Self-Incompatibility of Camellia weiningensis Y.K. Li.
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Methods
2.2.1. Self- and Cross-Pollination
2.2.2. Fluorescence Microscopy
2.2.3. Scanning Electron Microscopy
2.2.4. Stereoscopy
2.2.5. Field Pollination Experiment
2.3. Statistical Analysis
3. Results
3.1. Structural Characteristics of Flower Organs
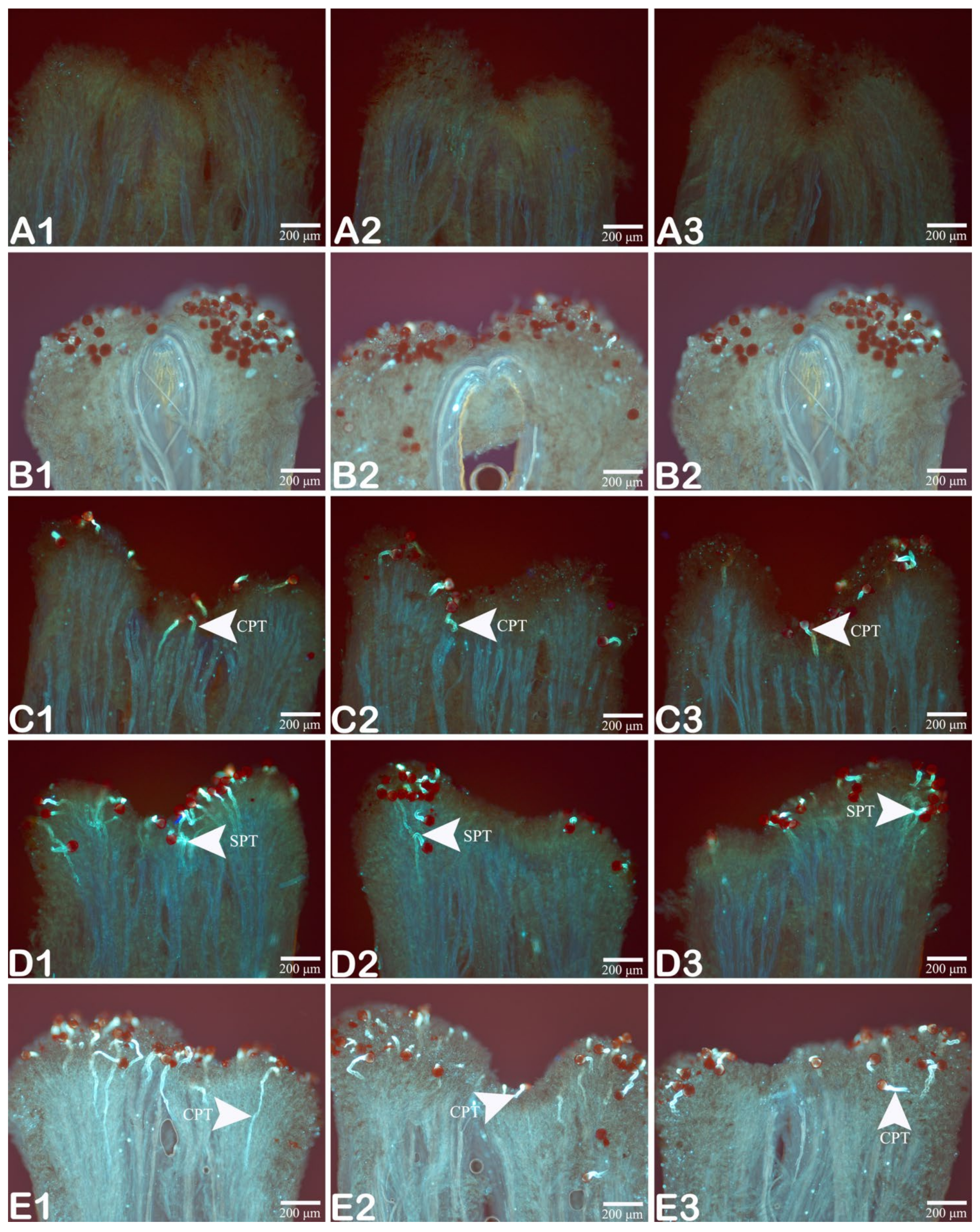
3.2. Pollen Germination
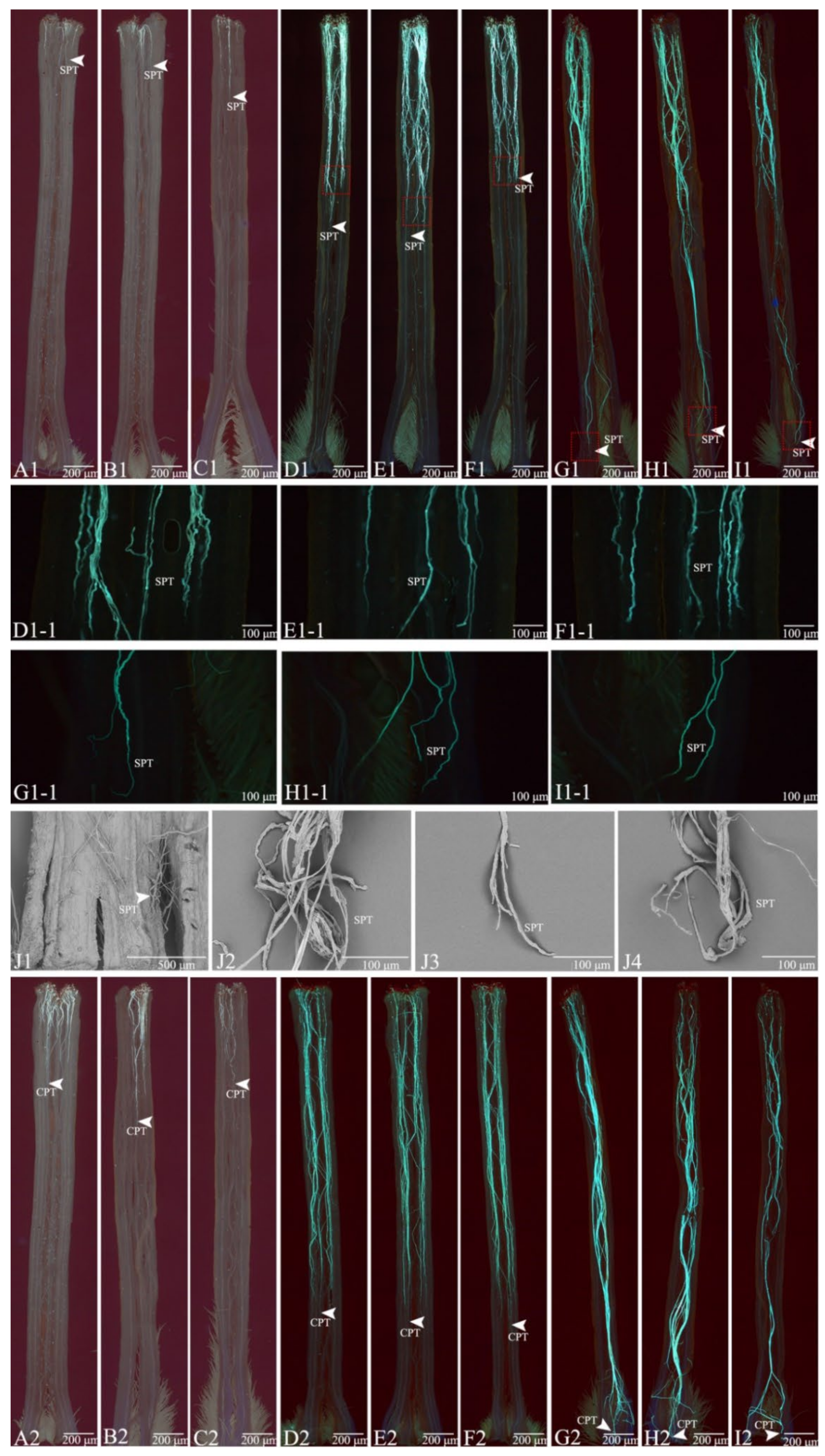
3.3. Growth Behavior of the Pollen Tube
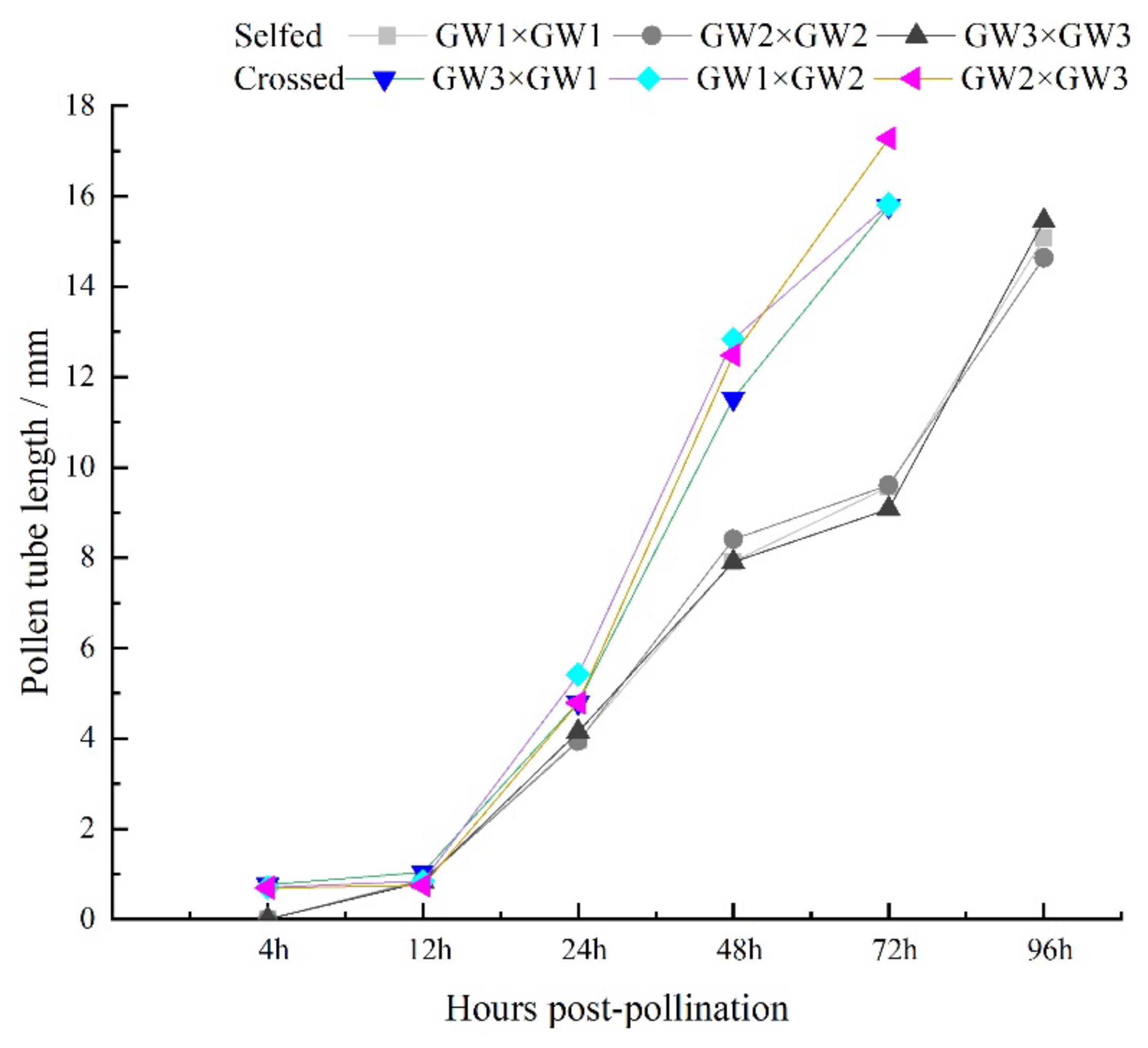
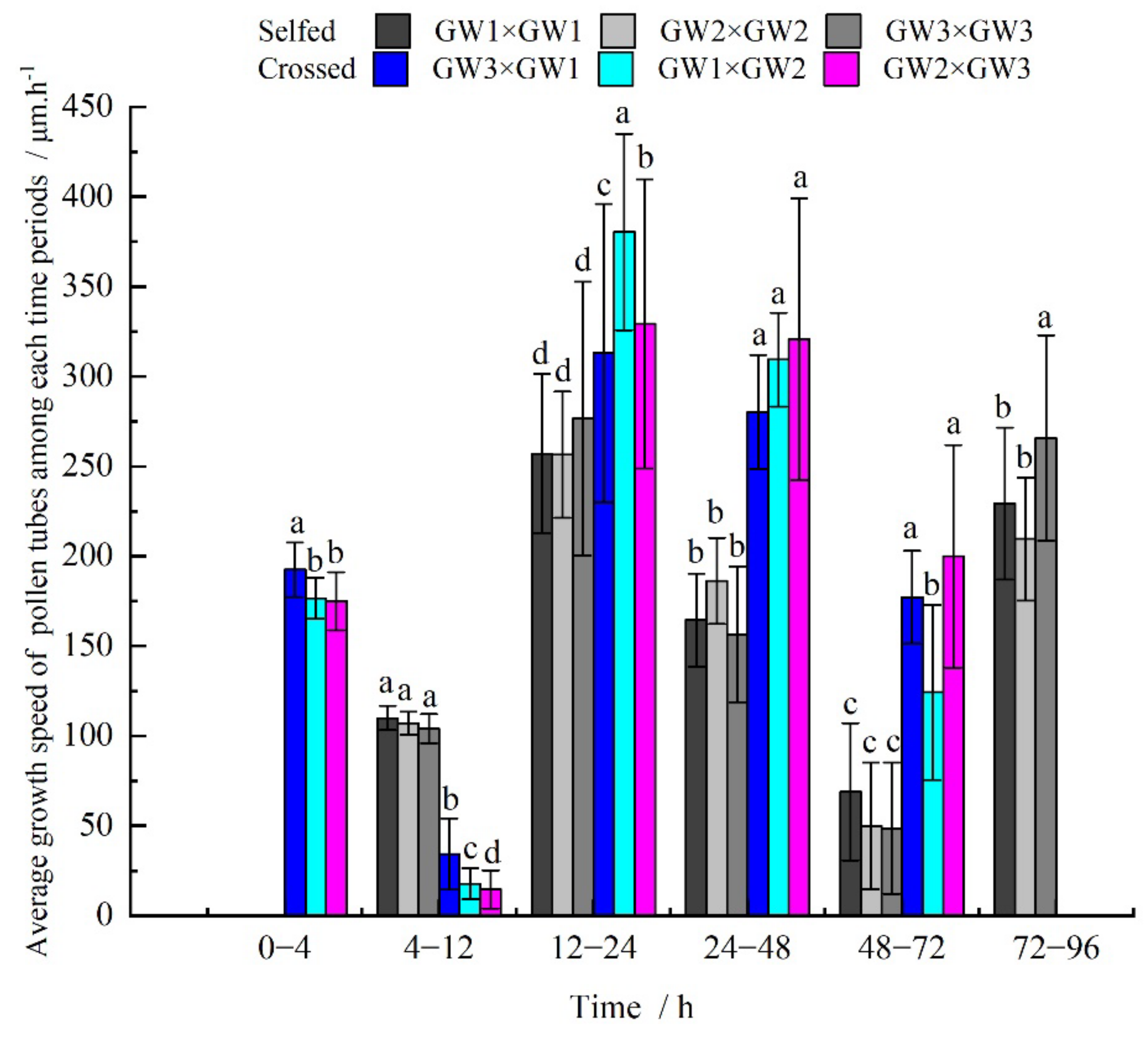
3.4. Comparison of Pollen Tube Growth between the Selfed and Crossed Groups
3.5. Field Experiment: Fruit Setting Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xavier, V.; Vincent, C. When the genetic architecture matters: Evolutionary and ecological implications of self versus nonself recognition in plant self-incompatibility. New Phytol. 2021, 231, 1304–1307. [Google Scholar]
- Chen, X.D. Analysis on the development and improvement of Zhejiang Safflower Camellia resources in Qingtian County. J. Agric. Catastrophol. 2021, 11, 188–189, 192. [Google Scholar]
- Sayyad-Amin, P.; Davarynejad, G.; Abedy, B. The effects of self-incompatibility control substances on yield and fruit growth traits of pear Pyrus communis L. Cultivar ‘Williams’. Erwerbs-Obstbau 2021, 63, 117–123. [Google Scholar] [CrossRef]
- Hou, S.; Hao, Z.Z.; Li, Y.H. Observation of pollen tubes behavior and early embryogenesis following self and cross-pollination in Chinese jujube. J. Fruit Sci. 2019, 36, 1515–1523. [Google Scholar] [CrossRef]
- Nettancourt, D.D. Incompatibility in Angiosperms; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Allen, A.M.; Hiscock, S.J.; Franklin-Tong, V.E. Evolution and phylogeny of self-incompatibility systems in angiosperms. In Self-Incompatibility in Flowering Plants; Springer: Berlin/Heidelberg, Germany, 2008; pp. 73–101. [Google Scholar]
- Maskromo, I.; Larekeng, S.H.; Novarianto, H.; Sudarsono, S. Xenia negatively affecting kopyor nut yield in Kalianda Tall kopyor and Pati Dwarf kopyor coconuts. Emir. J. Food Agric. 2016, 28, 644–652. [Google Scholar] [CrossRef]
- Franklin-Tong, V.E.; Ride, J.P.; Franklin, F.C.H. Recombinant stigmatic self-incompatibility (S-) protein elicits a Ca2+ transient in pollen of Papaver rhoeas. Plant J. 1995, 8, 299–307. [Google Scholar] [CrossRef]
- He, Y.F.; Song, Q.Q.; Wu, Y.F.; Ye, S.T. TMT-based quantitative proteomic analysis reveals the crucial biological pathways involved in self-incompatibility responses in Camellia oleifera. Int. J. Mol. Sci. 2020, 21, 1987. [Google Scholar] [CrossRef]
- Duarte, M.O.; Oliveira, D.M.T.; Borba, E.L. Two self-incompatibility sites occur simultaneously in the same acianthera species (Orchidaceae, pleurothallidinae). Plants 2020, 9, 1758. [Google Scholar] [CrossRef]
- Johnson, S.D.; Butler, H.C.; Robertson, A. Breeding systems in Cyrtanthus (Amaryllidaceae): Variation in self-sterility and potential for ovule discounting. Plant Biol. 2019, 21, 1008–1015. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.R.; Liu, M.J. Fluorescent microscope observation on pollen germination in different cross combinations of Chinese Jujube. Acta Agric. Boreali-Sin. 2008, 2008, 46–49. [Google Scholar]
- Gao, C.; Yuan, D.Y.; Yang, Y. Anatomical characteristics of self-Incompatibility in Camellia Oleifera. Sci. Silvae Sin. 2015, 51, 60–68. [Google Scholar]
- Hu, G.X.; Gao, C.; Fan, X.M. Pollination compatibility and xenia in Camellia oleifera. Hortscience 2020, 55, 898–905. [Google Scholar] [CrossRef]
- Fattahi, R.; Mohammadzedeh, M.; Khadivi-Khub, A. Influence of different pollen sources on nut and kernel characteristics of hazelnut. Sci. Hortic. 2014, 173, 15–19. [Google Scholar] [CrossRef]
- Casiva, P.V.; Vilardi, J.C.; Cialdella, A.M.; Saidman, B.O. Mating system and population structure of Acacia aroma and A. macracantha (Fabaceae). Am. J. Bot. 2004, 91, 58–64. [Google Scholar] [CrossRef]
- Kenrick, J. Review of pollen–pistil interactions and their relevance to the reproductive biology of Acacia. Aust. Syst. Bot. 2003, 16, 119–130. [Google Scholar] [CrossRef]
- Zhang, R.P.; Zhan, N.; Huang, L.J. Control pollination of Acacia mangium and Acacia auriculiformis. Mol. Plant Breed. 2020, 18, 5877–5882. [Google Scholar]
- Geng, J.B.; Xu, J.; Wang, J. Resource Survey on Camellia weiningensis Y.K. Li. sp. nov in Weining County, Guizhou Province and utilization status. J. Anhui Agric. Sci. 2019, 47, 128–131. [Google Scholar]
- Mondal, T.K.; Bhattacharya, A.; Laxmikumaran, M.; Ahuja, P.S. Recent advances of tea (Camellia sinensis) biotechnology. Plant Cell Tissue Organ Cult. 2004, 76, 195–254. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.X.; Yang, Y.J. Genetic improvement and breeding of tea plant (Camellia sinensis) in China: From individual selection to hybridization and molecular breeding. Euphytica 2007, 154, 239–248. [Google Scholar] [CrossRef]
- Wei, K.; Wang, L.Y.; Wu, L.Y. Transcriptome analysis of indole-3-butyric acid-induced adventitious rootformation in nodal cuttings of Camellia sinensis (L.). PLoS ONE 2014, 9, e107201. [Google Scholar] [CrossRef]
- Tan, L.Q.; Wang, L.Y.; Wei, K. Floral transcriptome sequencing for SSR markerdevelopment and linkage map construction in the tea plant (Camellia sinensis). PLoS ONE 2013, 8, e81611. [Google Scholar] [CrossRef]
- Chen, X.; Liu, T.K.; Hao, S. Differential gene expression analysis of self incompatible lines in tea by cDNA-AFLP. Acad. J. 2011, 10, 1684–5315. [Google Scholar]
- Huang, X.N.; Qi, Y.; Ye, P.M. The breeding of two new yellow Camellia hybrid. J. Anhui Agric. Sci. 2021, 49, 54–56, 66. [Google Scholar]
- Kaundun, S.S.; Matsumoto, S. Heterologous nuclear and chloroplast microsatellite amplification and variation in tea, Camellia sinensis. Genome 2002, 45, 1041–1048. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, L.P.; Gao, Q.K. Generation and analysis of expressed sequence tags from the tender shoots cDNA library of tea plant (Camellia sinensis). Plant Sci. 2005, 168, 359–363. [Google Scholar] [CrossRef]
- Zhao, L.P.; Liu, Z.; Chen, L. Generation and characterization of 24 novel EST derived microsatellite from tea plant (Camellia sinensis) and cross-species amplification in its closely related species and varieties. Conserv. Genet. 2008, 9, 1327–1331. [Google Scholar] [CrossRef]
- Seth, R.; Bhandawat, A.; Parmar, R.; Singh, P.; Kumar, S.; Sharma, R.K. Global transcriptional insights of pollen-pistil interactions commencing self-incompatibility and fertilization in tea [Camellia sinensis (L.) O. Kuntze]. Int. J. Mol. Sci. 2019, 20, 539. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Lu, M.Q.; Yu, S.S.; Liu, Y.Y.; Yang, J.; Tan, X.F. In-depth understanding of Camellia oleifera self-incompatibility by comparative transcriptome, proteome and metabolome. Int. J. Mol. Sci. 2020, 21, 1600. [Google Scholar] [CrossRef] [PubMed]
- McClure, B. New views of S-RNase-based self-incompatibility. Curr. Opin. Plant Biol. 2006, 9, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Yuan, D.Y.; Zou, F. Self-sterility in Camellia oleifera may be due to the prezygotic late-acting self-incompatibility. PLoS ONE 2014, 9, e99639. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hao, S.; Wang, L. Late-acting self-incompatibility in tea plant (Camellia sinensis). Sect. Bot. 2012, 67, 347–351. [Google Scholar] [CrossRef]
- Wachira, F.N.; Kamunya, S.K. Pseudo-self incompatibility in some tea clones (Camellia sinensis (L.) O. Kuntze). J. Hortic. Sci. Biotechnol. 2005, 80, 716–720. [Google Scholar] [CrossRef]
- Liao, T.; Yuan, D.Y.; Gao, C. Pollination, fertilization and early embryonic development of Camellia oleifera. Sci. Silvae Sin. 2014, 50, 50–55. [Google Scholar]
- Wei, H.L.; Gao, C.; Jie, Q. Flowering biological characteristics of Camellia weiningensis Y.K. Li. HortScience 2021, 56, 1331–1339. [Google Scholar] [CrossRef]
- Huo, H.L.; Zhang, R.Q. Stigma receptivity and characteristics of pollen tube growth of Corylus kweichowensis. For. Res. 2014, 27, 403–409. [Google Scholar]
- Ciampolini, F.; Cresti, M. The structure and cytochemistry of the stigma-style complex of Corylus avellana L., ‘Tonda Gentile delle Langhe’ (Corylaceae). Ann. Bot. 1998, 81, 513–518. [Google Scholar] [CrossRef][Green Version]
- Gao, Q.G.; Lei, Z.Z.; Liang, Y.F. Screening of Brassica oleracea MLPK-interacting PUB proteins based on gene family analysis. Acta Hortic. Sin. 2019, 46, 714–722. [Google Scholar]
- Heslop-Harrison, Y.; Shivanna, K.R. The receptive surface of the angiosperm stigma. Ann. Bot. 1997, 41, 1233–1258. [Google Scholar] [CrossRef]
- Hiscock, S.J.; McInnis, S.M. Pollen recognition and rejection duringthe sporophytic self-incompatibility response: Brassica and beyond. Trends Plant Sci. 2003, 8, 606–613. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.L.; Liu, W.Z. Pollination effects on the structure of tobacco’s (Nicotiana tabacum) transmitting tissue. Acta Bot. Boreali-Occident. Sin. 2016, 36, 486–492. [Google Scholar]
- Jahromi, H.A.; Zarei, A.; Mohammadkhani, A. Analysis the effects of pollen grain sources on the fruits set and their characteristics of ‘Clementine’ mandarin using microscopic and molecular approaches. Sci. Hortic. 2019, 249, 347–354. [Google Scholar] [CrossRef]
- Selak, G.V.; Cuevas, J.; Ban, S.G.; Perica, S. Pollen tube performance in assessment of compatibility in olive (Olea europaea L.) cultivars. Sci. Hortic. 2014, 165, 36–43. [Google Scholar] [CrossRef]
- Tangitcharoen, S.; Owens, J.N. Pollen viability and pollen-tube growth following controlled pollination and their relation to low fruit production in teak (Tectona grandis Linn. f.). Ann. Bot. 1997, 80, 401–410. [Google Scholar] [CrossRef]
- Pérez, V.; Herrero, M.; Hormaza, J.I. Self-fertility and preferential cross-fertilization in mango (Mangifera indica). Sci. Hortic. 2016, 213, 373–378. [Google Scholar] [CrossRef]
- Wang, G.M.; Gu, C.; Qiao, X.; Zhao, B.Y.; Ke, Y.Q.; Guo, B.B.; Hao, P.P.; Qi, K.J.; Zhang, S.L. Characteristic of pollen tube that grew into self-style in pear cultivar and parent assignment for cross-pollination. Sci. Hortic. 2017, 216, 226–233. [Google Scholar] [CrossRef]
- Mhanna, M.A.; Douay, F.; Rajab, M. Identifying the optimal pollinizer for ‘Dermlali’ olive cultivar under syrian coast conditions. Int. J. Fruit Sci. 2020, 20, 1984–1993. [Google Scholar] [CrossRef]

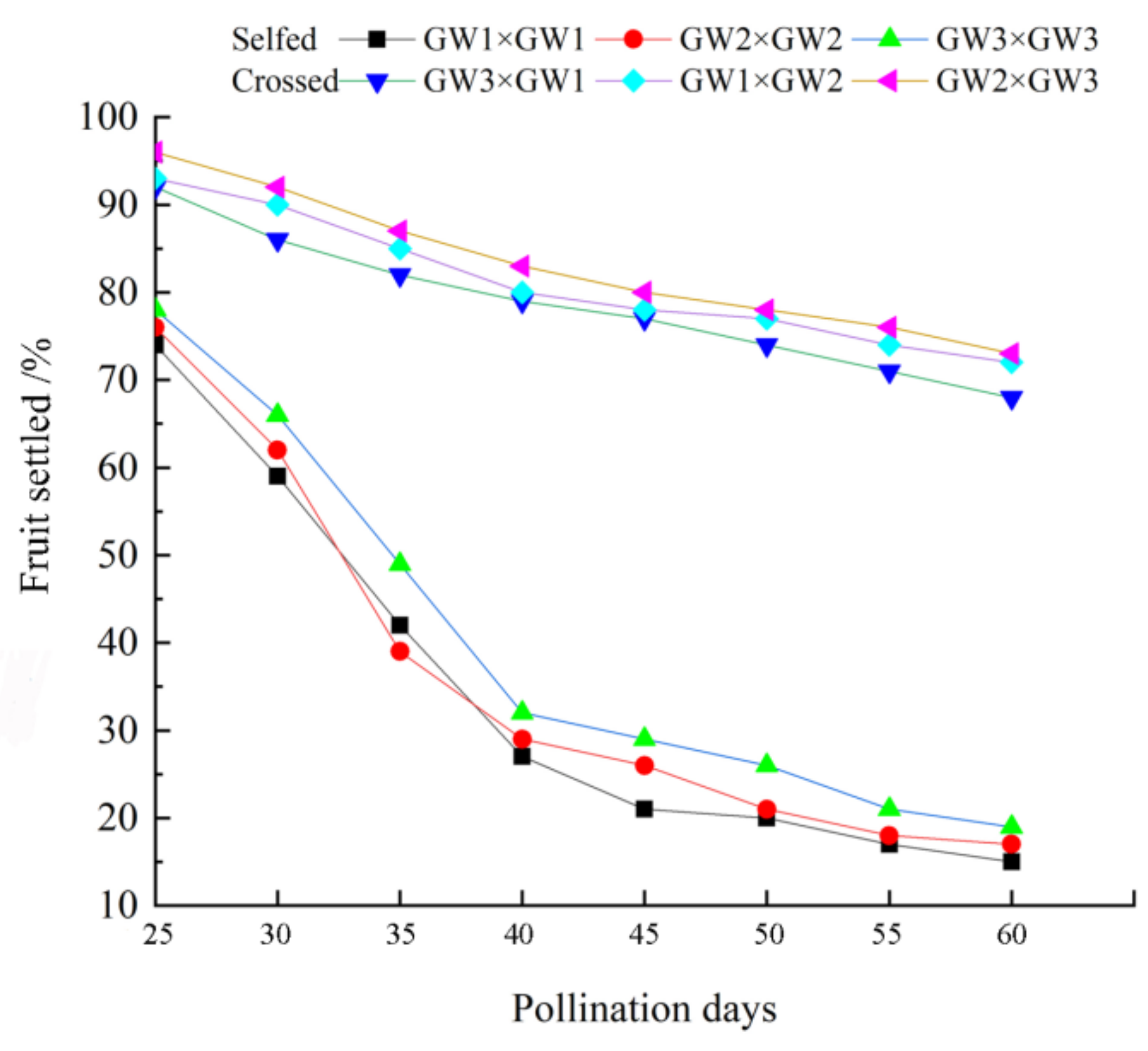
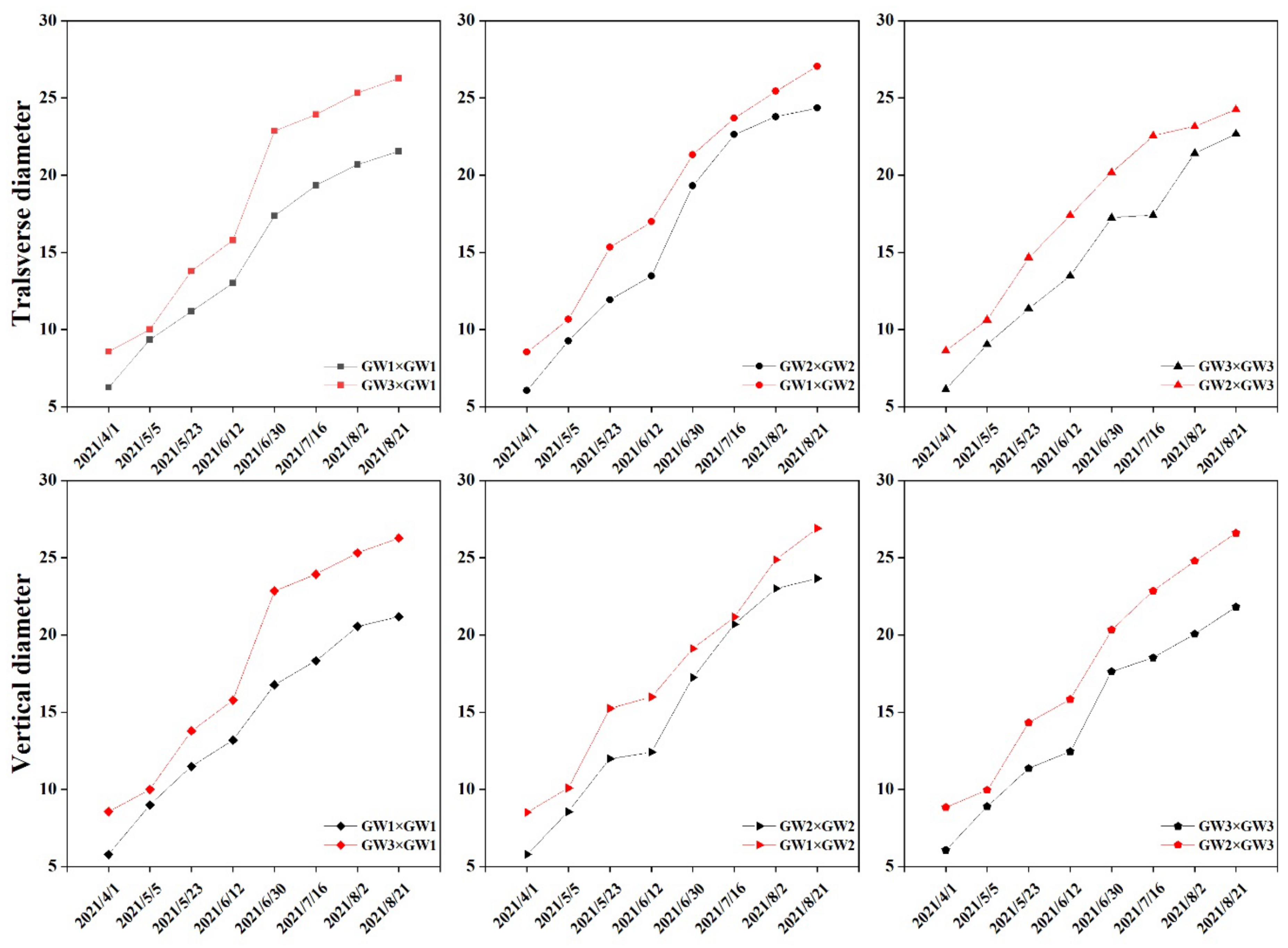
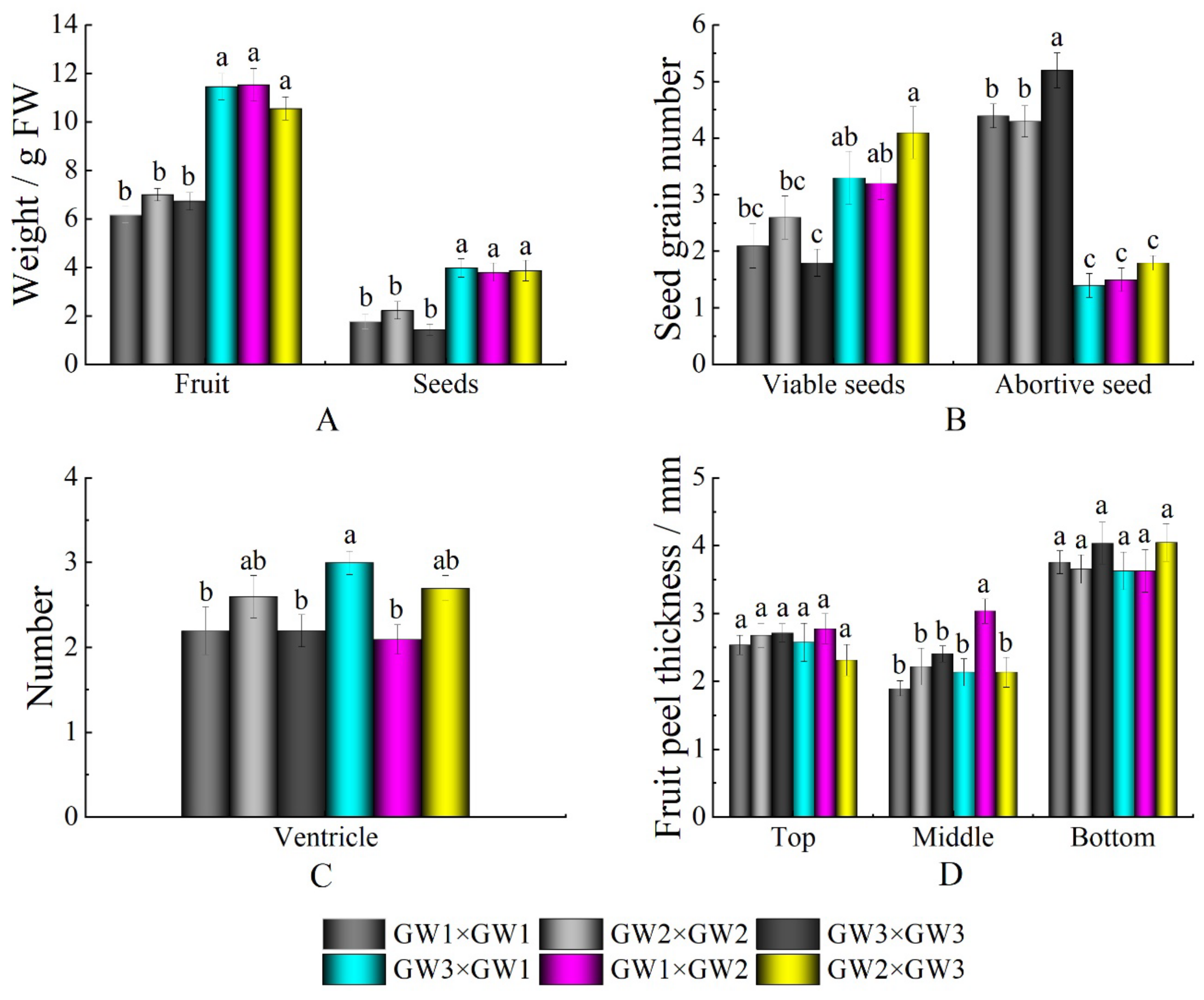
| Cross-Pollination Combination | Self-Pollination Combination |
|---|---|
| Male parent × Female parent | Male parent × Female parent |
| GW3 × GW1 | GW1 × GW1 |
| GW1 × GW2 | GW2 × GW2 |
| GW2 × GW3 | GW3 × GW3 |
| Material | Petal Length (mm) | Petal Width (mm) | Corolla Diameter (mm) | Filament Length (mm) | Style Length (mm) | Number of Stamens | Number of Ovules |
|---|---|---|---|---|---|---|---|
| GW1 | 41.21 ± 0.78b | 29.24 ± 0.65b | 71.18 ± 1.24c | 22.39 ± 0.72b | 16.58 ± 0.84ab | 78 ± 0.83b | 9 ± 0.71 |
| GW2 | 40.59 ± 1.88b | 29.57 ± 0.73b | 75.65 ± 0.90b | 19.23 ± 1.09c | 16.02 ± 0.86b | 77 ± 1.30b | 8 ± 0.71 |
| GW3 | 46.86 ± 1.18a | 31.31 ± 0.72a | 79.02 ± 1.48a | 23.75 ± 0.83a | 17.93 ± 0.66a | 81 ± 0.84a | 9 ± 1.14 |
| Pollination Combination | Pollen Tube Length (μm) | |||||
|---|---|---|---|---|---|---|
| 4 h | 12 h | 24 h | 48 h | 72 h | 96 h | |
| GW1 × GW1 | 0 | 880 ± 5.77b | 3965 ± 75.06 | 7913 ± 46.93c | 9566 ± 60.48d | 15,069 ± 39.19ab |
| GW2 × GW2 | 0 | 857 ± 8.82bc | 3935 ± 70.24c | 8407 ± 62.74c | 9605 ± 50.58d | 14,636 ± 326.67b |
| GW3 × GW3 | 0 | 832 ± 3.33c | 4150 ± 45.83c | 7907 ± 58.02c | 9075 ± 56.86e | 15,453 ± 318.35a |
| GW3 × GW1 | 770 ± 8.66a | 1044 ± 29.19a | 4800 ± 59.32b | 11,526 ± 299.82b | 16,479 ± 263.27b | 16,479 ± 263.27b |
| GW1 × GW2 | 706 ± 9.35b | 848 ± 12.92bc | 5412 ± 108.10a | 12,836 ± 470.61a | 16,019 ± 52.87c | 16,019 ± 52.87c |
| GW2 × GW3 | 700 ± 5.77b | 746 ± 7.37d | 4787 ± 75.92b | 12,485 ± 287.33ab | 17,382 ± 32.52a | 17,382 ± 32.52a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Wei, H.; Qiu, J.; Long, L.; Yang, L. Self-Incompatibility of Camellia weiningensis Y.K. Li. Horticulturae 2022, 8, 297. https://doi.org/10.3390/horticulturae8040297
Gao C, Wei H, Qiu J, Long L, Yang L. Self-Incompatibility of Camellia weiningensis Y.K. Li. Horticulturae. 2022; 8(4):297. https://doi.org/10.3390/horticulturae8040297
Chicago/Turabian StyleGao, Chao, Hongli Wei, Jie Qiu, Li Long, and Lu Yang. 2022. "Self-Incompatibility of Camellia weiningensis Y.K. Li." Horticulturae 8, no. 4: 297. https://doi.org/10.3390/horticulturae8040297
APA StyleGao, C., Wei, H., Qiu, J., Long, L., & Yang, L. (2022). Self-Incompatibility of Camellia weiningensis Y.K. Li. Horticulturae, 8(4), 297. https://doi.org/10.3390/horticulturae8040297





