Effects of Girdling and Foliar Fertilization with K on Physicochemical Parameters, Phenolic and Volatile Composition in ‘Hanxiangmi’ Table Grape
Abstract
:1. Introduction
2. Material and Method
2.1. Plant Materials
2.2. Measurement of General Properties
2.3. Extraction and Determination of Phenolic Composition and Concentrations
2.4. Aroma Compounds Extraction and Analysis by GC-MS
2.5. RNA Extraction and qRT-PCR
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Result
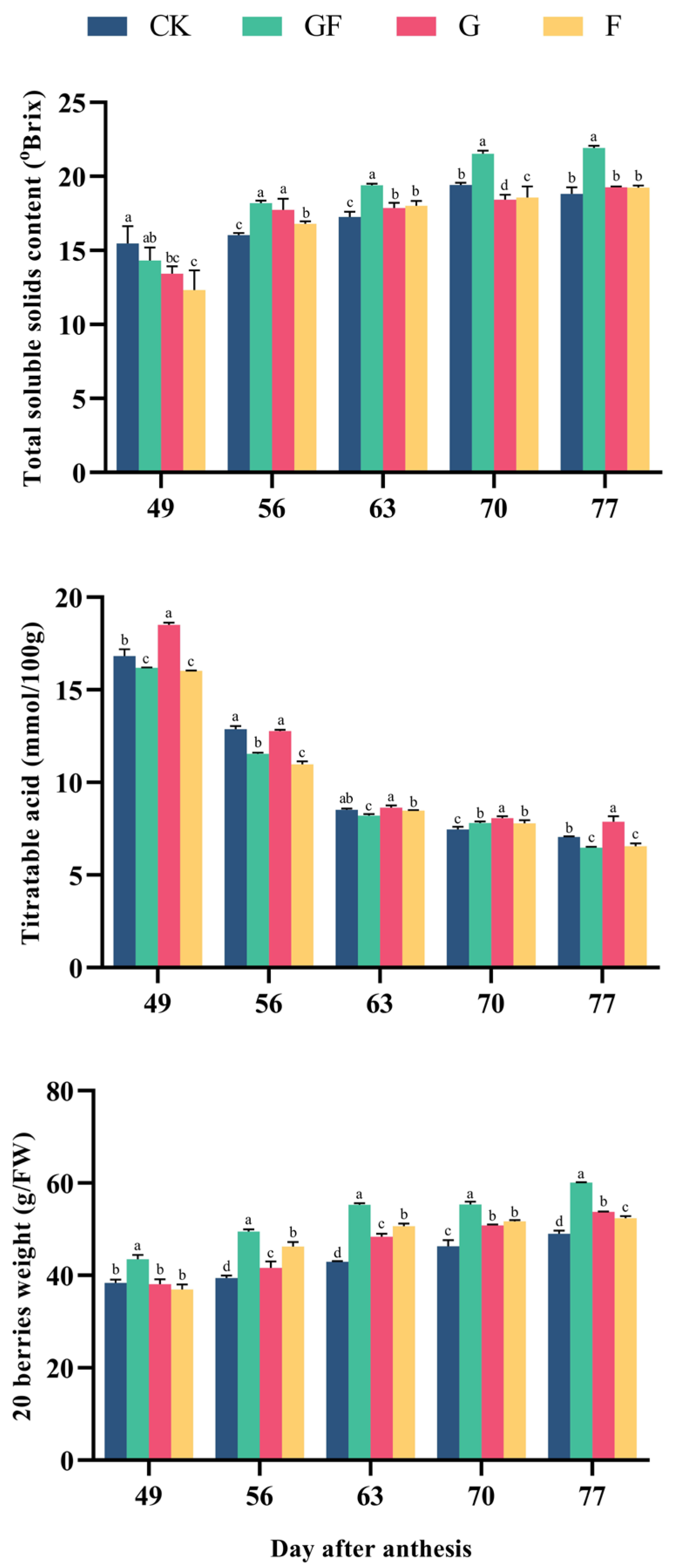
3.1. General Properties of Different Treatments
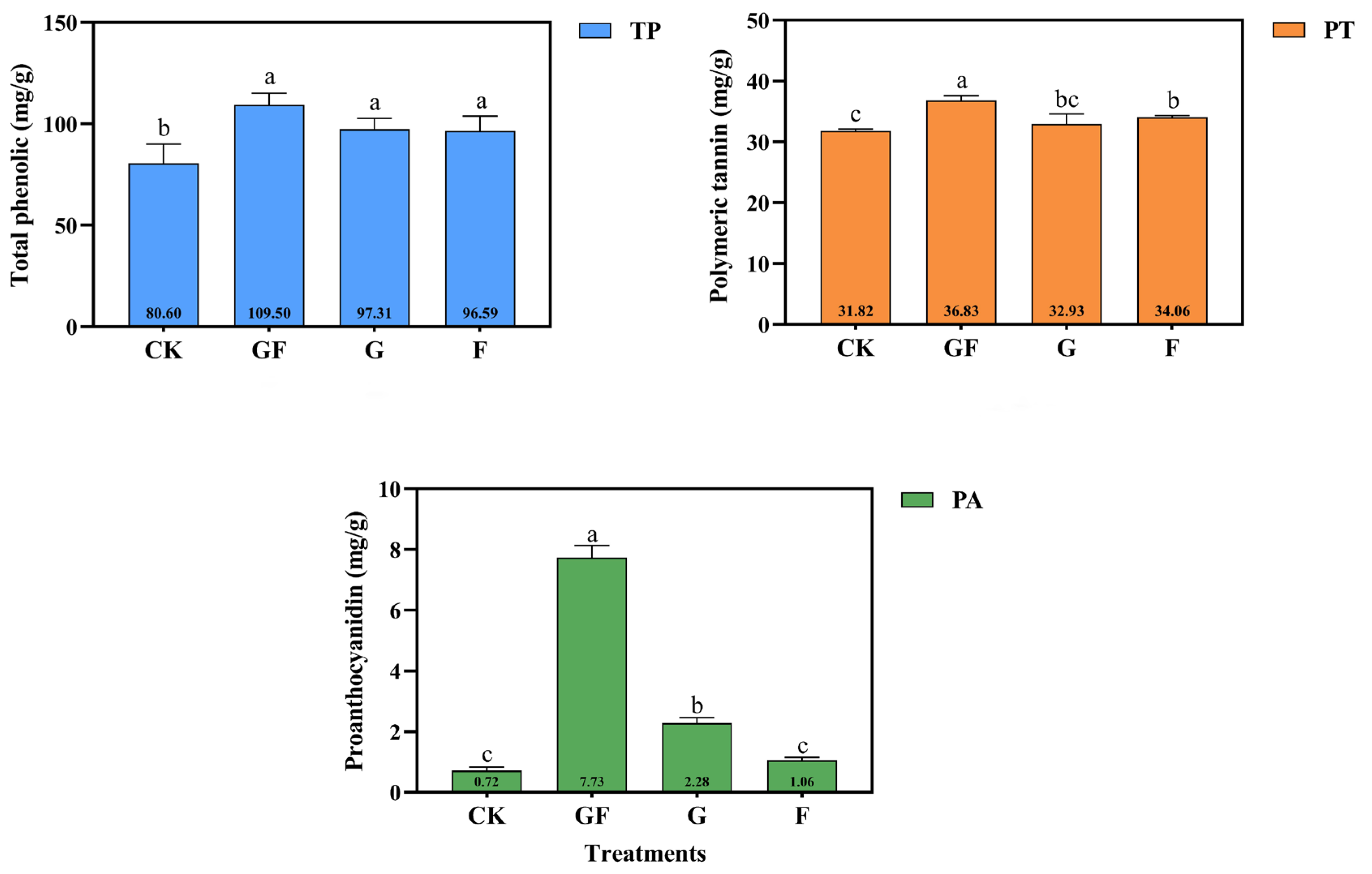
3.2. Phenolic Compound and Concentration
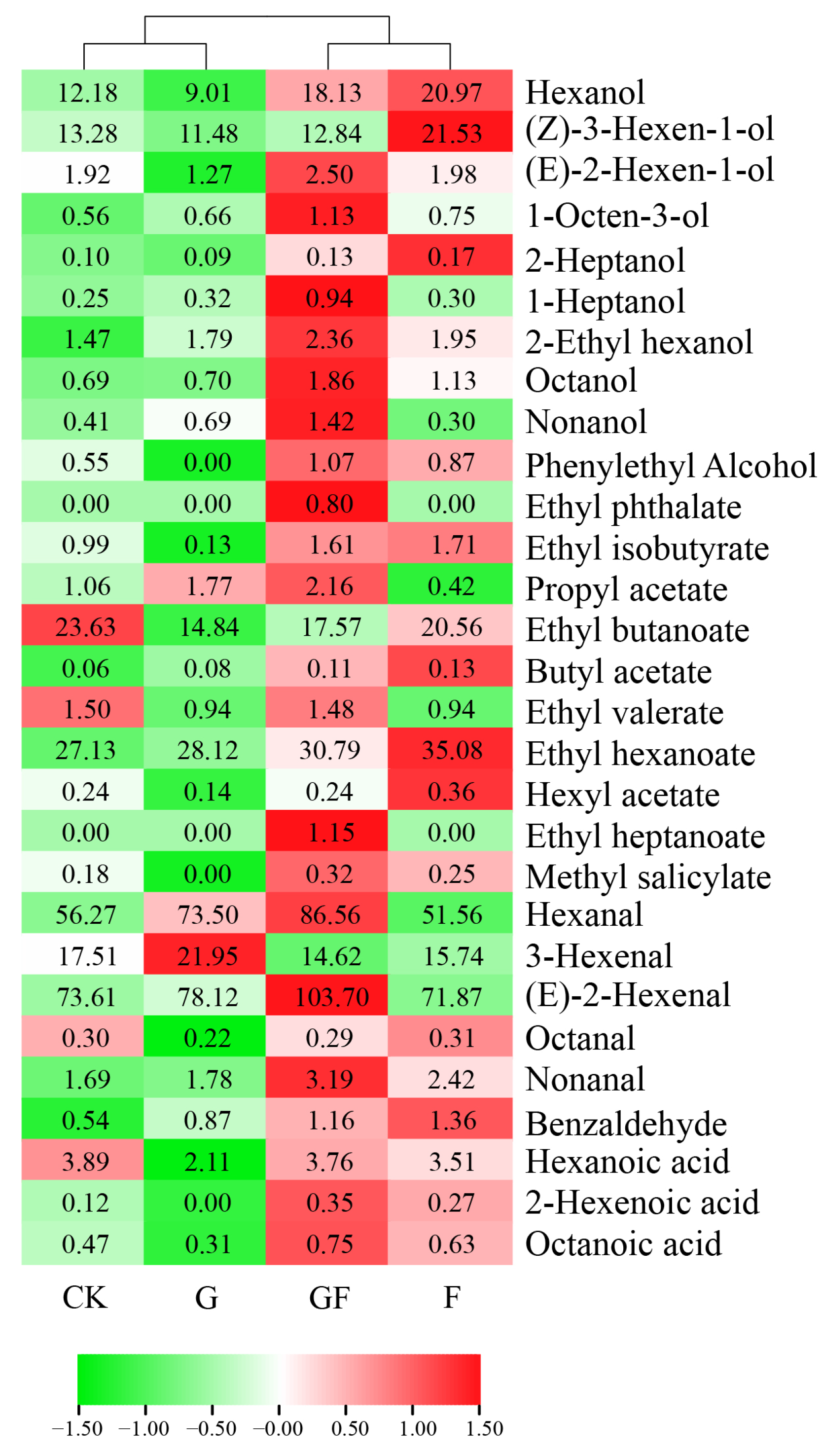
3.3. Volatile Free Aroma Composition
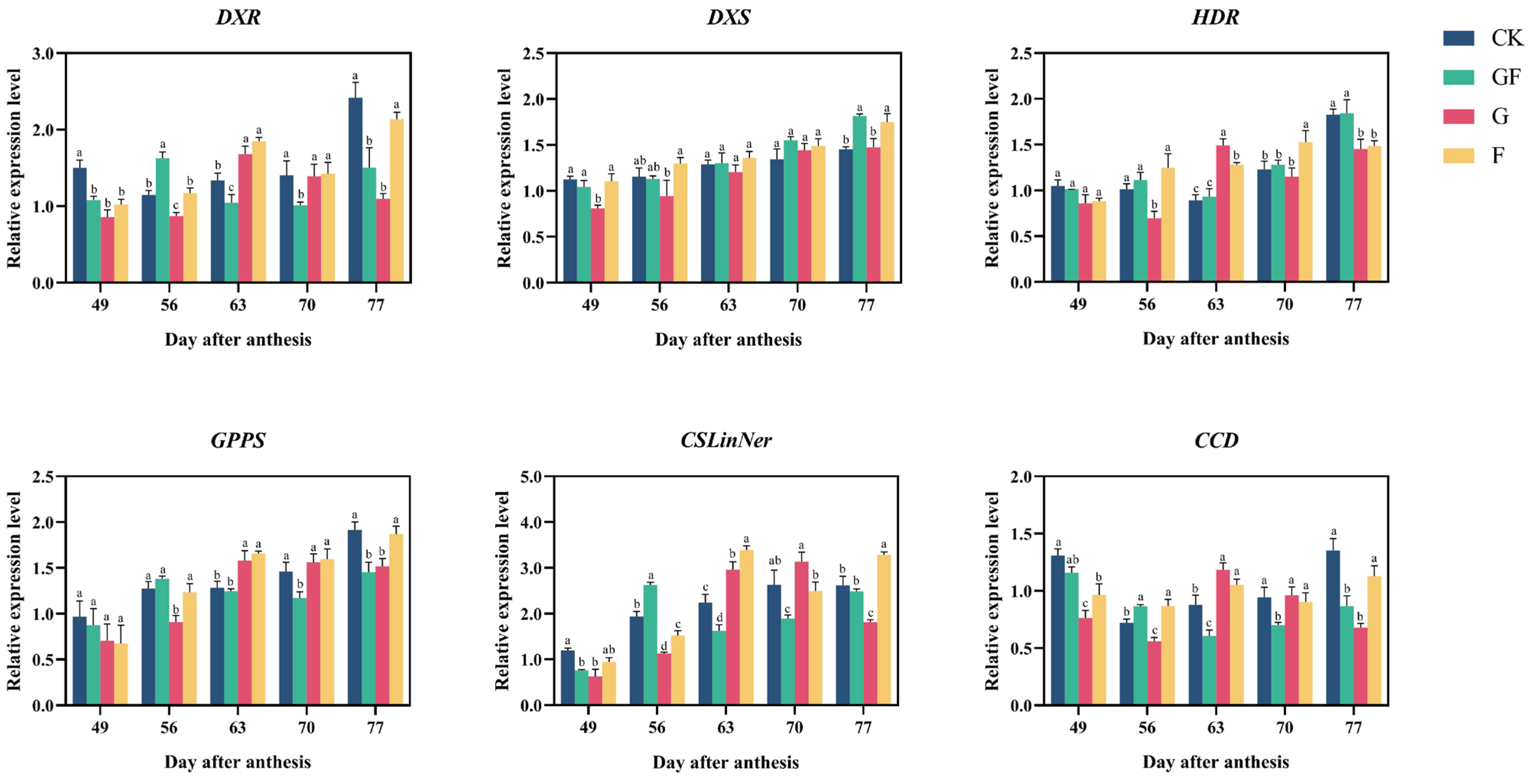
3.4. Spatial and Temporal Expression Analysis of Terpene-Related Genes
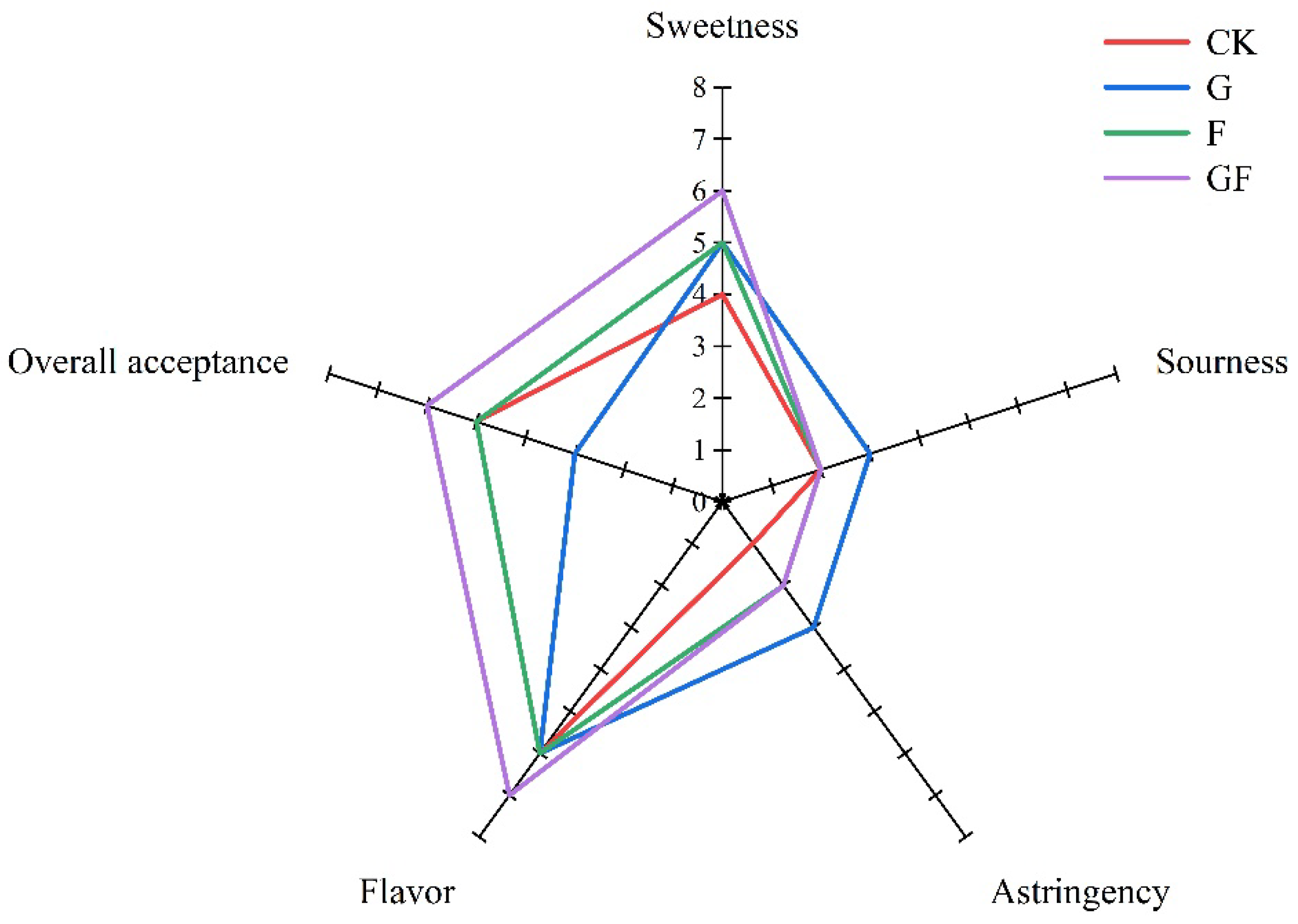
3.5. Sensory Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Feng, J.; Wei, L.; Khalil-Ur-Rehman, M.; Nieuwenhuizen, N.J.; Yang, L.; Zheng, H.; Tao, J. Transcriptomics Integrated with Free and Bound Terpenoid Aroma Profiling during “Shine Muscat” (Vitis labrusca × V. vinifera) Grape Berry Development Reveals Coordinate Regulation of MEP Pathway and Terpene Synthase Gene Expression. J. Agric. Food Chem. 2021, 69, 1413–1429. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, T.; Zhang, T.; Liu, T.; Shen, L.; Chen, Z.; Wu, Y.; Yang, J. Genome-Wide Identification and Characterization of DnaJ Gene Family in Grape (Vitis vinifera L.). Horticulturae 2021, 7, 589. [Google Scholar] [CrossRef]
- Li, X.; He, L.; An, X.; Yu, K.; Meng, N.; Duan, C.; Pan, Q.-H. VviWRKY40, a WRKY Transcription Factor, Regulates Glycosylated Monoterpenoid Production by VviGT14 in Grape Berry. Genes 2020, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Mariño, N.C.; Tamames, E.L.; Jares, Y.C.G. Contribution to the Study of the Aromatic Potential of Three Muscat Vitis Vinifera Varieties: Identification of New Compounds. Food Sci. Technol. Int. 1995, 1, 105–116. [Google Scholar] [CrossRef]
- Rocha, S.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile composition of Baga red wine: Assessment of the identification of the would-be impact odourants. Anal. Chim. Acta 2004, 513, 257–262. [Google Scholar] [CrossRef]
- Fenoll, J.; Manso, A.; Hellin, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Capone, S.; Tufariello, M.; Siciliano, P. Analytical characterisation of Negroamaro red wines by “Aroma Wheels”. Food Chem. 2013, 141, 2906–2915. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Evolution of Volatile Compounds during the Development of Cabernet Sauvignon Grapes (Vitis vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2018, 99, 975–985. [Google Scholar] [CrossRef]
- Ojeda, H.; Andary, C.; Kraeva, E.; Carbonneau, A.; Deloire, A. Influence of Pre- and Postveraison Water Deficit on Synthesis and Concentration of Skin Phenolic Compounds during Berry Growth of Vitis vinifera cv. Shiraz. Am. J. Enol. Vitic. 2002, 53, 261–267. [Google Scholar]
- Fragasso, M.; Antonacci, D.; Pati, S.; Tufariello, M.; Baiano, A.; Forleo, L.R.; Caputo, A.R.; La Notte, E.; Hirson, G.D.; Heymann, H.; et al. Influence of Training System on Volatile and Sensory Profiles of Primitivo Grapes and Wines. Am. J. Enol. Vitic. 2012, 63, 477–486. [Google Scholar] [CrossRef]
- Cincotta, F.; Verzera, A.; Prestia, O.; Tripodi, G.; Lechhab, W.; Sparacio, A.; Condurso, C. Influence of leaf removal on grape, wine and aroma compounds of Vitis vinifera L. cv. Merlot under Mediterranean climate. Eur. Food Res. Technol. 2021, 248, 403–413. [Google Scholar] [CrossRef]
- Buesa, I.; Intrigliolo, D.S.; Castel, J.R.; Vilanova, M. Influence of water regime on grape aromatic composition of Muscat of Alexandria in a semiarid climate. Sci. Hortic. 2021, 290, 110525. [Google Scholar] [CrossRef]
- Kok, D. Influences of pre- and post-veraison cluster thinning treatments on grape composition variables and monoterpene levels of Vitis vinifera L. cv. Sauvignon Blanc. J. Food Agric. Environ. 2011, 9, 22–26. [Google Scholar]
- Reynolds, A.G.; De Savigny, C. Influence of Girdling and Gibberellic Acid on Yield Components, Fruit Composition, and Vestigial Seed Formation of ‘Sovereign Coronation’ Table Grapes. HortScience 2004, 39, 541–544. [Google Scholar] [CrossRef] [Green Version]
- Roper, T.R.; Williams, L.E. Net CO2 Assimilation and Carbohydrate Partitioning of Grapevine Leaves in Response to Trunk Girdling and Gibberellic Acid Application. Plant Physiol. 1989, 89, 1136–1140. [Google Scholar] [CrossRef] [Green Version]
- El-Otmani, M.; Ait-Oubahou, A.; Zacarías, L. 21-Citrus spp.: Orange, mandarin, tangerine, clementine, grapefruit, pomelo, lemon and lime. In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 437–516. [Google Scholar]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A Review of Current Status and Future Perspectives. J. Soil Sci. Plant Nutr. 2020, 21, 104–118. [Google Scholar] [CrossRef]
- Song, C.-Z.; Liu, M.-Y.; Meng, J.-F.; Shi, P.-B.; Zhang, Z.-W.; Xi, Z.-M. Influence of foliage-sprayed zinc sulfate on grape quality and wine aroma characteristics of Merlot. Eur. Food Res. Technol. 2015, 242, 609–623. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Gamboa, G.G.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Influence of methyl jasmonate foliar application to vineyard on grape volatile composition over three consecutive vintages. Food Res. Int. 2018, 112, 274–283. [Google Scholar] [CrossRef]
- Martins, V.; Teixeira, A.; Gerós, H. Changes in the volatile composition of wine from grapes treated with Bordeaux mixture: A laboratory-scale study. Aust. J. Grape Wine Res. 2015, 21, 425–429. [Google Scholar] [CrossRef]
- Río Segade, S.; Vilanova, M.; Giacosa, S.; Perrone, I.; Chitarra, W.; Pollon, M.; Torchio, F.; Boccacci, P.; Gambino, G.; Gerbi, V.; et al. Ozone Improves the Aromatic Fingerprint of White Grapes. Sci. Rep. 2017, 7, 16301. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gao, S.; He, X.; Li, Y.; Zhang, Y.; Chen, W. Response of total phenols, flavonoids, minerals, and amino acids of four edible fern species to four shading treatments. PeerJ 2020, 8, e8354. [Google Scholar] [CrossRef]
- Woo, A.; Lindsay, R. Method for the Routine Quantitative Gas Chromatographic Analysis of Major Free Fatty Acids in Butter and Cream. J. Dairy Sci. 1980, 63, 1058–1064. [Google Scholar] [CrossRef]
- Xi, X.; Zha, Q.; He, Y.; Tian, Y.; Jiang, A. Influence of cluster thinning and girdling on aroma composition in ‘Jumeigui’ table grape. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.M.; Chiang, A.; Lund, S.T.; Bohlmann, J. Biosynthesis of wine aroma: Transcript profiles of hydroxymethylbutenyl diphosphate reductase, geranyl diphosphate synthase, and linalool/nerolidol synthase parallel monoterpenol glycoside accumulation in Gewürztraminer grapes. Planta 2012, 236, 919–929. [Google Scholar] [CrossRef]
- Battilana, J.; Emanuelli, F.; Gambino, G.; Gribaudo, I.; Gasperi, F.; Boss, P.K.; Grando, M.S. Functional effect of grapevine 1-deoxy-D-xylulose 5-phosphate synthase substitution K284N on Muscat flavour formation. J. Exp. Bot. 2011, 62, 5497–5508. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, S.; Terrier, N.; Procureur, J.; Bigey, F.; Günata, Z. A Carotenoid Cleavage Dioxygenase from Vitis vinifera L.: Functional characterization and expression during grape berry development in relation to C13-norisoprenoid accumulation. J. Exp. Bot. 2005, 56, 2721–2731. [Google Scholar] [CrossRef]
- Choi, K.-O.; Im, D.; Park, S.J.; Lee, D.H.; Kim, S.J.; Hur, Y.Y. Effects of Berry Thinning on the Physicochemical, Aromatic, and Sensory Properties of Shine Muscat Grapes. Horticulturae 2021, 7, 487. [Google Scholar] [CrossRef]
- Granato, D.; Masson, M.L.; Ribeiro, J.C.B. Sensory acceptability and physical stability evaluation of a prebiotic soy-based dessert developed with passion fruit juice. Food Sci. Technol. 2012, 32, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.-H.; Wang, B.-L.; Wang, X.-D.; Liu, F.-Z.; Wang, H.-B. Differences of aroma development and metabolic pathway gene expression between Kyoho and 87-1 grapes. J. Integr. Agric. 2021, 20, 1525–1539. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016, 6, 31116. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Yang, H.; Kim, D.; Yang, M.; Park, T.H.; Hong, S. Human-like smelling of a rose scent using an olfactory receptor nanodisc-based bioelectronic nose. Sci. Rep. 2018, 8, 13945. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.-O.; Lee, D.H.; Park, S.J.; Im, D.; Hur, Y.Y.; Kim, S.J. Changes in Biochemical and Volatile Flavor Compounds of Shine Muscat at Different Ripening Stages. Appl. Sci. 2020, 10, 5661. [Google Scholar] [CrossRef]
- Gunata, Y.; Bayonove, C.; Baumes, R.; Cordonnier, R. The aroma of grapes I. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J. Chromatogr. A 1985, 331, 83–90. [Google Scholar] [CrossRef]
- Genovese, A.; Lamorte, S.A.; Gambuti, A.; Moio, L. Aroma of Aglianico and Uva di Troia grapes by aromatic series. Food Res. Int. 2013, 53, 15–23. [Google Scholar] [CrossRef]
- Guth, H. Identification of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3022–3026. [Google Scholar] [CrossRef]
- Genovese, A.; Dimaggio, R.; Lisanti, M.T.; Piombino, P.; Moio, L. Aroma Composition of Red Wines by Different Extraction Methods and Gas Chromatography-SIM/Mass Spectrometry Analysis. Ann. Chim. 2005, 95, 383–394. [Google Scholar] [CrossRef]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Analytical Study of Aromatic Series in Sherry Wines Subjected to Biological Aging. J. Agric. Food Chem. 2002, 50, 7356–7361. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Franco, M.; Peinado, R.A.; Medina, A.M.; Moreno, J. Off-Vine Grape Drying Effect on Volatile Compounds and Aromatic Series in Must from Pedro Ximénez Grape Variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef]
- Du, X.; Finn, C.E.; Qian, M.C. Volatile composition and odour-activity value of thornless ‘Black Diamond’ and ‘Marion’ blackberries. Food Chem. 2010, 119, 1127–1134. [Google Scholar] [CrossRef]
- Yang, C.; Luo, L.; Zhang, H.; Yang, X.; Lv, Y.; Song, H. Common aroma-active components of propolis from 23 regions of China. J. Sci. Food Agric. 2010, 90, 1268–1282. [Google Scholar] [CrossRef]
- Pino, J.A.; Queris, O. Analysis of volatile compounds of mango wine. Food Chem. 2011, 125, 1141–1146. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z. Volatile Compounds of Young Wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay Varieties Grown in the Loess Plateau Region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef] [Green Version]
- Meng, N.; Wei, Y.; Gao, Y.; Yu, K.; Cheng, J.; Li, X.-Y.; Duan, C.-Q.; Pan, Q.-H. Characterization of Transcriptional Expression and Regulation of Carotenoid Cleavage Dioxygenase 4b in Grapes. Front. Plant Sci. 2020, 11, 483. [Google Scholar] [CrossRef]
- McFadyen, L.; Robertson, D.; Sedgley, M.; Kristiansen, P.; Olesen, T. Effects of girdling on fruit abscission, yield and shoot growth in macadamia. Sci. Hortic. 2013, 164, 172–177. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Serrano, M.; Guillén, F. Preharvest Salicylate Treatments Enhance Antioxidant Compounds, Color and Crop Yield in Low Pigmented-Table Grape Cultivars and Preserve Quality Traits during Storage. Antioxidants 2020, 9, 832. [Google Scholar] [CrossRef]
- Topalović, A.; Slatnar, A.; Štampar, F.; Knežević, M.; Veberič, R. Influence of Foliar Fertilization with P and K on Chemical Constituents of Grape cv. ‘Cardinal’. J. Agric. Food Chem. 2011, 59, 10303–10310. [Google Scholar] [CrossRef]
- Koshita, Y.; Yamane, T.; Yakushiji, H.; Azuma, A.; Mitani, N. Regulation of skin color in ‘Aki Queen’ grapes: Interactive effects of temperature, girdling, and leaf shading treatments on coloration and total soluble solids. Sci. Hortic. 2011, 129, 98–101. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, J.; Xu, Y.; Yang, G. Effect of Girdling on Anthocyanin Content and Quality of Spine Grape Berries. J. Plant Growth Regul. 2021, 41, 65–73. [Google Scholar] [CrossRef]
- Zhong, H.; Xue, Y.; Lu, X.; Shao, Q.; Cao, Y.; Wu, Z.; Chen, G. The Effects of Different Degrees of Procyanidin Polymerization on the Nutrient Absorption and Digestive Enzyme Activity in Mice. Molecules 2018, 23, 2916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, K.; Maoz, I.; Lewinsohn, E.; Lerno, L.; Ebeler, S.E.; Lichter, A. Girdling of table grapes at fruit set can divert the phenylpropanoid pathway towards accumulation of proanthocyanidins and change the volatile composition. Plant Sci. 2020, 296, 110495. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Schlosser, J.; Sorokowsky, D.; Roberts, R.; Willwerth, J.; de Savigny, C. Magnitude of Viticultural and Enological Effects. II. Relative Impacts of Cluster Thinning and Yeast Strain on Composition and Sensory Attributes of Chardonnay Musque. Am. J. Enol. Vitic. 2007, 58, 25–41. [Google Scholar]
- Boss, P.K.; Böttcher, C.; Davies, C. Various Influences of Harvest Date and Fruit Sugar Content on Different Wine Flavor and Aroma Compounds. Am. J. Enol. Vitic. 2014, 65, 341–353. [Google Scholar] [CrossRef]
- Rohmer, M.; Knani, M.; Simonin, P.; Sutter, B.; Sahm, H. Isoprenoid biosynthesis in bacteria: A novel pathway for the early steps leading to isopentenyl diphosphate. Biochem. J. 1993, 295, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Battilana, J.; Costantini, L.; Emanuelli, F.; Sevini, F.; Segala, C.; Moser, S.; Velasco, R.; Versini, G.; Grando, M.S. The 1-deoxy-d-xylulose 5-phosphate synthase gene co-localizes with a major QTL affecting monoterpene content in grapevine. Theor. Appl. Genet. 2008, 118, 653–669. [Google Scholar] [CrossRef]
- Li, Y.; He, C.; Yu, X.; Zhou, J.; Ntezimana, B.; Yu, Z.; Chen, Y.; Ni, D. Study on improving aroma quality of summer-autumn black tea by red-light irradiation during withering. LWT 2021, 154, 112597. [Google Scholar] [CrossRef]
- Morcia, C.; Tumino, G.; Raimondi, S.; Schneider, A.; Terzi, V. Muscat Flavor in Grapevine: A Digital PCR Assay to Track Allelic Variation in VvDXS Gene. Genes 2021, 12, 747. [Google Scholar] [CrossRef]
- Emanuelli, F.; Battilana, J.; Costantini, L.; Le Cunff, L.; Boursiquot, J.-M.; This, P.; Grando, M.S. A candidate gene association study on muscat flavor in grapevine (Vitis vinifera L.). BMC Plant Biol. 2010, 10, 241. [Google Scholar] [CrossRef] [Green Version]
- Soares, S.; Soares, S.; Brandão, E.; Guerreiro, C.; Mateus, N.; De Freitas, V. Oral interactions between a green tea flavanol extract and red wine anthocyanin extract using a new cell-based model: Insights on the effect of different oral epithelia. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]

| Compounds | DAA | Treatment | |||
|---|---|---|---|---|---|
| CK | GF | G | F | ||
| Nerol | 49 | N.D | N.D | N.D | N.D |
| 56 | N.D | 0.0997 ± 0.0150 | N.D | N.D | |
| 63 | N.D | 0.4667 ± 0.0632 a | 0.2206 ± 0.0349 b | 0.1361 ± 0.0145 b | |
| 70 | 0.2813 ± 0.0079 b | 0.7899 ± 0.0455 a | 0.2143 ± 0.0167 c | 0.2745 ± 0.0147 b | |
| 77 | 0.5610 ± 0.0292 b | 1.1219 ± 0.0942 a | 0.3800 ± 0.0530 c | 0.3708 ± 0.0560 c | |
| Linalool | 49 | 0.3869 ± 0.0420 a | 0.2228 ± 0.0162 d | 0.3005 ± 0.0280 b | 0.2858 ± 0.0297 b |
| 56 | 0.4724 ± 0.0551 a | 0.3936 ± 0.0288 a | 0.4582 ± 0.0129 a | 0.4341 ± 0.0560 a | |
| 63 | 0.9081 ± 0.0793 a | 0.7322 ± 0.0318 b | 0.9479 ± 0.0519 a | 0.7878 ± 0.0155 b | |
| 70 | 0.7761 ± 0.1207 b | 1.1705 ± 0.0115 a | 0.8042 ± 0.0933 b | 1.0628 ± 0.0660 a | |
| 77 | 1.5796 ± 0.1439 a b | 1.8683 ± 0.1646 a | 1.2967 ± 0.1013 b | 1.7188 ± 0.1798 a | |
| Myrcenol | 49 | 0.3359 ± 0.0468 b | 0.8604 ± 0.1113 a | N.D | N.D |
| 56 | 5.1900 ± 0.2021 b | 5.5554 ± 0.2781 b | 5.2314 ± 0.3910 b | 6.8505 ± 0.2181 a | |
| 63 | 5.5970 ± 0.1675 d | 8.2370 ± 0.3866 b | 6.4270 ± 0.3199 c | 9.3834 ± 0.5000 a | |
| 70 | 9.2388 ± 0.3783 c | 14.4505 ± 0.7998 a | 7.3326 ± 0.3538 d | 10.6854 ± 0.6543 b | |
| 77 | 14.0684 ± 0.4185 a | 15.2128 ± 0.5467 a | 12.4245 ± 1.0899 b | 11.5641 ± 0.8580 b | |
| Hotrienol | 49 | N.D | N.D | N.D | N.D |
| 56 | N.D | N.D | N.D | N.D | |
| 63 | N.D | N.D | N.D | N.D | |
| 70 | N.D | N.D | N.D | N.D | |
| 77 | 0.8900 ± 0.0521 b | 1.2072 ± 0.0844 a | 0.4167 ± 0.0053 c | 0.8986 ± 0.0565 b | |
| 4-Terpineol | 49 | N.D | N.D | N.D | N.D |
| 56 | N.D | N.D | N.D | N.D | |
| 63 | N.D | N.D | N.D | N.D | |
| 70 | 4.0766 ± 0.1007 a | 3.8199 ± 0.1371 b | 1.5359 ± 0.0817 c | 1.5628 ± 0.0481 c | |
| 77 | 5.2349 ± 0.0887 a | 5.0718 ± 0.1418 a | 1.6770 ± 0.1033 b | 1.7623 ± 0.0588 b | |
| Cineole | 49 | N.D | N.D | N.D | N.D |
| 56 | N.D | N.D | N.D | N.D | |
| 63 | N.D | N.D | N.D | N.D | |
| 70 | 0.3215 ± 0.0134 | N.D | N.D | N.D | |
| 77 | 0.6350 ± 0.0220 c | 0.3371 ± 0.0181 d | 1.6611 ± 0.0766 a | 1.1865 ± 0.0054 b | |
| Geraniol | 49 | N.D | N.D | N.D | N.D |
| 56 | N.D | N.D | N.D | N.D | |
| 63 | N.D | N.D | N.D | N.D | |
| 70 | 1.1726 ± 0.0529 b | 1.3391 ± 0.0250 a | 0.8352 ± 0.0333 c | 1.1490 ± 0.0812 b | |
| 77 | 1.3768 ± 0.0582 b | 1.5375 ± 0.1008 a | 0.9769 ± 0.0851 c | 1.4495 ± 0.0464 a b | |
| Citronellol | 49 | N.D | N.D | N.D | N.D |
| 56 | N.D | N.D | N.D | N.D | |
| 63 | N.D | N.D | N.D | N.D | |
| 70 | N.D | N.D | N.D | N.D | |
| 77 | 1.9764 ± 0.1442 b | 2.4003 ± 0.0496 a | 1.4818 ± 0.1188 c | 1.7002 ± 0.0714 d | |
| Phellandrene | 49 | 0.0486 ± 0.0037 b | 0.0434 ± 0.0058 b | 0.0399 ± 0.0039 b | 0.0672 ± 0.0064 a |
| 56 | 0.0983 ± 0.0150 a | 0.0681 ± 0.0103 b | 0.0732 ± 0.0041 b | 0.0822 ± 0.0051 a b | |
| 63 | 0.1173 ± 0.0138 a | 0.0627 ± 0.0010 b | 0.0755 ± 0.0091 b | 0.1269 ± 0.0184 a | |
| 70 | 0.1826 ± 0.0086 a | 0.1104 ± 0.0131 b | 0.0753 ± 0.0056 c | 0.1759 ± 0.0038 a | |
| 77 | 0.1949 ± 0.0109 c | 0.2912 ± 0.0082 a | 0.1124 ± 0.0150 d | 0.2222 ± 0.0073 b | |
| α-Thujene | 49 | N.D | N.D | N.D | N.D |
| 56 | N.D | N.D | N.D | N.D | |
| 63 | N.D | N.D | N.D | N.D | |
| 70 | N.D | N.D | 0.1042 ± 0.0091 a | 0.1160 ± 0.0090 a | |
| 77 | 0.1042 ± 0.0086 c | 0.3875 ± 0.0537 a | 0.1320 ± 0.0092 c | 0.1888 ± 0.0165 b | |
| Geranic acid | 49 | N.D | N.D | N.D | N.D |
| 56 | N.D | N.D | N.D | N.D | |
| 63 | N.D | N.D | N.D | N.D | |
| 70 | N.D | N.D | N.D | N.D | |
| 77 | N.D | 0.2152 ± 0.0055 a | 0.0297 ± 0.0007 b | N.D | |
| Compounds | Odor Threshold | Odorant Series | Compounds | Odor Threshold | Odorant Series |
|---|---|---|---|---|---|
| C6 compounds | Esters | ||||
| Hexanal | 16b [36] | 1 | Ethyl isobutyrate | 15b [37] | 3 |
| 3-Hexenal | 0.25a [38] | 1 | Ethyl butanoate | 600b [39] | 5 |
| (E)-2-Hexenal | 17a [36] | 1 | Butyl acetate | 66a [40] | 3 |
| Hexanol | 1300b [36] | 1, 2 | Ethyl Hexanoate | 100b [39] | 3 |
| (Z)-3-Hexen-1-ol | 400b [36] | 1, 6 | Hexyl acetate | 670b [41] | 1, 2, 3 |
| (E)-2-Hexen-1-ol | 400b [36] | 1 | Ethyl heptanoate | 300b [39] | 3 |
| Alcohols | Methyl salicylate | 40a [42] | 1, 4 | ||
| 1-Octen-3-ol | 1a [43] | 8 | Terpenes | ||
| 2-Heptanol | 70a [42] | 3 | Nerol | 500b [36] | 1, 2 |
| 2-Ethyl hexanol | 270a [44] | 2 | Linalool | 15b [36] | 2, 3, 5 |
| Octanol | 120b [45] | 2 | Geraniol | 30b [36] | 2 |
| Nonanol | 1000a [42] | 2 | Citronellol | 100b [39] | 2 |
| Phenylethyl Alcohol | 1100a [6] | 2 | 4-Terpineol | 500b [41] | 1 |
| Aldehydes | Acids | ||||
| Octanal | 50b [39] | 1, 2, 3, 6 | Hexanoic acid | 8500b [39] | 6 |
| Nonanal | 1a [45] | 1, 3 | Octanoic acid | 10,000b [41] | 5, 6 |
| Benzaldehyde | 2000b [45] | 2, 3, 5, 7 |
| Treatments | Aldehydes | Acids | Esters | Alcohols | Terpenes | Total |
|---|---|---|---|---|---|---|
| CK | 6 | 3 | 8 | 10 | 8 | 35 |
| GF | 6 | 3 | 10 | 10 | 11 | 40 |
| G | 6 | 2 | 7 | 9 | 9 | 33 |
| F | 6 | 3 | 8 | 10 | 8 | 35 |
| Treatments | Aldehydes | Acids | Esters | Alcohols | Terpenes | Total |
|---|---|---|---|---|---|---|
| CK | 149.9252 | 4.4743 | 54.7845 | 31.4012 | 26.6212 | 267.2065 |
| GF | 209.5317 | 4.8707 | 56.2325 | 42.3891 | 29.6508 | 342.6749 |
| G | 176.4324 | 2.4130 | 46.0202 | 26.0214 | 20.5888 | 271.4758 |
| F | 143.2634 | 4.4158 | 59.4534 | 49.9531 | 21.0618 | 278.1475 |
| Gene | Linalool | Total Terpenes | ||||||
|---|---|---|---|---|---|---|---|---|
| CK | GF | G | F | CK | GF | G | F | |
| DXR | 0.860 | 0.181 | 0.536 | 0.868 | 0.803 | 0.166 | 0.242 | 0.838 |
| DXS | 0.936 * | 0.997 ** | 0.889 * | 0.981 ** | 0.939 * | 0.994 ** | 0.886 * | 0.991 ** |
| HDR | 0.826 | 0.899 * | 0.891 * | 0.805 | 0.911 * | 0.882 * | 0.667 | 0.927 * |
| GPPS | 0.929 * | 0.609 | 0.869 | 0.854 | 0.979 ** | 0.604 | 0.726 | 0.935 * |
| CS | 0.735 | 0.512 | 0.585 | 0.781 | 0.828 | 0.548 | 0.405 | 0.813 |
| CCD | 0.421 | −0.360 | 0.266 | 0.664 | 0.353 | 0.005 | −0.069 | 0.508 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Xu, T.; Shen, L.; Zhang, T.; Wang, L.; Chen, Z.; Wu, Y.; Yang, J. Effects of Girdling and Foliar Fertilization with K on Physicochemical Parameters, Phenolic and Volatile Composition in ‘Hanxiangmi’ Table Grape. Horticulturae 2022, 8, 388. https://doi.org/10.3390/horticulturae8050388
Chen T, Xu T, Shen L, Zhang T, Wang L, Chen Z, Wu Y, Yang J. Effects of Girdling and Foliar Fertilization with K on Physicochemical Parameters, Phenolic and Volatile Composition in ‘Hanxiangmi’ Table Grape. Horticulturae. 2022; 8(5):388. https://doi.org/10.3390/horticulturae8050388
Chicago/Turabian StyleChen, Tianchi, Tao Xu, Leyi Shen, Tianye Zhang, Liru Wang, Zhihui Chen, Yueyan Wu, and Jian Yang. 2022. "Effects of Girdling and Foliar Fertilization with K on Physicochemical Parameters, Phenolic and Volatile Composition in ‘Hanxiangmi’ Table Grape" Horticulturae 8, no. 5: 388. https://doi.org/10.3390/horticulturae8050388
APA StyleChen, T., Xu, T., Shen, L., Zhang, T., Wang, L., Chen, Z., Wu, Y., & Yang, J. (2022). Effects of Girdling and Foliar Fertilization with K on Physicochemical Parameters, Phenolic and Volatile Composition in ‘Hanxiangmi’ Table Grape. Horticulturae, 8(5), 388. https://doi.org/10.3390/horticulturae8050388







