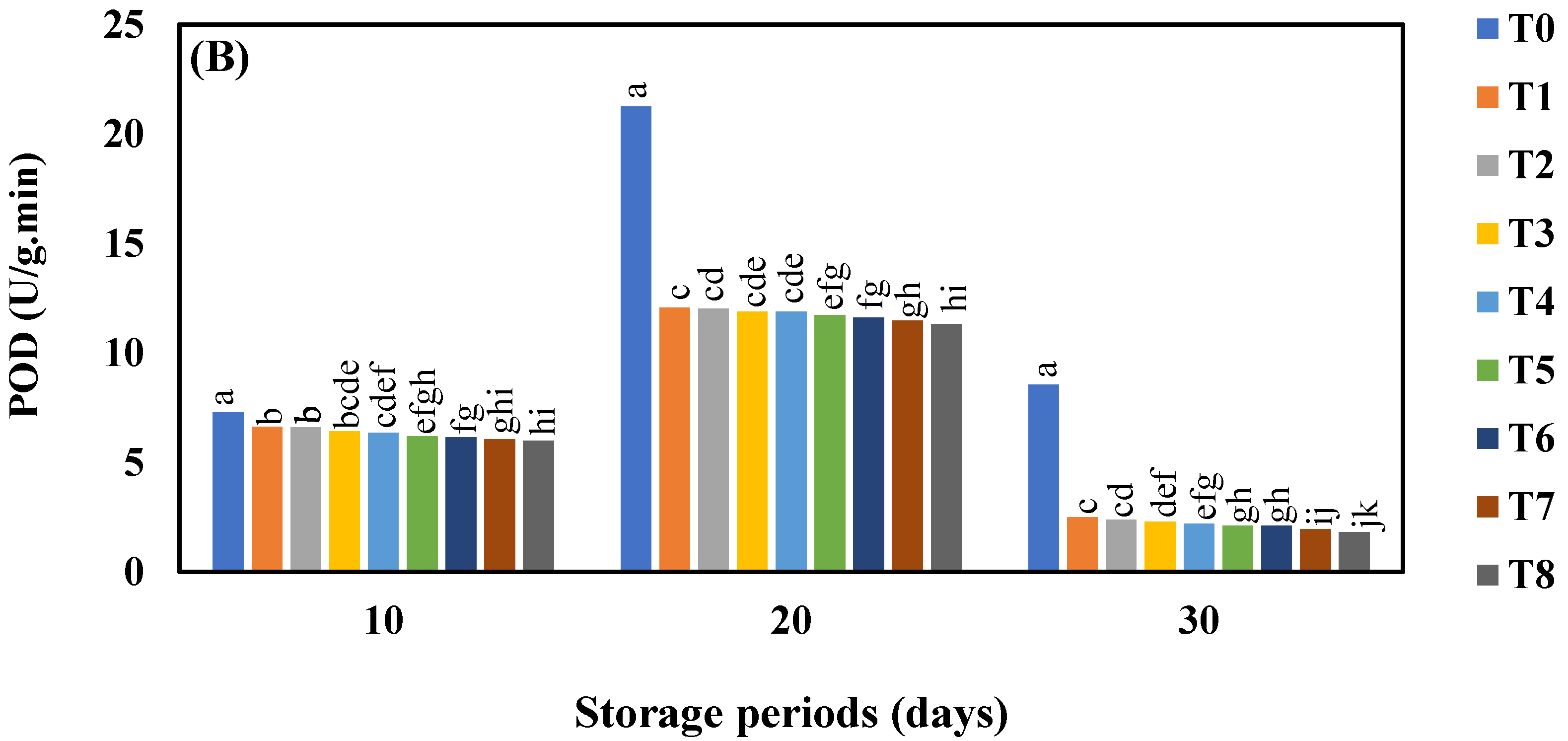
3.7. Effect of AgNO3 and Ag-NPs on Pyrogallol Peroxidase (POD) Activity in Shine Muscat and Kyoho Grape Varieties during 30 Days Cold Storage
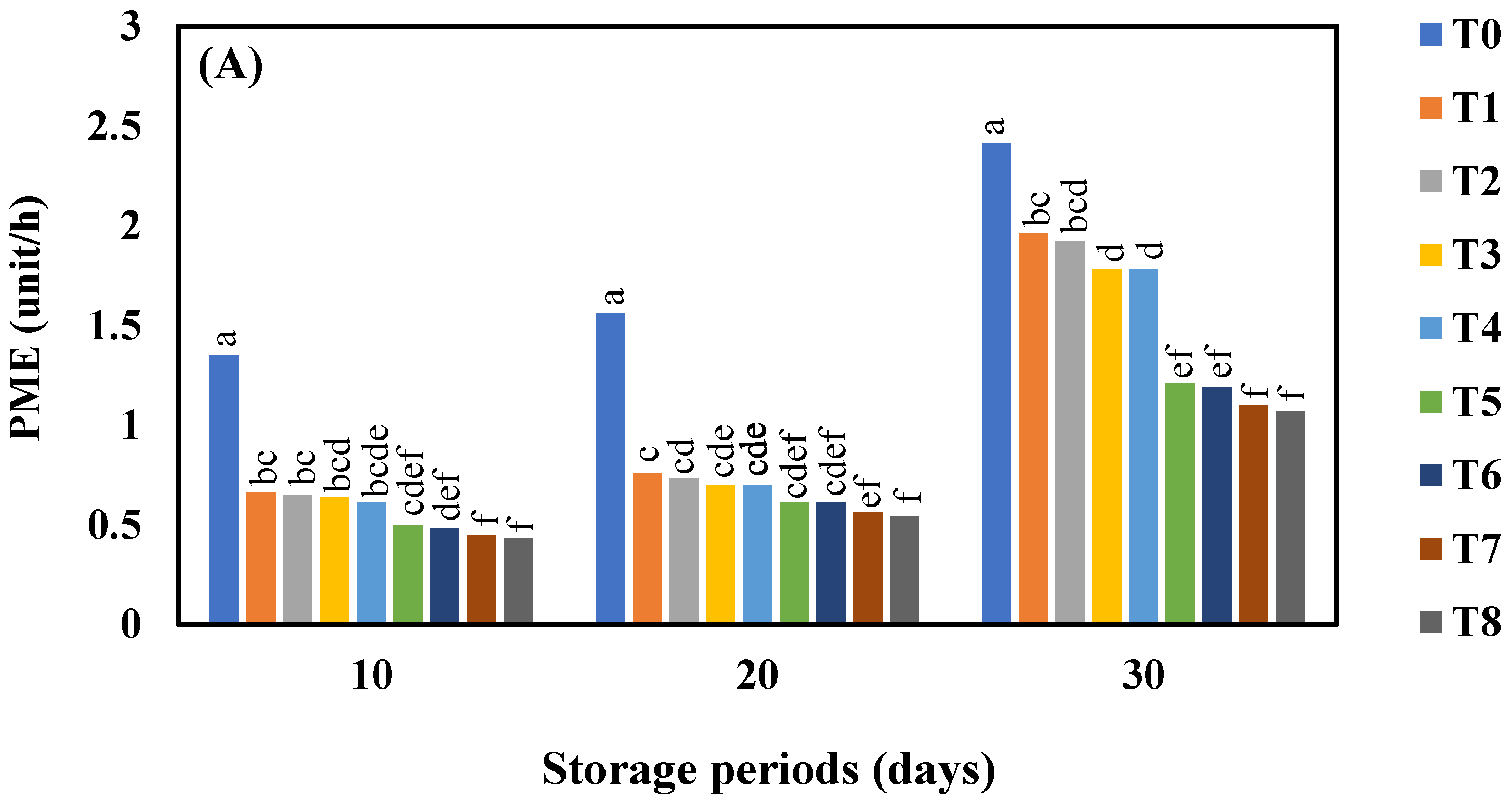
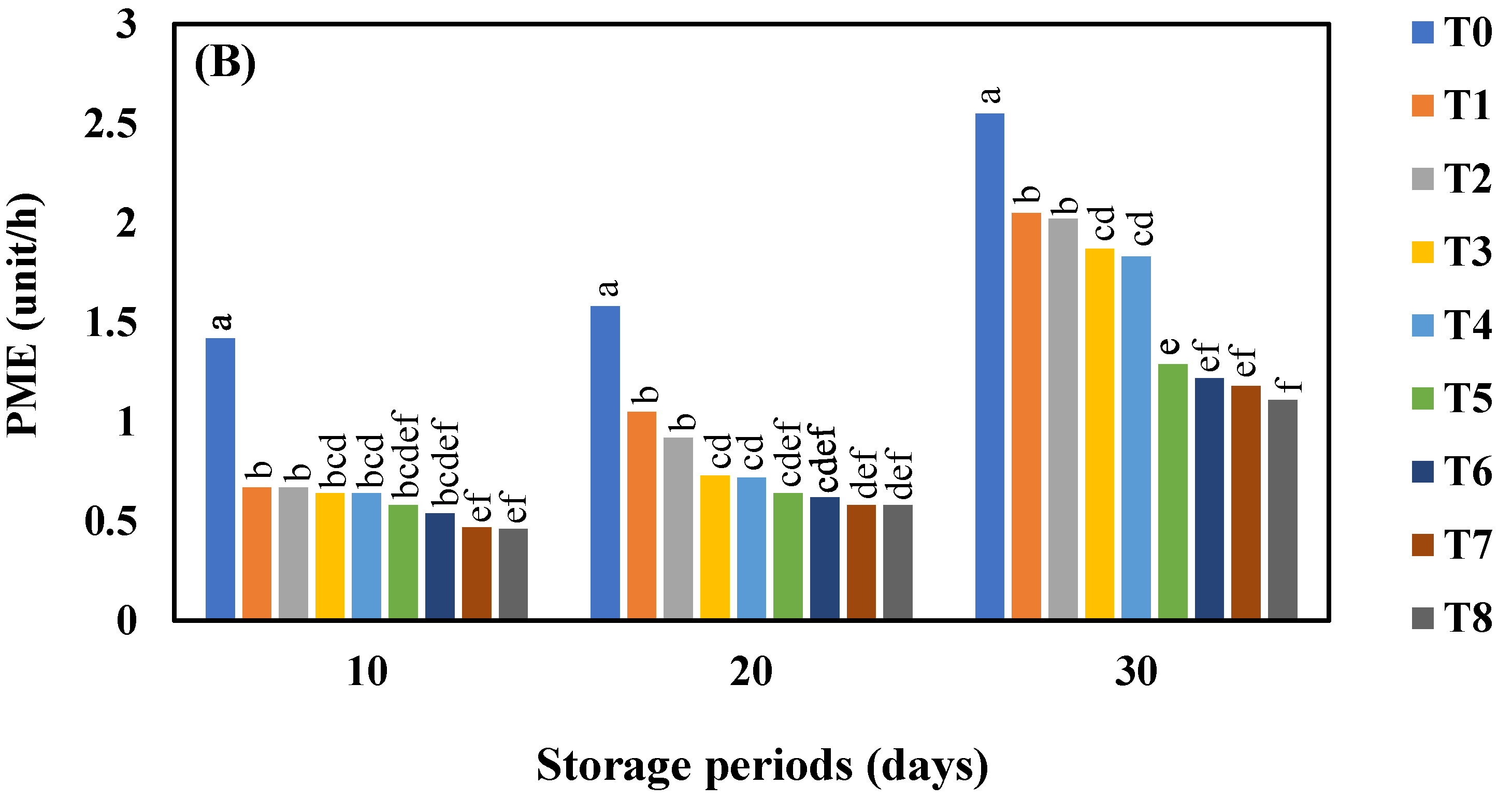
3.8. Effect of AgNO3 and Ag-NPs on Pectin Methylestraese Activity (PME) Activity in Shine Muscat and Kyoho Grape Varieties during 30 Days Cold Storage
4. Discussions
4.1. Effect of AgNO3 and Ag-NPs on the Weight Loss (%) in Shine Muscat (A) and Kyoho (B) Grape Varieties during 30 Days Cold Storage
4.2. Effect of AgNO3 and Ag-NPs on Firmness in Shine Muscat (A) and Kyoho (B) Grape Varieties during 30 Days Cold Storage
4.3. Effect of AgNO3 and Ag-NPs on Soluble Solids Content (°Brix) in Shine Muscat (A) and Kyoho (B) Grape Varieties during 30 Days Cold Storage
4.4. Effect of AgNO3 and Ag-NPs on Titratable Acidity (%) in Shine Muscat (A) and Kyoho (B) Grape Varieties during 30 Days Cold Storage
4.5. Effect of AgNO3 and Ag-NPs on Malondialdehyde (MDA) Content in Shine Muscat and Kyoho Grape Varieties during 30 Days Cold Storage
4.6. Effect of AgNO3 and Ag-NPs on Polyphenol Oxidase (PPO) Content in Shine Muscat and Kyoho Grape Varieties during 30 Days Cold Storage
4.7. Effect of AgNO3 and Ag-NPs on Pyrogallol Peroxidase (POD) Activity in Shine Muscat and Kyoho Grape Varieties during 30 Days Cold Storage
4.8. Effect of AgNO3 and Ag-NPs on Pectin Methylestraese (PME) Activity in Shine Muscat and Kyoho Grape Varieties during 30 Days Cold Storage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Min, Z.; Chunli, L.; Yanjun, H.; Qian, T.; Haiou, W. Preservation of fresh grapes at ice-temperature-high-humidity. Int. Agrophysics 2001, 15, 139–143. [Google Scholar]
- Paton, F.; Schwabe, W. Storage of cuttings of Pelargonium × hortorum Bailey. J. Hortic. Sci. 1987, 62, 79–87. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A.; Singh, H.; Pratap, S.; Singh, R. Effect of Potassium Permanganate and Silver Nitrate Chemicals on the Shelf Life of Guava Fruits. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 987–992. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.; Kim, T.; Kim, J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. Int. J. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Jiang, H.; Manolache, S.; Wong, A.C.L.; Denes, F.S. Plasma-enhanced deposition of silver nanoparticles onto polymer and metal surfaces for the generation of antimicrobial characteristics. J. Appl. Polym. Sci. 2004, 93, 1411–1422. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef]
- Ketsa, S.; Piyasaengthong, Y.; Prathuangwong, S. Mode of action of AgNO3 in maximizing vase life of Dendrobium ‘Pompadour’ flowers. Postharvest Biol. Technol. 1995, 5, 109–117. [Google Scholar] [CrossRef]
- Reddy, B.; Singh, K.; Singh, A. Effect of sucrose, citric acid and 8-hydroxyquinoline sulphate on the postharvest physiology of tuberose cv. single. Adv. Agric. Res. 1996, 3, 161–167. [Google Scholar]
- Kim, J.-H.; Lee, A.-K.; Suh, J.-K. Effect of certain pre-treatment substances on vase life and physiological character in Lilium spp. In IX International Symposium on Flower Bulbs; Acta Horticulturae 673; 2004; pp. 307–314. Available online: https://www.actahort.org/books/673/673_39.htm (accessed on 7 March 2022).
- Liu, J.; Ratnayake, K.; Joyce, D.C.; He, S.; Zhang, Z. Effects of three different nano-silver formulations on cut Acacia holosericea vase life. Postharvest Biol. Technol. 2012, 66, 8–15. [Google Scholar] [CrossRef]
- Furno, F.; Morley, K.S.; Wong, B.; Sharp, B.L.; Arnold, P.L.; Howdle, S.M.; Bayston, R.; Brown, P.D.; Winship, P.D.; Reid, H.J. Silver nanoparticles and polymeric medical devices: A new approach to prevention of infection? J. Antimicrob. Chemother. 2004, 54, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Bhusare, S.; Kadam, S. Applications of Nanotechnology in Fruits and Vegetables. Food Agric. Spectr. J. 2021, 2, 231–236. [Google Scholar]
- Rana, R.A.; Siddiqui, M.; Skalicky, M.; Brestic, M.; Hossain, A.; Kayesh, E.; Popov, M.; Hejnak, V.; Gupta, D.R.; Mahmud, N.U. Prospects of Nanotechnology in Improving the Productivity and Quality of Horticultural Crops. Horticulturae 2021, 7, 332. [Google Scholar] [CrossRef]
- Kazemi, M.; Ameri, A. Postharvest life of cut gerbera flowers as affected by Nano-Silver and acetylsalicylic acid. Asian J. Biochem. 2012, 7, 106–111. [Google Scholar] [CrossRef]
- Sah, S.; Sorooshzadeh, A.; Rezazadehs, H.; Naghdibadi, H. Effect of nano silver and silver nitrate on seed yield of borage. J. Med. Plants Res. 2011, 5, 171–175. [Google Scholar]
- Vinković, T.; Novák, O.; Strnad, M.; Goessler, W.; Jurašin, D.D.; Parađiković, N.; Vrček, I.V. Cytokinin response in pepper plants (Capsicum annuum L.) exposed to silver nanoparticles. Environ. Res. 2017, 156, 10–18. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Hatata, M.M.; Al-Huqail, A.A.; Ibrahim, M.M. Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J. Biol. Sci. 2018, 25, 313–319. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef]
- Ghasemnezhad, A.; Ghorbanpour, M.; Sohrabi, O.; Ashnavar, M. A general overview on application of nanoparticles in agriculture and plant science. Compr. Anal. Chem. 2019, 87, 85–110. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists International; Official Methods: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Dipierro, S.; De Leonardis, S. The ascorbate system and lipid peroxidation in stored potato (Solanum tuberosum L.) tubers. J. Exp. Bot. 1997, 48, 779–783. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Y.; Jiang, A.; Gong, Q. Physiological and quality responses of longan fruit to high O2 or high CO2 atmospheres in storage. Postharvest Biol. Technol. 2002, 24, 335–340. [Google Scholar] [CrossRef]
- Vicente, A.R.; Martínez, G.A.; Chaves, A.R.; Civello, P.M. Effect of heat treatment on strawberry fruit damage and oxidative metabolism during storage. Postharvest Biol. Technol. 2006, 40, 116–122. [Google Scholar] [CrossRef]
- Anthon, G.E.; Barrett, D.M. Characterization of the temperature activation of pectin methylesterase in green beans and tomatoes. J. Agric. Food Chem. 2006, 54, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M. Production and Storage of Fruits (in Bangla); Mondal, A., Ed.; BAU Campus: Mymsningh, Bangladesh, 2000; p. 312. [Google Scholar]
- CoStat. Microcomputer Program Analysis, Version 4-20; CoHort Software: Berkly, CA, USA, 1990. [Google Scholar]
- Snedecor, G.; Cochran, W. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Zaied, S.F. Silver Nanoparticles in Polymeric Matrices for Keeping Quality of Dates Packaging. J. Microb. Biochem. Technol. 2021, 13, 193. [Google Scholar]
- Abu Salha, B.; Gedanken, A. Extending the shelf life of strawberries by the sonochemical coating of their surface with nanoparticles of an edible anti-bacterial compound. Appl. Nano 2021, 2, 14–24. [Google Scholar] [CrossRef]
- Gao, L.; Li, Q.; Zhao, Y.; Wang, H.; Liu, Y.; Sun, Y.; Wang, F.; Jia, W.; Hou, X. Silver nanoparticles biologically synthesised using tea leaf extracts and their use for extension of fruit shelf life. IET Nanobiotechnol. 2017, 11, 637–643. [Google Scholar] [CrossRef]
- Gu, R.; Yun, H.; Chen, L.; Wang, Q.; Huang, X. Regenerated cellulose films with amino-terminated hyperbranched polyamic anchored nanosilver for active food packaging. ACS Appl. Bio Mater. 2019, 3, 602–610. [Google Scholar] [CrossRef]
- Liu, R.; Liu, D.; Liu, Y.; Song, Y.; Wu, T.; Zhang, M. Using soy protein SiOx nanocomposite film coating to extend the shelf life of apple fruit. Int. J. Food Sci. Technol. 2017, 52, 2018–2030. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer: Berlin/Heidelberg, Germany, 2010; Volume 2. [Google Scholar]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Andronescu, E. Smart food packaging designed by nanotechnological and drug delivery approaches. Coatings 2020, 10, 806. [Google Scholar] [CrossRef]
- Shah, S.W.A.; Jahangir, M.; Qaisar, M.; Khan, S.A.; Mahmood, T.; Saeed, M.; Farid, A.; Liaquat, M. Storage stability of kinnow fruit (Citrus reticulata) as affected by CMC and guar gum-based silver nanoparticle coatings. Molecules 2015, 20, 22645–22661. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, H.; Li, F.; Xin, Z.; Zhao, L.; Zheng, Y.; Hu, Q. Effect of nano-packing on preservation quality of fresh strawberry (Fragaria ananassa Duch. cv Fengxiang) during storage at 4 C. J. Food Sci. 2010, 75, C236–C240. [Google Scholar] [CrossRef] [PubMed]
- Svagan, A.J.; Hedenqvist, M.S.; Berglund, L. Reduced water vapour sorption in cellulose nanocomposites with starch matrix. Compos. Sci. Technol. 2009, 69, 500–506. [Google Scholar] [CrossRef]
- SINGH, J.; BAKSHI, M.; MIRZA, A. A Study on Post-harvest Application of Silver Nitrate and Potassium Permangnate on Physico-chemical Aspects of Guava (Psidium guajava L.). Ann. Biol. 2018, 34, 138–142. [Google Scholar]
- Khaleel, R.; Al-Samarrai, G.F.; Mohammed, A. Coating of Orange Fruit With Nano-Silver Particles to Minimizing Harmful Environmental Pollution by Chemical Fungicide. Iraqi J. Agric. Sci. 2019, 50, 1668–1673. [Google Scholar]
- Bairwa, L.; Dashora, L. Effect of KM1104 and AgNO3 on post harvest shelf life of banana; Advances Hort. Frost 1999, 7, 9–15. [Google Scholar]
- Atta-Aly, M.A.; Saltveit, M.E., Jr.; Hobson, G.E. Effect of silver ions on ethylene biosynthesis by tomato fruit tissue. Plant Physiol. 1987, 83, 44–48. [Google Scholar] [CrossRef]
- Salem, E.A.; Nawito, M.A.S.; Abd El-Raouf, A.E.-R. Effect of silver nano-particles on gray mold of tomato fruits. J. Nanotechnol. Res. 2019, 1, 108–118. [Google Scholar]
- Naibaho, J.; Elisa, J.; Era, Y. Penyimpanan Buah Terung Belanda dengan Kemasan Aktif Menggunakan Bahan Penjerap Oksigen, Karbondioksida, Uap Air dan Etilen, Skripsi Program Studi Ilmu dan Teknologi Pangan; Fakultas Pertanian, Universitas Sumatera Utara: Kota Medan, Indonesia, 2013. [Google Scholar]
- Triyono, A.; Ardiansyah, R.E.; Hapsari, M. Studying the Effects Of Inhibitor Solution Soaking Time On Guava (Psidiumguajava L.) Storability. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2019; p. 012033. [Google Scholar]
- El-Khalek, A.; Fathy, A.; El-Abbasy, U.; Ismail, M. Postharvest applications of 1-methylcyclopropene and salicylic acid for maintaining quality and enhancing antioxidant enzyme activity of apricot fruits cv.‘Canino’During cold storage. Egypt. J. Hortic. 2018, 45, 1–23. [Google Scholar] [CrossRef]
- Ali, M.; Ahmed, A.; Shah, S.W.A.; Mehmood, T.; Abbasi, K.S. Effect of silver nanoparticle coatings on physicochemical and nutraceutical properties of loquat during postharvest storage. J. Food Processing Preserv. 2020, 44, e14808. [Google Scholar] [CrossRef]
- Huang, Q.; Qian, X.; Jiang, T.; Zheng, X. Effect of chitosan and guar gum based composite edible coating on quality of mushroom (Lentinus edodes) during postharvest storage. Sci. Hortic. 2019, 253, 382–389. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Sun, Y.; Chen, B.; Wu, X.; Le, T. Improvement of banana postharvest quality using a novel soybean protein isolate/cinnamaldehyde/zinc oxide bionanocomposite coating strategy. Sci. Hortic. 2019, 258, 108786. [Google Scholar] [CrossRef]
- Gholami, R.; Ahmadi, E.; Farris, S. Shelf life extension of white mushrooms (Agaricus bisporus) by low temperatures conditioning, modified atmosphere, and nanocomposite packaging material. Food Packag. Shelf Life 2017, 14, 88–95. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Rao, T.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Shahat, M.; Ibrahim, M.; Osheba, A.; Taha, I. Preparation and characterization of silver nanoparticles and their use for improving the quality of apricot fruits. Al-Azhar J. Agric. Res. 2020, 45, 38–55. [Google Scholar] [CrossRef]
- Wani, S.M.; Gull, A.; Wani, T.A.; Masoodi, F.A.; Ganaie, T. Effect of edible coating on the shelf life enhancement of apricot (Prunus armeniaca L.). J. Postharvest Technol 2019, 5, 26–34. [Google Scholar]
- Fagundes, C.; Moraes, K.; Pérez-Gago, M.B.; Palou, L.; Maraschin, M.; Monteiro, A. Effect of active modified atmosphere and cold storage on the postharvest quality of cherry tomatoes. Postharvest Biol. Technol. 2015, 109, 73–81. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Castillo, S.; Guillén, F.; Paladine, D.; Zapata, P.J.; Valero, D.; Serrano, M. Rosehip oil coating delays postharvest ripening and maintains quality of European and Japanese plum cultivars. Postharvest Biol. Technol. 2019, 155, 29–36. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Ding, C.-K.; Chachin, K.; Ueda, Y.; Imahori, Y. Purification and properties of polyphenol oxidase from loquat fruit. J. Agric. Food Chem. 1998, 46, 4144–4149. [Google Scholar] [CrossRef]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization of polyphenol oxidase and peroxidase and influence on browning of cold stored strawberry fruit. J. Agric. Food Chem. 2007, 55, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Takahama, U. Changes in peroxidase activity and in peroxidase isozymes in carrot callus. Physiol. Plant. 1993, 88, 167–171. [Google Scholar] [CrossRef]
- Biles, C.L.; Peroxidase, M.R. polyphenoloxidase and shikimate dehydrogenase isoenzymes in relation to tissue type, maturity and pathogen induction of watermelon seedlings. Plant Physiol. Biochem. 1993, 31, 499–506. [Google Scholar]
- Garcia-Betanzos, C.; Hernández-Sánchez, H.; Quintanar-Guerrero, D.; Galindo-Pérez, M.; Zambrano-Zaragoza, M. Influence of solid lipid nanoparticle/xanthan gum coatings on compositional and enzymatic changes in guava (Psidium guajava L.) during ripening. Acta Hortic. 2018, 289–295. [Google Scholar] [CrossRef]



| Groups | Treatments |
|---|---|
| T0 | Control (water without chemical) |
| T1 | AgNO3 5000 ppm for 3 min |
| T2 | AgNO3 5000 ppm for 5 min |
| T3 | AgNO3 10,000 ppm for 3 min |
| T4 | AgNO3 10,000 ppm for 5 min |
| T5 | Ag-NPs 50 ppm for 3 min |
| T6 | Ag-NPs 50 ppm for 5 min |
| T7 | Ag-NPs 100 ppm for 3 min |
| T8 | Ag-NPs 100 ppm for 5 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elatafi, E.; Fang, J. Effect of Silver Nitrate (AgNO3) and Nano-Silver (Ag-NPs) on Physiological Characteristics of Grapes and Quality during Storage Period. Horticulturae 2022, 8, 419. https://doi.org/10.3390/horticulturae8050419
Elatafi E, Fang J. Effect of Silver Nitrate (AgNO3) and Nano-Silver (Ag-NPs) on Physiological Characteristics of Grapes and Quality during Storage Period. Horticulturae. 2022; 8(5):419. https://doi.org/10.3390/horticulturae8050419
Chicago/Turabian StyleElatafi, Essam, and Jinggui Fang. 2022. "Effect of Silver Nitrate (AgNO3) and Nano-Silver (Ag-NPs) on Physiological Characteristics of Grapes and Quality during Storage Period" Horticulturae 8, no. 5: 419. https://doi.org/10.3390/horticulturae8050419
APA StyleElatafi, E., & Fang, J. (2022). Effect of Silver Nitrate (AgNO3) and Nano-Silver (Ag-NPs) on Physiological Characteristics of Grapes and Quality during Storage Period. Horticulturae, 8(5), 419. https://doi.org/10.3390/horticulturae8050419







