Abstract
As an endophytic fungus, the growth-promoting effects of Piriformospora indica have been widely confirmed in many of its host plants. In this study, we investigated the influences of P. indica colonization on the growth of the daughter plants of two strawberry cultivars, ‘Benihoppe’ and ‘Sweet Charlie.’ The results showed that the fungus colonization significantly promoted the growth of the daughter plants of both of the two strawberry varieties. Its colonization greatly improved almost all of the growth parameters of the ‘Benihoppe’ daughter plants, including the above-ground fresh weight, above-ground dry weight, root fresh weight, root dry weight, plant height, petiole length, leaf area, number of roots and chlorophyll content. However, the fungus colonization showed significant improving effects on only the above-ground fresh weight, root fresh weight and root dry weight of ‘Sweet Charlie.’ Surprisingly, the average root length of ‘Benihoppe’ and ‘Sweet Charlie’ was suppressed by about 14.3% and 24.6%, respectively, by P. indica. Moreover, after P. indica colonization, the leaf nitrate reductase activity and root activity upregulated by 30.12% and 12.74%, and 21.85% and 21.16%, respectively, for the ‘Benihoppe’ and ‘Sweet Charlie’ daughter plants. Our study indicated that P. indica could promote the growth of strawberry daughter plants by improving rooting, strengthening photosynthetic pigments production and nutrient absorption and accelerating biomass accumulation. The fungus shows great potential to be used in the strawberry industry, especially in the breeding of daughter plants.
1. Introduction
Plant roots are inhabited or colonized by a large population of microorganisms, including fungi, bacteria and so on [1]. Among these microorganisms, some fungi were identified to have profound effects on plant growth and development [2]. The inoculation of plants with these growth-beneficial endophytic fungi has been proposed as a promising biological approach to promote plant growth [3]. For example, the arbuscular mycorrhizal fungi (AMF) have been identified to play significant roles in promoting plant nutrient absorption [4], strengthening plant growth potential [5], improving agronomic traits [6,7,8] and enhancing abiotic and biotic stress resistance of their host plants [9,10,11], thus drawing great attention from scientists and farmers. Recently, another endophytic plant-growth-beneficial fungus, Piriformospora indica (also called Serendipita indica), has become a new research hotspot because of its AMF-like plant growth promoting functions [10,11], even wider host ranges [12] and axenically cultivable characteristics [13]. It has been reported that P. indica can colonize the roots of plants from more than 30 families [12]. Moreover, the fungus has been successfully applied in the fields of seedling breeding, growth promotion, stress resistance enhancement and fruit quality improvement of many horticultural crops [12].
Strawberry (Fragaria × ananassa Duch.) is a perennial dicotyledonous herb plant belonging to the Fragaria genus of the Rosaceae Family. It is one of the most important economic fruit crops widely cultivated in the world. Strawberry fruits are of very high economic and nutritional value. Noteworthily, the annual fruit production of strawberries ranked the first among all the berries [14]. To improve the production and fruit quality of the strawberry, many growth-beneficial microorganisms have been applied to strawberry plants, and the interactions between strawberry plants and several fungi have been well studied. The symbiosis between AMF and strawberry roots was first reported in 1953 [15]. Recent studies have shown that AMF treatment could increase the yield of strawberry fruits under drought and low nitrogen stress conditions [16]. Cordeiro et al. reported that the AMF colonization could improve the fruit quality of strawberries [17]. Consistently, Trichoderma application to strawberry plants has also been reported to result in promoted growth and improved fruit yield and quality [18]. P. indica inoculation experiments have also been performed in tissue-cultured seedlings of some strawberry varieties [19,20]. The P. indica colonized tissue-cultured seedlings of strawberry ‘Chandler’ displayed shorter root length, but a significantly increased root number, compared with the noncolonized controls. In addition, after transplanting these tissue-culture seedlings into culture substrates, the P. indica colonized ‘Chandler’ seedlings were found to be much taller and stronger than the noncolonized controls, and their leaves were a deeper green color [19]. In strawberry variety ‘Toyonoka,’ in addition to the growth-promoting effect of this fungus on tissue-cultured seedlings, Chien and Lin also reported that P. indica colonization improved the anthracnose resistance of strawberry plants [20].
Previously, the interactions between P. indica and strawberry plants were mainly investigated using tissue-cultured seedlings, which are generally utilized as original seedling sources, but not for seedling production. Daughter plants, deriving from the stolons of the stock strawberry plants, are mainly utilized as the major strawberry propagation materials. Daughter plants are divided from the stolon connected to the stock plants and their root system is not well developed. Therefore, improving the rooting and growth of strawberry daughter plants is vital for the strawberry production. In our present study, to determine whether P. indica colonization can promote the growth of strawberry daughter plants or not, we compared the growth of the P. indica colonized and noncolonized ‘Benihoppe’ and ‘Sweet Charlie’ daughter plants by observing and measuring many growth-related parameters, including above-ground fresh weight and dry weight, root fresh weight and dry weight, plant height, petiole length, leaf area, root length, root number and so on. Moreover, photosynthetic pigment (including chlorophyll a, chlorophyll b, total chlorophyll and carotenoids) contents, leaf nitrate reductase activity (representing the nitrogen assimilation activity in plant leaf [21]) and root activity (representing the plant root water and nutrients absorption capacity [22]) were also determined to explore the possible mechanisms underlying the promoting effects of P. indica in strawberry daughter plants. The results obtained in this study will provide a basis for the future application of the endophytic growth-promoting fungus in the seedling breeding and production of strawberries.
2. Materials and Methods
2.1. Plant Materials and Fungi Preparation
In this study, daughter plants of two strawberry varieties, ‘Benihoppe’ (one of the most widely grown fresh strawberry varieties in China) and ‘Sweet Charlie’ (an early-maturing strawberry variety often used for food processing), were used as experimental materials. The P. indica (DSM11827 strain) used in this study was kept in our laboratory. The spore suspension used for the P. indica inoculation was prepared according to the method described by Cheng et al. [23].
Unique and healthy daughter plants were harvested from the stock strawberry plants and pruned, leaving only two expanded leaves, and then divided into two groups. One group was subjected to P. indica inoculation by immersing their roots in the P. indica spore suspension (2 × 105 spores/mL) for 5 h. Roots of the daughter plants from the other group were immersed in an equal volume of potato glucose broth (PDB) and were used as controls. For each group, 24 seedlings were used. After P. indica inoculation, daughter plants were wrapped with plastic film, kept at 4 °C in the dark for 3 days to promote rooting, and then transplanted into seedling-raising plug plates (50.8 cm × 30.8 cm × 12.1 cm, 24 holes per plate, and the volume of each hole is 146 mL) containing nutrient soil and grown in a greenhouse at 20~25 °C. In the first two weeks, for the adaption of transplanted daughter plants, shading was performed using a black sun-shading net, and the relative humidity was set at more than 85%.
2.2. Detection of Piriformospora indica Colonization in Strawberry Roots
Two weeks after transplanting, the roots of P. indica treated strawberry daughter plants were collected for fungus colonization detection. After removing the attached nutrient soil under tap water, roots were cut into 0.5 cm segments, soaked in 10% KOH in boiling water for 20 min, washed with sterile water 3 times, soaked in 1% hydrochloric acid solution for 1 min, stained using 0.05% Trypan blue solution for 20 min and then washed with sterile water 3 times. The colonization of P. indica in strawberry roots was observed under an optical microscope (Motic BA410E, Xiamen, China) [24]. At least three root segments were observed for each strawberry daughter plant. The colonization rate was calculated according to the description of Sharma et al. [25]. Only strawberry daughter plants that shown to be colonized by P. indica were used for further studies.
2.3. Measurement of Plant Growth-Related Parameters
At 50 days post P. indica inoculation, growth-related parameters, including plant height (cm), petiole length (cm), leaf area (cm2), root length (cm), root number, above-ground fresh weight (g), root fresh weight (g), total fresh weight (g), above-ground dry weight (g), root dry weight (g) and total dry weight (g) of P. indica colonized and noncolonized control daughter plants were separately measured or calculated. For the measurement of leaf area, leaves were scanned on a HP LaserJet M1005 MFP scanner (Shanghai, China) and measurements were determined using software Image J 1.8.0. For fresh weight measurement, samples from different strawberry parts were washed in running water to remove dirt or soil attachments, and blotted dry with filter paper before weighing. Before dry weight measurement, samples were kept in a drying oven at 70 °C to constant weight. For each parameter, eight replications were made for the strawberry daughter plants from each group.
2.4. Determination of Photosynthetic Pigments Contents
The contents of chlorophyll a, chlorophyll b, total chlorophyll and carotenoids in the leaves of P. indica colonized and noncolonized control plants were determined with three replications. Briefly, fully expanded leaves were collected, cut into pieces, and 0.3 g leaf samples were added to 10 mL of acetone–ethanol mixed extract (acetone: ethanol: water = 4.5:4.5:1) in the dark until they turned completely white. The mixture was then filtered using filter paper and diluted into a final volume of 25 mL using ethanol. The absorbance values of the obtained solution at 665 nm, 649 nm and 470 nm wavelengths were measured using a UV VIS spectrophotometer, and the concentrations of various pigments were calculated based on the following formulas [26]: chlorophyll a (Ca) = 13.95 A665 − 6.88 A649; chlorophyll b (Cb) = 24.96 A649 − 7.32 A665; total chlorophyll content = Ca + Cb; chlorophyll a/chlorophyll b = Ca/Cb; and carotenoids (Cx) = (1000 A470 − 2.05 Ca − 104 Cb)/245.
2.5. Determination of Leaf Nitrate Reductase Activity and Root Activity
At 50 days post P. indica inoculation, the leaf nitrate reductase activity of P. indica colonized and noncolonized strawberry daughter plants was measured using the modified in vivo assay method [27]. Briefly, the strawberry leaves were first washed with distilled water, blotted dry using filter paper and punched into circles of about 0.5 cm in diameter. Then, 0.5 g leaf samples were placed into a conical flask and submerged with 10 mL of a composite assay buffer containing 0.05 mol/L phosphate buffer (pH 7.5) and 0.1 mol/L KNO3, placed under vacuum for 3 min and then incubated at 30 °C for 30 min. A total of 1 mL reaction solution, 2 mL 1% sulfonamide and 2 mL 0.2% naphthylamine were mixed and reacted at 30 °C for 1 h. The absorbance value of the obtained solution at the 540 nm wavelength was measured using a UV VIS spectrophotometer and used for the calculation of the nitrate reductase activity [21]. The root activity of P. indica colonized and noncolonized strawberry daughter plants was measured using the triphenyl tetrazolium chloride (TTC) method. For the detection of leaf nitrate reductase activity and root activity, three replications were made for each treatment group.
2.6. Statistics Analysis
The results of the obtained growth-related parameters, photosynthetic pigments contents, leaf nitrate reductase and root activity were all expressed as mean ± standard deviation (SD) of at least three replications. For the analysis of the significance of the difference of these parameters or indexes between the P. indica colonized and noncolonized strawberry daughter plants, IBM® SPSS® statistical software version 24.0 (IBM Corp., Armonk, NY, USA) was applied using the Student’s t-test method at the 5% and 1% levels, and GraphPad Prism 8.0 software was used for figure drawing.
3. Results
3.1. P. indica Colonization Detection Results in Roots of Strawberry Daughter Plants
Two weeks after P. indica inoculation, the fungus colonization in the roots of strawberry daughter plants was detected using the trypan blue staining method and observed under a microscope (Figure 1). The results showed that 70.83% of the root segments of ‘Benihoppe’ and 66.67% of the root segments of ‘Sweet Charlie’ were identified to be colonized by P. indica, indicating that, as observed in the tissue-cultured seedlings [19], P. indica can easily colonize into the roots of daughter plants of both the two strawberry varieties (Figure 1c,d).
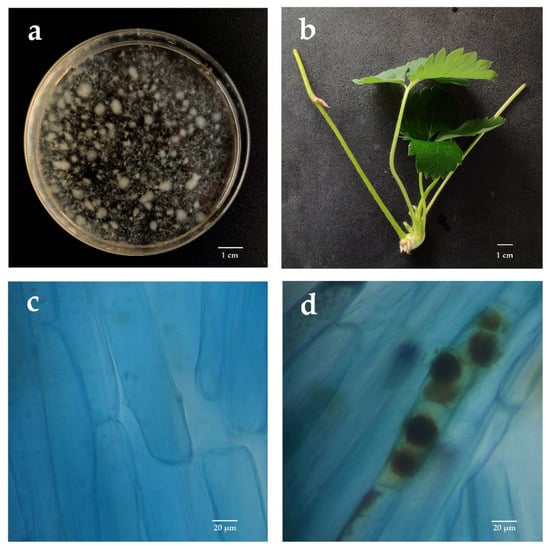
Figure 1.
P. indica colonization in roots of strawberry cutting seedings. (a) Inoculation solution of P. indica; (b) Typical strawberry daughter plants used in this study; (c) Root cells without P. indica colonization; (d) Root cells with P. indica colonization.
3.2. Effects of P. indica Colonization on the Growth of Strawberry Daughter Plants
The colonization of P. indica significantly influenced the growth of the daughter plants of the two strawberry varieties (Table 1, Figure 2). Interestingly, the plant height of the fungus colonized ‘Benihoppe’ daughter plants was obviously greater than the noncolonized control plants (Figure 2a,b), and all their growth-related parameters, except for root length, were found to be significantly increased by P. indica colonization (p < 0.05). The above-ground fresh weight, above-ground dry weight, root fresh weight, root dry weight, plant height, petiole length, leaf area and root number accounted for about 1.47-, 1.49-, 1.43-, 1.54-, 1.17-, 1.39-, 1.15- and 1.64-fold of the noncolonized controls, respectively. However, the average root length of P. indica colonized ‘Benihoppe’ daughter plants was only about 85.7% of the controls.

Table 1.
Effects of P. indica on the growth-related parameters of ‘Benihoppe’ and ‘Sweet Charlie’ daughter plants. CK: noncolonized control strawberry daughter plants; Pi: P. indica colonized strawberry seedlings; ‘*’ represents that the difference between P. indica colonized and noncolonized strawberry seedlings was significant (Student’s t-test, *, p < 0.05; n = 8).
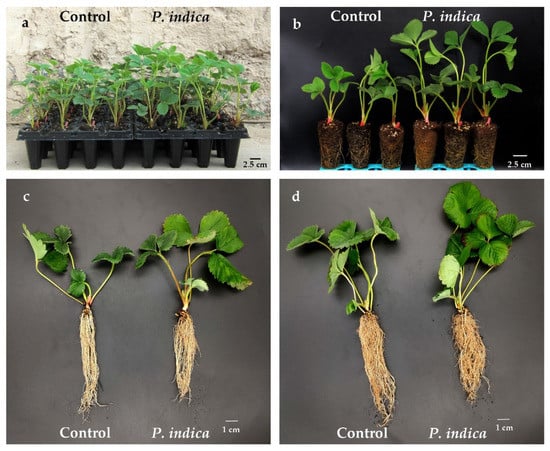
Figure 2.
Typical phenotypes of P. indica colonized and noncolonized control strawberry daughter plants at 50 days post P. indica inoculation. (a,b) Typical phenotypes of ‘Benihoppe’ daughter plants; (c) P. indica colonized and noncolonized control ‘Benihoppe’ daughter plants; (d) P. indica colonized and noncolonized control ‘Sweet Charlie’ daughter plants.
The above-ground fresh weight, root fresh weight and root dry weight of P. indica colonized ‘Sweet Charlie’ seedlings were also significantly higher than those of their corresponding controls (p < 0.05). The above-ground dry weight, plant height and petiole length of ‘Sweet Charlie’ seedlings colonized by P. indica were also greater than those of the control group, but no significant difference was identified. Similar to ‘Benihoppe,’ the root length of the P. indica colonized ‘Sweet Charlie’ daughter plants was also found to be significantly shorter than that of their controls, accounting for only 75% of the controls.
3.3. Effects of P. indica on Photosynthetic Pigments Accumulations in Leaves of Strawberry Daughter Plants
It was noticed that the leaves of P. indica colonized ‘Benihoppe’ and ‘Sweet Charlie’ daughter plants were both a deeper green color than their corresponding controls (Figure 3). To explore the possible mechanism underlying this event, contents of chlorophyll a, chlorophyll b, total chlorophyll and carotenoids in the leaves of P. indica colonized and noncolonized daughter plants of the two varieties were measured. The contents of chlorophyll a, chlorophyll b, total chlorophyll and carotenoids in leaves of P. indica colonized ‘Benihoppe’ daughter plants were all found to be very significantly higher than those of their corresponding controls (p < 0.01), and the chlorophyll a/chlorophyll b ratio in the leaves of P. indica colonized ‘Benihoppe’ daughter plants was found to be very significantly lower than that in the control group (Figure 3a) (p < 0.01).
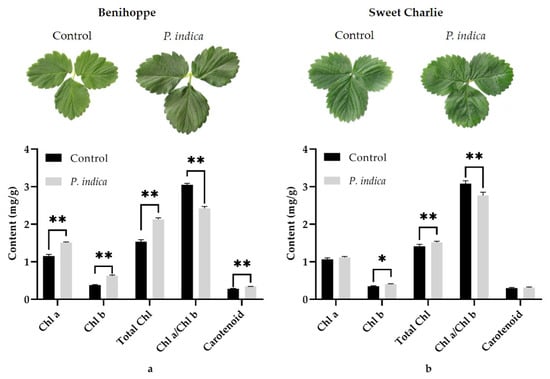
Figure 3.
The influences of P. indica on the leaf color and photosynthetic pigments content in strawberry leaves. (a) The photosynthetic pigments content in leaves of strawberry variety ‘Benihoppe;’ (b) The photosynthetic pigment contents in leaves of strawberry variety ‘Sweet Charlie.’ Values were presented as mean ± SD (standard deviation) of three replications. *, p < 0.05; **, p < 0.01.
In P. indica colonized ‘Sweet Charlie’ daughter plants, the chlorophyll b content was also found to be significantly higher than that of their controls (p < 0.05, accounting for 1.16-fold of the control group), and the total chlorophyll content was very significantly higher than that of control group (p < 0.01, accounting for 1.07-fold of the control group). The chlorophyll a/chlorophyll b ratio in leaves of P. indica colonized ‘Sweet Charlie’ daughter plants was also very significantly lower than that of the control group (p < 0.01). However, no significant difference in chlorophyll a and carotenoid content was identified between the P. indica colonized and nonconlonized ‘Sweet Charlie’ daughter plants (Figure 3b).
3.4. Effects of P. indica on Nitrate Reductase Activity and Root Activity
To further explore how the P. indica colonization promoted the growth of the strawberry daughter plants, the leaf nitrate reductase activity and root activity of the P. indica colonized and noncolonized control ‘Benihoppe’ and ‘Sweet Charlie’ daughter plants were measured and compared at 50 days post the fungus inoculation. The results showed that the leaf nitrate reductase activities of P. indica colonized ‘Benihoppe’ and ‘Sweet Charlie’ daughter plants were both found to be very significantly higher than those of their corresponding controls (p < 0.01) (Figure 4a), accounting for 1.3- and 1.12-fold of their controls, respectively (Figure 4a). Similarly, the root activities of the ‘Benihoppe’ and ‘Sweet Charlie’ seedlings were also found to be very significantly increased by P. indica colonization (p < 0.01), which was 21.85% and 21.16% higher than their corresponding controls, respectively (Figure 4b).
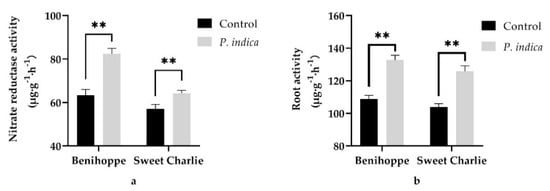
Figure 4.
The influences of P. indica on the leaf nitrate reductase activity and root activity of strawberry daughter plants. (a) Leaf nitrate reductase activities of P. indica colonized and noncolonized strawberry daughter plants; (b) Root activities of P. indica colonized and noncolonized strawberry daughter plants. Values were presented as mean ± SD of three replicates. **, p < 0.01.
4. Discussion
As an important economic horticultural crop, the annual fruit yield of strawberry plants ranks the first among all the berries [14], and its fruits are greatly valued by people from around the world for their great flavor and high nutrition. Seedling breeding and production is critical for the healthy and sustainable development of the strawberry industry. As a main source of strawberry propagation materials, the rooting condition and growth of daughter plants greatly influences the production of strawberry fruit. The endophytic fungus P. indica has been used in tissue-cultured strawberry seedlings, and its colonization has shown significant growth-promoting effects [19,20]. In this study, we investigated the influences of this beneficial fungus on the growth of the daughter plants of two strawberry varieties, ‘Benihoppe’ and ‘Sweet Charlie.’ The obtained results were as follows.
4.1. Colonization of P. indica Promoted the Growth of Strawberry Daughter Plants, and Its Promoting Effects Varied in Different Varieties
Extensive evidence has demonstrated that P. indica colonization in the root system of host plants can not only promote rooting, but also stimulate the growth and development of the above-ground plant parts. In horticultural crops, the inoculation of P. indica has been confirmed to have the ability to increase biomass accumulations of many woody plants, such as Feronia limonia [28], Azadirachta indica [29] and trifoliate orange [30,31], as well as herbaceous crops such as bananas [32], sweet potatoes [33] and tomatoes [34]. Generally, the plant root promoting effect of P. indica can be achieved by increasing the length and number of roots [35]. However, according to the previous reports on strawberries, P. indica colonization would increase the biomass, but inhibit the root elongation of strawberry plants [19]. In our present study, we also found that P. indica colonization significantly increased the above-ground and root biomass of two strawberry varieties, indicating that the fungus could promote the growth of strawberry seedlings. Consistent with previous reports [19], the suppression of strawberry root elongation caused by P. indica colonization was also found in the two strawberry varieties. However, P. indica colonization significantly improved the root number, root weight and root activity of strawberry daughter plants. These results suggested that, although the fungus inhibited root elongation, the root biomass, volume and activity were greatly heightened.
Moreover, we found that the fungus colonization improved almost all the growth-related parameters of the ‘Benihoppe’ daughter plants, but for the ‘Sweet Charlie’ variety, only three parameters, including above-ground fresh weight, root fresh weight and root dry weight, were found to be significantly increased by the fungus. Thus, it was suggested that the growth-promoting effects of P. indica varied among different strawberry varieties.
4.2. P. indica Colonization Significantly Induces the Accumulation of Photosynthetic Pigments in Strawberry Leaves
Photosynthetic pigments are important substances involved in plant photosynthesis. Moreover, the content of photosynthetic pigments is often considered as an important indicator of plant health status. Accumulating evidence has shown that P. indica colonization could increase chlorophyll content in host plants such as bananas [32], sweet potatoes [33], and rice [36]. In this study, we found that the leaf color of P. indica colonized strawberry seedlings was an obviously deeper green than the noncolonized controls. By measuring the contents of chlorophyll a, chlorophyll b, total chlorophyll and carotenoids, we found that the leaf chlorophyll content of P. indica colonized strawberry seedlings was significantly higher than that of the control group. This suggested that P. indica colonization enhanced the photosynthesis ability of strawberries by increasing their photosynthetic pigments contents.
4.3. P. indica Colonization Enhanced the Nutrient Uptake Ability of Strawberry Daughter Plants
The growth-promoting effects of P. indica were reported to be achieved by enhancing the nutrient uptake ability of host plants to absorb sufficient mineral substances from the soil [37,38]. It was reported that P. indica could increase the nitrogen content in plants, as well as enhance the expression of the nitrate reductase gene [39]. In this study, the nitrate reductase activity in the leaves of P. indica colonized strawberry seedlings was identified to be significantly higher than in the noncolonized controls, indicating that P. indica colonization enhanced the nitrogen assimilation ability of strawberry daughter plants. Additionally, the root activity was also found to be very significantly upregulated by P. indica colonization in daughter plants of both the two strawberry varieties. Therefore, it could be concluded that the growth-promoting effects of P. indica on strawberry daughter plants, to some extent, were achieved by enhancing the nutrient uptake ability in both the root and above-ground parts of the strawberry plants.
Increasing evidence has confirmed that P. indica has a tremendous potential to be used as a production improvement agent, mycofertilizer and biotizer [12,40,41]. Given the enhancement effect of P. indica on the nutrient uptake ability of strawberry daughter plants, we deduced that the fungus colonization in strawberry roots or the addition of this fungus to the strawberry culture substrates might be helpful in promoting strawberry seedling growth and may contribute to decreasing the usage of chemical fertilizer during strawberry culture in the future.
5. Conclusions
P. indica colonization showed significant growth-promoting effects on strawberry daughter plants. From the aspect of the root, although the fungus colonization shortened the root length to some extent, it significantly increased the root number, upregulated the root activity and promoted nutrient uptake ability of the root system of strawberry daughter plants. From the aspect of the above-ground plant parts, P. indica colonization stimulated the accumulation of photosynthetic pigments and increased the nitrate reductase activity in strawberry leaves, thus enhancing the photosynthesis and nitrogen assimilation capacity of strawberry seedlings. Our study indicated that P. indica had great potential to be used in the strawberry industry, especially in daughter plant breeding.
Author Contributions
Conceptualization, C.C., W.L. (Wei Liu) and X.F.; methodology, C.C. and W.L. (Wei Liu); software, W.L. (Wei Liu); validation, W.L. (Wei Liu), M.T. and P.Q.; formal analysis, W.L. (Wei Liu); investigation, W.L. (Wei Liu); resources, W.L. (Wenjie Liang) and X.F.; data curation, C.H., W.L. (Wenjie Liang), R.L. and Y.J.; writing—original draft preparation, W.L. (Wei Liu) and C.C.; writing—review and editing, C.C.; visualization, W.L. (Wei Liu); supervision, C.C.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fund for High-Level Talents of Shanxi Agricultural University (2021XG010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Hrynkiewicz, K.; Baum, C. The potential of rhizosphere microorganisms to promote the plant growth in disturbed soils. In Environmental Protection Strategies for Sustainable Development; Malik, A., Grohmann, E., Eds.; Springer: Berlin, Germany, 2012; pp. 35–64. [Google Scholar]
- Jain, A.; Chakraborty, J.; Das, S. Underlying mechanism of plant-microbe crosstalk in shaping microbial ecology of the rhizosphere. Acta Physiol. Plant. 2020, 42, 8. [Google Scholar] [CrossRef]
- Shao, Y.D.; Zhang, D.J.; Hu, X.C.; Wu, Q.S.; Jiang, C.J.; Xia, T.J.; Gao, X.B.; Kuča, K. Mycorrhiza-induced changes in root growth and nutrient absorption of tea plants. Plant Soil Environ. 2018, 64, 283–289. [Google Scholar]
- Rocha, I.; Ma, Y.; Carvalho, M.F.; Magalhães, C.; Janoušková, M.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating with inocula of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria for nutritional enhancement of maize under different fertilisation regimes. Arch. Agron. Soil Sci. 2019, 65, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Cobb, A.B.; Wilson, G.W.T.; Goad, C.L.; Grusak, M.A. Influence of alternative soil amendments on mycorrhizal fungi and cowpea production. Heliyon 2018, 4, e00704. [Google Scholar] [CrossRef] [Green Version]
- Sabatino, L.; Iapichino, G.; Consentino, B.B.; D’Anna, F.; Rouphael, Y. Rootstock and arbuscular mycorrhiza combinatorial effects on eggplant crop performance and fruit quality under greenhouse conditions. Agronomy 2020, 10, 693. [Google Scholar] [CrossRef]
- Hart, M.; Ehret, D.L.; Krumbein, A.; Leung, C.; Murch, S.; Turi, C.; Franken, P. Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 2015, 25, 359–376. [Google Scholar] [CrossRef]
- Li, F.; Hao, Z.P.; Chen, B.D. Molecular mechanism for the adaption of arbuscular mycorrhizal symbiosis to phosphorus deficiency. J. Plant Nutr. Fertil. 2019, 25, 1989–1997. [Google Scholar]
- Rivero, J.; Álvarez, D.; Flors, V.; Azcón-Aguilar, C.; Pozo, M.J. Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol. 2018, 220, 1322–1336. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Hu, Y.; Zhang, K.; Tian, C.; Guo, J. Arbuscular mycorrhizal fungi improve plant growth of Ricinus communis by altering photosynthetic properties and increasing pigments under drought and salt stress. Ind. Crop. Prod. 2018, 117, 13–19. [Google Scholar] [CrossRef]
- Mensah, R.A.; Li, D.; Liu, F.; Tian, N.; Sun, X.; Hao, X.; Lai, Z.; Cheng, C. Versatile Piriformospora indica and its potential applications in horticultural crops. Hortic. Plant J. 2020, 6, 111–121. [Google Scholar] [CrossRef]
- Varma, A.; Verma, S.; Sudha; Sahay, N.; Bütehorn, B.; Franken, P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl. Environ. Microbiol. 1999, 65, 2741–2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, F.; Wang, B.; Wu, H.; Wu, J.; Liu, J.; Sun, Y.; Cheng, C.; Qiu, D. Identification, characterization and expression analysis of anthocyanin biosynthesis-related bHLH genes in blueberry (Vaccinium corymbosum L.). Int. J. Mol. Sci. 2021, 22, 13274. [Google Scholar] [CrossRef] [PubMed]
- Mosse, B. Fructifications associated with mycorrhizal strawberry roots. Nature 1953, 171, 974. [Google Scholar] [CrossRef]
- Boyer, L.R.; Feng, W.; Gulbis, N.; Hajdu, K.; Harrison, R.J.; Jeffries, P.; Xu, X. The use of arbuscular mycorrhizal fungi to improve strawberry production in coir substrate. Front. Plant Sci. 2016, 7, 1237. [Google Scholar]
- Cordeiro, E.C.N.; De Resende, J.T.V.; Córdova, K.R.V.; Nascimento, D.A.; Júnior, O.J.S.; Zeist, A.R.; Favaro, R. Arbuscular mycorrhizal fungi action on the quality of strawberry fruits. Hortic. Bras. 2019, 37, 437–444. [Google Scholar] [CrossRef]
- Lombardi, N.; Caira, S.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Salzano, A.M.; Lorito, M.; Woo, S.L. Trichoderma applications on strawberry plants modulate the physiological processes positively affecting fruit production and quality. Front. Microbiol. 2020, 11, 1364. [Google Scholar] [CrossRef]
- Husaini, A.M.; Abdin, M.Z.; Khan, S.; Xu, Y.W.; Aquil, S.; Anis, M. Modifying strawberry for better adaptability to adverse impact of climate change. Curr. Sci. 2012, 102, 1660–1673. [Google Scholar]
- Chien, Y.; Lin, N. Effects of biohardening with Serendipita indica on strawberry growth and resistance to Colletotrichum gloeosporioides. J. Plant Med. 2018, 60, 1–8. [Google Scholar]
- Riens, B.; Heldt, H.W. Decrease of nitrate reductase activity in spinach leaves during a light-dark transition. Plant Physiol. 1992, 98, 573–577. [Google Scholar] [CrossRef]
- Lv, T.; Yang, H.; Zhang, R.; Fan, W.; Xu, Y.; Cao, H.; Ning, L.; Zhou, C.; Wang, L. Effects of lignin on root activity and soil nutrients of Malus hupehensis. var. pingyiensis under the use of organic fertilizer. Agric. Sci. 2017, 8, 341–347. [Google Scholar]
- Cheng, C.; Li, D.; Qi, Q.; Sun, X.; Anue, M.R.; David, B.M.; Zhang, Y.; Hao, X.; Zhang, Z.; Lai, Z. The root endophytic fungus Serendipita indica improves resistance of Banana to Fusarium oxysporum f. sp. cubense tropical race 4. Eur. J. Plant Pathol. 2020, 156, 87–100. [Google Scholar] [CrossRef]
- Rai, M.; Acharya, D.; Singh, A.; Varma, A. Positive growth responses of the medicinal plants Spilanthes calva and Withania somnifera to inoculation by Piriformospora indica in a field trial. Mycorrhiza 2001, 11, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kharkwal, A.C.; Abdin, M.Z.; Varma, A. Piriformospora indica improves micropropagation, growth and phytochemical content of Aloe vera L. plants. Symbiosis 2014, 64, 11–23. [Google Scholar] [CrossRef]
- Salah, S.M.; Yajing, G.; Dongdong, C.; Jie, L.; Aamir, N.; Qijuan, H.; Weimin, H.; Mingyu, N.; Jin, H. Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci. Rep. 2015, 5, 14278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Gharbi, A.; Hipkin, C.R. Studies on nitrate reductase in British angiosperms: I. A comparison of nitrate reductase activity in ruderal, woodland-edge and woody species. New Phytol. 1984, 97, 629–639. [Google Scholar] [CrossRef]
- Vyas, S.; Nagori, R.; Purohit, S. Root colonization and growth enhancement of micropropagated Feronia limonia (L.) swingle by Piriformospora indica—A cultivable root endophyte. Int. J. Plant Dev. Biol. 2008, 2, 128–132. [Google Scholar]
- Bagde, U.S.; Prasad, R.; Varma, A. Interaction of mycobiont: Piriformospora indica with medicinal plants and plants of economic importance. Afr. J. Biotechnol. 2010, 9, 9214–9226. [Google Scholar]
- Yang, L.; Zou, Y.N.; Tian, Z.H.; Wu, Q.S.; Kuča, K. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci. Hortic. 2021, 277, 109815. [Google Scholar] [CrossRef]
- Meng, L.L.; Liu, R.C.; Yang, L.; Zou, Y.N.; Srivastava, A.K.; Kuča, K.; Hashem, A.; Abd-Allah, E.F.; Giri, B.; Wu, Q.S. The change in fatty acids and sugars reveals the association between trifoliate orange and endophytic fungi. J. Fungi 2021, 7, 716. [Google Scholar] [CrossRef]
- Li, D.; Bodjrenou, D.M.; Zhang, S.; Wang, B.; Pan, H.; Yeh, K.W.; Lai, Z.; Cheng, C. The endophytic fungus Piriformospora indica reprograms banana to cold resistance. Int. J. Mol. Sci. 2021, 22, 4973. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kuo, Y.W.; Lin, K.H.; Huang, W.; Deng, C.; Yeh, K.W.; Chen, S.P. Piriformospora indica colonization increases the growth, development, and herbivory resistance of sweet potato (Ipomoea batatas L.). Plant Cell Rep. 2021, 40, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.E.; Abdelsattar, M.; Abdeldaym, E.A.; Atia, M.A.M.; Mahmoud, A.W.M.; Saad, M.M.; Hirt, H. Piriformospora indica alters Na+/K+ homeostasis, antioxidant enzymes and LeNHX1 expression of greenhouse tomato grown under salt stress. Sci. Hortic. 2019, 256, 108532. [Google Scholar] [CrossRef]
- Dong, S.; Tian, Z.; Chen, P.J.; Kumar, R.S.; Shen, C.H.; Cai, D.; Oelmüller, R.; Yeh, K.W. The maturation zone is an important target of Piriformospora indica in Chinese cabbage roots. J. Exp. Bot. 2013, 64, 4529–4540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jogawat, A.; Saha, S.; Bakshi, M.; Dayaman, V.; Kumar, M.; Dua, M.; Varma, A.; Oelmüller, R.; Tuteja, N.; Johri, A.K. Piriformospora indica rescues growth diminution of rice seedlings during high salt stress. Plant Signal. Behav. 2013, 8, e26891. [Google Scholar] [CrossRef] [Green Version]
- Varma, A.; Bakshi, M.; Lou, B.; Hartmann, A.; Oelmueller, R. Piriformospora indica: A novel plant growth-promoting mycorrhizal fungus. Agric. Res. 2012, 1, 117–131. [Google Scholar] [CrossRef]
- Moreira, B.C.; Mendes, F.C.; Mendes, I.R.; Paula, T.A.; Prates Junior, P.; Salomão, L.C.C.; Stürmer, S.L.; Otoni, W.C.; Guarçoni, M.A.; Kasuya, M.C.M. The interaction between arbuscular mycorrhizal fungi and Piriformospora indica improves the growth and nutrient uptake in micropropagation-derived pineapple plantlets. Sci. Hortic. 2015, 197, 183–192. [Google Scholar] [CrossRef]
- Sherameti, I.; Shahollari, B.; Venus, Y.; Altschmied, L.; Varma, A.; Oelmüller, R. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J. Biol. Chem. 2005, 280, 26241–26247. [Google Scholar]
- Cheng, C.; Liu, F.; Wang, B.; Qu, P.; Liu, J.; Zhang, Y.; Liu, W.; Tong, Z.; Deng, G. Influences of Serendipita indica and Dictyophorae echinovolvata on the growth and Fusarium wilt disease resistance of banana. Biology 2022, 11, 393. [Google Scholar] [CrossRef]
- Shende, S.; Bhagwat, K.; Wadegaonkar, P.; Rai, M.; Varma, A.; Rai, M.K. Piriformospora indica as a new and emerging mycofertilizer and biotizer: Potentials and prospects in sustainable agriculture. In Handbook of Microbial Biofertilizers, 1st ed.; Food Products Press: Binghamton, NY, USA, 2005; pp. 477–496. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).