Resistance Management through Brassica Crop–TuMV–Aphid Interactions: Retrospect and Prospects
Abstract
1. Introduction
2. Interactions between Brassica Crops and TuMV
3. Interactions between Brassica Crops and Aphids
3.1. Sensitive Olfactory System Facilitating Aphids Invading Brassica Crops
3.2. Two Ways for Brassica Crops’ Defense against Aphids
3.3. Special Volatiles Released after Being Attacked by Aphids
4. Interactions between TuMV and Aphids
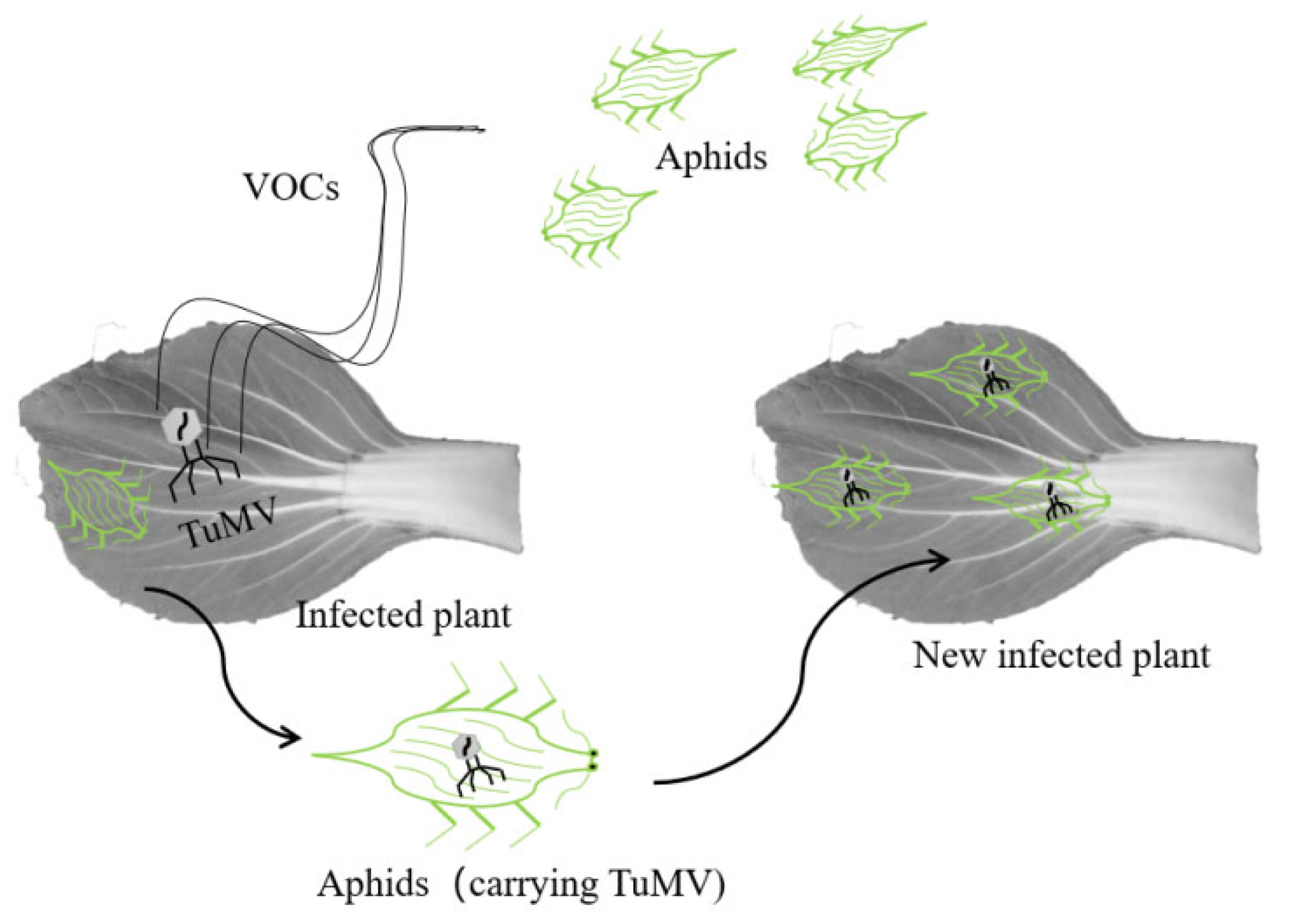
4.1. Aphids Were the Main Mode of TuMV Transmission among Brassica Crops
4.2. The Effect of Virus on Aphids
5. Brassica Crop–TuMV–Aphid Interactions
5.1. The Effect of Phytohormone on Brassica Crop–TuMV–Aphid Interactions
5.2. The Resistance Mechanism between Virus and Host Plants
5.3. The Effect of Environmental Factors on Brassica Crop–TuMV–Aphid Interactions
5.3.1. Light Conditions Affecting Brassica Crop–TuMV–Aphid Interactions
5.3.2. Temperature Affecting Brassica Crop–TuMV–Aphid Interactions
5.3.3. CO2 Concentration Affecting Brassica Crop–TuMV–Aphid Interactions
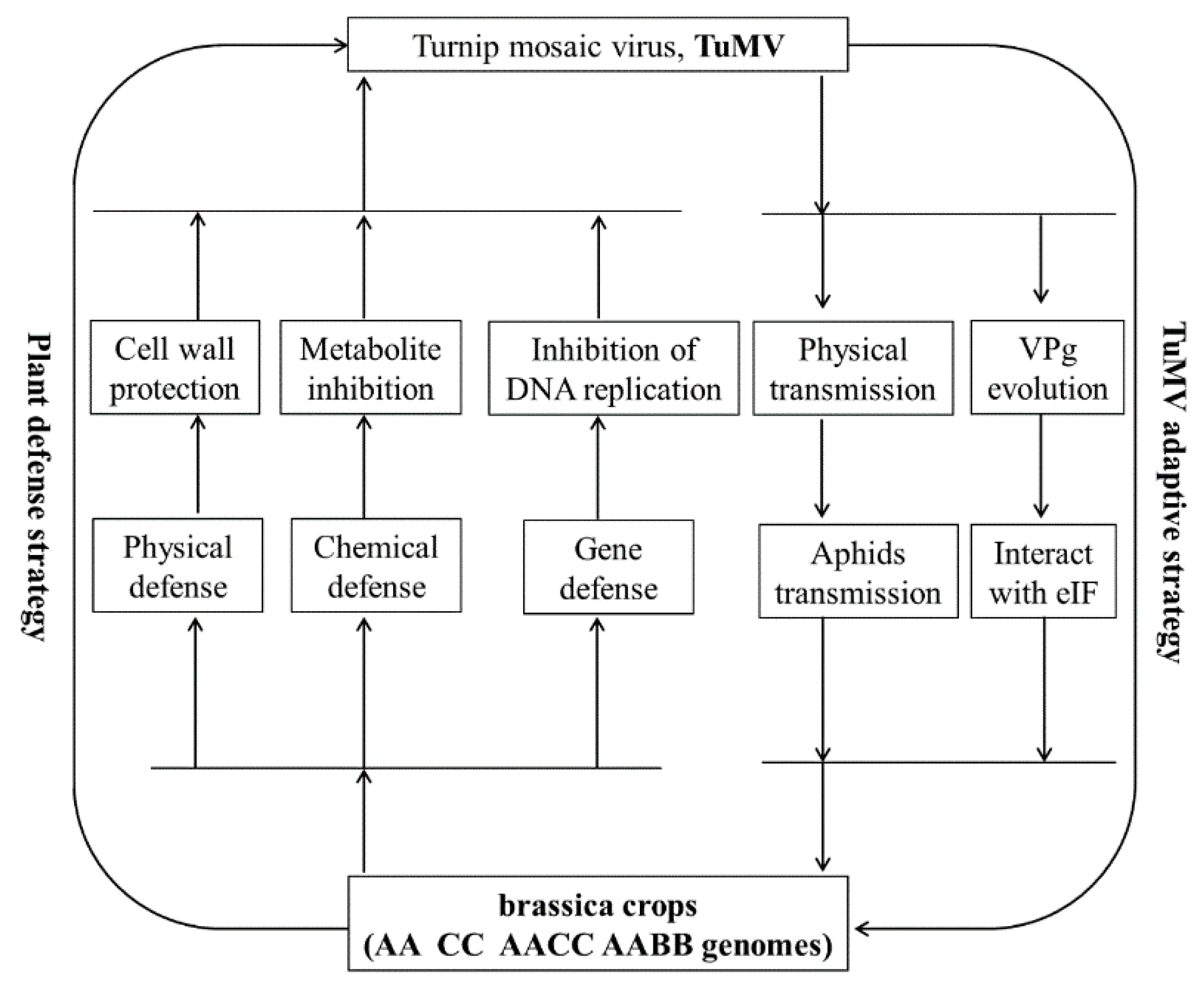
6. Prospect
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomlinson, J.A. Epidemiology and control of virus diseases of vegetables. Ann. Appl. Biol. 1987, 110, 661–681. [Google Scholar] [CrossRef]
- Hunter, P.J.; Jones, J.E.; Walsh, J.A. Involvement of Beet western yellows virus, Cauliflower mosaic virus, and Turnip mosaic virus in internal disorders of stored white cabbage. Phytopathology 2002, 92, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.A.; Jenner, C.E. Turnip mosaic virus and the quest for durable resistance. Mol. Plant Pathol. 2002, 3, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Casteel, C.L.; Hansen, A.K. Evaluating Insect-Microbiomes at the Plant-Insect Interface. J. Chem. Ecol. 2014, 40, 836–847. [Google Scholar] [CrossRef]
- Yan, F.M. Plant pathogen-insect vector interactions: Research progress and prospects. Acta Hortic. Sin. 2020, 63, 123–130. (In Chinese) [Google Scholar] [CrossRef]
- Liu, S.S.; Barro, P.J.D.; Xu, J.; Luan, J.B.; Zang, L.S.; Ruan, Y.M.; Wan, F.H. Asymmetric Mating Interactions Drive Widespread Invasion and Displacement in a Whitefly. Science 2007, 318, 1769–1772. [Google Scholar] [CrossRef]
- Li, R.; Weldegergis, B.T.; Li, J.; Jung, C.; Qu, J. Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promot evector performance. Plant Cell 2016, 26, 4991–5008. [Google Scholar] [CrossRef]
- Mauck, K.E.; Moraes, C.M.D.; Mescher, M.C. Biochemical and physiological mechanisms underlying effects of Cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant Cell Environ. 2014, 37, 1427–1439. [Google Scholar] [CrossRef]
- Li, G.L.; Lv, H.H.; Zhang, S.; Zhang, S.F.; Li, F.; Zhang, H.; Qian, W.; Fang, Z.Y.; Sun, R.F. TuMV management for brassica crops through host resistance: Retrospect and prospects. Plant Pathol. 2019, 68, 1035–1044. [Google Scholar] [CrossRef]
- Gardner, M.W.; Kendrick, J.B. Turnip mosaic. J. Agric. Res. 1921, 22, 123–124. [Google Scholar]
- Hull, R. The movement of viruses in plants. Ann. Rev. Phytopathol. 1989, 27, 2–6. [Google Scholar] [CrossRef]
- Doem, R.M.; Oliver, M.J. The 30-kilodalton gene product of TMV Potentiates virus movement. Science 1987, 237, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.A.; Sharpe, A.G.; Jenner, C.E.; Lydiate, D.J. Characterisation of resistance to turnip mosaic virus in oilseed rape (Brassica napus) and genetic mapping of TuRB01. Theor. Appl. Genet. 1999, 99, 1149–1154. [Google Scholar] [CrossRef]
- Hughes, S.L.; Hunter, P.J.; Sharpe, A.G.; Kearsey, M.J.; Lydiate, D.J.; Walsh, J.A. Genetic mapping of the novel Turnip mosaic virus resistance gene TuRB03 in Brassica napus. Theor. Appl. Genet. 2003, 107, 1169–1173. [Google Scholar] [CrossRef]
- Jenner, C.E.; Tomimura, K.; Ohshima, K.; Hughes, S.L.; Walsh, J.A. Mutations in Turnip mosaic virus P3 and cylindrical inclusion proteins are separately required to overcome two Brassica napus resistance genes. Virology 2002, 300, 50–59. [Google Scholar] [CrossRef][Green Version]
- Rusholme, R.L.; Higgins, E.E.; Walsh, J.A.; Lydiate, D.J. Genetic control of broad-spectrum resistance to Turnip mosaic virus in Brassica rapa (Chinese cabbage). J. Gen. Virol. 2007, 88, 3177–3186. [Google Scholar] [CrossRef]
- Zhang, F.L.; Wang, M.; Liu, X.C.; Zhao, X.Y.; Yang, J.P. Quantitative trait loci analysis for resistance against Turnip mosaic virus based on a doubled-haploid population in Chinese cabbage. Plant Breed. 2008, 127, 82–86. [Google Scholar] [CrossRef]
- Zhang, X.W.; Yuan, Y.X.; Wang, X.W. QTL mapping for TuMV resistance in Chinese cabbage [Brassica campestris L. ssp. pekinensis (Lour.) Olssom]. Acta Hortic. Sin. 2009, 36, 731–736. [Google Scholar]
- Ma, J.F.; Hou, X.L.; Xiao, D.; Li, Q.; Wang, F. Cloning and characterization of the BcTUR3, gene related to resistance to Turnip mosaic virus (TuMV) from non-heading Chinese cabbage. Plant Mol. Biol. Rep. 2010, 28, 588–596. [Google Scholar] [CrossRef]
- Fujiwara, A.; Inukai, T.; Kim, B.M.; Chikara, M. Combinations of a host resistance gene and the CI gene of Turnip mosaic virus differentially regulate symptom expression in Brassica rapa cultivars. Arch. Virol. 2011, 156, 1575–1581. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, Y.; Chen, H.Y. A linkage map of pak-choi (Brassica rapa ssp. chinensis) based on AFLP and SSR markers and identification of AFLP markers for resistance to TuMV. Plant Breed. 2011, 130, 275–277. [Google Scholar]
- Qian, W.; Zhang, S.J.; Zhang, S.F.; Li, F.; Zhang, H.; Wu, J.; Wang, X.W.; Walsh, J.A.; Sun, R.F. Mapping and candidate-gene screening of the novel Turnip mosaic virus resistance gene retr02 in Chinese cabbage (Brassica rapa L.). Theor. Appl. Genet. 2013, 126, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, W.H.; Hwang, J.; Yang, H.; Dosun, K.; Oh, C.S.; Kang, B.C. Transgenic Brassica rapa plants over-expressing eIF(iso)4E variants show broad-spectrum Turnip mosaic virus (TuMV) resistance. Mol. Plant Pathol. 2014, 15, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Jeong, Y.M.; Mun, J.H.; Lee, S.S.; Chung, W.H.; Yu, H.J. Construction of a genetic map based on high-throughput SNP genotyping and genetic mapping of a TuMV resistance locus in Brassica rapa. Mol. Genet. Genom. 2014, 289, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Lydiate, D.J.; Pilcher, R.L.; Higgins, E.E.; Walsh, J.A. Genetic control of immunity to Turnip mosaic virus (TuMV) pathotype 1 in Brassica rapa (Chinese cabbage). Genome 2014, 57, 419–425. [Google Scholar] [CrossRef]
- Jin, M.; Lee, S.S.; Ke, L.; Kim, J.S.; Seo, M. Identification and mapping of a novel dominant resistance gene, TuRB07 to Turnip mosaic virus in Brassica rapa. Theor. Appl. Genet. 2014, 127, 127,509–519. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Zeng, Q.; Zhang, Z.; Liu, S.; Pei, Y.; Wang, S.; Liu, X.; Xu, W.; Fu, W.; et al. Identification and mapping of a novel Turnip mosaic virus resistance gene TuRBCS01 in Chinese cabbage (Brassica rapa L.). Plant Breed. 2015, 134, 221–225. [Google Scholar] [CrossRef]
- Nyalugwe, E.P.; Barbetti, M.J.; Jones, R.A.C. Preliminary studies on resistance phenotypes to Turnip mosaic virus, in B. napus, and B. carinata, from different continents and effects of temperature on their expression. Eur. J. Plant Pathol. 2014, 139, 687–706. [Google Scholar] [CrossRef]
- Nyalugwe, E.P.; Barbetti, M.J.; Jones, R.A.C. Studies on resistance phenotypes to Turnip mosaic virus, in five species of Brassicaceae, and identification of a virus resistance gene in Brassica juncea. Eur. J. Plant Pathol. 2015, 141, 647–666. [Google Scholar] [CrossRef]
- Shopan, J.; Mou, H.; Zhang, L.L.; Zhang, C.T.; Ma, W.W.; Walsh, J.A. Eukaryotic translation initiation factor 2B-beta (eIF 2Bβ), a new class of plant virus resistance gene. Plant J. 2017, 90, 929–940. [Google Scholar] [CrossRef]
- Kang, B.C.; Yeam, I.; Frantz, J.D.; Murphy, J.F.; Jahn, M.M. The pvr1 locus in Capsicum encodes a translation initiation factor eIF4E that interacts with Tobacco etch virus VPg. Plant J. 2005, 42, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Lellis, A.D.; Kasschau, K.D.; Whitham, S.A.; Carrington, J.C. Loss-of-susceptibility mutants of Arabidopsis thaliana reveal an essential role for eIF(iso)4E during potyvirus infection. Curr. Biol. 2002, 12, 1046–1051. [Google Scholar] [CrossRef]
- Joshi, B.; Lee, K.; Maeder, D.L.; Jagus, R. Phylogenetic analysis of eIF4E-family members. BMC Evol Biol. 2005, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Mayberry, L.K.; Allen, M.L.; Dennis, M.D.; Browning, K.S. Evidence for variation in the optimal translation initiation complex: Plant eIF4B, eIF4F, and eIF(iso)4F differentially promote translation of mRNAs. Plant Physiol. 2009, 150, 1844–1854. [Google Scholar] [CrossRef]
- Jenner, C.E.; Nellist, C.F.; Barker, G.C.; Walsh, J.A. Turnip mosaic virus (TuMV) is able to use alleles of both eIF4E and eIF(iso)4E from multiple loci of the diploid Brassica rapa. Mol. Plant Microbe Interact 2010, 23, 1498–1505. [Google Scholar] [CrossRef]
- Li, G.L.; Zhang, S.F.; Li, F.; Zhang, H.; Zhang, S.J.; Zhao, J.J.; Sun, R.F. Variability in the Viral Protein Linked to the Genome of Turnip Mosaic Virus Influences Interactions with eIF(iso)4Es in Brassica rapa. Plant Pathol. J. 2021, 37, 47–56. [Google Scholar] [CrossRef]
- Li, G.L.; Yue, L.X.; Li, F.; Zhang, S.F.; Zhang, H.; Qian, W.; Fang, Z.Y.; Wu, J.; Wang, X.W.; Zhang, S.J.; et al. Research Progress on Agrobacterium tumefaciens-based Transgenic Technology in Brassica rapa. Hortic. Plant J. 2018, 4, 126–132. [Google Scholar] [CrossRef]
- Knolhoff, L.M.; Heckel, D.G. Behavioral assays for studies of host plant choice and adaptation in herbivorous insects. Annu. Rev. Entomol. 2014, 59, 263–278. [Google Scholar] [CrossRef]
- Natalia, D.; Florence, N.; Dinesh, A.; Nagegowda, I.O. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar]
- Hatanaka, A. The biogeneration of green odour by green leaves. Pergamon 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2018, 220, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jin, Y.; Liu, J.; Tang, Y.; Cao, S.; Qi, H. The phylogeny and expression profiles of the lipoxygenase (LOX) family genes in the melon (Cucumis melo L.) genome. Sci. Hortic. 2014, 170, 94–102. [Google Scholar] [CrossRef]
- Sanfaçon, H. Plant translation factors and virus resistance. Viruses 2015, 7, 3392–3419. [Google Scholar] [CrossRef] [PubMed]
- Jassy, D.; Olubukola, A.; Ranjan, S.; Toby, B.; Rumiana, V.R. Aphid Infestation Increases Fusarium langsethiae and T-2 and HT-2 Mycotoxins in Wheat. Appl. Environ. Microbiol. 2016, 82, 6548–6556. [Google Scholar]
- Boissot, N.; Thomas, S.; Chovelon, V.; Lecoq, H. NBS-LRR-mediated resistance triggered by aphids: Viruses do not adapt; aphids adapt via different mechanisms. BMC Plant Biol. 2016, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Clement, S.L. Molecular Bases of Plant Resistance to Arthropods. Annu. Rev. Entomol. 2012, 57, 309–328. [Google Scholar] [CrossRef]
- Santamaria, M.E.; Martínez, E.; Cambra, I.; Grbic, V.; Diaz, I. Understanding plant defence responses against herbivore attacks: An essential first step towards the development of sustainable resistance against pests. Transgenic Res. 2013, 22, 697–708. [Google Scholar] [CrossRef]
- Müller, C.; Riederer, M. Plant Surface Properties in Chemical Ecology. J. Chem. Ecol. 2005, 31, 2621–2651. [Google Scholar] [CrossRef]
- Werker, E. Trichome diversity and development. Adv. Bot. Res. 2000, 31, 1–35. [Google Scholar]
- Simmons, A.T.; Gurr, G.M. Trichome-based host plant resistance of Lycopersicon species and the biocontrol agent Mallada signata: Are they compatible? Entomol. Exp. Appl. 2004, 113, 95–101. [Google Scholar] [CrossRef]
- Broekgaarden, C.; Snoeren, T.A.; Dicke, M.; Vosman, B. Exploiting natural variation to identify insect-resistance genes. Plant Biotechnol. J. 2011, 9, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Vanetten, H.D.; Mansfield, J.W.; Farmer, B. Two Classes of Plant Antibiotics: Phytoalexins versus “Phytoanticipins”. Plant Cell 1994, 6, 1191–1192. [Google Scholar] [CrossRef] [PubMed]
- Browne, L.M.; Conn, K.L.; Ayert, W.A.; Tewari, J.P. The camalexins: New phytoalexins produced in the leaves of camelina sativa (cruciferae). Tetrahedron 1991, 47, 3909–3914. [Google Scholar] [CrossRef]
- Ferrari, S.; Galletti, R.; Denoux, C.; Lorenzo, G.D.; Ausubel, F.M.; Dewdney, J. Resistance to botrytis cinerea induced in arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires phytoalexin deficient3. Plant Physiol. 2007, 144, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Sellam, A.; Iacomi-Vasilescu, B.; Hudhomme, P.; Simoneau, P. In vitro antifungal activity of brassinin, camalexin and two isothiocyanates against the crucifer pathogens alternaria brassicicola and alternaria brassicae. Plant Pathol. 2010, 56, 296–301. [Google Scholar] [CrossRef]
- Kuśnierczyk, A.; Winge, P.; Jrstad, T.S.; Troczyska, J.; Rossiter, J.T.; Bones, A.M. Towards global understanding of plant defence against aphids–timing and dynamics of early arabidopsis defence responses to cabbage aphid (Brevicoryne brassicae) attack. Plant Cell Environ. 2008, 31, 1097–1115. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Turlings, T.C.J. Advances and challenges in the identification of volatiles that mediate interactions among plants and arthropods. Analyst 2006, 131, 24–32. [Google Scholar] [CrossRef]
- Schuman, M.C.; Heinzel, N.; Gaquerel, E.; Svatos, A.; Baldwin, I.T. Polymorphism in Jasmonate Signaling Partially Accounts for the Variety of Volatiles Produced by Nicotiana Attenuata Plants in a Native Population. New Phytol. 2009, 183, 1134–1148. [Google Scholar] [CrossRef]
- Snoeren, T.A.L.; Kappers, I.F.; Broekgaarden, C.; Mumm, R.; Dicke, M.; Bouwmeester, H.J. Natural variation in herbivore-induced volatiles in Arabidopsis thaliana. J. Exp. Bot. 2010, 61, 3041–3056. [Google Scholar] [CrossRef]
- Webster, B. The role of olfaction in aphid host location. Physiol. Entomol. 2012, 37, 10–18. [Google Scholar] [CrossRef]
- Visser, J.H. Host Odor Perception in Phytophagous Insects. Annu. Rev. Entomol. 1986, 31, 121–144. [Google Scholar] [CrossRef]
- Fereres, A.; Peñaflor, M.; Favaro, C.; Azevedo, K.; Landi, C.; Maluta, N.; Bento, J.; Lopes, J. Tomato Infection by Whitefly-Transmitted Circulative and Non-Circulative Viruses Induce Contrasting Changes in Plant Volatiles and Vector Behaviour. Viruses 2016, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.S.; Wu, Y.; Eigenbrode, S.D. The Effects of Bean Leafroll Virus on Life History Traits and Host Selection Behavior of Specialized Pea Aphid (Acyrthosiphon pisum, Hemiptera: Aphididae) Genotypes. Environ. Entomol. 2017, 46, 68–74. [Google Scholar]
- Weeraddana, C.D.S.; Manobii, V.P.; Strelkov, S.E.; Mata, A.P.L.; Harynuk, J.J.; Evenden, M.L. Infection of canola by the root pathogen plasmodiophora brassicae increases resistance to aboveground herbivory by bertha armyworm, mamestra configurata walker (lepidoptera: Noctuidae). Plant Sci. 2020, 300, 110625. [Google Scholar] [CrossRef]
- Hare, J.D. Ecological Role of Volatiles Produced by Plants in Response to Damage by Herbivorous Insects. Annu. Rev. Entomol. 2011, 56, 161–180. [Google Scholar] [CrossRef]
- Danner, H.; Brown, P.; Cator, E.A.; Harren, F.J.M.; Van, D.N.M.; Cristescu, S.M. Aboveground and Belowground Herbivores Synergistically Induce Volatile Organic Sulfur Compound Emissions from Shoots but Not from Roots. J. Chem. Ecol. 2015, 41, 631–640. [Google Scholar] [CrossRef]
- Read, D.P.; Feeny, P.P.; Root, R.B. Habit selection by the aphid parasite Diaeretiella rapae (hymenopter: Brassicae) and hyperparasite charips brassicae (hymenoptera: Cynipidae). Can. Entomol. 1970, 102, 1567–1578. [Google Scholar] [CrossRef]
- Niinemets, U.; Loreto, F.; Reichstein, M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 2004, 9, 180–186. [Google Scholar] [CrossRef]
- Blaakmeer, A.; Geervliet, J.; Geervliet, J.B.F.; Van, L.J.J.A.; Posthumus, M.A.; Van, B.T.A.; De, G.A. Comparative headspace analysis of cabbage plants damaged by two species of Pieris caterpillars: Consequences for in-flight host location by Cotesia parasitoids. Entomol. Exp. Appl. 1994, 73, 175–182. [Google Scholar] [CrossRef]
- Meiners, T.; Hilker, M. Induction of plant synomones by oviposition of a phytophagous insect. J. Chem. Ecol. 2000, 26, 221–232. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Matthias, E. Tritrophic Interactions Mediated by Herbivore-Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2011, 410, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.A.M.; Erb, M.; Duployer, M.; Zwahlen, C.; Doyen, G.R.; Turlings, T.J.C. Herbivore-induced plant volatiles mediate host selection by a root herbivore. New Phytol. 2012, 194, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.Y.; Hu, X.Y.; Peng, Y.F.; Wu, K.M.; Romeis, J.; Li, Y.H. Bt rice plants may protect neighbouring non-Bt rice plants against the striped stem borer, Chilo suppressalis. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181283. [Google Scholar] [CrossRef]
- Halitschke, R.; Stenberg, J.A.; Kessler, D.; Kessler, A.; Baldwin, I.T. Shared signals—‘alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecol. Lett. 2008, 11, 24–34. [Google Scholar] [CrossRef]
- Allmann, S.; Baldwin, I.T. Insects Betray Themselves in Nature to Predators by Rapid Isomerization of Green Leaf Volatiles. Science 2010, 329, 1075–1078. [Google Scholar] [CrossRef]
- Ye, M.; Veyrat, N.; Xu, H.; Hu, L.F.; Turlings, T.C.J.; Erb, M. An herbivore-induced plant volatile reduces parasitoid attraction by changing the smell of caterpillars. Sci. Adv. 2018, 4, eaar4767. [Google Scholar] [CrossRef]
- Karban, R.; Yang, L.H.; Edwards, K.F. Volatile communication between plants that affects herbivory: A meta-analysis. Ecol. Lett. 2014, 17, 44–52. [Google Scholar] [CrossRef]
- Ali, M.; Sugimoto, K.; Ramadan, A.; Arimura, G. Memory of plant communications for priming anti-herbivore responses. Sci Rep. 2013, 3, 1642–1649. [Google Scholar] [CrossRef]
- Ingwell, L.L.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Plant viruses alter insect behavior to enhance their spread. Sci Rep. 2012, 2, 578. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D.; Bosque-Pérez, N.A.; Davis, T.S. Insect-Borne Plant Pathogens and Their Vectors: Ecology, Evolution, and Complex Interactions. Annu. Rev. Entomol. 2018, 63, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Johansen, E.; Martin, R.R.; Hampton, R.O. Potyvirus Genome-Linked Protein (VPg) Determines Pea Seed-Borne Mosaic Virus Pathotype-Specific Virulence in Pisum sativum. Mol. Plant Microbe Interact. 1998, 11, 124–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ng, J.C.K.; Zhou, J.S. Insect vector-plant virus interactions associated with non-circulative, semi-persistent transmission: Current perspectives and future challenges. Curr. Pin. Virol. 2015, 15, 48–55. [Google Scholar] [CrossRef]
- Weeraddana, C.D.S.; Kandasamy, S.; Cutler, G.C.; Shukla, P.S.; Critchley, A.T.; Prithiviraj, B. An alkali-extracted biostimulant prepared from Ascophyllum nodosum alters the susceptibility of Arabidopsis thaliana to the green peach aphid. J. Appl. Phycol. 2021, 33, 3319–3329. [Google Scholar] [CrossRef]
- Mauck, K.E. Variation in virus effects on host plant phenotypes and insect vector behavior: What can it teach us about virus evolution? Curr. Opin. Virol. 2016, 21, 114–123. [Google Scholar] [CrossRef]
- Mauck, K.E.; Moraes, C.M.D.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef]
- Lu, X.X.; Zhang, L.; Huang, W.Y.; Zhang, S.J.; Zhang, S.F.; Li, F.; Zhang, H.; Sun, R.F.; Zhao, J.J.; Li, G.L. Integrated Volatile Metabolomics and Transcriptomics Analyses Reveal the Influence of Infection TuMV to Volatile Organic Compounds in Brassica rapa. Horticulturae 2022, 8, 57. [Google Scholar] [CrossRef]
- Mauc, K.K.; Bosque-Perez, N.A.; Eigenbrode, S.D.; Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host-vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Casteel, C.L.; Yang, C.L.; Nanduri, A.C.; Jong, H.N.D.; Whitham, S.A.; Jander, G. The NIa-Pro protein of Turnip mosaic virus improves growth and reproduction of the aphid vector, Myzus persicae (green peach aphid). Plant J. 2014, 77, 653–663. [Google Scholar] [CrossRef]
- Tamara, D.C.; James, N.C. The impact of phytohormones on virus infection and disease. Curr. Opin. Virol. 2016, 17, 25–31. [Google Scholar]
- Guo, H.J.; Gu, L.Y.; Liu, F.Q.; Chen, F.J.; Ge, F.; Sun, Y.C. Aphid-borne Viral Spread Is Enhanced by Virus-induced Accumulation of Plant Reactive Oxygen Species. Plant Physiol. 2019, 179, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Alazem, M.; Lin, N.S. Roles of plant hormones in the regulation of host-virus interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Holly, D.; Christoph, R.; Fouad, D. Signaling cross-talk in plant disease resistance. Plant Sci. 2013, 207, 79–87. [Google Scholar]
- Alexandre, R.; Murray, G.; Jonathan, D.G.J. Hormone Crosstalk in Plant Disease and Defense: More Than Just JASMONATE-SALICYLATE Antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar]
- Thompson, G.A.; Goggin, F.L. Transcriptomics and functional genomics of plant defence induction by phloem-feeding insects. J. Exp. Bot. 2006, 57, 755–766. [Google Scholar] [CrossRef]
- Pegadaraju, V.; Louis, J.; Singh, V.; Reese, J.C.; Bautor, J.; Feys, B.J.; Cook, G.; Parker, J.E.; Shah, J. Phloem-based resistance to green peach aphid is controlled by Arabidopsis Phytoalexin Deficient4 without its signaling partner Enhanced Disease Susceptibility1. Plant J. 2007, 52, 332–341. [Google Scholar] [CrossRef]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf Whitefly Induces Salicylic Acid Defenses and Suppresses Effectual Jasmonic Acid Defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef]
- Sugio, A.; Kingdom, H.N.; MacLean, A.M.; Grieve, V.M.; Hogenhout, S.A. Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proc. Natl. Acad. Sci. USA 2011, 108, E1254–E1263. [Google Scholar] [CrossRef]
- Casteel, C.L.; De, A.M.; Bak, A.; Dong, H.; Whitham, S.A.; Jander, G. Disruption of ethylene responses by Turnip mosaic virus mediates suppression of plant defense against the green peach aphid vector. Plant Physiol. 2015, 169, 209–218. [Google Scholar] [CrossRef]
- Wu, D.W.; Qi, T.C.; Li, W.X.; Tian, H.X.; Gao, H. Viral effector protein manipulates host hormone signaling to attract insect vectors. Cell Res. 2017, 27, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Matthias, E.; Stefan, M.; Gregg, A.H. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar]
- Pieterse, C.; Does, D.; Zamioudis, C.; Leon-Reyes, A.; Wees, S. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting Mechanisms of Defense Against Biotrophic and Necrotrophic Pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Carr, J.P.; Lewsey, M.G.; Palukaitis, P. Signaling in Induced Resistance. Adv. Virus Res. 2010, 76, 57–121. [Google Scholar]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Ent, S.; Wees, S.; Pieterse, C. Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 2009, 70, 1581–1588. [Google Scholar]
- Lewsey, M.G.; Murphy, A.M.; Maclean, D.; Dalchau, N.; Westwood, J.H.; Macaulay, K.; Bennett, M.H.; Moulin, M.; Hanke, D.E.; Powell, G.; et al. Disruption of two defensive signaling pathways by a viral RNA silencing suppressor. Mol. Plant Microbe Interact. 2010, 23, 835–845. [Google Scholar] [CrossRef]
- Wei, T.Y.; Zhang, C.W.; Hong, J.; Xiong, R.Y.; Kasschau, K.D.; Zhou, X.P.; Carrington, J.C.; Wang, A.M. Formation of complexes at plasmodesmata for potyvirus intercellular movement is mediated by the viral protein P3N-PIPO. PLoS Pathog. 2010, 6, e1000962. [Google Scholar] [CrossRef]
- Dáder, B.; Fereres, A.; Moreno, A.; Trębicki, P. Elevated CO2 impacts bell pepper growth with consequences to Myzus persicae life history, feeding behaviour and virus transmission ability. Sci. Rep. 2016, 6, 19120. [Google Scholar] [CrossRef] [PubMed]
- Casteel, C.L.; Jander, G. New Synthesis: Investigating Mutualisms in Virus-Vector Interactions. J. Chem. Ecol. 2013, 39, 809. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, K.; Peng, J.J.; Zhao, J.P.; Jiang, L.L.; Lu, Y.W.; Zheng, H.Y.; Lin, L.; Chen, J.P.; Yan, F. NbALD1 mediates resistance to turnip mosaic virus by regulating the accumulation of salicylic acid and the ethylene pathway in Nicotiana benthamiana. Mol. Plant Pathol. 2019, 20, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Ganeshamoorthi, P.T.; Pandiaraj, P. Potential impacts of recent climate change on biological control agents in agro-ecosystem: A review. Annu. Rev. Ecol. Syst. 2013, 5, 845–852. [Google Scholar]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef]
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Prasch, C.M.; Sonnewald, U. Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol. 2013, 162, 1849–1866. [Google Scholar] [CrossRef]
- Roberts, M.R.; Paul, N.D. Seduced by the dark side: Integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol. 2006, 170, 677–699. [Google Scholar] [CrossRef]
- Faigon-Soverna, A.; Harmon, F.G.; Storani, L.; Karayekov, E.; Staneloni, R.J.; Walter, G.; Paloma, M.; Casal, J.J.; Kay, S.A.; Yanovsky, M.J. A Constitutive Shade-Avoidance Mutant Implicates TIR-NBS-LRR Proteins in Arabidopsis Photomorphogenic Development. Plant Cell 2016, 18, 2919–2928. [Google Scholar] [CrossRef]
- Wu, J.Q.; Baldwin, I.T. New Insights into Plant Responses to the Attack from Insect Herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Ibarra-Laclette, E.; Adame-Álvarez, R.M.; Martínez, O.; Ramirez-Chávez, E.; Molina-Torres, J.; Herrera-Estrella, L. How Plants Sense Wounds: Damaged-Self Recognition Is Based on Plant-Derived Elicitors and Induces Octadecanoid Signaling. PLoS ONE. 2012, 7, e30537. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, G. Perception of insect feeding by plants. Plant Biol. 2012, 14, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Mazza, C.A.; Izaguirre, M.M.; Zavala, J.; Scopel, A.L.; Ballaré, C.L. Insect perception of ambient ultraviolet-B radiation. Ecol. Lett. 2002, 5, 722–726. [Google Scholar] [CrossRef]
- Yang, C.S. Progress in aphid transmission and control of Chinese cabbage virus disease. Tianjin Agric. Sci. 1993, 37–40. [Google Scholar]
- Honjo, M.N.; Emura, N.; Kawagoe, T.; Sugisaka, J.; Kudoh, H. Seasonality of interactions between a plant virus and its host during persistent infection in a natural environment. ISME J. 2019, 14, 506–518. [Google Scholar] [CrossRef]
- Kangasjärvi, J.; Jaspers, P.; Kollist, H. Signalling and cell death inozone-exposed plants. Plant Cell Env. 2005, 28, 1021–1036. [Google Scholar] [CrossRef]
- Sun, Y.C.; Guo, H.J.; Zhu-Salzman, K.; Feng, G. Elevated CO2 increases the abundance of the peach aphid on Arabidopsis by reducing jasmonic acid defenses. Plant Sci. 2013, 210, 128–140. [Google Scholar] [CrossRef]
- Zavala, J.A.; Nabity, P.D.; DeLucia, E.H. An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annu. Rev. Entomol. 2013, 58, 79–97. [Google Scholar] [CrossRef]
- Baltes, N.J.; Hummel, A.W.; Konecna, E.; Cegan, R.; Bruns, A.N.; Bisaro, D.M.; Voytas, D.F. Conferring resistance to geminiviruses with the CRISPR–Cas prokaryotic immune system. Nat. Plants 2015, 1, 15145. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.; Ali, Z.; Butt, H.; Mahas, A.; Aljedaani, F.; Khan, M.Z.; Ding, S.; Mahfouz, M. RNA virus interference via CRISPR/Cas13a system in plants. Genome Biol. 2018, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zheng, Q.; Yi, X.; An, H.; Zhao, Y.; Ma, S.; Zhou, G. Establishing RNA virus resistance in plants by harnessing CRISPR immune system. Plant Biotechnol. J. 2018, 16, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.; Ohshima, K. Potyviruses and the digital revolution. Annu. Rev. Phytopathol. 2010, 48, 205–223. [Google Scholar] [CrossRef]
- Mahas, A.; Stewart, C.N.; Mahfouz, M.M. Harnessing CRISPR/Cas systems for programmable transcriptional and post-transcriptional regulation. Biotechnol. Adv. 2017, 36, 295–310. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Huang, W.; Zhang, S.; Li, F.; Zhang, H.; Sun, R.; Li, G.; Zhang, S. Resistance Management through Brassica Crop–TuMV–Aphid Interactions: Retrospect and Prospects. Horticulturae 2022, 8, 247. https://doi.org/10.3390/horticulturae8030247
Lu X, Huang W, Zhang S, Li F, Zhang H, Sun R, Li G, Zhang S. Resistance Management through Brassica Crop–TuMV–Aphid Interactions: Retrospect and Prospects. Horticulturae. 2022; 8(3):247. https://doi.org/10.3390/horticulturae8030247
Chicago/Turabian StyleLu, Xinxin, Wenyue Huang, Shifan Zhang, Fei Li, Hui Zhang, Rifei Sun, Guoliang Li, and Shujiang Zhang. 2022. "Resistance Management through Brassica Crop–TuMV–Aphid Interactions: Retrospect and Prospects" Horticulturae 8, no. 3: 247. https://doi.org/10.3390/horticulturae8030247
APA StyleLu, X., Huang, W., Zhang, S., Li, F., Zhang, H., Sun, R., Li, G., & Zhang, S. (2022). Resistance Management through Brassica Crop–TuMV–Aphid Interactions: Retrospect and Prospects. Horticulturae, 8(3), 247. https://doi.org/10.3390/horticulturae8030247





