Abstract
This research was designed to provide the first protocol to establish an efficient solution for direct organogenesis regeneration in Passiflora quadrangularis using nodal explants from young shoots. Passifloraceae tissue culture has been associated with problems such as recalcitrance, sensitivity to ethylene accumulation and browning of explants due to the presence of phenols in the tissues. Due to the high rate of endogenous contamination of the explants, a preliminary experiment was performed. The best results of surface sterilization were obtained using the pretreatment with 70% EtOH, 1 min and 50% NaOCl, 10 min along with the treatment of Rifampicin 15 µg/mL and Benomyl 2 g/L. The effects of plant growth regulators on the induction of direct organogenesis, multiplication of shoots in subcultures and in vitro rooting were evaluated. Additional compounds such as AgNO3 and Pluronic F-68 were added to the culture media in order to reduce the effects of phenols and the sudden browning of the explants. Shoot proliferation increased to the sixth subculture after which it decreased. A maximum of 7.17 shoots were obtained from one shoot on Murashige and Skoog (MS) medium supplemented with 2 mg/L 6-benzylaminopurine and 1 mg/L thidiazuron. Supplementation of ½ MS medium with 1 mg/L 1-naphthaleneacetic acid was conducing to root formation in 61.11% of shoots. After acclimatization, the plants showed vigorous growth, green leaves, and well-developed roots. Although this species has previously shown difficulty in in vitro propagation, this protocol established based on the results proved to be efficient and reproducible.
1. Introduction
The genus Passiflora is the largest of the Passifloraceae family [1], consisting of 576 species [2], of which only 50 are edible [3]. Passiflora quadrangularis (giant granadilla) native to the Andean Region, South America [4] is a vine that produces big fruits [5]. In recent years, growers have paid attention to the species P. quadrangularis due to its ornamental value, fragrant flowers [6], special fruit aroma, phytocompounds present in various organs of the plant [7,8,9] and phytotherapeutic properties [10], being used in traditional medicine [11,12]. Passiflora is an allogamous plant with self-incompatible flowers for most species belonging to the genus [13].
The production of planting material of P. quadrangularis by conventional methods has proven to be problematic due to the low germination rate of the seeds. At the same time, the presence of disease-causing microorganisms significantly reduces the efficiency of propagation by cuttings [14,15]. Passiflora spp. secretes a sweet sap that is a nutrient medium for many microorganisms [14]. Passionflower is often infected with Passion fruit Woodiness Virus [16,17,18] and Xanthomonas axonopodis pv. passiflorae [19,20], two pathogens that decrease their exploitation duration and decorative utility. Regarding the possibilities of in vitro multiplication, the contamination of the vegetal material used for the initiation of cultures remains the biggest problem [21,22]. Over time, researchers have tried several protocols for plant material asepsis to initiate in vitro cultures. For seed disinfection, asepsis with NaOCl in various concentrations is the most commonly used method [23]. In the case of Passiflora explants, solutions based on NaOCl were most commonly used [24,25], but also HgCl2 [26].
Plant regeneration can be achieved by organogenesis and somatic embryogenesis. For the induction of organogenesis, the main pathway for in vitro regeneration in Passiflora [21] used mainly Murashige and Skoog (MS) media [27] supplemented with cytokinins: 6-benzyladenine (BA) only or in combination with thidiazuron (TDZ). For the proliferation of axillary shoots, kinetin (KIN) is effective [28,29,30,31]. In addition, supplementing the culture media with compounds that stimulate regeneration processes, such as AgNO3 or Pluronic F-68 (PF-68), plays an important role in the proliferation and regeneration of shoots.
PF-68 non-ionic surfactant stimulates in vitro growth and differentiation of tissues and organs in several plant species [32]. Supplementation of culture media with PF-68 in concentrations below 1.0% (w/v) stimulated the growth and regeneration of Arabidopsis thaliana shoots and the production of cotyledon shoots of Corchorus capsularis [33]. The mechanism of in vitro growth stimulation of PF-68 is achieved by increased sugar accumulation (alpha amylase), protein biosynthesis (ribosomal proteins) and nitrogen metabolism (glutamate synthase), which are crucial for plant growth and development [34]. De-Xian Kok et al. [34] demonstrated in their study that PF-68 has growth-stimulating effects without being dependent on the presence of plant hormones. The optimal concentration of PF-68 has improved the absorption of nutrients in plant tissue demonstrating the many beneficial effects of tissue regeneration and proliferation in recalcitrant species in vitro [34,35,36,37] as well as in P. edulis, P. giberti and P. mollissima [32].
Passifloraceae tissue cultures have also been associated with problems such as recalcitrance [5,38], sensitivity to ethylene accumulation [39] and browning of explants due to the presence of phenols in the tissues [40]. Regarding Passiflora edulis f. flavicarpa, it was stated that the mature tissue is recalcitrant to in vitro regeneration [41,42] in addition, the problem of tissue infection was found. Passionflower plantlets showed difficulty in vitro growing, due to the reduced gas exchange [43,44]. Although culture vessels allow the exchange of ethylene, its production at the plantlet level is often higher than its consumption, which leads to its accumulation. [45]. Using AgNO3 as an ethylene inhibitor [46], various protocols have been developed to improve the regeneration rate. Thus, Huh et al. [40] and Trevisan and Mendes [39] added AgNO3 to the culture medium in P. edulis f. flavicarpa and Pinto et al. [47] at the initiation of P. alata culture. In each of these experiments, the results obtained on culture media supplemented with AgNO3 were significant. Oxidation of phenols in tissues leads to browning of the explants and thus to the failure of in vitro culture [48]. In this case, too, Huh et al. [40] state that the culture medium supplemented with AgNO3 reduces the negative effect of phenols on the regeneration of explants. As the stage of initiation and stabilization of the culture took a long time, encountering along the way each of the problems described above, an attempt was made to find solutions to achieve the expected results, some being effective, others not.
In vitro multiplication is becoming a powerful tool applied in research, for commercial production, and plant conservation. In terms of plant regeneration, recalcitrance may be a major limiting factor for the exploitation of medicinal plant species [49]. Recalcitrance is the inability of plant cells, tissues, and organs to respond to the regeneration program [50]. Some recalcitrant species can be established and propagated in vitro. Due to the physiology of donor plants and the physiological stress of in vitro plants [51], different cellular skills to respond by somatic embryogenesis and/or oxidative stress by organogenesis may contribute to the recalcitrance of P. quadrangularis culture.
To date, no complete in vitro regeneration protocol for P. quadrangularis has been reported. Regarding the regeneration studies by direct organogenesis in P. quadrangularis, so far, Otahola and Diaz [25] conducted a study in which they were used explants from leaf discs and axillary buds, and the present research was preceded by a preliminary study in which four culture media and two types of explants (nodal segments and leaf fragments) were tested [52].
The present research aims to establish an efficient solution for regeneration by direct organogenesis, using nodal explants from young shoots and the characterization of morphogenic responses, results confirmed by anatomical analysis.
The objectives of this study were to establish the optimal disinfection treatment of explants from nodal segments in P. quadrangularis, to inspect the influence of cytokinins and, as the case, auxins, at each stage of micropropagation. The study also analyzed whether the supplementation of culture media with AgNO3 or PF-68 reduces recalcitrance, manifested by in vitro tissue browning in P. quadrangularis.
2. Materials and Methods
2.1. Source of Plant Materials and Explants
The biological material came from one-year-old P. quadrangularis plants, which were in the vegetative growth stage. The experimental plants were grown in the Didactic and Experimental Greenhouse of the discipline of Ornamental Plants, at the University of Agricultural Sciences and Veterinary Medicine (UASVM) Cluj-Napoca, Romania. These plants were obtained from cuttings from Naples Botanical Garden, USA.
The biological material used to initiate the in vitro culture were nodal segments taken from nodes 3–8 in the apical part of the young shoots.
2.2. Culture Media and Growth Conditions
In the case of each experiment, the explants were cultured in MS medium including vitamins, 30 g/L sucrose and 2.5 g/L Gelrite. Depending on the purpose, plant growth regulators (PGRs) (Table 1) or phenolic compound inhibitors have been added. Thus, AgNO3 [0, 1 or 2 mg/L)] was added to the culture medium from the stock solution before autoclaving and PF-68 ((0, 0.2 and 0.4% (w/v)) by filter sterilization using Millipore filters (0.22 µm), described below for each step.

Table 1.
Composition of culture media used for micropropagation of P. quadrangularis species.
The pH was adjusted with 1N NaOH or 1N HCl to 5.8. Distribution of the solution for culture initiation was performed in test tubes (10 mL/tube) and for subculturing and rooting in culture flasks (30–50 mL). The sterilization was performed by autoclaving at 121 °C, under a pressure of 101,325–121,590 Pa, for 20 min.
All cultures were maintained in the growth chamber at a photoperiod of 16 h light/8 h dark and a temperature of 25 ± 2 °C. Gro-Lux F36W/GRO fluorescent tubes (Sylvania, Germany) with an irradiance capacity of 100–112 µmol/m2/s were used.
2.3. Explant Asepsis
For asepsis of explants from nodal segments, 15 treatments were tested (Table 2). A total of 450 explants were initiated in order to identify the optimal disinfection treatment.

Table 2.
Composition of asepsis treatments for nodal explants of P. quadrangularis.
The stems were shaped to prepare the nodal explants and washed in a continuous stream of water for 20 min. Then, in the case of T1–T9 treatments (Table 2), they were kept in a 70% EtOH solution with 2–3 drops of Tween 20 for 1 min and rinsed with sterile distilled water three times. This was followed by treatment with NaClO solution in different concentrations (15%, 25%, 50%) at three exposure times (10, 15 and 20 min) then three rinses with sterile distilled water were performed. In the case of T10–T15 treatments, surface disinfection of explants was preceded by treatment with the antibiotic Rifampicin 15 µg/mL, or with the fungicide Benomyl 2 g/L or a combination of Rifampicin + Benomyl at two exposure times, 15 and 30 min. The aseptic treatment consisted of maintaining the explants in 70% EtOH for 1 min, then in 50% NaClO solution, 10 min applying the protocol described above. In the preparation of pretreatments, Rifampicin was first solubilized using dimethylsulfoxide, followed by dilution in sterile distilled water. Benomyl fungicide was solubilized directly in sterile distilled water.
After rinsing with autoclaved water, the stem fragments were shaped into 1 cm nodal segments with latent axillary buds. The explants were inoculated on MS-0 medium (without phytohormones). All operations were performed in aseptic conditions, in the laminar flow hood. Contamination data were registered 2–14 days after inoculation of the explants.
2.4. Initiation and Stabilization of In Vitro Plantlets
The experimental model of the initiation phase aims to determine the influence of cytokinins, ethylene inhibitors and the position of the explant on the regeneration axillary shoots. A total of 1020 nodal explants were initiated in order to stabilize the in vitro culture of P. quadrangularis in the form of three bifactorial experiments. All these explants were surface sterilized using the T14 treatment. The first study was on the influence of cytokinins on axillary shooting, then a study on the influence of the explant position and the culture medium, and the third experiment was on the influence of culture medium and additional compounds on axillary shooting. From an experimental point of view, the initiation phase was divided into two stages. In the preliminary initiation step (identified as the first experiment), the MS culture medium was supplemented with 6-benzylaminopurine (BAP) only (0.5–3 mg/L) (MS-I.1-MS-I.6) or in combination with KIN (1 or 2 mg/L) (MS-I.7-MS-I.12). Because in this first stage explants were regenerated in very low percentages, the experiment was continued with a secondary initiation stage, using the cytokinin concentrations that obtained the highest regeneration rate. Thus, MS + 2 mg/L BAP became MS-1, and MS + 2 mg/L BAP + 1 mg/L KIN became MS-2. Additional compounds such as AgNO3 (0, 1 or 2 mg/L) and PF-68 (0, 0.2 and 0.4% (w/v)) were added to MS-1 and MS-2, in order to reduce the effects of leached phenols and sudden browning of explants. The explants from the nodal segments, 10 mm in thickness, were inoculated on the medium in a vertical and horizontal position. After 6 weeks, the following parameters were determined: regeneration frequency (%), callus incidence (%), browning (%), phenolic secretion (%), number of shoots per explant and shoot length (cm).
2.5. Shoot Subculturing
In vitro regenerated shoots were multiplied by repeated subcultures on fresh culture medium every 4 weeks. In the multiplication stage, three types of culture medium indicated in the literature were tested [53] for good results in the multiplication of passionflowers. Multiplication was continued on the MS-2 (MS + 2 mg/L BAP + 1 mg/L KIN) medium, on MS-3 represented by MS medium supplemented with 2 mg/L BAP and 1 mg/L TDZ and MS-4 being the MS medium with 2 mg/L BAP and 0.5 mg/L 1-naphthaleneacetic acid (NAA).
All three culture media were supplemented with PF-68 0.2% (w/v) in order to reduce the effects of leached phenols. The antibiotic Kanamycin was added to the culture medium at concentrations of 50–150 mg/mL to control endogenous infections. Callusing frequency and biometric parameters were determined for each subculture, statistical analysis was performed for subcultures 2 and 6.
2.6. In Vitro Rooting of Regenerants
The microshoots obtained after the sixth subculture represented the biological material used for the study of the in vitro rooting capacity. To induce rooting, the culture medium MS was reduced to half concentration, supplemented with auxins: 1 mg/L NAA and 1 mg/L indole-3-butyric acid (IBA). Each medium was supplemented with PF-68 0.2% (w/v). After 4 weeks, the regenerative response was determined (expressed as the number of days from inoculation to the appearance of the first root), the rate of rhizogenesis (expressed as a percentage) and the number of roots (calculated average value/plant).
2.7. Acclimatization of Regenerants
After in vitro rooting, the seedlings were removed from the culture flasks and washed with distilled water to remove residual culture media from the roots. These were transferred into pots containing a mixture of peat and perlite 1:1 or peat and vermiculite 1:1. Initially, the plants were covered with polyethylene bags to maintain 100% relative humidity. Then, for 2 weeks at 25 ± 2 °C, 70% humidity and a photoperiod of 16 h light/8 h darkness were maintained in climate-controlled storage rooms for 2 weeks. Further, the plants were transferred to greenhouse conditions and watered every 2 days. After a period of 4 months of acclimatization, the plants’ survival rate was determined.
2.8. Experimental Design and Data Analysis
To determine the importance of disinfection treatments, growth phytoregulators and additional compounds (AgNO3 and PF-68), a completely randomized experimental design was chosen. Each treatment was applied in 3 repetitions and each repetition consisted of 10 samples/replicate. To test the degree of endogenous infection, simple tests (smears) were performed. A sample from an infected plantlet was fixed on a microscope slide in the presence of a coloring agent (basic fuchsin). Specimens of bacteria and fungi from infected plants were then viewed under a binocular microscope (Motic B1-252 Binocular Microscope, with 100X objective lenses).
Histological studies were performed to determine the regenerative cell layers. For this purpose, the nodal regenerative tissues were fixed in Carnoy no. 2 solution [54] (6:3:1 EtOH:chloroform:acetic acid) for 3 h. The fixed material in Carnoy was washed and dehydrated in 90% EtOH, then in absolute EtOH twice. Clarification was performed in a mixture of absolute EtOH + chloroform (3:1; 1:1 and 1:3) than in pure chloroform followed by inclusion in paraffin. The vegetal parts were left in the above mixtures and pure chloroform until they became submerged [55]. Sections of 7–8 μm thickness were cut with a rotative microtome (Sakura Accu-Cut SRM 200) and fixed on glass slides then observed under an Olympus CKX41SF microscope with a 40X objective. The observations regarding the formation of the shoots were made with a binocular stereo magnifier Motic DM 143.
Data were tested using the analysis of variance procedure in the POLIFACT (UASVM Cluj-Napoca, Romania) statistical software, and the Duncan’s Multiple Range Test (Duncan’s MRT, p < 0.05) was used as a post hoc test for comparison among treatment means.
3. Results
3.1. Explant Asepsis
Following the application of nodal explant asepsis treatments to P. quadrangularis (Table 3), the most effective formula proved to be T14: the pretreatment with Rifampicin 15 μg/mL in combination with Benomyl 2 g/L, followed by the treatment with EtOH 70%, 1 min and NaClO 50%, 10 min.

Table 3.
Disinfection treatments influence on decontamination of explants from nodal segments in P. quadrangularis.
Through the T14 method of disinfection, 61.67% of the explants remained uncontaminated and viable for regeneration. Treatments based on 15% and 25% NaClO for 10–20 min (T1–T6) were shown to be ineffective (Figure 1a–c). A longer exposure time (20 min) caused phytotoxicity (Figure 1f). The incubation period of the pathogens represents the time when contamination was first observed and it varies on average from 2 to 4 days in the case of T1–T4, T7, and T8 treatments and 5 to 7 days in the case of T12 and T13 treatments. This shows the influence of fungicide treatment on the number of days until the first symptoms of contamination. The pathogens have been shown to be both fungi and bacteria (Figure 1d,e).
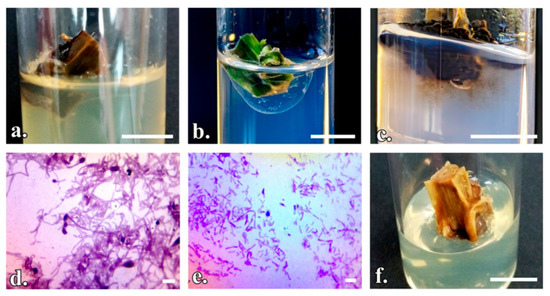
Figure 1.
The behavior of P. quadrangularis explants after asepsis treatments: (a) Endogenous bacterial and fungal contamination (T1–T6); (b) Endogenous bacterial contamination (T10–T13); (c) Fungal contamination and the presence of phenols (T10–T13); (d,e) Bacterial and fungal contamination, 100× magnification; (f) Explant affected by phytotoxicity. (a–c,f) Scale bar represents 1 cm; (d,e) Scale bar represents 10 µm.
3.2. Initiation and Stabilization of In Vitro Plantlets
The development of in vitro axillary shooting began with swelling of the nodal explant. The organogenic structure proliferated after about 3 weeks (Figure 2a,b). Table 4 summarizes the data on the in vitro regeneration of shoots on nodal explants of P. quadrangularis under conditions where different concentrations of cytokinins were applied. The best results regarding the regeneration rate and the number of shoots per explant were registered on the culture medium MS supplemented with 2 mg/L BAP (MS-I.4) and, respectively, the one with 2 mg/L BAP + 1 mg/L KIN (MS-I.8) (Figure 3a). Thus, on the MS-I.4 medium, the regeneration frequency was 33.33% of the explants, with an average number of 2.52 shoots per explant. On the MS-I.8 medium, the regeneration frequency was 24.44%, which is lower than that on MS-I.9 (25.55%), but the regenerants formed an average of 3.19 shoots per explant. The longest shoots, with an average of 2.54 cm and a higher absolute value of 3.8 cm, were obtained on the MS-I.4 medium. The callus incidence was over 50% in the case of MS-I.4 and MS-I.5 treatments, with the callus obtained being friable and green.
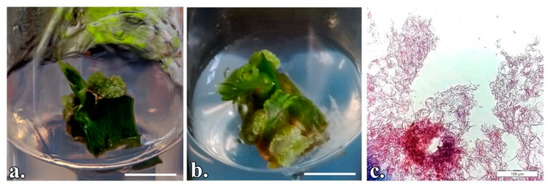
Figure 2.
P. quadrangularis nodal segments cultured in vitro to induce organogenesis: (a) Vertically initiated explant; (b) Horizontally initiated explant; (c) Longitudinal section of the adventitious shoot. (a,b) Scale bar represents 1 cm; c- Scale bar represents 100 µm.

Table 4.
Cytokinin influence on axillary shooting in P. quadrangularis (the first stage of initiation).
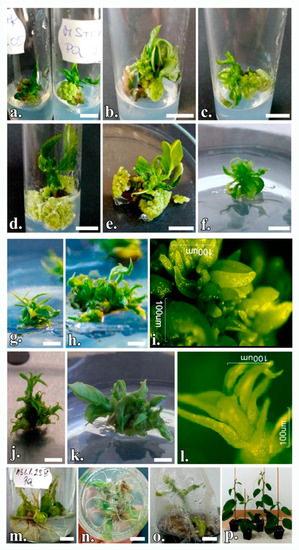
Figure 3.
Regenerative process from nodal segments in P. quadrangularis: (a) On the MS-I.4 and MS-I.8 culture medium in the absence of phenolic inhibitors; (b) On the MS-1 culture medium supplemented with 1 mg/L AgNO3; (c) On MS-1 culture medium supplemented with 2 mg/L AgNO3; (d) On MS-1 culture medium supplemented with PF-68 0.2%; (e) On MS-1 culture medium supplemented with PF-68 0.4%; (f) Microshoot on multiplication medium in the first subculture; (g) Microshoots cluster in the second subculture on MS-2 medium and (h) on MS-3 medium; (i) Proliferation of adventitious buds; (j) Cluster of microshoots in the sixth subculture on MS-2 medium and (k) on MS-3 medium; (l) de novo shoot organogenesis; (m) P. quadrangularis seedlings in vitro rooted; (n) rhizogenesis on MS-5 medium; (o) Acclimatization of plants; (p) Acclimatized plants. The scale bar represents 1 cm.
To improve the regeneration frequency (%), the influence of the explant position was tested. Nodal segments were inoculated in either a vertical or horizontal position on the culture medium. Horizontally initiated explants obtained statistically higher regeneration rates compared to vertically initiated explants on each culture medium analyzed (Table 5). Additionally, biometric parameters registered higher average values in the case of explants inoculated in a vertical position, but without statistically significant differences. The callus incidence was higher in the case of explants inoculated in a horizontal position, of 82.22%, 71.11% and 70%, the differences being statistically significant.

Table 5.
Explant position and culture medium influence on axillary shooting in P. quadrangularis.
To reduce the effects of leached phenols and sudden browning of explants, the media that obtained the best regeneration rates were supplemented with AgNO3 and PF-68. The results of the present research demonstrated the essential role of AgNO3 and PF-68 surfactant in the regeneration by direct organogenesis of nodal explants of P. quadrangularis, with specific cytokinin ratios (Table 6).

Table 6.
Phenolic inhibitors and culture medium influence on axillary shooting in P. quadrangularis.
The additional compounds act synergistically and beneficially in order to induce organogenesis, with the highest regeneration rate of 84.44% for the treatment with PF-68 0.2% on MS medium supplemented with 2 mg/L BAP (Figure 3d), which is statistically superior to the other experimental treatments. The growth-stimulating mechanism of PF-68 is concentration-dependent, and the incorporation of PF-68 at 0.2% or 0.4% supports plant growth. Thus, the treatment with PF-68 0.4% registered a regeneration rate of 76.67% and the one with PF-68% 0.2% of 73.33% on the MS-2 culture medium supplemented with BAP and KIN, the differences being statistically significant. On this culture medium, too, the treatment with 2 mg/L AgNO3 registered the best shooting rate of 71.11%.
The regenerative response occurs on average after 3 days, but in some explants, it can be seen after as many as 5 days. AgNO3 treatments demonstrated higher values than the control treatment, with the differences being statistically significant (Figure 3b,c). Regarding the browning of the explants, the treatment with PF-68 0.4% controlled this phenomenon best, leading to an average browning rate of only 8.52%, a statistically significant value. This treatment showed statistically significant positive differences in the number of shoots per explant (4.71 on MS + BAP + KIN and 4.36 on MS + BAP (Figure 3e)) and in the length of the main shoot (3.06 cm on MS + BAP). Analyzing the unilateral influence of the treatments, it appears that the anionic surfactant PF-68 led to the optimization of the in vitro culture initiation, with an efficiency of over 50% (Figure 4).
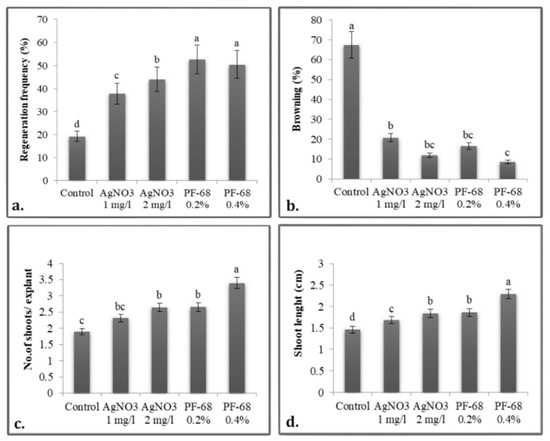
Figure 4.
Unilateral influence of phenolic inhibitors on: (a) Regenerative frequency; (b) Necrosis rate; (c) Number of shoots per explant, (d) Shoot length in P. quadrangularis. The values shown are means ± SE (n = 3). Differences among treatments indicated by different letters are significant according to Duncan’s MRT test (p < 0.05).
3.3. Shoot Subculturing
For mass propagation, the shoots were propagated by subculturing clusters of proliferated shoots. The shooting rate increased until the sixth subculture, after which it decreased. Table 7 summarizes the data on the subcultivation of shoots in the second and sixth subcultures under the influence of PGRs. In the first subculture, the shoots showed insignificant proliferations (Figure 3f). The shoots subculturing leads to obtaining a green, friable callus. The incidence of callus is much reduced in the sixth subculture compared to the second subculture. In the second subculture, there was an average callus incidence of over 69.99%, while in the sixth subculture only 22.22%. In the second subculture, on the MS-2 medium (Figure 3g), an average of 4.45 shoots were obtained in a cluster, compared to the MS-3 medium (Figure 3h), where the clusters were on average composed of 5.01 shoots. A maximum of 7.17 shoots could be obtained from one shoot on MS medium supplemented with 2 mg/L BAP and 1 mg/L TDZ (MS-3) in the sixth subculture (Figure 3k). Here, too, on the MS-2 medium, an average of 5.83 shoots were obtained (Figure 3j), the differences between the treatments being statistically significant. On the MS-3 culture medium, in the analyzed subcultures, average shoot lengths of 3.76 cm and 4.48 cm, respectively, were registered, which is statistically superior to the other experimental treatments. Even after the third subculture, the presence of endogenous bacterial infections continued to occur, affecting up to 30% of the explants. This infection is manifested in the form of haloes dispersed in the culture medium. The addition of Kanamycin to the culture medium at concentrations of 50, 100, and 150 mg/mL, respectively, did not affect the viability of the micro-shoots.

Table 7.
Culture medium and subculture influence on the multiplication of microshoots.
Rhizogenesis began after the sixth subculture on the MS culture medium supplemented with 0.5 mg/L NAA (MS-4) (Figure 5), when 48.89% of the shoots developed roots, a statistically superior value to the other treatments. Because root vigor was low, the in vitro rooting induction step prior to the acclimatization step was required.
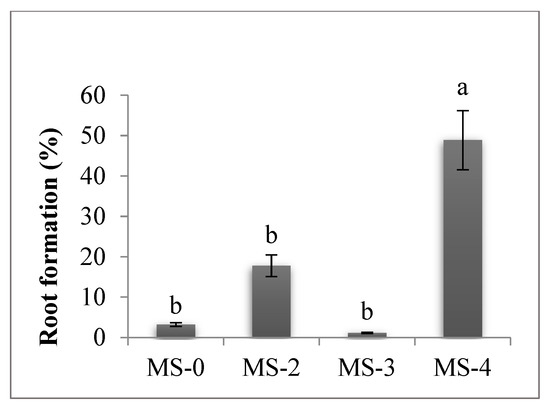
Figure 5.
Culture medium influence in the sixth subculture on root formation. The values shown are means ± SE (n = 3). Differences among treatments indicated by different letters are significant according to Duncan’s MRT test (p < 0.05).
3.4. In Vitro Rooting of Regenerants
Supplementation of ½ MS medium with 1 mg/L NAA (MS-5) proved to be the most prolific for stimulating root formation in 61.11% of shoots (Table 8). The rooting of P. quadrangularis shoots on the ½ MS culture medium in the absence of auxins led to a low percentage of only 11.11%, and lengths of only 2–3 mm. Supplementing the ½ MS medium with 1 mg/L IBA (MS-6) led to an average of 9.61 roots per seedling (Figure 3m,n), with the differences being significantly positive, but not statistically assured. In terms of the number of days until the onset of rhizogenesis, the two auxins proved to have about the same degree of effectiveness. The roots obtained at this stage were of low vigor and a large number were broken when the plants were washed or planted in the acclimatization substrate. Additionally, the regenerated plants showed reduced vigor.

Table 8.
Culture medium influence on P. quadrangularis rhizogenesis.
3.5. Acclimatization of Regenerants
Well-developed and well-rooted plants survived in the acclimatization phase. For this stage, the substrate has an important role in the survival rate of regenerated plants. On the peat + vermiculite (1:1) mix substrate (Figure 3o), the percentage of seedlings that survived was 73.33%, while it was 66.67% on the peat + perlite (1:1) substrate. Regarding plant growth, the substrate did not show any influence. The acclimatized plants showed vigorous growth, green leaves, and well-developed roots (Figure 3p).
4. Discussion
The main objective of this study was to establish a complete in vitro direct organogenesis regeneration protocol in P. quadrangularis using nodal explants. Among the previous investigations of other authors, we did not find any reports containing results regarding a complete in vitro regeneration protocol in P. quadrangularis from nodal explants.
To date, the micropropagation of Passiflora species has been a field of research of interest, with a significant number of studies being conducted. The first study was performed in 1966 on P. caerulea, using nodal explants as the initiation material [56]. Since then, studies showing various micropropagation techniques in Passiflora have been on the rise [22,29,39,42,57,58,59,60,61]. All these studies show that organogenesis remains the main regeneration path for this species [53].
Several studies have reported difficulties in the decontamination of explants [61,62], which is the most important step in the micropropagation protocol. Generally, there are four possible sources of contaminants: internal or external contamination of the parent plant, insufficiently sterilized nutrient media, laboratory air, and inaccuracies on the part of the researcher [63]. Contamination with bacteria, fungi, yeasts, or viruses has been recognized as the most important cause of in vitro culture failure [64,65]. One of the most widely used disinfectants is NaClO [66,67,68,69], applied as a disinfection treatment in research on P. caerulea [70], P. edulis [40] and P. suberosa [31]. The treatment with NaClO solution preceded by EtOH [71] has also been used in the case of the P. miniata species [59]. To eliminate the possibility of contamination of explants, several experiments have been performed to evaluate the efficacy of various antibiotics and fungicides [72,73] during the sterilization stage of explants. In the present study, in the case of P. quadrangularis, since the sterilizing agents did not succeed in achieving total decontamination, the presence of endogenous saprophytic microorganisms could be involved. These pathogens, which have been shown to be both fungi and bacteria, could be responsible for the prolonged contamination of P. quadrangularis explants in the current experiments. Thus, after the subsequent subcultures, the appearance of endogenous infections persisted, with the rate of aseptic explants decreasing by up to 30% after the third subculture. Kanamycin has a general spectrum of action, so an accurate identification of microorganism species would allow the use of an antibiotic with a specific range of action and, consequently, with improved control of endogenous infections. The control of endogenous fungal or bacterial infections has been a problem in many plant micropropagation studies.
In the preliminary study conducted by Boboc et al. [52], the initiation was performed on four culture medium treatments: MS + 0.5 mg/L indole-3-acetic acid (IAA) + 1 mg/L BAP, 1/2 MS without PGRs, Anderson Rhododendron Medium without PGRs, and McCown Woody Plant without PGRs. Starting from nodal segments, a regeneration rate of 23.3% on the McCown Woody Plant culture medium was obtained. The leaf fragments did not obtain any regenerant. In their study, Otahola and Diaz [25] showed that a dose of 2 mg/L BAP led to the highest survival rate of the explants, 76.33% in P. quadrangularis and 80% in P. edulis f flavicarpa. At a concentration of 2 mg/L BAP, P. quadrangularis resulted in an average of 7.37 shoots per explant, compared to P. edulis f. flavicarpa, which resulted in the formation of 1.83 shoots per explant. Initiation of tissue cultures in Passiflora has been shown to be effective on media supplemented with BA in various concentrations, alone or in combination with other PGRs [30]. Nodal explants or leaf fragments are most often used as initiation explants. The initiation of other types of explants in a horizontal position has been recommended in P. tenuifila and P. setacea [58], and P. edulis [42].
Inorganic substances such as AgNO3 and PF-68 have been used to improve the regenerative capacity of explants. The way in which AgNO3 acts on the in vitro culture of plants is still ambiguous, but it has been shown that this compound can antagonize the action of ethylene by reducing the potential of the receptor to bind the gas molecule to ethylene [46]. Currently, scientific research indicates that AgNO3 has beneficial effects on the regeneration and clonal propagation of several economically important plants [46,56]. The properties of AgNO3, such as water solubility, easy availability, specificity, and stability, have been found to be effective for in vitro regeneration. Silver ions in the form of nitrate play an important role in germination, rhizogenesis and somatic embryogenesis, being able to block the action of ethylene which causes abscission, senescence, and growth retardation [40]. The results obtained by Pinto et al. [47] demonstrated that AgNO3 treatment is essential for the induction of adventitious buds in P. alata. The culture medium supplemented with cytokinins and AgNO3 at a photoperiod of 16 h favored direct organogenesis in this species.
To minimize the negative effect of phenols on the regeneration of P. edulis f. flavicarpa explants and to induce robust shoot growth, Huh et al. [40] tested several antioxidant compounds. AgNO3 was shown to be effective in reducing browning and sudden death of explants. Also in this species, Trevisan et al. [39] reported that induction of axillary shoots was improved with AgNO3 on MS culture media supplemented with TDZ. Several researchers have reported the effect of AgNO3 and PF-68 on the regeneration of recalcitrant species. The incorporation of AgNO3 into the initiating medium led to improvements in the regeneration of the species Tagetes erecta [74] and Prosopis cineraria [75].
In the research conducted by Prammanee et al. [76], in the repeated subcultures of P. edulis, the highest number of shoots was obtained on the culture media supplemented with 1 mg/L BA and 1.5 mg/L BA. For P. tenuifila and P. setacea species, the subculture medium contained 2.5 mg/L IBA and 2 g/L Phytagel. Subcultures were performed at 8 weeks, for 24 months without reducing the proliferation of shoots [58]. In the subculture of P. edulis shoots, changing the position of the explants in the subculture on fresh medium led to a decrease in the browning rate due to polyphenols [77].
Numerous studies have shown that supplementation of culture media with auxins plays an important role in rhizogenesis. Moreover, the efficiency of different auxins differs from species to species. Effective rooting on ½ MS medium with 1 mg/L IBA was registered for P. caerulea [70,71], Spemacoce hispida [78] and Lupinus albus [79]. Supplementation with NAA has been shown to be effective in rooting P. caerulea [23,26] and P. edulis f. flavicarpa [80] species. To reduce costs and minimize time to acclimatization, numerous studies have developed the possibility of ex vitro rooting, while acclimatizing using in vitro microshoots. In the case of P. setacea, shoots at least 30 mm long were removed and then immersed in a 9.94 mg/L IBA solution for 10 s to stimulate rooting. The shoots were then transferred to plastic cups containing a substrate for acclimatization [30]. This technique has also been applied to Pseudostellaria heterophylla [81] and Lawsonia inermis [82].
The results obtained in the acclimatization stage are consistent with the results of other studies performed on P. caerulea [26,70] and P. edulis [38]. Additionally, upon the acclimatization of P. foetida plants, a survival rate of 85% was registered on 1:1 vermiculite substrate and sterilized soil [83]. This protocol can be used for the regeneration of shoots, elongation, rooting and acclimatization of other species of interest in the genus Passiflora.
5. Conclusions
The present study led to the first establishment of a complete regeneration protocol by direct organogenesis in P. quadrangularis. Starting from nodal segments, the protocol proved to be efficient and reproducible. For the asepsis of nodal explants the most effective formula proved to be the pretreatment with Rifampicin 15 μg/mL in combination with Benomyl 2 g/L, followed by the treatment with EtOH 70%, 1 min and NaClO 50%, 10 min. Regarding the initiation and stabilization of the culture, the best morphogenic responses were obtained on the MS culture medium supplemented with 2 mg/L BAP. Supplementation of the culture medium with PF-68 0.2% (w/v) proved to have a multiple shooting capacity of 84.44%, and PF-68 0.4% (w/v) led to the maximization of biometric parameters like shoot length and the number of shoots per explant. Supplementing the basal medium with AgNO3 and PF-68 decreased phenolic secretion, and improved plantlet quality and survival rate. Biometric parameters registered higher average values in the case of explants inoculated in a horizontal position on the analyzed culture media. The addition of phytoregulators BAP and TDZ in subcultures induced the maximum multiplication of shoots. To stimulate rhizogenesis, supplementation of ½ MS medium with 1 mg/L NAA was shown to be the most prolific. For acclimatization, the substrate proved to be important, thus, peat + vermiculite 1:1 mixing substrate registered the highest plant survival rate. Although this species has been shown to be recalcitrant, in vitro propagation is recommended as a method of propagation on an industrial scale, especially in areas where this plant is not found in the natural area, as in Romania. On the basis of this study, it is recommended to apply the protocol both for the large-scale production of genetically uniform plant material, due to the ensured genetic stability, and also for the germplasm collections of P. quadrangularis species.
Author Contributions
Conceptualization, P.B.O., M.C. and C.C.; methodology, C.C.; software, P.B.O.; validation, P.B.O., M.C., M.I.C. and C.C.; formal analysis, M.C. and C.C.; investigation, P.B.O.; data curation, P.B.O.; writing—original draft preparation, P.B.O.; writing—review and editing, M.C., M.I.C. and C.C.; visualization, M.C., M.I.C. and C.C.; supervision, M.C. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was supported by funds from the National Research Development Projects to finance excellence (PFE)-14/2022-2024 granted by the Romanian Ministry of Research and Innovation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Institute of Advance Horticulture Research of Transylvania (ICHAT), Center for Biodiversity and Conservation (CBC), University of Agricultural Science and Veterinary Medicine of Cluj–Napoca.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dhawan, K.; Dhawan, S.; Sharma, A. Passiflora: A review update. J. Ethnopharmacol. 2004, 94, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, P.M.; Nicoli, C.F.; Alexandre, R.S.; Guilhen, J.H.S.; Praça-Fontes, M.M.; Ferreira, A.; da Silva Ferreira, M.F. Vegetative and reproductive performance of species of the genus Passiflora. Sci. Hortic. 2020, 265, 109193. [Google Scholar] [CrossRef]
- Vijay, A.; Nizam, A.; Radhakrishnan, A.M.; Anju, T.; Kashyap, A.K.; Kumar, N.; Kumar, A. Comparative study of ovule development between wild (Passiflora foetida L.) and cultivated (P. edulis Sims) species of Passiflora L. provide insights into its differential developmental patterns. J. Zool. Bot. Gard. 2021, 2, 502–516. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Passion fruits. Encycl. Food Sci. Nutr. 2003, 2, 4368–4373. [Google Scholar] [CrossRef]
- Faleiro, F.G.; Junqueira, N.T.V.; Junghans, T.G.; de Jesus, O.N.; Miranda, D.; Otoni, W.C. Advances in passion fruit (Passiflora spp.) propagation. Rev. Bras. Frutic. 2019, 41, 1–17. [Google Scholar] [CrossRef]
- Torsten, U.; MacDougal, J.M. Passiflora: Passionflowers of the World, 1st ed.; Timber Press: Portland, OR, USA, 2004. [Google Scholar]
- Guevara, M.; Tejera, E.; Granda-Albuja, M.G.; Iturralde, G.; Chisaguano-Tonato, M.; Granda-Albuja, S.; Jaramillo-Vivanco, T.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Chemical composition and antioxidant activity of the main fruits consumed in the western coastal region of Ecuador as a source of health-promoting compounds. Antioxidants 2019, 8, 387. [Google Scholar] [CrossRef]
- Costa, G.M.; Gazola, A.C.; Zucolotto, S.M.; Castellanos, L.; Ramos, F.A.; Reginatto, F.H.; Schenkel, E.P. Chemical profiles of traditional preparations of four South American Passiflora Species by chromatographic and capillary electrophoretic techniques. Braz. J. Pharmacogn. 2016, 26, 451–458. [Google Scholar] [CrossRef]
- Antognoni, F.; Zheng, S.; Pagnucco, C.; Baraldi, R.; Poli, F.; Biondi, S. Induction of flavonoid production by UV-B Radiation in Passiflora quadrangularis callus cultures. Fitoterapia 2007, 78, 345–352. [Google Scholar] [CrossRef]
- Shahbani, N.S.; Ramaiya, S.D.; Saupi, N.; Bujang, J.S.; Zakaria, M.H. Reproductive Biology and fruit setting of Passiflora quadrangularis L. (giant granadilla) in East Malaysia. Pertanika J. Trop. Agric. Sci. 2020, 43, 637–652. [Google Scholar] [CrossRef]
- Ramaiya, S.D.; Bujang, J.S.; Zakaria, M.H. Assessment of total phenolic, antioxidant, and antibacterial activities of Passiflora species. Sci. World J. 2014, 2014, 167309. [Google Scholar] [CrossRef]
- Yuldasheva, L.N.; Carvalho, E.B.; Catanho, M.T.J.A.; Krasilnikov, O.V. Cholesterol-dependent hemolytic activity of Passiflora quadrangularis leaves. Braz. J. Med. Biol. Res. 2005, 38, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, R.S.; Júnior, A.W.; Rondinelli, J. Germinação de sementes de genótipos de maracujazeiro. Pesqui. Agropecu. Bras. 2004, 39, 1239–1245. [Google Scholar] [CrossRef]
- Ożarowski, M.; Thiem, B. Development and optimization of a low-cost system for micropropagation of valuable medicinal plants of Passiflora species. In Proceedings of the Conference: I International Scientific and Practical Internet Conference "Investigations of medicinal plants—Theoretical and practical aspects", Kharkiv, Ukraine, 20–21 March 2014; At: Abstract book. National University of Pharmacy: Kharkiv, Ukraine, 2014; pp. 270–271. [Google Scholar]
- Boboc Oros, P.; Hitter Buru, T.; Cătană, C.; Cantor, M. In vitro plant tissue culture: Means for production of Passiflora species. Int. J. Innov. Approaches Agric. Res. 2020, 4, 505–523. [Google Scholar] [CrossRef]
- Rodríguez, A.; Fábio, C.; Faleiro, G.; Parra, M.; Ana, M.; Costa, M. Pasifloras Especies Cultivadas En El Mundo; ProImpress Cepass: Brasília, Brazil, 2020. [Google Scholar]
- Arogundade, O.; Oyekanmi, J.; Oresanya, A.; Ogunsanya, P.; Akinyemi, S.O.S.; Lava Kumar, P. First report of Passion Fruit Woodiness Virus associated with Passion Fruit Woodiness disease of passion fruit in Nigeria. Plant Dis. 2018, 102, 1181. [Google Scholar] [CrossRef]
- Drew, R.A. Micropropagation of Passiflora species (passionfruit). In High-Tech and Micropropagation V; Springer: Berlin/Heidelberg, Germany, 1997; Volume 39, pp. 135–149. [Google Scholar] [CrossRef]
- Boro, M.C.; Beriam, L.O.S.; Guzzo, S.D. Induced resistance against Xanthomonas axonopodis pv. Passiforae in passion fruit plants. Trop. Plant Pathol. 2011, 36, 74–80. [Google Scholar] [CrossRef]
- Hadaś, E.; Ozarowski, M.; Derda, M.; Thiem, B.; Cholewiński, M.; Skrzypczak, Ł.; Gryszczyńska, A.; Piasecka, A. The Use of extracts from Passiflora spp. in helping the treatment of acanthamoebiasis. Acta Pol. Pharm.-Drug Res. 2017, 74, 921–928. [Google Scholar]
- Otoni, W.C.; Pinto, D.L.P.; Rocha, D.I.; Vieira, L.M.; Dias, L.L.C.; da Silva, M.L.; da Silva, C.V.; Lani, E.R.G.; da Silva, L.C.; Tanaka, F.A.O. Organogenesis and somatic embryogenesis in passionfruit (Passiflora sps.). Somat. Embryog. Gene Expr. 2013, 1, 1–17. [Google Scholar]
- Fernando, J.A.; Vieira, M.L.C.; MacHado, S.R.; Appezzato-Da-Glória, B. New insights into the in vitro organogenesis process: The case of Passiflora. Plant Cell. Tissue Organ Cult. 2007, 91, 37–44. [Google Scholar] [CrossRef]
- Jafari, M.; Daneshvar, M.; Lotfi-Jalalabadi, A. Control of in vitro contamination of Passiflora caerulea by using of sodium hypochlorite. Indo-Am. J. Agric. Vet. Sci. 2016, 4, 8–15. [Google Scholar]
- Rathod, H.P.; Pohare, M.B.; Bhor, S.A.; Jadhav, K.P.; Batule, B.S.; Shahakar, S.; Wagh, S.G.; Wadekar, H.B.; Kelatkar, S.K.; Kulkarni, M.R. In vitro micropropagation of blue passion flower (Passiflora caerulea L.). Trends Biosci. 2014, 7, 3079–3082. [Google Scholar]
- Otahola, V.; Diaz, M. Regeneracion in vitro de Passiflora edulis f. flavicarpa y Passiflora quadrangularis utilizando dos tipos de explante provenientes de plantas adultas y bencilaminopurina. Udo 2010, 10, 23–28. [Google Scholar]
- Prithviraj, H.S.; Hemanth, K.; Prakasha, N.K.; Shobha, J. An efficient in vitro regeneration of multiple shoots from leaf explant of Passiflora caerulea L. an important medicinal plant. Int. J. Recent Sci. Res. 2015, 6, 7263–7265. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mikosvki, A.I.; Silva, N.T.; Souza, C.S.; Machado, M.D.; Otoni, W.C.; Carvalho, I.F.; Rocha, D.I.; Silva, M.L. Tissue culture and biotechnological techniques applied to passion fruit with ornamental potential: An overview. Ornam. Hortic. 2019, 25, 189–199. [Google Scholar] [CrossRef]
- de Faria, R.B.; de Carvalho, I.F.; Rossi, A.A.B.; de Matos, E.M.; Rocha, D.I.; Paim Pinto, D.L.; Otoni, W.C.; da Silva, M.L. High responsiveness in de novo shoot organogenesis induction of Passiflora cristalina (Passifloraceae), a wild Amazonian passion fruit species. Vitr. Cell. Dev. Biol.-Plant 2018, 54, 166–174. [Google Scholar] [CrossRef]
- Vieira, L.M.; Rocha, D.I.; Taquetti, M.F.; da Silva, L.C.; de Campos, J.M.S.; Viccini, L.F.; Otoni, W.C. In vitro plant regeneration of Passiflora setacea D.C. (Passifloraceae): The Influence of explant type, growth regulators, and incubation conditions. Vitr. Cell. Dev. Biol.-Plant 2014, 50, 738–745. [Google Scholar] [CrossRef]
- Garcia, R.; Pacheco, G.; Falcão, E.; Borges, G.; Mansur, E. Influence of type of explant, plant growth regulators, salt composition of basal medium, and light on callogenesis and regeneration in Passiflora suberosa L. (Passifloraceae). Plant Cell. Tissue Organ Cult. 2011, 106, 47–54. [Google Scholar] [CrossRef]
- Davey, M.R.; Cancino, G.O.; Gill, M.I.S.; Anthony, P.; Power, J.B. Micropropagation of tropical fruits: Beneficial effects of non-ionic surfactants. ISHS Acta Hortic. 2003, 616, 353–358. [Google Scholar] [CrossRef]
- Lowe, K.C.; Davey, M.R.; Power, J.B.; Mulligan, B. Surfactant supplements systems in plant culture. Agrofoodindustry High-Tech. 1993, 4, 9–13. [Google Scholar]
- Kok, A.D.X.; Mohd Yusoff, N.F.; Sekeli, R.; Wee, C.Y.; Lamasudin, D.U.; Ong-Abdullah, J.; Lai, K.S. Pluronic F-68 improves callus proliferation of recalcitrant rice cultivar via enhanced carbon and nitrogen metabolism and nutrients uptake. Front. Plant Sci. 2021, 12, 667434. [Google Scholar] [CrossRef]
- Kok, A.D.X.; Wan Abdullah, W.M.A.N.; Tan, N.P.; Ong-Abdullah, J.; Sekeli, R.; Wee, C.Y.; Lai, K.S. Growth promoting effects of Pluronic f-68 on callus proliferation of recalcitrant rice cultivar. 3 Biotech 2020, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Rizwan, H.M.; Debnath, B.; Anwar, M.; Li, M.; Liu, S.; He, B.; Qiu, D. Ascorbic acid controls lethal browning and Pluronic F-68 promotes high-frequency multiple shoot regeneration from cotyldonary node explant of okra (Abelmoschus sculentus L.). HortScience 2018, 53, 183–190. [Google Scholar] [CrossRef]
- Barbulescu, D.M.; Burton, W.A.; Salisbury, P.A. Pluronic F-68: An answer for shoot regeneration recalcitrance in microspore-derived Brassica napus embryos. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 282–288. [Google Scholar] [CrossRef]
- Khas, M.E.; Abbasifar, A.; Valizadehkaji, B. Optimization of in vitro propagation of purple passion fruit (Passiflora edulis), an important medicinal and ornamental plant. Int. J. Hortic. Sci. Technol. 2020, 7, 305–314. [Google Scholar] [CrossRef]
- Trevisan, F.; Mendes, B.M.J. Optimization of in vitro organogenesis in passion fruit (Passiflora edulis f. flavicarpa). Sci. Agric. 2005, 62, 346–350. [Google Scholar] [CrossRef]
- Huh, Y.S.; Lee, J.K.; Nam, S.Y. Effect of plant growth regulators and antioxidants on in vitro plant regeneration and callus induction from leaf explants of purple passion fruit (Passiflora edulis Sims). J. Plant Biotechnol. 2017, 44, 335–342. [Google Scholar] [CrossRef]
- Tuhaise, S.; Nakavuma, J.L.; Adriko, J.; Ssekatawa, K.; Kiggundu, A. In vitro regeneration of Ugandan passion fruit cultivars from leaf discs. BMC Res. Notes 2019, 12, 425. [Google Scholar] [CrossRef]
- Drew, R.A. In vitro culture of adult and juvenile bud explants of Passiflora species. Plant Cell. Tissue Organ Cult. 1991, 26, 23–27. [Google Scholar] [CrossRef]
- Reis, L.B.; Paiva Neto, V.B.; Toledo Picoli, E.A.; Costa, M.G.C.; Rego, M.M.; Carvalho, C.R.; Finger, F.L.; Otoni, W.C. Axillary bud development of passionfruit as affected by ethylene precursor. Vitr. Cell. Dev. Biol.-Plant 2003, 39, 618–622. [Google Scholar] [CrossRef]
- Faria, J.L.C.; Segura, J.; de Biolog, D.; de Farmacia, F.; de Valencia, U.; Vicent, A.; Estell, A.; August, R.; Smith, M.A.L. In vitro control of adventitious bud differentiation by inorganic medium components and silver thiosulfate in explants of Passiflora edulis f. flavicarpa. Vitr. Cell. Dev. Biol.-Plant 1997, 33, 209–212. [Google Scholar] [CrossRef]
- Pua, E.-C. Morphogenesis in cell and tissue cultures: Role of ethylene and polyamines. In Morphogenesis in Plant Tissue Culture; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 255–303. [Google Scholar]
- Kumar, V.; Parvatam, G.; Ravishankar, G.A. AgNO3—A potential regulator of ethylene activity and plant growth modulator. Electron. J. Biotechnol. 2009, 12, 8–9. [Google Scholar] [CrossRef]
- Pinto, A.P.C.; Monteiro-Hara, A.C.B.A.; Stipp, L.C.L.; Mendes, B.M.J. In vitro organogenesis of Passiflora alata. Vitr. Cell. Dev. Biol.-Plant 2010, 46, 28–33. [Google Scholar] [CrossRef]
- Singh, C.R. Review article review on problems and its remedy in plant tissue culture. Asian J. Biol. Sci. 2018, 11, 165–172. [Google Scholar] [CrossRef]
- Buendía-González, L.; Orozco-Villafuerte, J.; Cruz-Sosa, F.; Chávez-Ávila, V.M.; Vernon-Carter, E.J. Clonal propagation of mesquite tree (Prosopis laevigata Humb. & Bonpl. Ex Willd. M.C. Johnston). I. via cotyledonary nodes. Vitr. Cell. Dev. Biol.-Plant 2007, 43, 260–266. [Google Scholar] [CrossRef]
- Benson, E.E. Special Symposium: In vitro plant recalcitrance: An introduction. Vitr. Cell. Dev. Biol.-Plant 2000, 36, 141–148. [Google Scholar] [CrossRef]
- Benson, E.E. Do free radicals have a role in plant tissue culture recalcitrance? Vitr. Cell. Dev. Biol.-Plant 2000, 36, 163–170. [Google Scholar] [CrossRef]
- Boboc, P.; Cantor, M.; Pop, R. Studies concerning in vivo and in vitro vegetative propagation of Passiflora quadrangularis species. J. Hortic. For. Biotechnol. 2017, 21, 69–76. [Google Scholar]
- Ozarowski, M.; Thiem, B. Progress in micropropagation of Passiflora spp. to produce medicinal plants: A mini-review. Rev. Bras. Farmacogn.-Braz. J. Pharmacogn. 2013, 23, 937–947. [Google Scholar] [CrossRef]
- Puchtler, H.; Waldrop, F.S.; Conner, H.M.; Terry, M.S. Carnoy Fixation: Practical and Theoretical Considerations. Histochemie 1968, 16, 361–371. [Google Scholar] [CrossRef]
- Șerbănescu-Jitariu, G.; Andrei, M.; Rădulescu-Mitroiu, N.; Petria, E. Practicum de Biologie Vegetal; Ceres: Bucharest, Romania, 1983; pp. 263–265. [Google Scholar]
- Nakayama, F. Cultivo in vitro de tejidos de Passiflora caerulea. Rev. Fac. Agron. Univ. Nac. Plata 1966, 42, 63–74. [Google Scholar]
- Becerra, D.C.; Forero, A.P.; Góngora, G.A. Age and physiological condition of donor plants affect in vitro morphogenesis in leaf explants of Passiflora edulis f. flavicarpa. Plant Cell. Tissue Organ Cult. 2004, 79, 87–90. [Google Scholar] [CrossRef]
- Sozo, J.S.; Cruz, D.C.; Pavei, A.F.; Medeiros, I.; Wolfart, M.; Ramlov, F.; Montagner, D.F.; Maraschin, M.; Viana, A.M. In vitro culture and phytochemical analysis of Passiflora tenuifila Killip and Passiflora setacea DC (Passifloraceae). In Protocols for In Vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants, 2nd ed.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1391. [Google Scholar] [CrossRef]
- de Carvalho, P.P.; Antoniazzi, C.A.; de Faria, R.B.; de Carvalho, I.F.; Rocha, D.I.; da Silva, M.L. In vitro organogenesis from root explants of Passiflora miniata Mast., an amazonian species with ornamental potential. Braz. Arch. Biol. Technol. 2019, 62, 1–9. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Song, S.J.; Landrein, S. In vitro organogenesis and plant regeneration of Passiflora xishuangbannaensis, a species with extremely small populations. Glob. Ecol. Conserv. 2021, 31, e01836. [Google Scholar] [CrossRef]
- Minipara, D.; Dhaduk, H.; Patil, G.; Narayanan, S.; Kumar, S. Identification of best surface sterilization treatment and control of endophytic bacterial contamination in Annona squamosa L. Int. J. Plant Soil Sci. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Ramanathan, S.; Sengodagounder, S.; Senniappan, C.; Shanmuganathan, R.; Brindhadevi, K.; Kaliannan, T. Optimizing the sterilization methods for initiation of the five different clones of the Eucalyptus hybrid species. Biocatal. Agric. Biotechnol. 2019, 22, 101361. [Google Scholar] [CrossRef]
- Onwubiko, N.C.; Nkogho, C.S.; Anyanwu, C.P.; Onyeishi, G.C. Effect of different concentration of sterilant and exposure time on sweet potato (Ipomoea batatas Lam) explants. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 14–20. [Google Scholar]
- Cassells, A.C. Problems in tissue culture: Culture contamination. In Micropropagation: Technology and Application; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 31–44. [Google Scholar] [CrossRef]
- Leifert, C.; Morris, C.E.; Waites, W.M. Ecology of Microbial saprophytes and pathogens in tissue culture and field-grown plants: Reasons for contamination problems in vitro. Crit. Rev. Plant Sci. 1994, 13, 139–183. [Google Scholar] [CrossRef]
- Ugur, R. Development of in vitro sterilization protocol for DO-1 (Prunus domestica) rootstock. Appl. Ecol. Environ. Res. 2020, 18, 2339–2349. [Google Scholar] [CrossRef]
- Azofeifa-Bolaños, J.B.; Rivera-Coto, G.; Paniagua-Vásquez, A.; Cordero-Solórzano, R.; Salas-Alvarado, E. Disinfection effect of nodal segments from Vanilla planifolia Andrews on the morphogenetic response of in vitro plants. Agron. Mesoam. 2019, 30, 33–49. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, D.; Liu, F.; Qin, M.; Tian, D. Micropropagation of Nelumbo nucifera ‘Weishan Hong’ through germfree mature embryos. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 305–312. [Google Scholar] [CrossRef]
- Hesami, M.; Daneshvar, M.H.; Lotfi-Jalalabadi, A. Effect of sodium hypochlorite on control of in vitro contamination and seed germination of Ficus religiosa. Iran. J. Plant Physiol. 2017, 7, 2157–2162. [Google Scholar] [CrossRef]
- Jafari, M.; Daneshvar, M.H.; Lotfi, A. In vitro shoot proliferation of Passiflora caerulea L. via cotyledonary node and shoot tip explants. Biotechnologia 2017, 98, 113–119. [Google Scholar] [CrossRef]
- Pipino, L.; Braglia, L.; Giovannini, A.; Fascella, G.; Mercuri, A. In vitro regeneration of Passiflora species with ornamental value. Propag. Ornam. Plants 2008, 8, 47–49. [Google Scholar]
- Eed, A.M.; Reddy, S.A.; Reddy, K.M.; Silva, J.A.T.; Reddy, P.V.; Beghum, H.; Venkatsubbaiah, P.Y. Effect of antibiotics and fungicides on the in vitro production of Citrus limonia Osbeck nodal segment and shoot tip explants. Asian Australas. J. Plant Sci. Biotechnol. 2010, 4, 66–70. [Google Scholar]
- Mng’omba, S.A.; du Toit, E.S.; Akinnifesi, F.K.; Sileshi, G. Efficacy and utilization of fungicides and other antibiotics for aseptic plant cultures. In Fungicides for Plant and Animal Diseases; InTech: Houston, TX, USA, 2012; pp. 245–254. [Google Scholar] [CrossRef][Green Version]
- Ravindra Kumar, K.; Singh, K.P.; Raju, D.; Bhatia, R.; Panwar, S. Influence of genotypes, growth regulators and basal media on direct differentiation of shoot buds from leaf segments of marigold (Tagetes spp.). Indian J. Exp. Biol. 2019, 57, 30–39. [Google Scholar]
- Venkatachalam, P.; Jinu, U.; Gomathi, M.; Mahendran, D.; Ahmad, N.; Geetha, N.; Sahi, S.V. Role of silver nitrate in plant regeneration from cotyledonary nodal segment explants of Prosopis cineraria (L.) Druce: A recalcitrant medicinal leguminous tree. Biocatal. Agric. Biotechnol. 2017, 12, 286–291. [Google Scholar] [CrossRef]
- Prammanee, S.; Thumjamras, S.; Chiemsombat, P.; Pipattanawong, N. Efficient shoot regeneration from direct apical meristem tissue to produce virus-free purple passion fruit plants. Crop Prot. 2011, 30, 1425–1429. [Google Scholar] [CrossRef]
- Muriithi, M.M. Analysis of Molecular Phylogeny of Kenyan Passion Fruit (Passiflora edulis) and Their Micropropagation to Establish Genetic Stability in Regenerants. Ph.D. Thesis, School of Biological Sciences College of Biological and Physical Sciences, University of Nairobi, Nairobi, Kenya, 2014. [Google Scholar] [CrossRef]
- Deepak, K.V.; Narayanan, G.S.; Prakash, M.; Murugan, S.; Anandan, R. Efficient plant regeneration and histological evaluations of regenerants through organogenesis and somatic embryogenesis in Spermacoce hispida L.—An underutilized medicinally important plant. Ind. Crops Prod. 2019, 134, 292–302. [Google Scholar] [CrossRef]
- Aslam, M.M.; Karanja, J.K.; Zhang, Q.; Lin, H.; Xia, T.; Akhtar, K.; Liu, J.; Miao, R.; Xu, F.; Xu, W. In vitro regeneration potential of white lupin (Lupinus albus) from cotyledonary nodes. Plants 2020, 9, 318. [Google Scholar] [CrossRef]
- Shekhawat, M.S.; Manokari, M.; Ravindran, C.P. An improved micropropagation protocol by ex vitro rooting of Passiflora edulis Sims. f. flavicarpa Deg. through nodal segment culture. Scientifica 2015, 2015, 578676. [Google Scholar] [CrossRef][Green Version]
- Wang, F.; Xin, X.; Wei, H.; Qiu, X.; Liu, B. In vitro regeneration, ex vitro rooting and foliar stoma studies of Pseudostellaria heterophylla (Miq.) Pax. Agronomy 2020, 10, 949. [Google Scholar] [CrossRef]
- Shiji, P.C.; Siril, E.A. An improved micropropagation and ex vitro rooting of a commercially important crop henna (Lawsonia Inermis L.). Physiol. Mol. Biol. Plants 2018, 24, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.P.; Jayakumar, E.; Jeyachandran, R.; Nandagobalan, V.; Doss, A. Direct organogenesis of Passiflora foetida L. through nodal explants. Plant Tissue Cult. Biotechnol. 2012, 22, 87–91. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).