Effect on the Growth and Photosynthetic Characteristics of Anthurium andreanum (‘Pink Champion’, ‘Alabama’) under Hydroponic Culture by Different LED Light Spectra
Abstract
1. Introduction
2. Materials and Methods
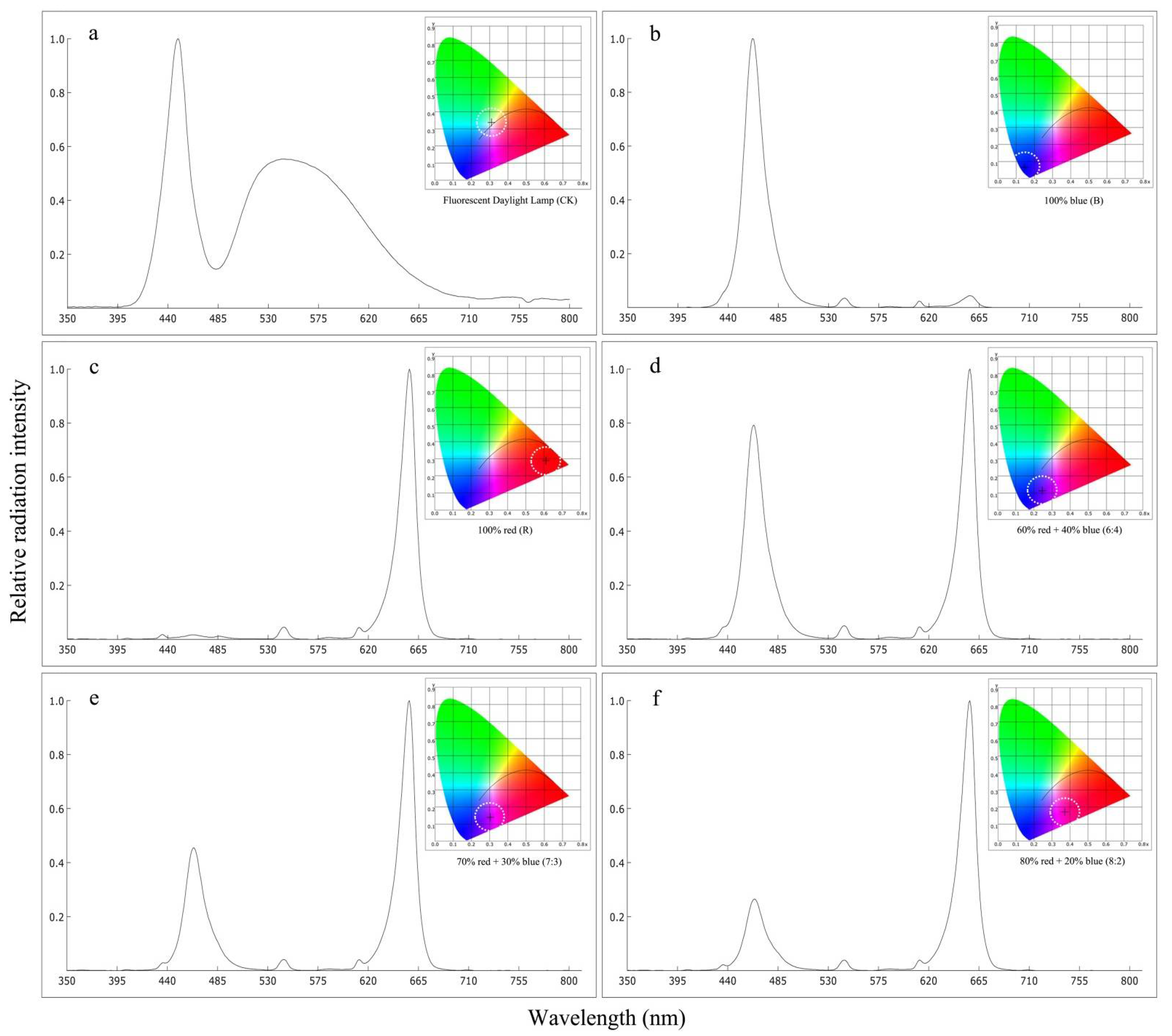
2.1. Plant Materials, Treatments, and Culture Conditions
2.2. Morphological Indicators and Root Activity Analysis
2.3. Chlorophyll, Chlorophyll Fluorescence, and Photosynthetic Parameters Analysis
2.4. Soluble Sugars and Soluble Proteins Analysis
2.5. Antioxidant Enzymes Activity Analysis
2.6. Statistical Analysis
3. Results
3.1. Plant Growth and Morphology
3.2. Root Activity
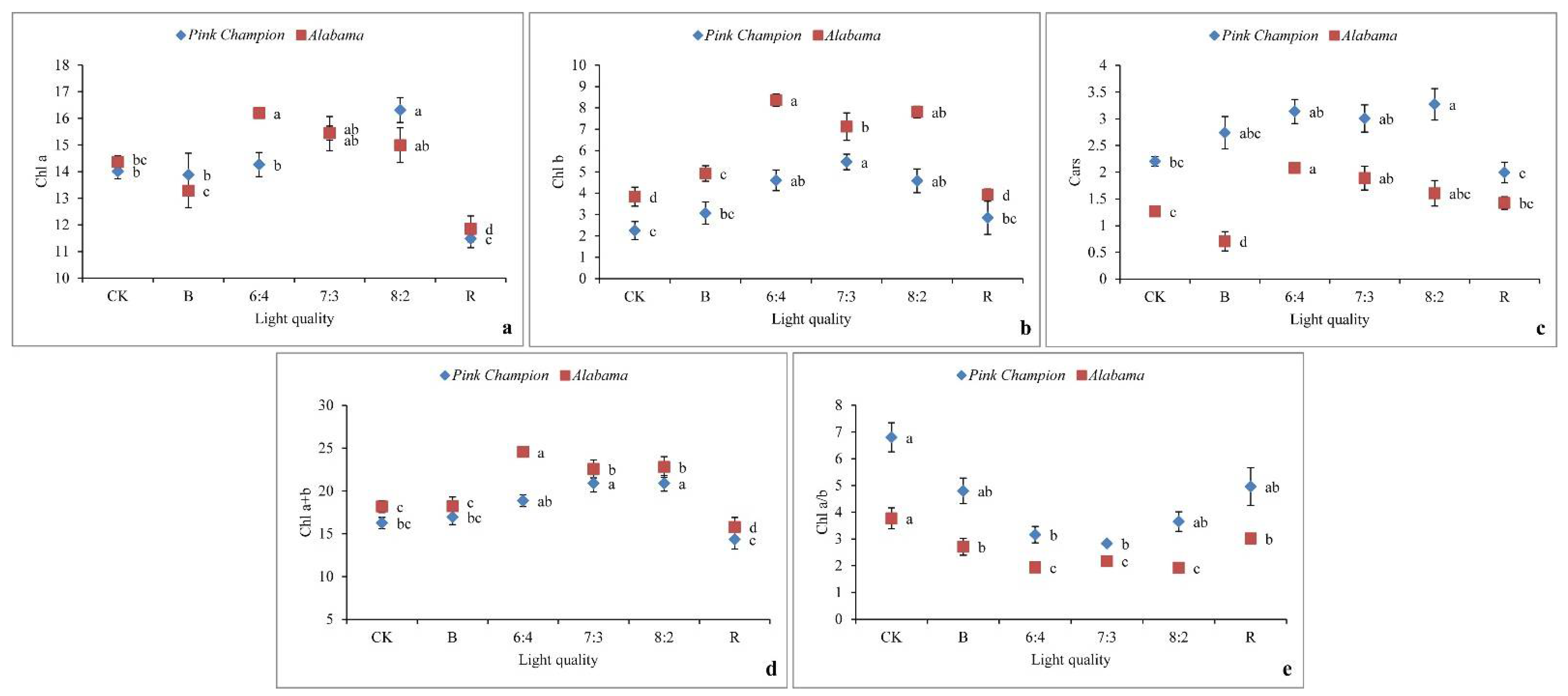
3.3. Pigment Contents
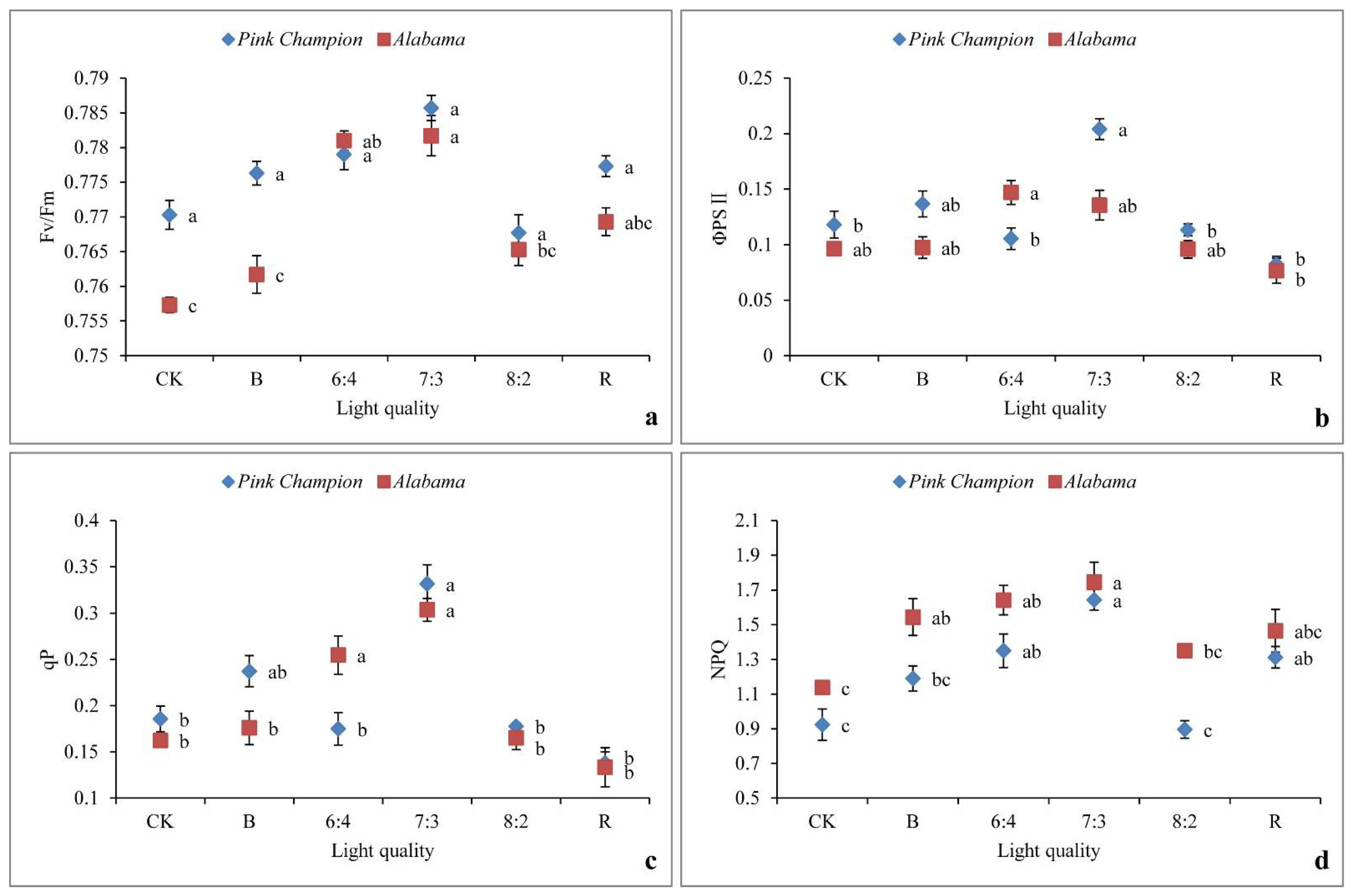
3.4. Chlorophyll Fluorescence
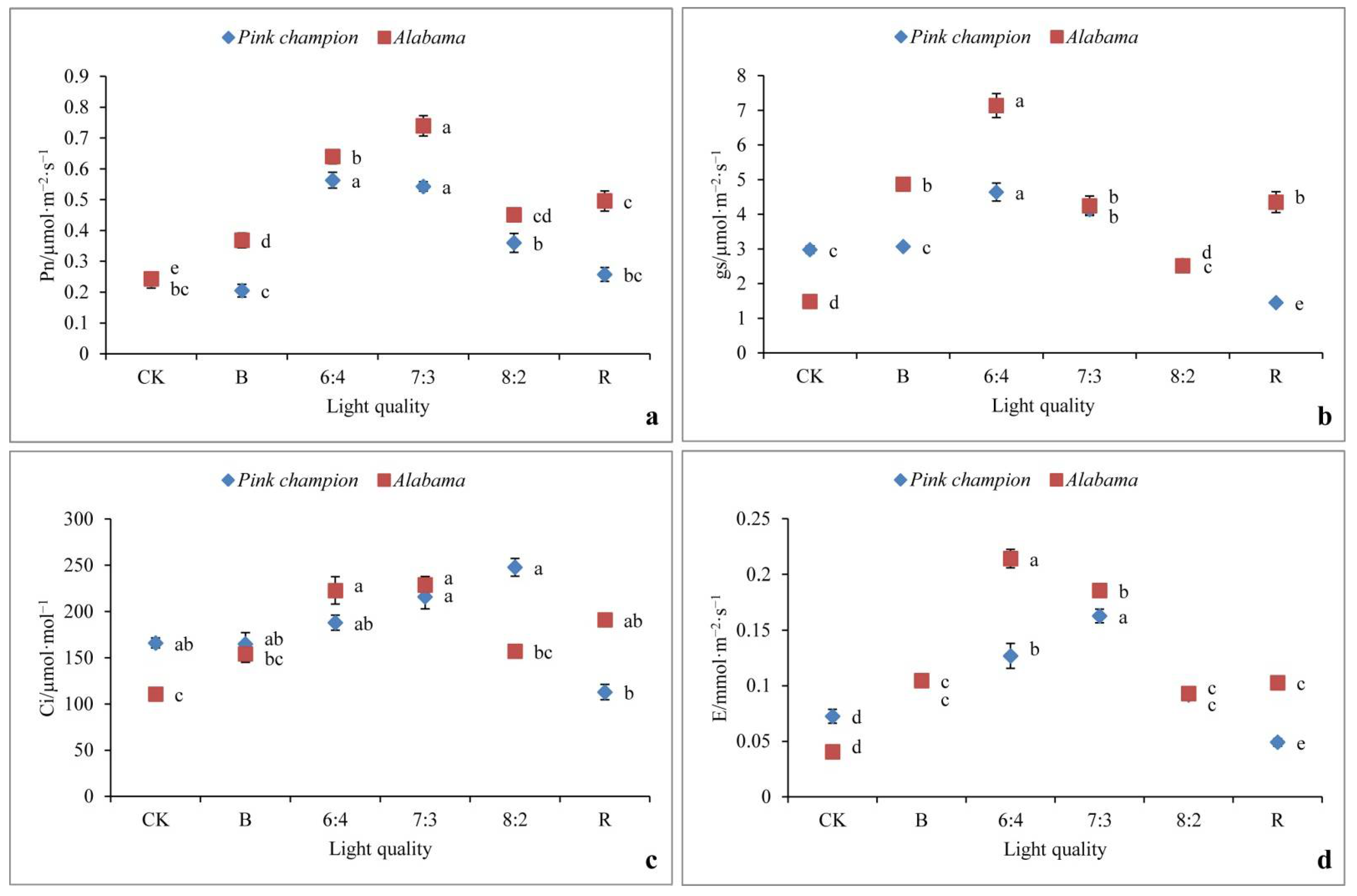
3.5. Photosynthesis
3.6. Soluble Sugars, Soluble Proteins
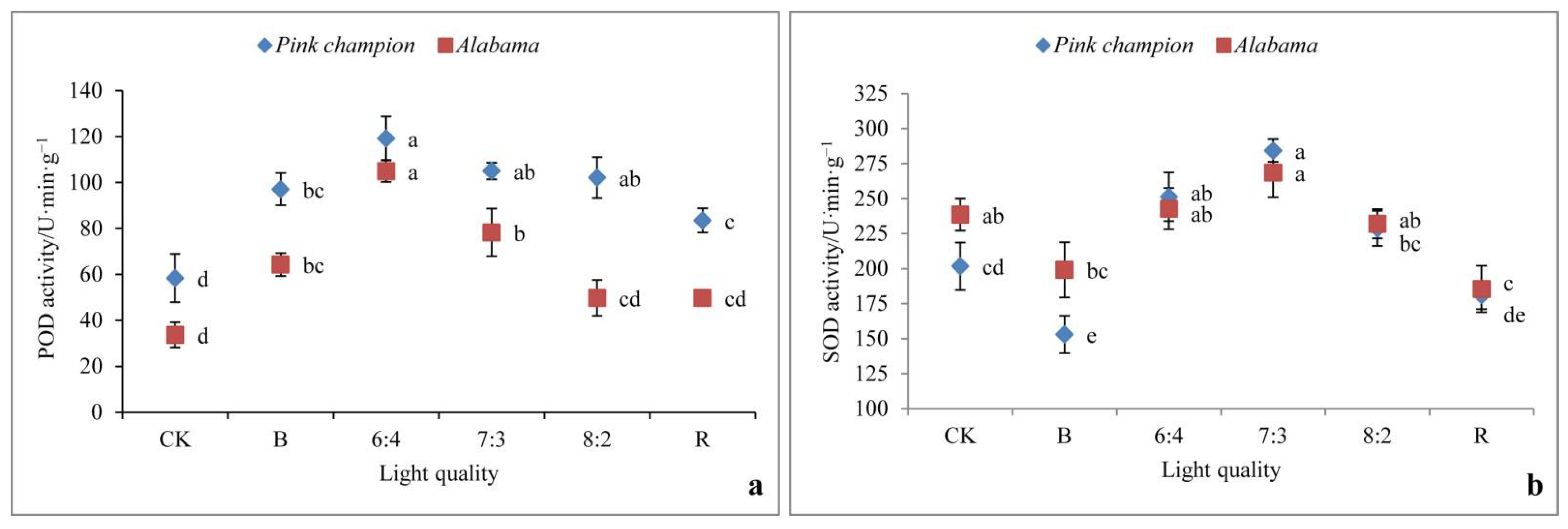
3.7. Antioxidant Enzyme Activity (POD, SOD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henny, R.J.; Chen, J.; Mellich, T.A. Tropical foliage plant development: Breeding techniques for Anthurium and Spathiphyllum. EDIS 2008, 5, ENH1102. [Google Scholar] [CrossRef]
- Zhang, H.X.; Wang, G.L.; Qiao, Y.X.; Chen, C. Plant regeneration from root segments of Anthurium andraeanum and assessment of genetic fidelity of in vitro regenerates. Vitr. Cell Dev.–PL 2021, 57, 954–964. [Google Scholar] [CrossRef]
- Elibox, W.; Umaharan, P. Cultivar differences in the deterioration of vase-life in cut-flowers of Anthurium andraeanum is determined by mechanisms that regulate water uptake. Sci. Hortic. 2010, 124, 102–108. [Google Scholar] [CrossRef]
- Assis, A.M.; Unemoto, L.K.; Faria, R.T.; Destro, D.; Takahashi, L.S.A.; Ruffo, S.; Sandra, R.; Prudêncio, H.; Fernando, A.; Tombolato, C. Adaptation of anthurium cultivars as cut flowers in subtropical area. Pesq. Agrop. Bras. 2011, 46, 161–166. [Google Scholar] [CrossRef]
- Habermann, G.; Cardoso, J.C. Adventitious shoot induction from leaf segments in Anthurium andraeanum is affected by age of explant, leaf orientation and plant growth regulator. Hortic. Environ. Biotechnol. 2014, 55, 56–62. [Google Scholar] [CrossRef]
- Cardoso, J.C. Silver nitrate enhances in vitro development and quality of shoots of Anthurium andraeanum. Sci. Hortic. 2019, 253, 358–363. [Google Scholar] [CrossRef]
- Li, S.G.; Li, J.K.; Li, X.F.; Guan, Y.; Chen, M.M.; Zhu, J. Comparative transcriptome analysis reveals molecular regulators underlying pluripotent cell induction and callus formation in Anthurium andraeanum “Alabama”. Vitr. Cell Dev.–PL 2021, 57, 235–247. [Google Scholar] [CrossRef]
- Suzuki, J.Y.; Bliss, B.; Clark, B.R.; Borris, R.; Gonsalves, D. Molecular and biochemical tools for characterization and cultivar improvement of Anthurium andraeanum Hort. Hort. Sci. 2011, 46, S171. [Google Scholar]
- Elibox, W.; Umaharan, P. Genetic basis for productivity in Anthurium andraeanum Hort. HortScience 2014, 49, 859–863. [Google Scholar] [CrossRef]
- Niu, J.H.; Leng, Q.Y.; Li, G.Y.; Huang, S.H.; Xu, S.S.; Lin, X. ‘Victory flag’: A new cut Anthurium cultivar. HortScience 2021, 56, 513. [Google Scholar] [CrossRef]
- Elibox, W.; Umaharan, P. The inheritance of systemic resistance to the bacterial blight pathogen (Xanthomonas axonopodis pv. dieffenbachiae) in Anthurium andraeanum (Hort.). Sci. Hortic. 2007, 115, 76–81. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Hui, X.; Wu, W.J.; Xu, C.L. Pin nematode slow decline of Anthurium andraeanum, a new disease caused by the pin nematode Paratylenchus shenzhenensis. Plant Dis. 2016, 100, 940–945. [Google Scholar] [CrossRef][Green Version]
- Ben, H.Y.; Zhao, Y.J.; Chai, A.L.; Shi, Y.X.; Xie, X.W.; Li, B. First report of Myrothecium roridum causing leaf spot on Anthurium andraeanum in China. J. Phytopathol. 2015, 163, 144–147. [Google Scholar] [CrossRef]
- Surassawadee, P.; Saichol, K.; Wouter, G.D. Salicylic acid alleviates chilling injury in anthurium (Anthurium andraeanum L.) flowers. Postharvest Biol. Technol. 2012, 64, 104–110. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, X.K.; Li, S.T.; Fu, Y.X.; Xu, J.J.; Wang, G. The AabHLH35 transcription factor identified from Anthurium andraeanum is involved in cold and drought tolerance. Plants 2019, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Aliniaeifard, S.; Falahi, Z.; Dianati, S.; Li, T. Postharvest spectral light composition affects chilling injury in anthurium cut flowers. Front. Plant Sci. 2020, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Yuan, Z.B.; Wang, B.; Zheng, L.P.; Tan, J.Z.; Chen, F. Physiological and transcriptome changes induced by exogenous putrescine in anthurium under chilling stress. Bot. Stud. 2020, 61, 28. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.S.; Liu, W.F.; Ma, C.; Cui, J.; Henny, R.J.; Chen, J. Regeneration of Anthurium andraeanum from leaf explants and evaluation of microcutting rooting and growth under different light qualities. HortScience 2012, 47, 88–92. [Google Scholar] [CrossRef]
- Chen, X.L.; Yang, Q.C. Effects of intermittent light exposure with red and blue light emitting diodes on growth and carbohydrate accumulation of lettuce. Sci. Hortic. 2018, 234, 220–226. [Google Scholar] [CrossRef]
- Favero, B.T.; Lutken, H.; Dole, J.M.; Lima, G.P.P. Anthurium andraeanum senescence in response to 6-benzylaminopurine: Vase life and biochemical aspects-ScienceDirect. Postharvest Biol. Technol. 2020, 161, 111084. [Google Scholar] [CrossRef]
- Sarah, E.; Aidan, D.F.; Winston, E.; Kathryn, D.A.; Pathmanathan, U. The impact of light on vase life in (Anthurium andraeanum Hort.) cut flowers. Postharvest Biol. Technol. 2020, 159, 110984. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.X.; Yang, Q.C. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Yi, C.Y.; Zhang, C.R.; Pan, F.; Xie, C.; Zhou, W.; Zhou, C. Effects of light quality on leaf growth and photosynthetic fluorescence of Brasenia schreberi seedlings. Heliyon 2021, 7, e06082. [Google Scholar] [CrossRef] [PubMed]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Yen, N.; Chung, J.P. High-brightness LEDs-energy efficient lighting sources and their potential in indoor plant cultivation. Renew. Sustain. Energy Rev. 2009, 13, 2175–2180. [Google Scholar] [CrossRef]
- Vänninen, I.; Pinto, D.M.; Nissinen, A.I.; Johansen, N.S.; Shipp, L. In the light of new greenhouse technologies: 1: Plant-mediated effects of artificial lighting on arthropods and tritrophic interactions. Ann. Appl. Biol. 2010, 157, 393–414. [Google Scholar] [CrossRef]
- Park, Y.G.; Muneer, S.; Jeong, B.R. Morphogenesis, flowering, and gene expression of Dendranthema grandiflorum in response to shift in light quality of night interruption. Int. J. Mol. Sci. 2015, 16, 16497–16513. [Google Scholar] [CrossRef]
- Urbonaviciute, A.; Pinho, P.; Samuoliene, G.; Duchovskis, P.; Vitta, P.; Stonkus, A.; Tamulaitis, G.; Žukauskas, A.; Halonen, L. Effect of short-wavelength light on lettuce growth and nutritional quality. Scientific works of the Lithuanian Institute of Horticulture and Lithuanian University of Agriculture. Sodininkystė Ir Daržininkystė 2007, 26, 157–165. [Google Scholar]
- Keyser, E.D.; Dhooghe, E.; Christiaens, A.; Labeke, M.C.V.; Huylenbroeck, J.V. LED light quality intensifies leaf pigmentation in ornamental pot plants. Sci. Hortic. 2019, 253, 270–275. [Google Scholar] [CrossRef]
- Heo, J.; Lee, C.; Chakrabarty, D.; Paek, K. Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a Light-Emitting Diode (LED). Plant Growth Regul. 2002, 38, 225–230. [Google Scholar] [CrossRef]
- Sakurako, H.; Shota, Y.; Haruki, K.; Saashia, F.; Shigekazu, K.; Ken-Ichiro, S.; Atsushi, T. A BLUS1 kinase signal and a decrease in intercellular CO2 concentration are necessary for stomatal opening in response to blue light. Plant Cell 2021, 33, 1813–1827. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; Van-Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.D.; Hong, C.H.; Jung, H.B.; Kim, S.K.; Ket, N.V.; Nam, M.-W.; Choi, D.-H.; Lee, H.-I. Growth and morphogenesis of encapsulated strawberry shoot tips under mixed LEDs. Sci. Hortic. 2015, 194, 194–200. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.H.; Kim, S.K.; Lee, K.H.; Park, J.Y.; Nam, M.-W.; Choi, D.-H.; Lee, H.-I. LED light for in vitro and ex vitro efficient growth of economically important highbush blueberry (Vaccinium corymbosum L.). Acta Physiol. Plant. 2016, 38, 1–9. [Google Scholar] [CrossRef]
- Izzo, L.G.; Mele, B.H.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Gad, A.G.; Chen, F. Light quality and quantity affect graft union formation of tomato plants. Sci. Rep. 2021, 11, 9870. [Google Scholar] [CrossRef]
- Folta, K.M.; Kos, L.L.; McMorrow, R.; Kim, H.H.; Kenitz, J.D.; Wheeler, R.; Sager, J.C. Design and fabrication of adjustable red-green-blue LED light arrays for plant research. BMC Plant Biol. 2005, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Bourget, C.M. An introduction to light-emitting diodes. HortScience 2008, 43, 1944–1946. [Google Scholar] [CrossRef]
- Morrow, R. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Hernandez, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 29, 66–74. [Google Scholar] [CrossRef]
- Shin, K.S.; Murthy, H.N.; Heo, J.W.; Hahn, E.J.; Park, K.Y. The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol. Plant. 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, X.-L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Xu, D.Q.; Gao, W.; Ruan, J. Effects of light quality on plant growth and development. Plant Physiol. J. 2015, 51, 1217–1234. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Shi, Q.H.; Yang, F.J.; Wei, M. Mixed red and blue light promotes ripening and improves quality of tomato fruit by influencing melatonin content. Environ. Exp. Bot. 2021, 185, 104407. [Google Scholar] [CrossRef]
- Ryssov-Nielsen, H. Measurement of the inhibition of respiration in activated sludge by a modified determination of the TTC-dehydrogenase activity. Water Res. 1975, 9, 1179–1185. [Google Scholar] [CrossRef]
- Trevors, J.T.; Roger, K. Electron transport system activity in soil, sediment, and pure cultures. Crit. Rev. Microbiol. 1984, 11, 83–100. [Google Scholar] [CrossRef]
- Holm, G. Chlorophyll mutation in barley. Hereditas 2009, 55, 79–120. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA-Gene Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Bjorkman, O. Temperature-dependence of violaxanthin deepoxidation and nonphotochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parvoflora L. Planta 1991, 184, 226–234. [Google Scholar] [CrossRef]
- Clegg, K.M. The application of the anthrone reagent to the estimation of starch in cereals. J. Sci. Food Agric. 1956, 7, 40–44. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhao, S.J.; Liu, H.S.; Dong, X.C. Experiment Guide of Plant Physiology; Agricultural Science and Technology Press: Beijing, China, 1998; pp. 98–99. [Google Scholar]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. B Biol. 2009, 96, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Xu, Q.C.; Li, F.L.; Feng, Y.Z.; Qin, F.F.; Fang, W. Applications of xerophytophysiology in plant production-LED blue light as a stimulus improved the tomato crop. Sci. Hortic. 2012, 148, 190–196. [Google Scholar] [CrossRef]
- Kurilčik, A.; Miklušytė-Čanova, R.; Dapkūnienė, S.; Žilinskaitė, S.; Kurilčik, G.; Tamulaitis, G.; Duchovskis, P.; Žukauskas, A. In vitro culture of Chrysanthemum plantlets using light-emitting diodes. Cent. Eur. J. Biol. 2008, 3, 161–167. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. BBA-Gen Subj. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Tang, D.W.; Zhang, G.B.; Zhang, F. Effect of different LED light qualities on growth and physiological and biochemical characteristics of cucumber seedlings. J. Gansu Agric. Univ. 2011, 46, 44–48. [Google Scholar] [CrossRef]
- Shang, W.Q.; Wang, Z.; He, S.L.; Meng, X.Y.; Song, Y.L. Effect of light quality ratio and intensity of red/blue light on growth of Hemerocallis middendorfii plantlets in vitro. J. Northwest A F Univ.—Nat. Sci. Ed. 2017, 45, 90–96. [Google Scholar]
- Pu, G.B.; Liu, S.Q.; Liu, L.; Ren, L.H. Effect of different light qualities on growth and physiological characteristics of tomato seedlings. Acta Hortic. Sin. 2005, 32, 420–425. [Google Scholar]
- Cao, G.; Zhang, G.B.; Hua, Y.J.; Ma, Y.X. Effects of different LED light qualities on cucumber seedling growth and chlorophyll fluorescence parameters. Sci. Agric. Sin. 2013, 46, 1297–1304. [Google Scholar]
- Shi, X.D.; Wang, Z.Z.; Man, X.L. Effects of different light qualities on growth and development and photosynthetic physiological characteristics of fle-cured tobacco seedlings in flating system. Acta Tab. Sin. 2013, 1, 43–46. [Google Scholar]
- Lichtenthaler, H.K.; Babani, F.; Langsdorf, G. Chlorophyll fluorescence imaging of photosynthetic activity in sun and shade leaves of trees. Photosynth. Res. 2007, 93, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Tadda, S.A.; Chen, F. Photosynthetic apparatus performance of tomato seedlings grown under various combinations of LED illumination. PLoS ONE 2021, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, Y.F.; Chu, H.L.; Chen, X.M.; Guo, H.P.; Yuan, H.; Yan, D.; Zheng, B. Effects of different light qualities on seedling growth and chlorophyll fluorescence parameters of Dendrobium officinale. Biologia 2017, 72, 735–744. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.A.; Liang, Y.Y. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolata. BMC Plant Biol. 2020, 20, 269. [Google Scholar] [CrossRef]
- Moe, R.; Grimstad, S.O.; Gislerod, H.R. The use of artificial light in year round production of greenhouse crops in Norway. Acta Hortic. 2006, 711, 35–42. [Google Scholar] [CrossRef]
- Husajn, S.; Iqbal, N.; Rahman, T.; Liu, T.; Brestic, M.; Safdar, M.E.; Asghar, M.A.; Farooq, M.U.; Shafiq, I.; Ali, A.; et al. Shade effect on carbohydrates dynamics and stem strength of soybean genotypes. Environ. Exp. Bot. 2019, 162, 374–382. [Google Scholar] [CrossRef]
- Kokalj, D.; Zlatić, E.; Cigić, B.; Kobav, M.B.; Vidrih, R. Postharvest flavonol and anthocyanin accumulation in three apple cultivars in response to blue-light-emitting diode light. Sci. Hortic. 2019, 257, 108711. [Google Scholar] [CrossRef]
- Wu, H. Effect of LED Light Quality on the Growth Characteristics and Nutritional Quality of Arugula. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2021. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.C.; He, S.L.; He, D.; Shang, W.Q.; Song, Y.L. Effect of different light quality ratios of LED red/blue light on growth and antioxidant enzyme activities of Photinia fraseri plantlets in vitro. J. Northwest A F Univ.—Nat. Sci. Ed. 2018, 46, 49–56. [Google Scholar] [CrossRef]
- Samuoliene, G.; Brazaityte, A.; Sirtautas, A.; Viršilė, A.; Sakalauskaitė, J.; Sakalauskienė, S.; Duchovskis, P. LED illumination affects bioactive compounds in romaine baby leaf lettuce. J. Sci. Food Agric. 2013, 93, 3286–3291. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, S.; Li, C.X.; Xin, S.P.; Wang, X.Q.; Tao, J. Effects of LED light quality on the photosynthetic properties and physiological indexes of ‘Summer black’ grape. J. Fruit Sci. 2015, 32, 879–884. [Google Scholar] [CrossRef]
- Normanly, J. Auxin metabolism. Physiol. Plant. 1997, 100, 431–442. [Google Scholar] [CrossRef]
- Kim, K.; Kook, H.S.; Jang, Y.J.; Lee, W.H.; Kamala-Kannan, S.; Chae, J.C.; Lee, K.J. The effect of blue-light-emitting diodes on antioxidant properties and resistance to Botrytis cinerea in tomato. J. Plant Pathol. Microbiol. 2013, 4, 203. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Simlat, M.; Ślęzak, P.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar] [CrossRef]


| Cultivar | Treatment | Plant Height (cm) | Leaf Number | Leaf Length (cm) | Leaf Width (cm) | Root Number | Root Length (cm) |
|---|---|---|---|---|---|---|---|
| Pink Champion | CK | 20.72 bc ± 0.39 | 8.20 b ± 0.58 | 6.90 ab ± 0.27 | 3.50 ab ± 0.11 | 9.60 c ± 0.82 | 8.46 b ± 0.56 |
| B | 22.06 ab ± 1.56 | 8.00 b ± 0.55 | 7.42 a ± 0.44 | 3.59 a ± 0.19 | 11.20 bc ± 0.93 | 8.78 b ± 0.55 | |
| 6:4 | 22.32 ab ± 0.54 | 9.00 ab ± 0.63 | 7.26 ab ± 0.57 | 4.02 a ± 0.30 | 13.60 ab ± 0.24 | 9.98 ab ± 0.29 | |
| 7:3 | 24.68 a ± 1.14 | 10.60 a ± 0.75 | 7.04 ab ± 0.24 | 3.62 a ± 0.10 | 16.60 a ± 0.75 | 11.28 a ± 0.48 | |
| 8:2 | 23.72 ab ± 0.96 | 9.40 ab ± 0.98 | 6.96 ab ± 0.43 | 3.72 a ± 0.17 | 13.80 ab ± 0.86 | 8.94 b ± 0.56 | |
| R | 18.76 c ± 1.01 | 8.00 b ± 0.84 | 6.02 b ± 0.26 | 2.98 b ± 0.18 | 13.20 b ± 0.66 | 8.88 b ± 0.66 | |
| Alabama | CK | 18.14 bc ± 1.35 | 6.80 a ± 0.58 | 6.18 b ± 0.29 | 3.86 bc ± 0.19 | 8.40 bc ± 0.51 | 9.16 b ± 0.65 |
| B | 16.22 c ± 0.89 | 7.20 a ± 0.73 | 6.50 ab ± 0.24 | 4.08 b ± 0.12 | 8.00 c ± 0.32 | 8.82 b ± 0.92 | |
| 6:4 | 20.48 b ± 0.67 | 7.80 a ± 0.49 | 6.60 ab ± 0.43 | 3.90 bc ± 0.15 | 11.00 ab ± 0.89 | 11.00 b ± 0.71 | |
| 7:3 | 24.06 a ± 0.57 | 7.40 a ± 0.24 | 7.32 a ± 0.44 | 4.72 a ± 0.24 | 11.80 a ± 0.21 | 14.28 a ± 0.61 | |
| 8:2 | 19.70 b ± 1.04 | 8.20 a ± 0.50 | 6.78 ab ± 0.28 | 4.16 b ± 0.24 | 9.00 abc ± 0.44 | 10.58 b ± 0.51 | |
| R | 18.40 bc ± 0.63 | 7.00 a ± 0.32 | 6.22 b ± 0.21 | 3.42 c ± 0.14 | 10.00 abc ± 0.52 | 10.72 b ± 0.40 |
| Cultivar | Treatment | Fresh Weight (g) | Dry Weight (g) | Dry Mass Rate (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Total | Shoot | Root | Total | |||
| Pink Champion | CK | 5.85 bc ± 0.43 | 2.02 c ± 0.21 | 7.87 c ± 0.62 | 0.83 a ± 0.07 | 0.13 b ± 0.01 | 0.96 b ± 0.08 | 11.68 ab ± 0.83 |
| B | 6.23 bc ± 0.47 | 2.27 c ± 0.31 | 8.50 bc ± 0.67 | 0.84 a ± 0.06 | 0.20 ab ± 0.03 | 1.05 ab ± 0.07 | 12.40 a ± 0.69 | |
| 6:4 | 6.70 b ± 0.56 | 3.15 bc ± 0.45 | 9.85 bc ± 0.59 | 0.87 a ± 0.07 | 0.24 ab ± 0.04 | 1.11 ab ± 0.09 | 11.48 ab ± 0.59 | |
| 7:3 | 8.60 a ± 0.64 | 5.00 a ± 0.51 | 13.60 a ± 0.91 | 1.02 a ± 0.09 | 0.31 a ± 0.02 | 1.33 a ± 0.10 | 9.73 ab ± 0.24 | |
| 8:2 | 7.07 ab ± 0.46 | 3.56 b ± 0.32 | 10.64 b ± 0.71 | 0.97 a ± 0.07 | 0.26 a ± 0.04 | 1.23 ab ± 0.05 | 11.71 ab ± 0.68 | |
| R | 4.89 c ± 0.64 | 2.76 bc ± 0.35 | 7.65 c ± 0.37 | 0.39 b ± 0.08 | 0.22 ab ± 0.03 | 0.62 c ± 0.03 | 8.57 b ± 0.65 | |
| Alabama | CK | 3.85 b ± 0.16 | 1.72 b ± 0.28 | 5.58 b ± 0.30 | 0.47 a ± 0.05 | 0.13 a ± 0.04 | 0.60 b ± 0.04 | 11.10 a ± 0.82 |
| B | 3.98 b ± 0.38 | 1.42 b ± 0.36 | 5.40 b ± 0.35 | 0.56 a ± 0.09 | 0.16 a ± 0.02 | 0.72 ab ± 0.04 | 14.64 a ± 0.57 | |
| 6:4 | 4.98 ab ± 0.31 | 2.23 b ± 0.33 | 7.21 b ± 0.54 | 0.63 a ± 0.05 | 0.23 a ± 0.03 | 0.86 ab ± 0.05 | 12.12 a ± 0.72 | |
| 7:3 | 5.69 a ± 0.25 | 3.26 a ± 0.39 | 8.95 a ± 0.38 | 0.68 a ± 0.09 | 0.28 a ± 0.03 | 0.96 a ± 0.05 | 10.81 a ± 0.65 | |
| 8:2 | 4.33 b ± 0.19 | 2.19 b ± 0.26 | 6.52 b ± 0.50 | 0.55 a ± 0.07 | 0.18 a ± 0.02 | 0.73 ab ± 0.06 | 11.68 a ± 0.13 | |
| R | 3.96 b ± 0.16 | 1.83 b ± 0.32 | 5.79 b ± 0.36 | 0.47 a ± 0.03 | 0.17 a ± 0.02 | 0.64 b ± 0.05 | 11.57 a ± 0.66 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Shang, W.; Ma, D.; Wang, Z.; He, S.; Shi, L.; Shen, Y.; He, D.; Wang, E.; Wang, X. Effect on the Growth and Photosynthetic Characteristics of Anthurium andreanum (‘Pink Champion’, ‘Alabama’) under Hydroponic Culture by Different LED Light Spectra. Horticulturae 2022, 8, 389. https://doi.org/10.3390/horticulturae8050389
Song Y, Shang W, Ma D, Wang Z, He S, Shi L, Shen Y, He D, Wang E, Wang X. Effect on the Growth and Photosynthetic Characteristics of Anthurium andreanum (‘Pink Champion’, ‘Alabama’) under Hydroponic Culture by Different LED Light Spectra. Horticulturae. 2022; 8(5):389. https://doi.org/10.3390/horticulturae8050389
Chicago/Turabian StyleSong, Yinglong, Wenqian Shang, Dandan Ma, Zheng Wang, Songlin He, Liyun Shi, Yuxiao Shen, Dan He, Erqiang Wang, and Xiaohui Wang. 2022. "Effect on the Growth and Photosynthetic Characteristics of Anthurium andreanum (‘Pink Champion’, ‘Alabama’) under Hydroponic Culture by Different LED Light Spectra" Horticulturae 8, no. 5: 389. https://doi.org/10.3390/horticulturae8050389
APA StyleSong, Y., Shang, W., Ma, D., Wang, Z., He, S., Shi, L., Shen, Y., He, D., Wang, E., & Wang, X. (2022). Effect on the Growth and Photosynthetic Characteristics of Anthurium andreanum (‘Pink Champion’, ‘Alabama’) under Hydroponic Culture by Different LED Light Spectra. Horticulturae, 8(5), 389. https://doi.org/10.3390/horticulturae8050389






