Volatiles Distinguishing the European ‘Conference’ and the Asian ‘Yali’ Pears Stored at Different Post-Harvest Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Volatile Compounds in Pear Fruits
2.3. Statistical Analysis
3. Results and Discussion
3.1. Aromatic Compounds Identified in Pears Stored in Different Regimes
3.2. Statistically Significant Volatile Substances Distinguishing the Cultivars and Post-Harvest Storage Regimes
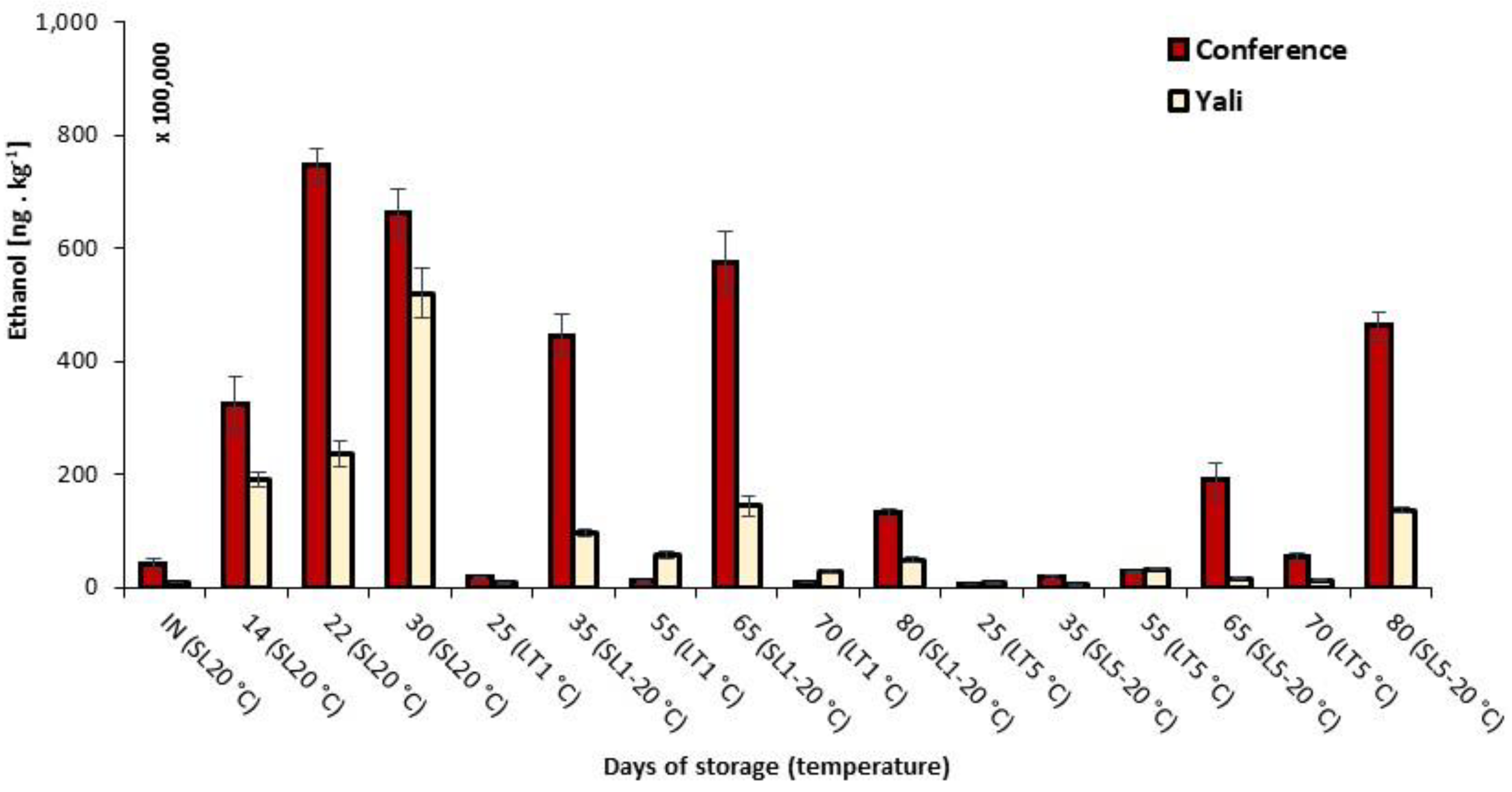
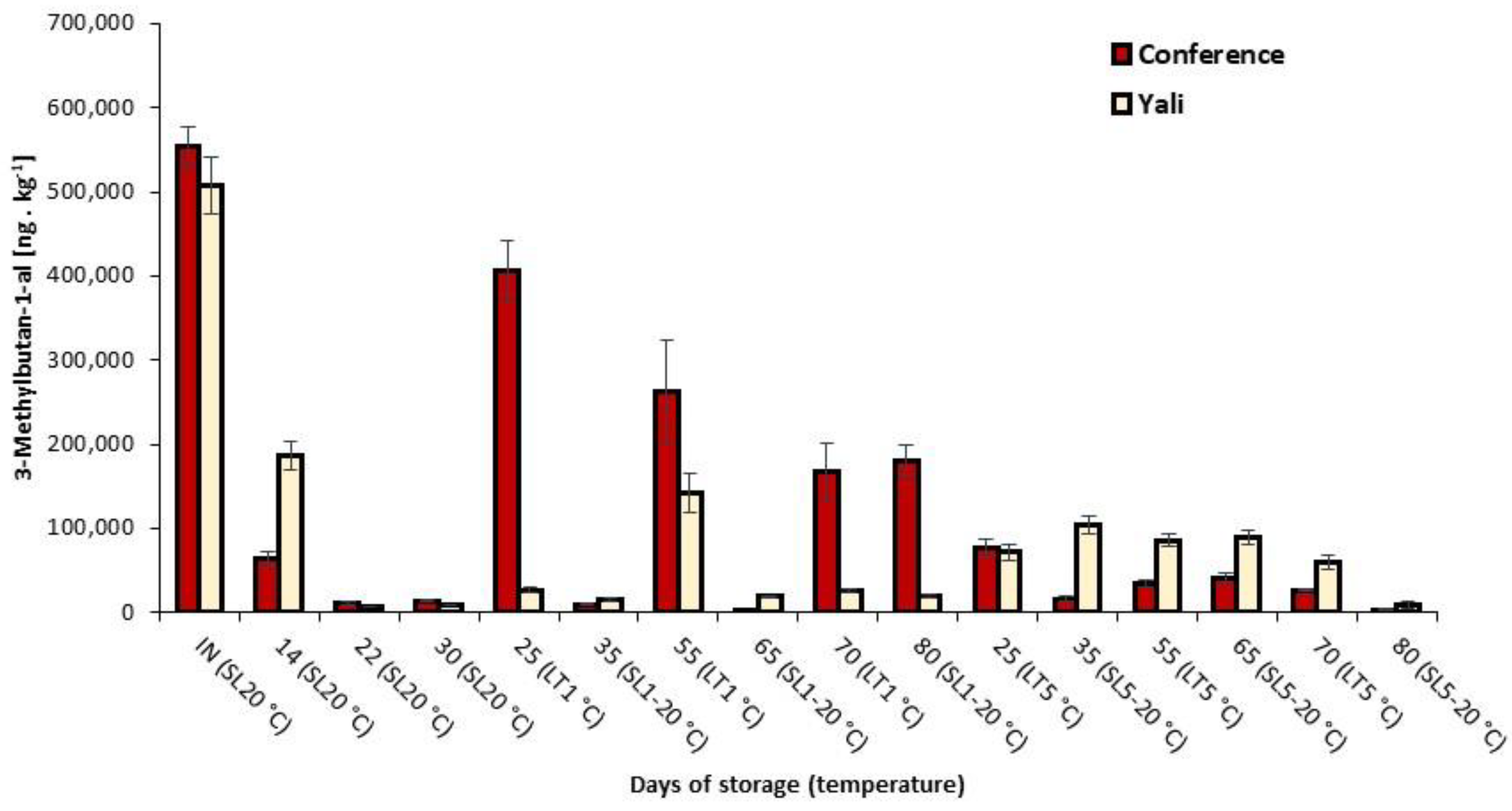
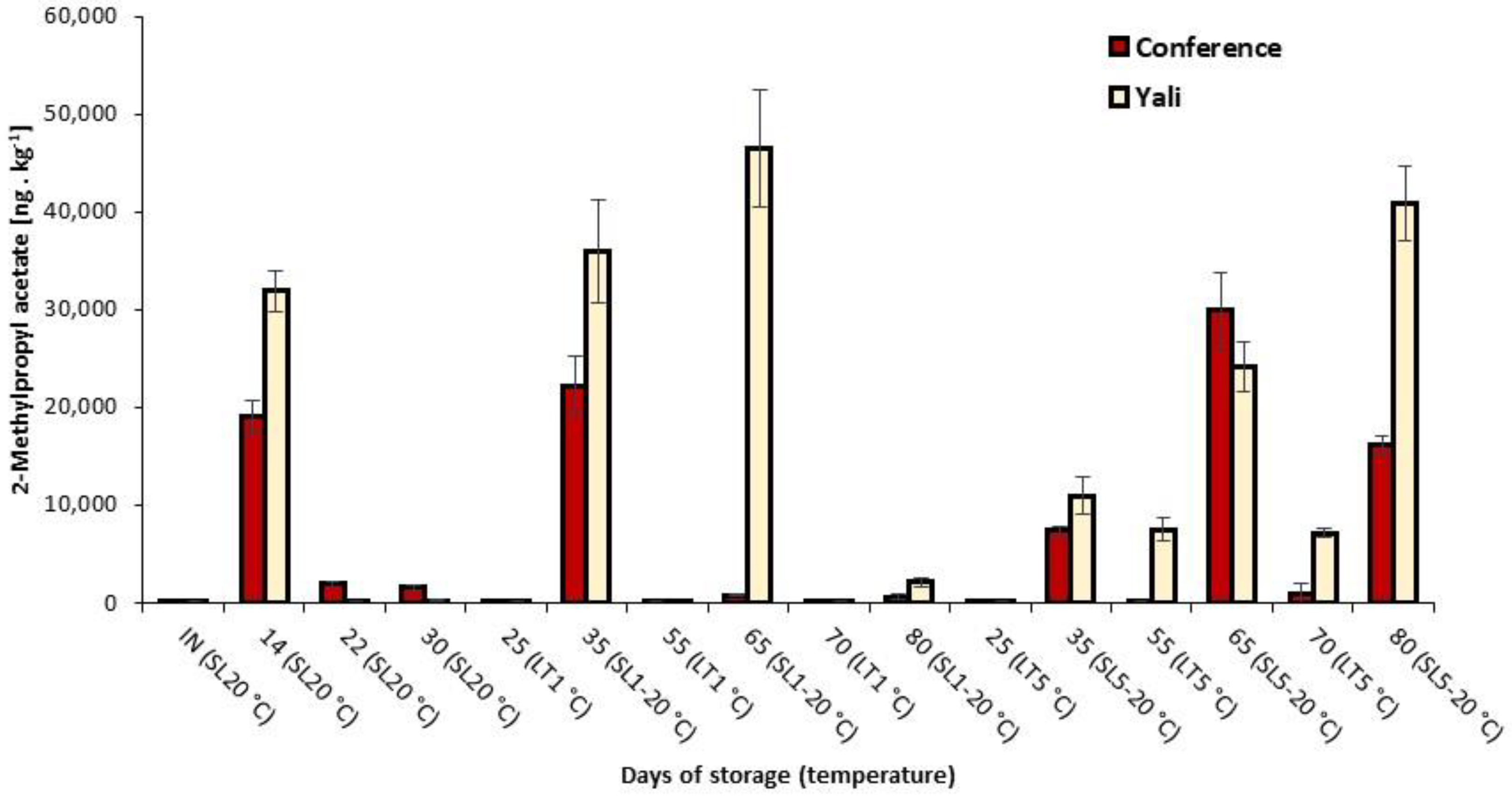
3.3. Concentration Changes of Significant Volatiles under Various Storage Conditions
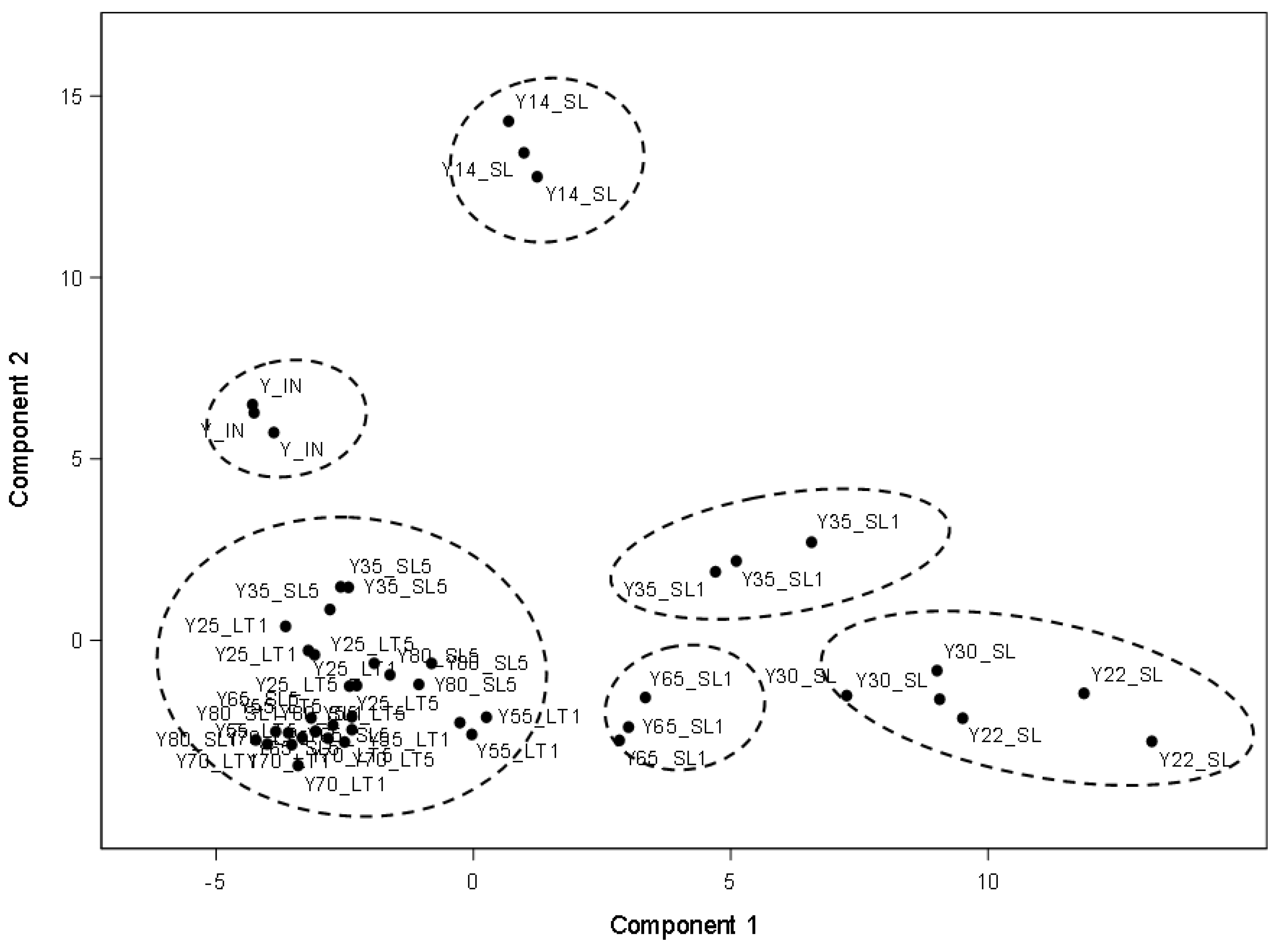
3.4. Graphic Expression of Principal Components Distinguishing the Different Storage Regimes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiriboga, M.-A.; Saladié, M.; Bordonaba, J.G.; Recasens, I.; Garcia-Mas, J.; Larrigaudière, C. Effect of cold storage and 1-MCP treatment on ethylene perception, signalling and synthesis: Influence on the development of the evergreen behaviour in ‘Conference’ pears. Postharvest Biol. Technol. 2013, 86, 212–220. [Google Scholar] [CrossRef]
- Faostat. Database FAO. 2019. Available online: http://faostat.fao.org/home/E (accessed on 10 December 2019).
- Dong, Y.; Liu, L.; Zhao, Z.; Zhi, H.; Guan, J. Effects of 1-MCP on reactive oxygen species, polyphenol oxidase activity, and cellular ultra-structure of core tissue in ‘Yali’ pear (Pyrus bretschneideri Rehd.) during Storage. Hortic. Environ. Biotechnol. 2015, 56, 207–215. [Google Scholar] [CrossRef]
- Necas, T.; Wolf, J.; Kiss, T.; Göttingerová, M.; Ivo, O.; Bieniasz, M. Evaluation of certain pomological and phenological traits of selected asian pear varieties growing in Middle European conditions. Hortic. Sci. 2020, 47, 81–92. [Google Scholar] [CrossRef]
- Chen, J.L.; Yan, S.; Feng, Z.; Xiao, L.; Hu, X.S. Changes in the volatile compounds and chemical and physical properties of Yali pear (Pyrus bertschneideri Reld.) during storage. Food Chem. 2006, 97, 248–255. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, H.; Wu, X.; Shi, X.; Qi, K.; Zhang, S. Comparative analysis of the volatile organic compounds in mature fruits of 12 Occidental pear (Pyrus communis L.) cultivars. Sci. Hortic. 2018, 240, 239–248. [Google Scholar] [CrossRef]
- Torregrosa, L.; Echeverria, G.; Illa, J.; Giné-Bordonaba, J. Ripening behaviour and consumer acceptance of ‘Conference’ pears during shelf life after long term DCA-storage. Postharvest Biol. Technol. 2019, 155, 94–101. [Google Scholar] [CrossRef]
- Makkumrai, W.; Sivertsen, H.; Sugar, D.; Ebeler, S.E.; Negre-Zakharov, F.; Mitcham, E.J. Effect of Ethylene and Temperature Conditioning on Sensory Attributes and Chemical Composition of ‘Comice’ Pears. J. Agric. Food Chem. 2014, 62, 4988–5004. [Google Scholar] [CrossRef]
- Heinz, D.E.; Jennings, W.G. Volatile Components of Bartlett Pear. V. J. Food Sci. 1966, 31, 69–80. [Google Scholar] [CrossRef]
- Suwanagul, A.; Richardson, D. Identification of headspace volatile compounds from different pear (Pyrus communis L.) varieties. Acta Hortic. 1998, 475, 605–624. [Google Scholar] [CrossRef]
- Qin, G.; Tao, S.; Cao, Y.; Wu, J.; Zhang, H.; Huang, W.; Zhang, S. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC–MS. Food Chem. 2012, 134, 2367–2382. [Google Scholar] [CrossRef]
- Jennings, W.G.; Sevenants, M.R. Volatile Esters of Bartlett Pear. III. J. Food Sci. 1964, 29, 158–163. [Google Scholar] [CrossRef]
- Heinz, D.E.; Creveling, R.K.; Jennings, W.G. Direct Determination of Aroma Compounds as an Index of Pear Maturity. J. Food Sci. 1965, 30, 641–643. [Google Scholar] [CrossRef]
- Kahle, K.; Preston, C.; Richling, E.; Heckel, F.; Schreier, P. On-line gas chromatography combustion/pyrolysis isotope ratio mass spectrometry (HRGC-C/P-IRMS) of major volatiles from pear fruit (Pyrus communis) and pear products. Food Chem. 2005, 91, 449–455. [Google Scholar] [CrossRef]
- Rizzolo, A.; Cambiaghi, P.; Grassi, M.; Zerbini, P.E. Influence of 1-Methylcyclopropene and Storage Atmosphere on Changes in Volatile Compounds and Fruit Quality of Conference Pears. J. Agric. Food Chem. 2005, 53, 9781–9789. [Google Scholar] [CrossRef]
- Tian, H.; Zhan, P.; Deng, Z.; Yan, H.; Zhu, X. Development of a flavour fingerprint by GC-MS and GC-O combined with chemometric methods for the quality control of Korla pear (Pyrus serotina Reld.). Int. J. Food Sci. Technol. 2014, 49, 2546–2552. [Google Scholar] [CrossRef]
- Zhong, H.-Y.; Zhang, C.-F.; Sun, H.-Z.; Xie, D.; Li, Z.-H.; Yuan, L.-J. The Volatile Profile of Selected Juices of Pyrus Pyrifolia Cultivars. Acta Hortic. 2008, 769, 189–194. [Google Scholar] [CrossRef]
- Rapparini, F.; Gatti, E.; Predieri, S.; Cavicchi, L. Effect of Pear Production System on Volatile Aroma Constituents of Fruits. Acta Hortic. 2008, 800, 1061–1068. [Google Scholar] [CrossRef]
- Predieri, S.; Gatti, E. Effects of cold storage and shelf-life on sensory quality and consumer acceptance of ‘Abate Fetel’ pears. Postharvest Biol. Technol. 2009, 51, 342–348. [Google Scholar] [CrossRef]
- Zlatić, E.; Zadnik, V.; Fellman, J.; Demšar, L.; Hribar, J.; Čejić, Ž; Vidrih, R. Comparative analysis of aroma compounds in ‘Bartlett’ pear in relation to harvest date, storage conditions, and shelf-life. Postharvest Biol. Technol. 2016, 117, 71–80. [Google Scholar] [CrossRef]
- Villalobos-Acuña, M.; Mitcham, E.J. Ripening of European pears: The chilling dilemma. Postharvest Biol. Technol. 2008, 49, 187–200. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, L.; Li, R.; Zhou, Q.; Wang, J.-W.; Ji, S.-J. Low temperature conditioning prevents loss of aroma-related esters from ‘Nanguo’ pears during ripening at room temperature. Postharvest Biol. Technol. 2015, 100, 23–32. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Li, H.; Mao, J.; Guo, C.; Ding, R.; Wang, Y.; Fang, L.; Chen, Z.; Yang, G. Analysis of Volatile Compounds in Pears by HS-SPME-GC×GC-TOFMS. Molecules 2019, 24, 1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Jia, H.; Wu, R.; Teng, Y. Changes in volatile organic compound composition during the ripening of ‘Nanguoli’ pears (Pyrus ussuriensis Maxim) harvested at different growing locations. J. Hortic. Sci. Biotechnol. 2013, 88, 563–570. [Google Scholar] [CrossRef]
- Yi, X.-K.; Liu, G.-F.; Rana, M.M.; Zhu, L.-W.; Jiang, S.-L.; Huang, Y.-F.; Lu, W.-M.; Wei, S. Volatile profiling of two pear genotypes with different potential for white pear aroma improvement. Sci. Hortic. 2016, 209, 221–228. [Google Scholar] [CrossRef]
- Taiti, C.; Marone, E.; Lanza, M.; Azzarello, E.; Masi, E.; Pandolfi, C.; Giordani, E.; Mancuso, S. Nashi or Williams pear fruits? Use of volatile organic compounds, physicochemical parameters, and sensory evaluation to understand the consumer’s preference. Eur. Food Res. Technol. 2017, 23, 396–1931. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.Y.; Jin, Z.; Hao, L.P.; Yan, S.J. Effects of Different Cooling Methods on Postharvest Physiology of Yali Pears during Ice Temperature Storage. Adv. Mater. Res. 2012, 554, 1072–1075. [Google Scholar] [CrossRef]
- Hendges, M.V.; Neuwald, D.A.; Steffens, C.A.; Vidrih, R.; Zlatić, E.; Amarante, C.V.T.D. 1-MCP and storage conditions on the ripening and production of aromatic compounds in Conference and Alexander Lucas pears harvested at different maturity stages. Postharvest Biol. Technol. 2018, 146, 18–25. [Google Scholar] [CrossRef]



| Volatile Compound | RI | Volatile Compound | RI | Volatile Compound | RI |
|---|---|---|---|---|---|
| Alcohols | Aldehydes | Terpenoids | |||
| ethanol | 443 | acetaldehyde | 381 | alpha-pinene | 931 |
| 2-propanol | 495 | 3-methylbutan-1-al | 628 | cymene | 1011 |
| 1-propanol | 524 | 2-methylbutan-1-al | 632 | limonene | 1020 |
| 1-butanol | 660 | hexanal | 769 | linalool | 1081 |
| 3-methyl-1-butanol | 706 | furfural | 794 | alpha-cyclocitral | 1096 |
| (2Z)-penten-1-ol | 743 | (E)-2-hexenal | 822 | thujone | 1123 |
| 2-methyl-2-buten-1-ol | 746 | heptanal | 882 | (-)-terpinen-4-ol | 1160 |
| 4-methyl-2-pentanol | 760 | 5-methylfurfural | 924 | (R)-(+)-alpha-citronellol | 1211 |
| 3-methyl-2-buten-1-ol | 762 | benzaldehyde | 927 | (Z)-citral | 1214 |
| 2-furanmethanol | 819 | (2E)-octenal | 1034 | nerol | 1215 |
| 2-methyl-1-pentanol | 822 | benzeneacetaldehyde | 1048 | (E)-geraniol | 1232 |
| 3-methyl-1-pentanol | 826 | nonanal | 1081 | alpha-ionene | 1255 |
| (2Z)-hexen-1-ol | 827 | (2E,6Z)-nonadienal | 1125 | eugenol | 1337 |
| 1-hexanol | 852 | (E)-2-nonenal | 1133 | damascenone | 1361 |
| 2-heptanol | 877 | decanal | 1183 | (D)-nerolidol | 1522 |
| phenol | 955 | hydrxoymethylfurfural | 1208 | (Z)-nerolidol | 1545 |
| 2-ethyl-1-hexanol | 1010 | (-)-menthol | 1596 | ||
| (3Z)-octen-1-ol | 1041 | ||||
| 2-ethyl-2-hexen-1-ol | 1051 | Hydrocarbons | Lactones | ||
| 1-octanol | 1054 | 1-methyl-1-cyclopentene | 641 | gama-octanolactone | 1220 |
| (3E)-octen-1-ol | 1066 | 2-pentylfuran | 977 | gama-nonanolactone | 1325 |
| phenethyl alcohol | 1082 | (Z)-alpha-farnesene | 1499 | gama-decanolactone | 1414 |
| (2E)-decenol | 1254 | alpha-dodecalactone | 1670 | ||
| 2-methoxy-4-vinylphenol | 1272 | Organic acids | |||
| farnesyl alcohol | 1658 | hexoic acid | 973 | ||
| 2-ethylhexoic acid | 1965 | ||||
| Esters | Esters | Ketones | |||
| methyl acetate | 517 | methyl octanoate | 1108 | diacetyl | 558 |
| ethyl acetate | 577 | ethyl benzoate | 1141 | 2,3-hexanedione | 757 |
| methyl propionate | 614 | benzyl acetate | 1142 | 2-heptanone | 871 |
| 2-methylpropyl acetate | 767 | diethyl succinate | 1149 | 3-methyl-2,4-pentanedione | 897 |
| butyl acetate | 805 | methyl (2E)-octenoate | 1164 | 6-methyl-5-heptene-2-one | 958 |
| ethyl 2-butenoate | 820 | butyl hexanoate | 1165 | 2-methyl-2-heptene-6-one | 960 |
| ethyl 2-methylbutanoate | 829 | hexyl butanoate | 1171 | 3-octanone | 963 |
| amyl acetate | 859 | ethyl octanoate | 1175 | 2-octanone | 964 |
| methyl hexanoate | 903 | methyl 2-hydroxybenzoate | 1176 | acetophenone | 1049 |
| ethyl 3E-hexenoate | 981 | octyl acetate | 1185 | 1-decen-3-one | 1485 |
| ethyl 3Z-hexenoate | 986 | hexyl 2-methylbutyrate | 1223 | ||
| (3Z)-3-hexenyl acetate | 987 | 2-phenethyl acetate | 1224 | ||
| hexyl acetate | 990 | ethyl (2E)-octenoate | 1275 | ||
| methyl (2E,4E)-hexadienoate | 998 | ethyl (4E)-decenoate | 1291 | ||
| methyl heptanoate | 1005 | geranyl acetate | 1360 | ||
| butyl 2-methylbutyrate | 1013 | ethyl decanoate | 1367 | ||
| (E,E)-ethyl 2,4-hexadienoate | 1071 | hexyl hexanoate | 1386 | ||
| amyl butyrate | 1079 | methyl (E,Z)-2,4- decadienoate | 1388 | ||
| ethyl (2S)-ethyl-(3S)-hydroxybutyrate | 1084 | ethyl (2E)-decenoate | 1389 | ||
| heptyl acetate | 1085 | ethyl (E,Z)-2,4-decadienoate | 1446 | ||
| hexyl propionate | 1089 | ethyl undecanoate | 1483 | ||
| 2-methylbutyl 2-methylbutanoate | 1090 | diethyl phthalate | 1543 | ||
| 3-methylbutyl 3-methylbutanoate | 1094 | ethyl hexadecanoate | 1968 | ||
| ethyl linolenate | 2166 |
| Variable | D.F. | Chi-Square | Pr > ChiSq |
|---|---|---|---|
| 3-Methylbutan-1-al | 1 | 30.8592 | <0.0001 |
| 2-Methylpropyl acetate | 1 | 26.9671 | <0.0001 |
| 2-Methoxy-4-vinylphenol | 1 | 16.0979 | <0.0001 |
| Ethanol | 1 | 16.9146 | <0.0001 |
| Eugenol * | 1 | 53.6200 | <0.0001 |
| Cultivar | ‘Conference’ | ‘Yali’ | ||||
|---|---|---|---|---|---|---|
| Variable | Comp1 | Comp2 | Comp3 | Comp1 | Comp2 | Comp3 |
| 3-Methylbutan-1-al | −0.6609 | −0.0049 | 0.4368 | −0.3678 | 0.5979 | 0.7059 |
| 2-Methylpropyl acetate | 0.5287 | −0.2910 | 0.7974 | 0.6046 | 0.3706 | −0.0923 |
| 2-Methoxy-4-vinylphenol | −0.1735 | 0.7720 | 0.3965 | 0.6083 | 0.3564 | 0.1088 |
| Ethanol | 0.5036 | 0.5651 | −0.1273 | 0.3593 | −0.6149 | 0.6938 |
| Comp. cumulative (%) | 91.7 | 89.3 | ||||
| Variable | ‘Conference’ | ‘Yali’ | ||
|---|---|---|---|---|
| Mean | ±S.D. * | Mean | ±S.D. * | |
| 3-Methylbutan-1-al | 553,025 a | 25,109 | 507,430 a | 33,282 |
| 2-Methylpropyl acetate | 57 b | 10 | 117 a | 18 |
| 2-Methoxy-4-vinylphenol | 2222 a | 308 | 1344 b | 181 |
| Ethanol | 4,236,735 a | 868,662 | 876,435 b | 75,366 |
| Eugenol | 172 b | 13 | 6571 a | 740 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goliáš, J.; Balík, J.; Létal, J. Volatiles Distinguishing the European ‘Conference’ and the Asian ‘Yali’ Pears Stored at Different Post-Harvest Temperatures. Horticulturae 2022, 8, 380. https://doi.org/10.3390/horticulturae8050380
Goliáš J, Balík J, Létal J. Volatiles Distinguishing the European ‘Conference’ and the Asian ‘Yali’ Pears Stored at Different Post-Harvest Temperatures. Horticulturae. 2022; 8(5):380. https://doi.org/10.3390/horticulturae8050380
Chicago/Turabian StyleGoliáš, Jan, Josef Balík, and Jiří Létal. 2022. "Volatiles Distinguishing the European ‘Conference’ and the Asian ‘Yali’ Pears Stored at Different Post-Harvest Temperatures" Horticulturae 8, no. 5: 380. https://doi.org/10.3390/horticulturae8050380
APA StyleGoliáš, J., Balík, J., & Létal, J. (2022). Volatiles Distinguishing the European ‘Conference’ and the Asian ‘Yali’ Pears Stored at Different Post-Harvest Temperatures. Horticulturae, 8(5), 380. https://doi.org/10.3390/horticulturae8050380






