Growth, Fruit Yield, and Bioactive Compounds of Cherry Tomato in Response to Specific White-Based Full-Spectrum Supplemental LED Lighting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Experimental Design and Light Treatment
2.3. Growth Characteristics and Harvest
2.4. Optical Properties (Absorbance and Transmittance)
2.5. Chlorophyll Fluorescence and Electron Transport Rate
2.6. Individual Phenolic Acid and Flavonol Analysis
2.7. Light and Energy Use Efficiency
2.8. Statistical Analysis
3. Results
3.1. Growth Characteristics
3.2. Absorbance and Transmittance
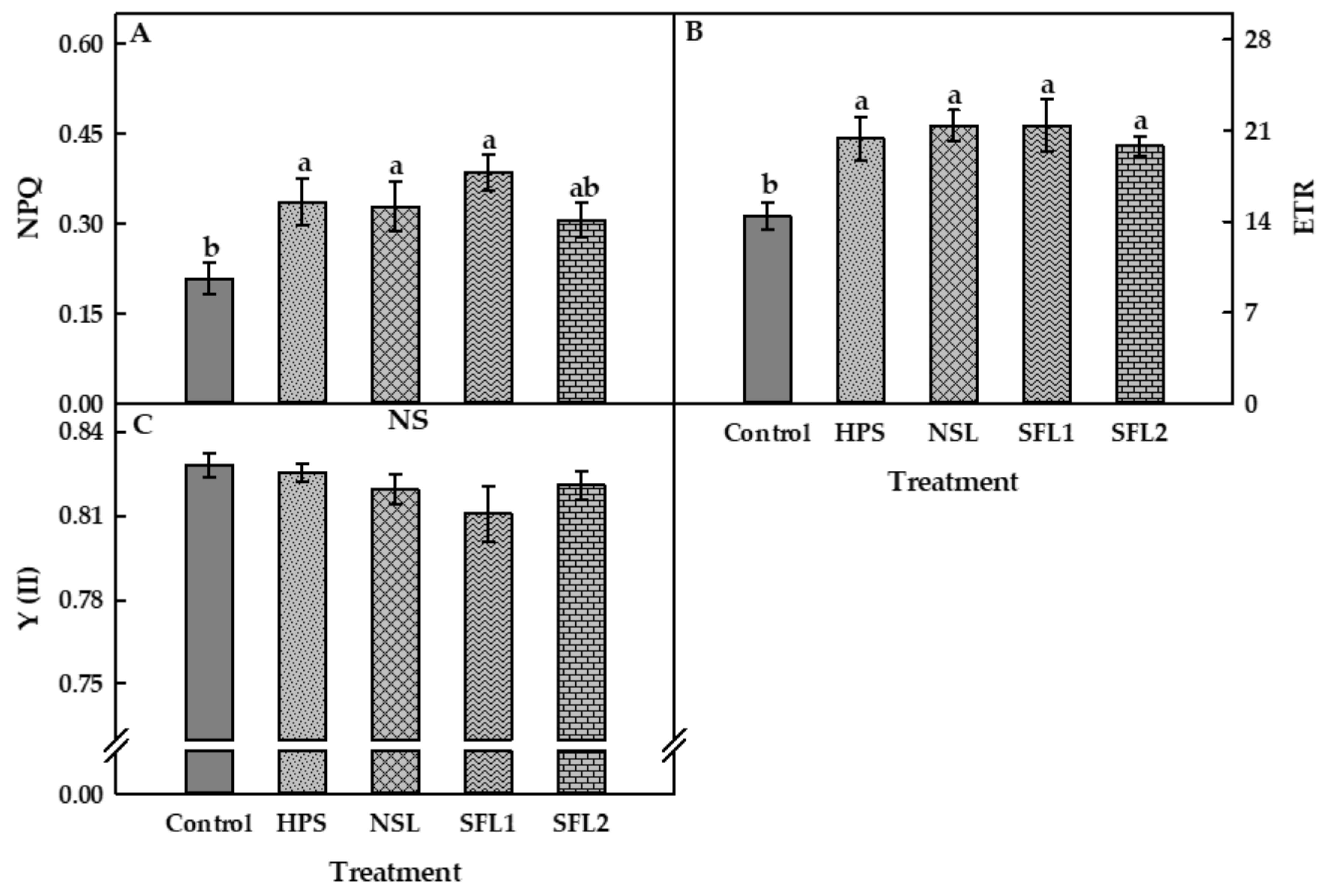
3.3. Chlorophyll Fluorescence and Electron Transport Rate
3.4. Light and Energy Use Efficiency
3.5. Individual Phenolic and Flavonol Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shamshiri, R.R.; Jones, J.W.; Thorp, K.R.; Ahmad, D.; Che Man, H.; Taheri, S. Review of optimum temperature, humidity, and vapour pressure deficit for microclimate evaluation and control in greenhouse cultivation of tomato: A review. Int. Agrophys. 2018, 32, 287–302. [Google Scholar] [CrossRef]
- Golzar, F.; Heeren, N.; Hellweg, S.; Roshandel, R. A comparative study on the environmental impact of greenhouses: A probabilistic approach. Sci. Total Environ. 2019, 675, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Critten, D.; Bailey, B. A review of greenhouse engineering developments during the 1990s. Agric. For. Meteorol. 2002, 112, 1–22. [Google Scholar] [CrossRef]
- Peet, M.; Welles, G. Greenhouse tomato production. Crop. Prod. Sci. Hortic. 2005, 13, 257. [Google Scholar]
- Trouwborst, G.; Oosterkamp, J.; Hogewoning, S.W.; Harbinson, J.; Van Ieperen, W. The responses of light interception, photosynthesis and fruit yield of cucumber to LED-lighting within the canopy. Physiol. Plant. 2010, 138, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Du, S.; van Willigenburg, G. Double closed-loop optimal control of greenhouse cultivation. Control Eng. Pract. 2019, 85, 90–99. [Google Scholar] [CrossRef]
- Tewolde, F.T.; Lu, N.; Shiina, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Yamori, W. Nighttime supplemental LED inter-lighting improves growth and yield of single-truss tomatoes by enhancing photosynthesis in both winter and summer. Front. Plant Sci. 2016, 7, 448. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A. Artificial lighting system for plant growth and development: Chronological advancement, working principles, and comparative assessment. In Light Emitting Diodes for Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–25. [Google Scholar]
- Wallace, C.; Both, A. Evaluating operating characteristics of light sources for horticultural applications. In Proceedings of the VIII International Symposium on Light in Horticulture, East Lansing, MI, USA, 22–26 May 2016. [Google Scholar]
- Gómez, C.; Mitchell, C. Supplemental lighting for greenhouse-grown tomatoes: Intracanopy LED towers vs. overhead HPS lamps. In Proceedings of the International Symposium on New Technologies for Environment Control, Energy-Saving and Crop Production in Greenhouse and Plant, Beijing, China, 20–24 August 2013. [Google Scholar]
- Joshi, N.C.; Ratner, K.; Eidelman, O.; Bednarczyk, D.; Zur, N.; Many, Y.; Shahak, Y.; Aviv-Sharon, E.; Achiam, M.; Gilad, Z. Effects of daytime intra-canopy LED illumination on photosynthesis and productivity of bell pepper grown in protected cultivation. Sci. Hortic. 2019, 250, 81–88. [Google Scholar] [CrossRef]
- Heuvelink, E. Tomatoes; CABI: Wallingford, UK, 2018. [Google Scholar]
- Gautier, H.; Diakou-Verdin, V.; Bénard, C.; Reich, M.; Buret, M.; Bourgaud, F.; Poëssel, J.L.; Caris-Veyrat, C.; Génard, M. How does tomato quality (sugar, acid, and nutritional quality) vary with ripening stage, temperature, and irradiance? J. Agric. Food Chem. 2008, 56, 1241–1250. [Google Scholar] [CrossRef]
- Kimura, S.; Sinha, N. Tomato (Solanum lycopersicum): A model fruit-bearing crop. Cold Spring Harb. Protoc. 2008, 2008, pdb-emo105. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Hao, X.; Khosla, S.; Guo, X.; Bennett, N. Comparison of HPS lighting and hybrid lighting with top HPS and intra-canopy LED lighting for high-wire mini-cucumber production. In Proceedings of the VIII International Symposium on Light in Horticulture, East Lansing, MI, USA, 22–26 May 2016. [Google Scholar]
- Tewolde, F.T.; Shiina, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Yamori, W. Supplemental LED inter-lighting compensates for a shortage of light for plant growth and yield under the lack of sunshine. PLoS ONE 2018, 13, e0206592. [Google Scholar] [CrossRef] [PubMed]
- Dzakovich, M.P.; Gómez, C.; Mitchell, C.A. Tomatoes grown with light-emitting diodes or high-pressure sodium supplemental lights have similar fruit-quality attributes. HortScience 2015, 50, 1498–1502. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Maruo, T.; Hohjo, M.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A. Effect of supplemental far-red light with blue and red LED lamps on leaf photosynthesis, stomatal regulation and plant development of protected cultivated tomato. In Proceedings of the International Symposium on New Technologies for Environment Control, Energy-Saving and Crop Production in Greenhouse and Plant, Beijing, China, 20–24 August 2017. [Google Scholar]
- Kim, H.-J.; Lin, M.-Y.; Mitchell, C.A. Light spectral and thermal properties govern biomass allocation in tomato through morphological and physiological changes. Environ. Exp. Bot. 2019, 157, 228–240. [Google Scholar] [CrossRef]
- Palmitessa, O.D.; Prinzenberg, A.E.; Kaiser, E.; Heuvelink, E. LED and HPS Supplementary Light Differentially Affect Gas Exchange in Tomato Leaves. Plants 2021, 10, 810. [Google Scholar] [CrossRef]
- Hao, X.; Zheng, J.; Little, C.; Khosla, S. Hybrid Lighting Configurations with Top HPS Lighting and LED Inter-lighting and N: K ratios in Nutrient Feedings Affected Plant Growth, Fruit Yield, and Light and Energy Use Efficiency in Greenhouse Tomato Production. In HORTSCIENCE; Amer Soc Horticultural Science: Alexandria, VA, USA, 2015. [Google Scholar]
- Kowalczyk, K.; Gajc-Wolska, J.; Metera, A.; Mazur, K.; Radzanowska, J.; Szatkowski, M. Effect of supplementary lighting on the quality of tomato fruit (Solanum lycopersicum L.) in autumn-winter cultivation. In Proceedings of the VII International Symposium on Light in Horticultural Systems, Wageningen, The Netherlands, 15–18 October 2012. [Google Scholar] [CrossRef]
- Paucek, I.; Pennisi, G.; Pistillo, A.; Appolloni, E.; Crepaldi, A.; Calegari, B.; Spinelli, F.; Cellini, A.; Gabarrell, X.; Orsini, F. Supplementary LED interlighting improves yield and precocity of greenhouse tomatoes in the Mediterranean. Agronomy 2020, 10, 1002. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Park, S.; Park, B.-J.; Lee, Y.; Oh, M.-M. Growth and antioxidant phenolic compounds in cherry tomato seedlings grown under monochromatic light-emitting diodes. Hortic. Environ. Biotechnol. 2014, 55, 506–513. [Google Scholar] [CrossRef]
- Ouzounis, T.; Heuvelink, E.; Ji, Y.; Schouten, H.; Visser, R.; Marcelis, L. Blue and red LED lighting effects on plant biomass, stomatal conductance, and metabolite content in nine tomato genotypes. In Proceedings of the VIII International Symposium on Light in Horticulture, East Lansing, MI, USA, 22–26 May 2016. [Google Scholar]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X.-l. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Lu, N.; Maruo, T.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ito, Y.; Ichimura, T.; Shinohara, Y. Effects of supplemental lighting with light-emitting diodes (LEDs) on tomato yield and quality of single-truss tomato plants grown at high planting density. Environ. Control Biol. 2012, 50, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.K.L.; Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Lee, G.O.; Jang, S.N.; Lee, Y.; Kim, D.; Son, K.-H. Effects of white LED lighting with specific shorter blue and/or green wavelength on the growth and quality of two lettuce cultivars in a vertical farming system. Agronomy 2021, 11, 2111. [Google Scholar] [CrossRef]
- Gajc-Wolska, J.; Kowalczyk, K.; Metera, A.; Mazur, K.; Bujalski, D.; Hemka, L. Effect of supplementary lighting on selected physiological parameters and yielding of tomato plants. Folia Hortic. 2013, 25, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Hernández, R.; Eguchi, T.; Kubota, C. (Eds.) Growth and morphology of vegetable seedlings under different blue and red photon flux ratios using light-emitting diodes as sole-source lighting. In Proceedings of the VIII International Symposium on Light in Horticulture, East Lansing, MI, USA, 22–26 May 2016. [Google Scholar]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Jullien, A.; Allirand, J.-M.; Mathieu, A.; Andrieu, B.; Ney, B. Variations in leaf mass per area according to N nutrition, plant age, and leaf position reflect ontogenetic plasticity in winter oilseed rape (Brassica napus L.). Field Crops Res. 2009, 114, 188–197. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massa, G.D.; Kim, H.-H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Oh, M.M. Physiological and biochemical responses of green and red perilla to LED-based light. J. Sci. Food Agric. 2021, 101, 240–252. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Kołton, A.; Długosz-Grochowska, O.; Kunicki, E.; Mrowiec, K.; Bathelt, P. LED lighting affected the growth and metabolism of eggplant and tomato transplants in a greenhouse. Hortic. Sci. 2020, 47, 150–157. [Google Scholar] [CrossRef]
- Samuolienė, G.; Sirtautas, R.; Brazaitytė, A.; Duchovskis, P. LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012, 134, 1494–1499. [Google Scholar] [CrossRef]
- Wang, H.; Qian, X.; Zhang, L.; Xu, S.; Li, H.; Xia, X.; Dai, L.; Xu, L.; Yu, J.; Liu, X. A method of high throughput monitoring crop physiology using chlorophyll fluorescence and multispectral imaging. Front. Plant Sci. 2018, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Chen, T.; Feng, B.; Zhang, C.; Peng, S.; Zhang, X.; Fu, G.; Tao, L. Non-photochemical quenching plays a key role in light acclimation of rice plants differing in leaf color. Front. Plant Sci. 2017, 7, 1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.; Zhu, X.-G. Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth. Res. 2019, 139, 107–121. [Google Scholar] [CrossRef]
- Nishio, J. Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement. Plant Cell Environ. 2000, 23, 539–548. [Google Scholar] [CrossRef]
- Trojak, M.; Skowron, E. Light quality-dependent regulation of non-photochemical quenching in tomato plants. Biology 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, W.; Hu, G.; Wang, X.C.; Zhang, Y.; Sun, G.X.; Fang, Z. Effects of light-emitting diode supplementary lighting on the winter growth of greenhouse plants in the Yangtze River Delta of China. Bot. Stud. 2016, 57, 2. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 49–70. [Google Scholar]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [Green Version]
- Bugbee, B. Toward an optimal spectral quality for plant growth and development: The importance of radiation capture. In Proceedings of the VIII International Symposium on Light in Horticulture, East Lansing, MI, USA, 22–26 May 2016. [Google Scholar]
- Peralta, I.E.; Peppi, D.; Sance, M.; Asis, R.; Asprelli, P.D.; Galmarini, C.R. Nutritional quality of orange tomatoes for fresh consumption and processing products. In Proceedings of the XIV International Symposium on Processing Tomato, Santiago, Chile, 6–9 March 2016. [Google Scholar]
- Borguini, R.G.; Ferraz da Silva Torres, E.A. Tomatoes and tomato products as dietary sources of antioxidants. Food Rev. Int. 2009, 25, 313–325. [Google Scholar] [CrossRef]
- García-Closas, R.; Berenguer, A.; Tormo, M.J.; Sánchez, M.J.; Quiros, J.R.; Navarro, C.; Arnaud, R.; Dorronsoro, M.; Chirlaque, M.D.; Barricarte, A. Dietary sources of vitamin C, vitamin E and specific carotenoids in Spain. Br. J. Nutr. 2004, 91, 1005–1011. [Google Scholar] [CrossRef] [Green Version]
- Taulavuori, K.; Hyöky, V.; Oksanen, J.; Taulavuori, E.; Julkunen-Tiitto, R. Species-specific differences in synthesis of flavonoids and phenolic acids under increasing periods of enhanced blue light. Environ. Exp. Bot. 2016, 121, 145–150. [Google Scholar] [CrossRef]
- Wang, W.; Su, M.; Li, H.; Zeng, B.; Chang, Q.; Lai, Z. Effects of supplemental lighting with different light qualities on growth and secondary metabolite content of Anoectochilus roxburghii. PeerJ 2018, 6, e5274. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in food: Cancer prevention and apoptosis induction. Curr. Med. Chem. 2018, 25, 4740–4757. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, M.; Shoji, K.; Kato, M.; Shimomura, K.; Goto, F.; Yoshihara, T. Supplementary ultraviolet radiation B together with blue light at night increased quercetin content and flavonol synthase gene expression in leaf lettuce (Lactuca sativa L.). Environ. Control Biol. 2008, 46, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ryan, K.G.; Swinny, E.E.; Markham, K.R.; Winefield, C. Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry 2002, 59, 23–32. [Google Scholar] [CrossRef]
- Cocetta, G.; Casciani, D.; Bulgari, R.; Musante, F.; Kołton, A.; Rossi, M.; Ferrante, A. Light use efficiency for vegetables production in protected and indoor environments. Eur. Phys. J. Plus 2017, 132, 43. [Google Scholar] [CrossRef]

| Type | Chemical | Amount (g 1000 L−1) |
|---|---|---|
| A | KNO3 | 20,200 |
| Ca(NO3)2·4H2O | 35,400 | |
| Fe-EDTA | 1500 | |
| B | KNO3 | 20,200 |
| NH4H2PO4 | 7600 | |
| MgSO4·7H2O | 24,600 | |
| H3BO3 | 114 | |
| MnSO4·4H2O | 81 | |
| ZnSO4·7H2O | 9 | |
| CuSO4·5H2O | 4 | |
| Na2MoO4·2H2O | 1 |
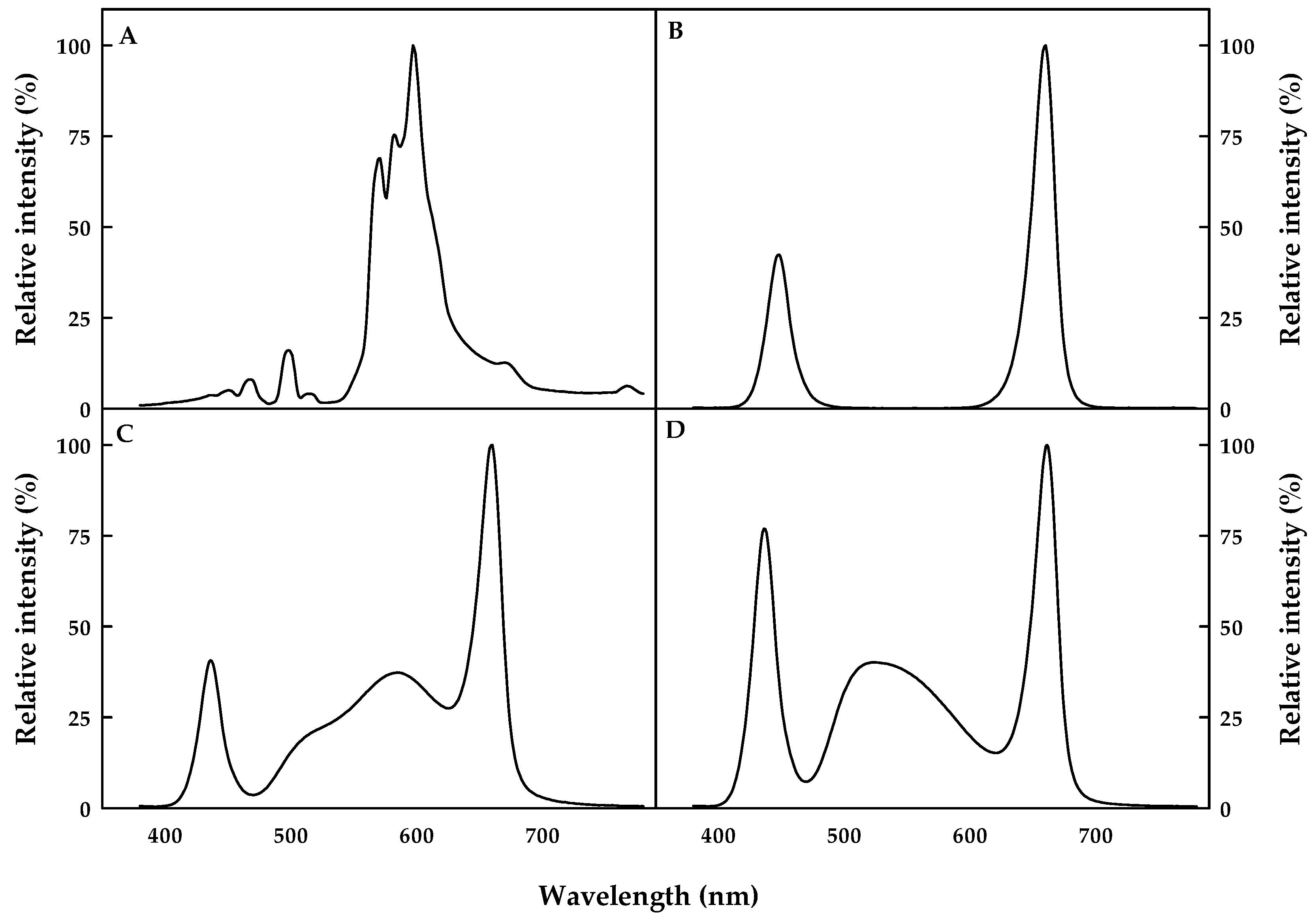
| Light Source z | Range (nm) | Peak Wavelength (nm) | Ratio (%) |
|---|---|---|---|
| HPS | Blue (380–499) | 497 | 7 |
| Green (500–599) | 597 | 49 | |
| Red (600–700) | 600 | 44 | |
| NSL | Blue (380–499) | 450 | 30 |
| Green (500–599) | 0 | 0 | |
| Red (600–700) | 660 | 70 | |
| SFL1 | Blue (380–499) | 436 | 16 |
| Green (500–599) | 585 | 36 | |
| Red (600–700) | 660 | 48 | |
| SFL2 | Blue (380–499) | 436 | 27 |
| Green (500–599) | 524 | 37 | |
| Red (600–700) | 660 | 36 |
| Light Sources z | Absorbance (%) | Transmittance (%) | |||||
|---|---|---|---|---|---|---|---|
| Blue (380–499 nm) | Green (500–599 nm) | Red (600–700 nm) | Blue (380–499 nm) | Green (500–599 nm) | Red (600–700 nm) | ||
| With sunlight | Control | 78 | 74 | 63 | 22 | 26 | 37 |
| HPS | 86 | 91 | 85 | 14 | 9 | 15 | |
| NSL | 91 | 71 | 90 | 9 | 29 | 10 | |
| SFL1 | 83 | 86 | 86 | 17 | 14 | 14 | |
| SFL2 | 92 | 91 | 86 | 8 | 9 | 14 | |
| Without sunlight | HPS | 96 | 94 | 91 | 4 | 6 | 9 |
| NSL | 99 | 95 | 98 | 1 | 5 | 2 | |
| SFL1 | 96 | 92 | 94 | 4 | 8 | 6 | |
| SFL2 | 96 | 92 | 94 | 4 | 8 | 6 | |
| Light Source | Energy Consumption (Watt) | Light Use Efficiency (g FW mol−1) | Energy Use Efficiency (g FW kWh−1) |
|---|---|---|---|
| HPS | 250 | 4.01 b z | 2.12 c |
| NSL | 104 | 4.14 b | 4.88 b |
| SFL1 | 100 | 5.72 a | 6.08 a |
| SFL2 | 100 | 5.72 a | 6.00 a |
| Individual Compound Contents (g plant−1) | Supplemental Light Source | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | HPS | NSL | SFL1 | SFL2 | |||||||
| Phenolic acids | Gallic acid | 7.07 | c z | 9.11 | bc | 8.62 | c | 10.97 | ab | 11.73 | a |
| Protocatechuic acid | 0.79 | b | 0.86 | b | 0.86 | b | 1.18 | a | 0.70 | b | |
| Chlorogenic acid | 32.80 | c | 62.29 | b | 64.06 | b | 64.25 | b | 78.93 | a | |
| p-Hydrobenzoic acid | 7.47 | b | 13.39 | a | 11.00 | a | 13.17 | a | 12.93 | a | |
| Vanillic acid | 1.36 | b | 3.02 | a | 2.90 | a | n.d. | n.d. | |||
| p-Coumaric acid | 0.78 | c | 1.91 | a | 1.17 | b | 1.12 | b | 0.77 | c | |
| Ferulic acid | 1.74 | c | 4.43 | a | 4.11 | ab | 3.57 | b | 4.20 | ab | |
| Ventaric acid | 1.93 | c | 4.73 | b | 6.92 | a | 6.48 | a | 6.51 | a | |
| Benzoic acid | 12.07 | c | 20.84 | b | 18.12 | b | 20.80 | b | 32.25 | a | |
| trans-Cinnamic acid | 2.53 | a | 3.16 | a | 0.47 | b | 3.00 | a | 2.84 | a | |
| Total | 68.54382 | 123.7343 | 118.2031 | 124.542 | 150.8515 | ||||||
| Flavonols | Epigallocatechin | 490.15 | c | 708.39 | b | 699.55 | b | 784.91 | b | 945.71 | a |
| Catechin | 317.55 | b | 537.12 | a | 473.16 | a | 566.82 | a | 540.95 | a | |
| Epicatechin | 71.10 | b | 118.25 | a | 97.67 | a | 117.09 | a | 107.56 | a | |
| Epigallocatechin gallate | 38.39 | c | 45.89 | c | 87.28 | a | 58.91 | b | 37.41 | c | |
| Vanillin | 6.06 | b | 5.48 | b | 5.25 | b | 12.52 | a | n.d. | ||
| Rutin | 319.69 | c | 551.42 | a | 416.01 | b | 459.14 | b | 377.43 | bc | |
| Catechin gallate | 35.08 | c | 62.87 | b | 54.91 | b | 54.42 | b | 80.23 | a | |
| Quercetin | 546.59 | b | 873.46 | a | 905.17 | a | 1012.09 | a | 1036.47 | a | |
| Naringin | 35.11 | b | 65.62 | a | 62.30 | a | 59.18 | a | 64.28 | a | |
| Naringenin | 263.87 | b | 347.12 | a | 327.31 | ab | 325.29 | ab | 318.66 | ab | |
| Formononetin | 21.36 | b | 39.49 | a | 27.34 | b | 41.54 | a | 38.46 | a | |
| Total | 2144.95 | 3355.10 | 3155.96 | 3491.90 | 3547.14 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.K.L.; Cho, K.M.; Lee, H.-Y.; Sim, H.-S.; Kim, J.-H.; Son, K.-H. Growth, Fruit Yield, and Bioactive Compounds of Cherry Tomato in Response to Specific White-Based Full-Spectrum Supplemental LED Lighting. Horticulturae 2022, 8, 319. https://doi.org/10.3390/horticulturae8040319
Nguyen TKL, Cho KM, Lee H-Y, Sim H-S, Kim J-H, Son K-H. Growth, Fruit Yield, and Bioactive Compounds of Cherry Tomato in Response to Specific White-Based Full-Spectrum Supplemental LED Lighting. Horticulturae. 2022; 8(4):319. https://doi.org/10.3390/horticulturae8040319
Chicago/Turabian StyleNguyen, Thi Kim Loan, Kye Man Cho, Hee-Yul Lee, Han-Sol Sim, Jin-Ha Kim, and Ki-Ho Son. 2022. "Growth, Fruit Yield, and Bioactive Compounds of Cherry Tomato in Response to Specific White-Based Full-Spectrum Supplemental LED Lighting" Horticulturae 8, no. 4: 319. https://doi.org/10.3390/horticulturae8040319
APA StyleNguyen, T. K. L., Cho, K. M., Lee, H.-Y., Sim, H.-S., Kim, J.-H., & Son, K.-H. (2022). Growth, Fruit Yield, and Bioactive Compounds of Cherry Tomato in Response to Specific White-Based Full-Spectrum Supplemental LED Lighting. Horticulturae, 8(4), 319. https://doi.org/10.3390/horticulturae8040319







