Effects of Metribuzin Herbicide on Some Morpho-Physiological Characteristics of Two Echinacea Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Plant Material
2.2. Leaf Area and Growth Parameters
2.3. Malondialdehyde (MDA) Content
2.4. Chlorophyll Fluorescence
2.5. Statistical Analysis
3. Results
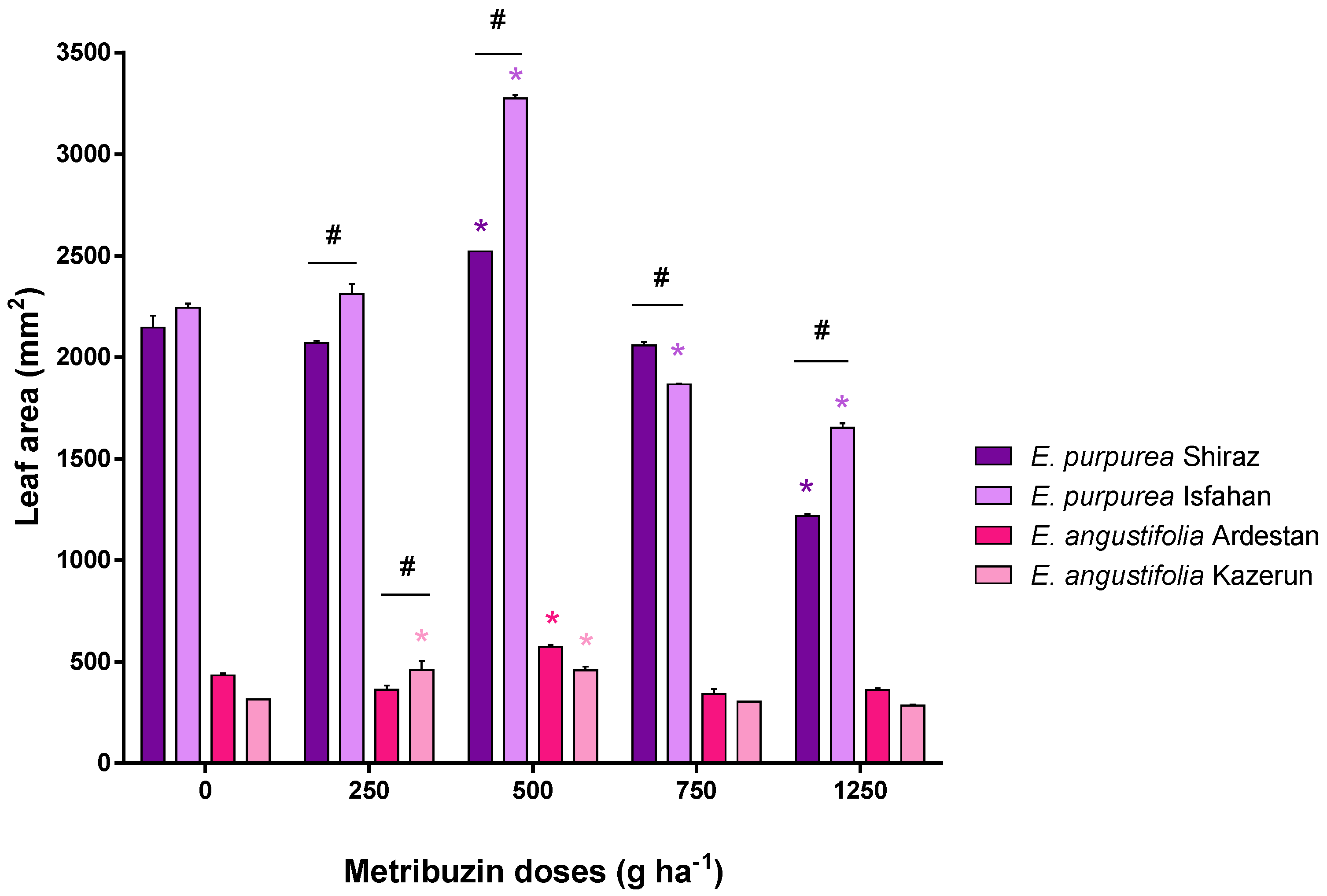
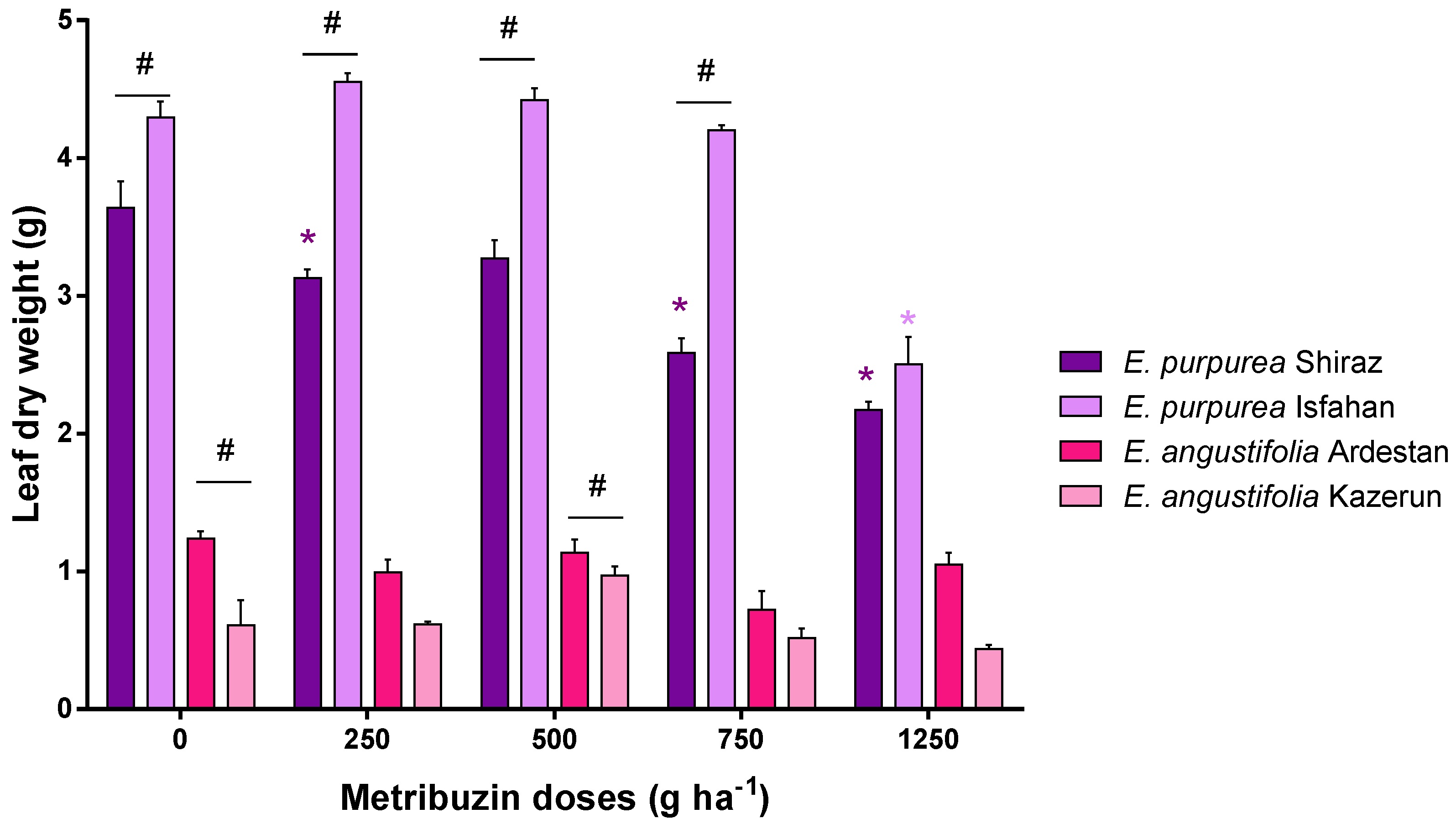
3.1. Leaf Parameters: Leaf Area and Leaf Dry Weight
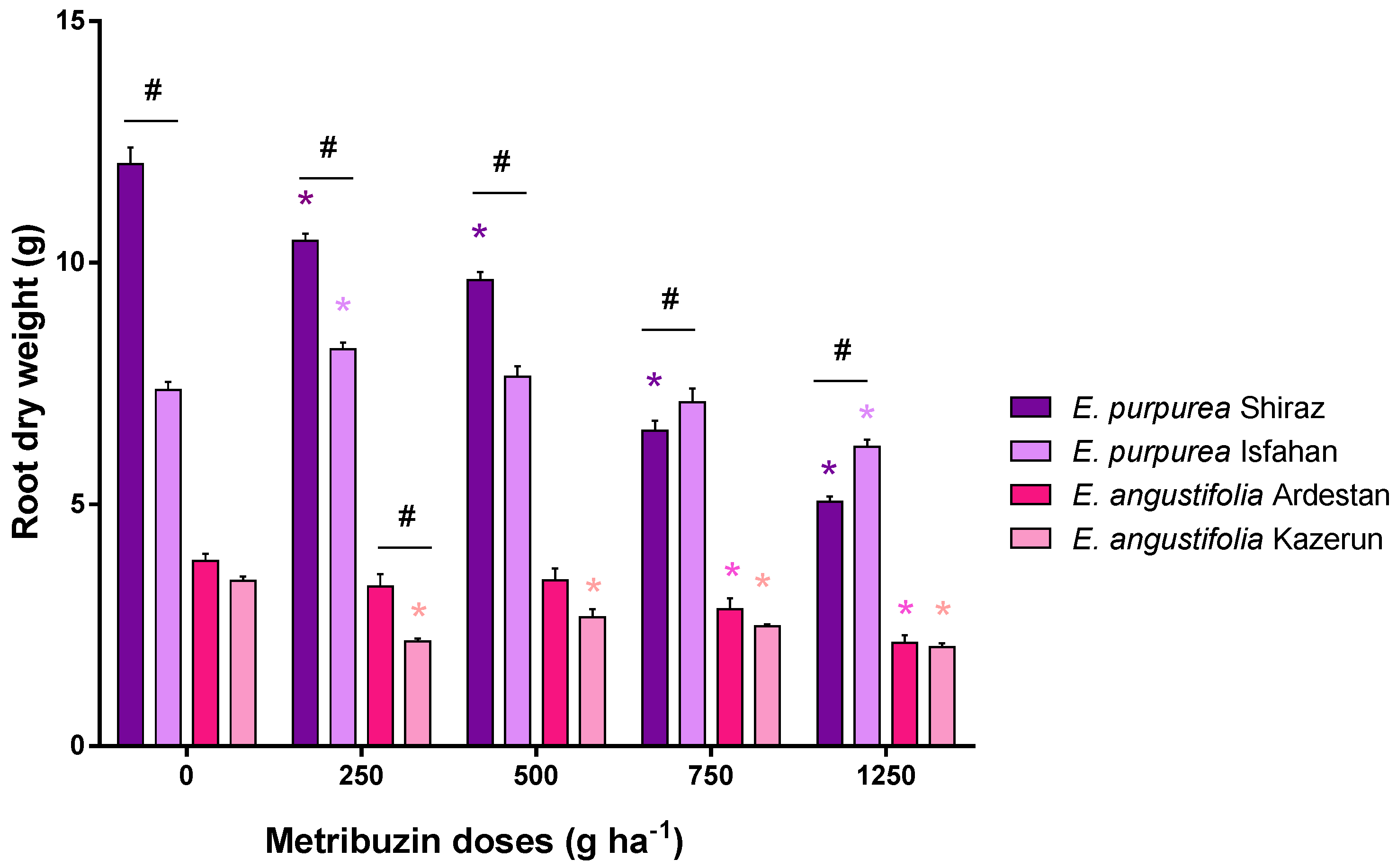
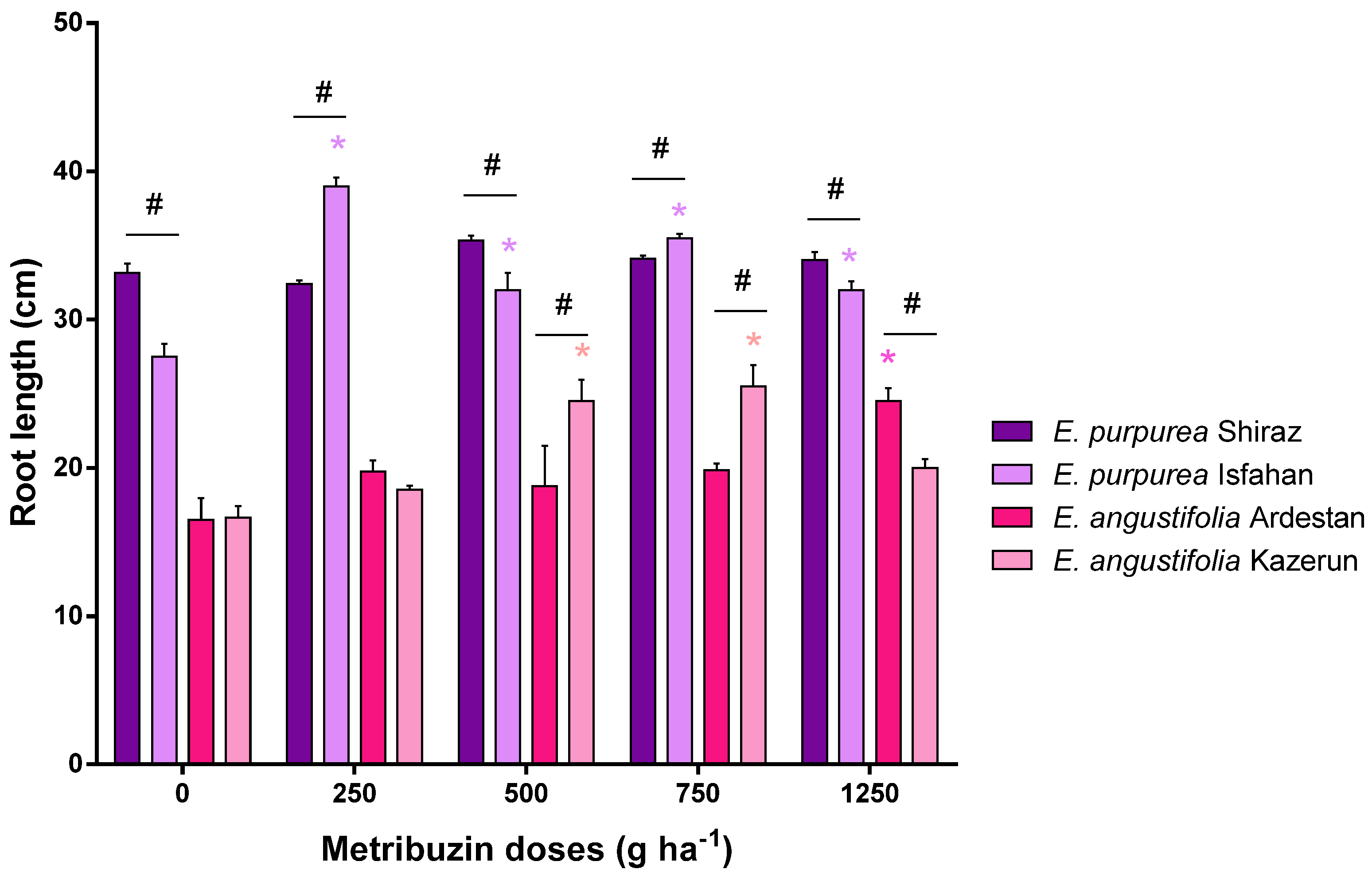
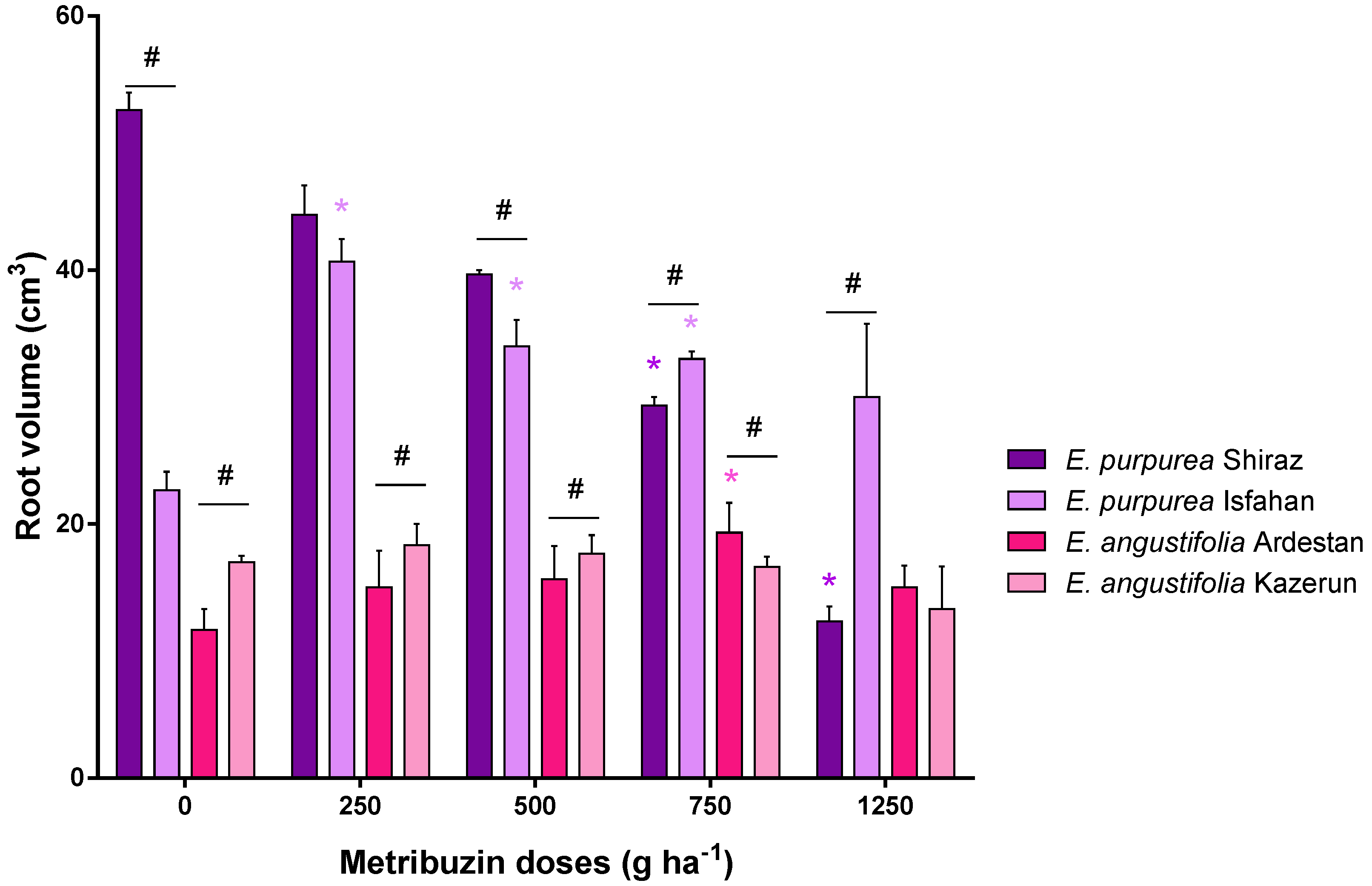
3.2. Root Parameters: Dry Weight, Length, and Volume
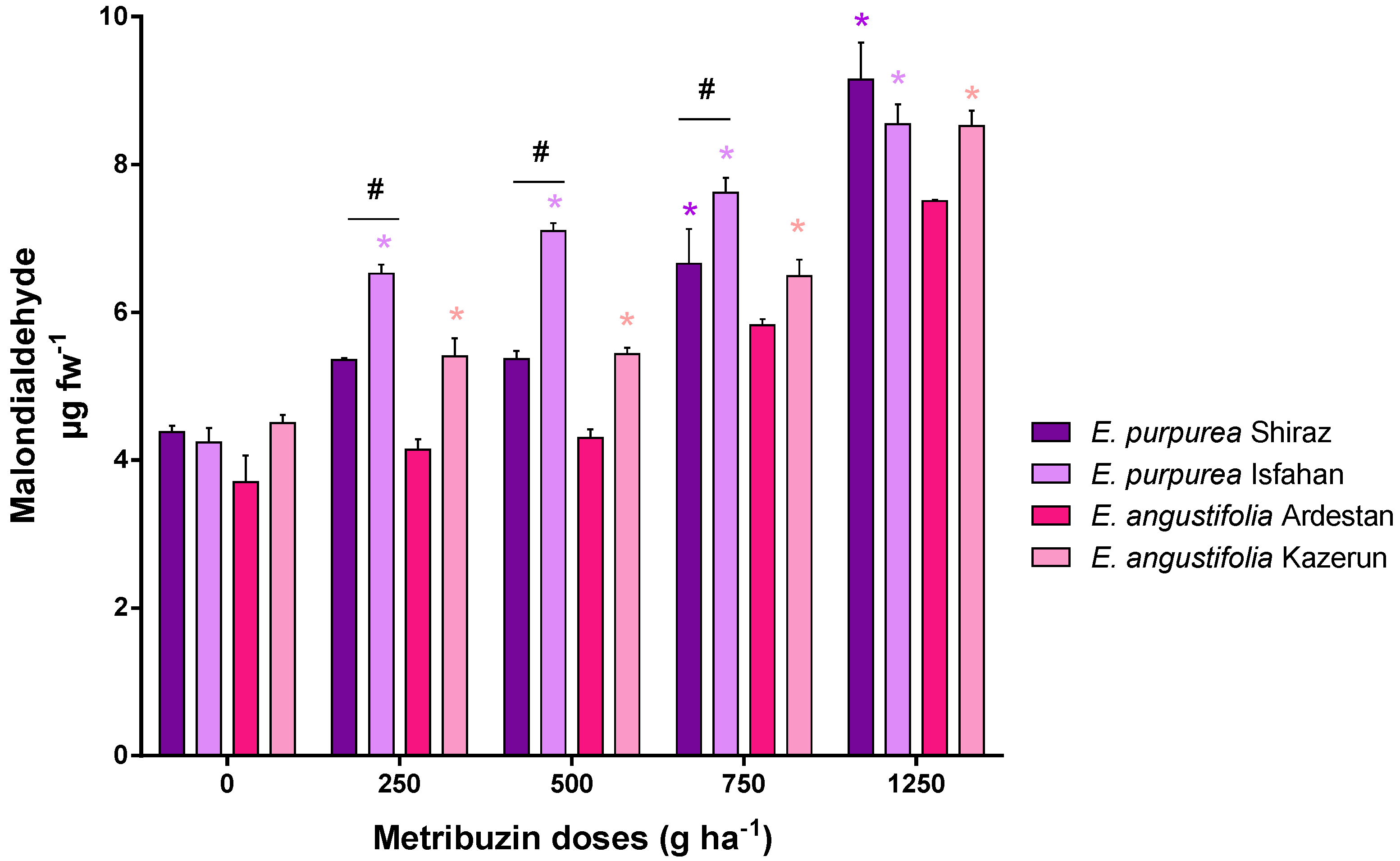
3.3. Oxidative Stress: Malondialdehyde (MDA)
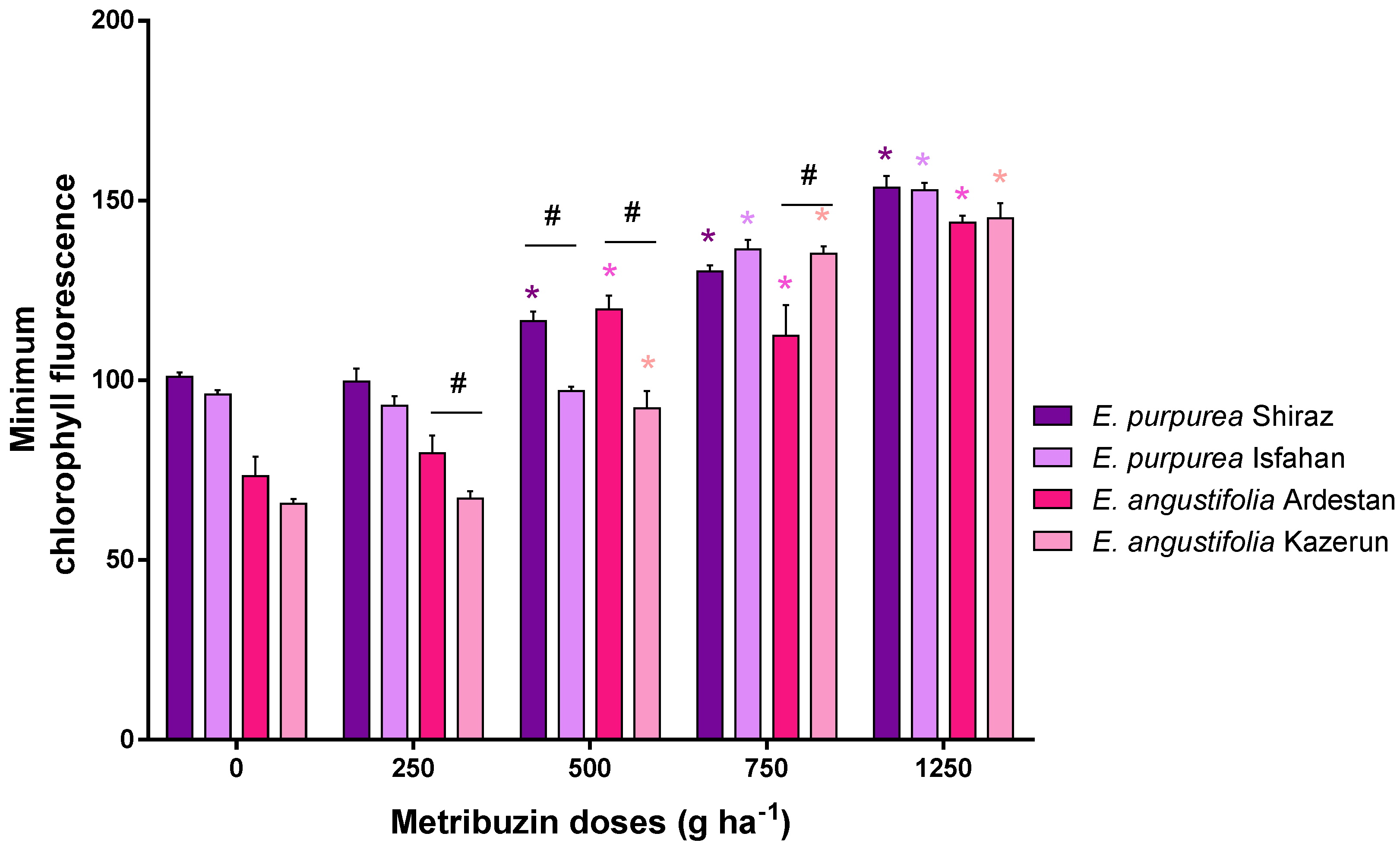
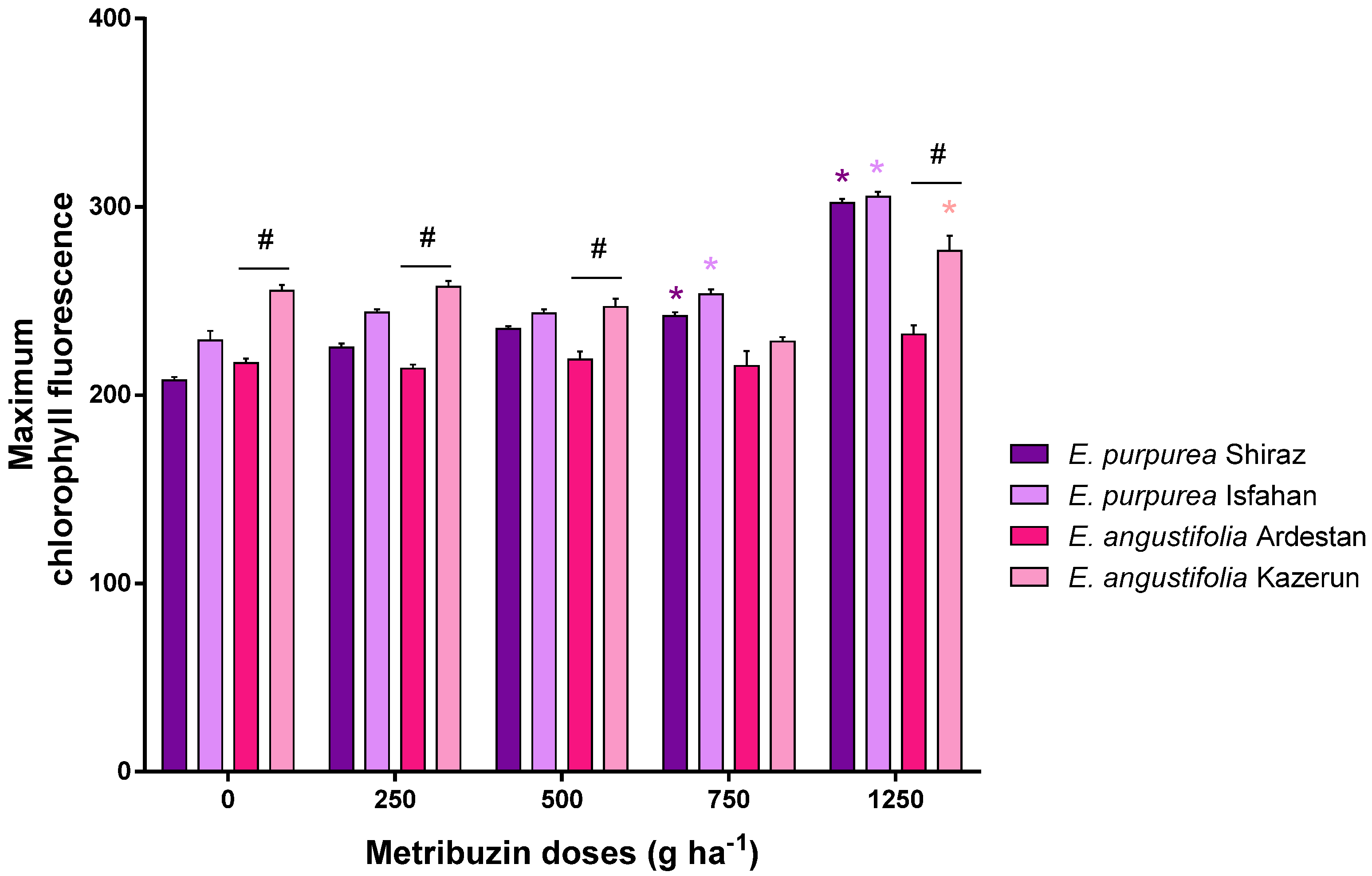
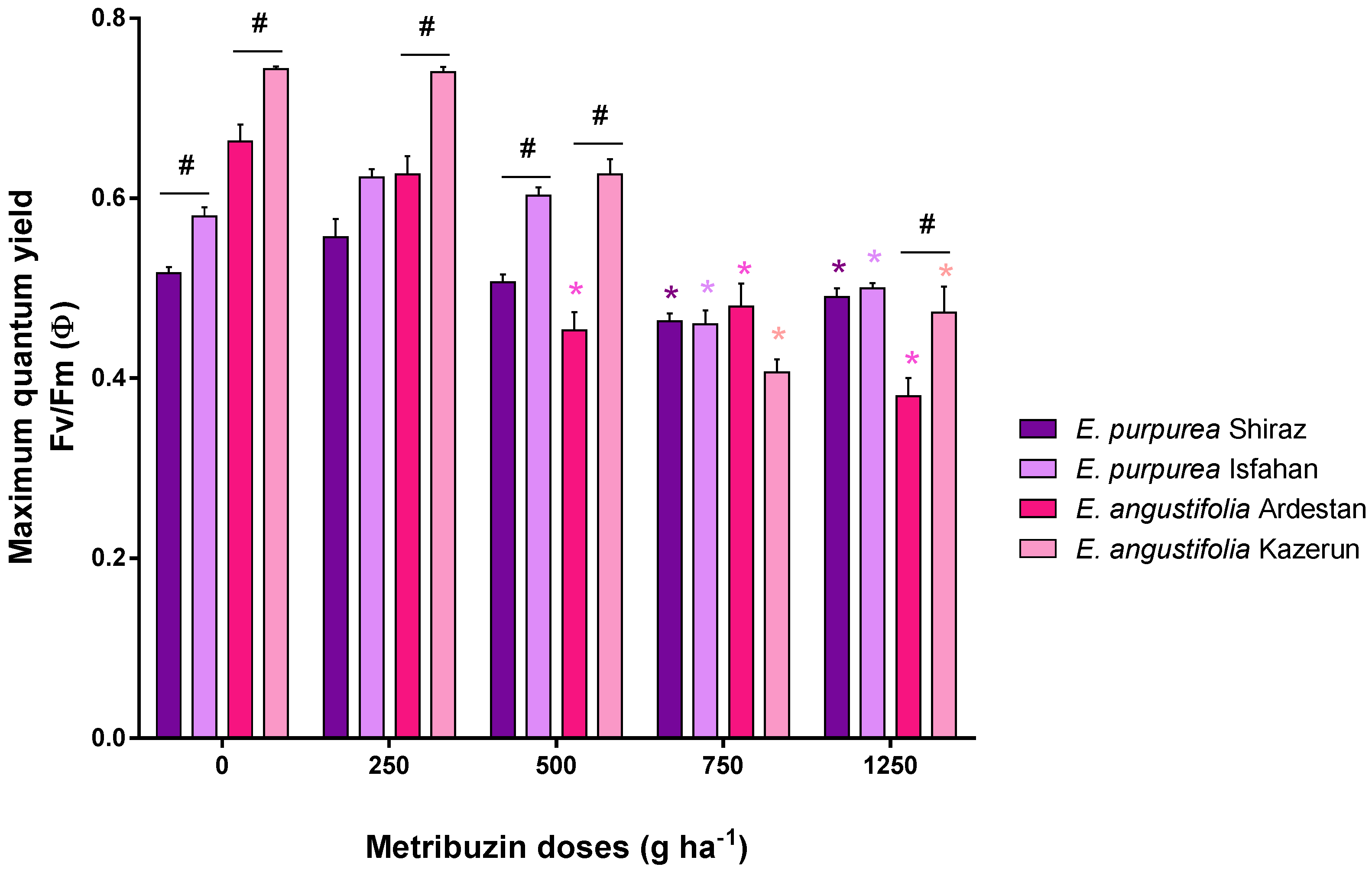
3.4. Chlorophyll Florescence and Maximum Quantum Yield
3.5. Correlation between Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binns, S.E.; Livesey, J.F.; Arnason, J.T.; Baum, B.R. Phytochemical Variation in Echinacea from Roots and Flowerheads of Wild and Cultivated Populations. J. Agric. Food Chem. 2002, 50, 3673–3687. [Google Scholar] [CrossRef]
- Mistríková, I.; Vaverková, Š. Morphology and anatomy of Echinacea purpurea, E. angustifolia, E. pallida and Parthenium integrifolium. Biologia 2007, 62, 2–5. [Google Scholar] [CrossRef]
- Haidu, D.; Parkanyi, D.; Moldovan, R.I.; Savii, C.; Pinzaru, I.; Dehelean, C.; Kurunczi, L. Elemental Characterization of Romanian Crop Medicinal Plants by Neutron Activation Analysis. J. Anal. Methods Chem. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Sloley, B.D.; Urichuk, L.J.; Tywin, C.; Coutts, R.T.; Pang, P.K.T.; Shan, J.J. Comparison of chemical components and antioxidant capacity of different Echinacea species. J. Pharm. Pharmacol. 2001, 53, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Loddo, D.; McElroy, J.S.; Giannini, V. Problems and perspectives in weed management. Ital. J. Agron. 2021, 16, 1854. [Google Scholar] [CrossRef]
- Chen, C.L.; Zhang, S.C.; Sung, J.M. Biomass and Caffeoyl Phenols Production Ofechinacea purpurea grown in Taiwan. Exp. Agric. 2008, 44, 497–507. [Google Scholar] [CrossRef]
- Kristiansen, P.; Sindel, B.M.; Jessop, R.S. Weed management in organic echinacea (Echinacea purpurea) and lettuce (Lactuca sativa) production. Renew. Agric. Food Syst. 2008, 23, 120–135. [Google Scholar] [CrossRef]
- Aghajanlou, F.; Mirdavoudi, H.; Shojaee, M.; Mac Sweeney, E.; Mastinu, A.; Moradi, P. Rangeland Management and Ecological Adaptation Analysis Model for Astragalus curvirostris Boiss. Horticulturae 2021, 7, 67. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rahimian, H.; Alizadeh, H.; Yousefi, A.R.; Gonzalez-Andujar, J.L.; Mac Sweeney, E.; Mastinu, A. Competitive Ability Effects of Datura stramonium L. and Xanthium strumarium L. on the Development of Maize (Zea mays) Seeds. Plants 2021, 10, 1922. [Google Scholar] [CrossRef]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef]
- Rad, S.V.; Valadabadi, S.A.R.; Pouryousef, M.; Saifzadeh, S.; Zakrin, H.R.; Mastinu, A. Quantitative and Qualitative Evaluation of Sorghum bicolor L. under Intercropping with Legumes and Different Weed Control Methods. Horticulturae 2020, 6, 78. [Google Scholar] [CrossRef]
- Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Beltrão, J.G.T.; Mastinu, A. Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae 2022, 8, 79. [Google Scholar] [CrossRef]
- Gharibvandi, A.; Karimmojeni, H.; Ehsanzadeh, P.; Maleki, M.R.; Mastinu, A. Weed management by allelopathic activity of Foeniculum vulgare essential oil. Plant Biosyst. 2022, 1–15. [Google Scholar] [CrossRef]
- Qasem, J.R.; Foy, C.L. Selective Weed Control in Syrian Marjoram (Origanum syriacum) with Oxadiazon and Oxyfluorfen Herbicides. Weed Technol. 2017, 20, 670–676. [Google Scholar] [CrossRef]
- Forcella, F.; Papiernik, S.K.; Gesch, R.W. Postemergence Herbicides for Calendula. Weed Technol. 2017, 26, 566–569. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Rahimi, M.R. Integration of soil-applied herbicides at the reduced rates with physical control for weed management in fennel (Foeniculum vulgare Mill.). Crop Prot. 2014, 63, 107–112. [Google Scholar] [CrossRef]
- Wang, X. Effect of quizalofop on protective enzymes and photosynthesis in Radix isatidis. J. Med. Plants Res. 2012, 6, 1770–1776. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, P.Y.; Yuan, X.Y.; Wang, J.Y.; Han, M.Q. Effect of paraquat on the antioxidative enzyme activities and lipid peroxidation in opium poppy (Papaver somniferum L.). J. Plant Dis. Prot. 2016, 117, 55–59. [Google Scholar] [CrossRef]
- Kong, L.; Sun, M.; Xie, Y.; Wang, F.; Zhao, Z. Photochemical and antioxidative responses of the glume and flag leaf to seasonal senescence in wheat. Front. Plant Sci. 2015, 6, 358. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, D.-G.; Lee, K.-W.; Alam, I.; Lee, S.-H.; Bahk, J.D.; Lee, B.-H. Glyphosate-induced oxidative stress in rice leaves revealed by proteomic approach. Plant Physiol. Biochem. 2008, 46, 1062–1070. [Google Scholar] [CrossRef]
- Harfouche, R.; Basu, S.; Soni, S.; Hentschel, D.M.; Mashelkar, R.A.; Sengupta, S. Nanoparticle-mediated targeting of phosphatidylinositol-3-kinase signaling inhibits angiogenesis. Angiogenesis 2009, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Collins, R.M.; Bowran, D.G. Efficacy of Interrow Weed Control Techniques in Wide Row Narrow-Leaf Lupin. Weed Technol. 2017, 25, 135–140. [Google Scholar] [CrossRef]
- Brown, H.M. Mode of action, crop selectivity, and soil relations of the sulfonylurea herbicides. Pestic. Sci. 1990, 29, 263–281. [Google Scholar] [CrossRef]
- Kostopoulou, S.; Ntatsi, G.; Arapis, G.; Aliferis, K.A. Assessment of the effects of metribuzin, glyphosate, and their mixtures on the metabolism of the model plant Lemna minor L. applying metabolomics. Chemosphere 2020, 239, 124582. [Google Scholar] [CrossRef] [PubMed]
- Ganugi, P.; Miras-Moreno, B.; Garcia-Perez, P.; Lucini, L.; Trevisan, M. Concealed metabolic reprogramming induced by different herbicides in tomato. Plant Sci. 2021, 303, 110727. [Google Scholar] [CrossRef]
- Si, P.; Sweetingham, M.W.; Buirchell, B.J.; Bowran, D.G.; Piper, T. Genotypic variation in metribuzin tolerance in narrow-leafed lupin (Lupinus angustifolius L.). Aust. J. Exp. Agric. 2006, 46, 85. [Google Scholar] [CrossRef]
- Si, P.; Buirchell, B.; Sweetingham, M. Improved metribuzin tolerance in narrow-leafed lupin (Lupinus angustifolius L.) by induced mutation and field selection. Field Crop Res. 2009, 113, 282–286. [Google Scholar] [CrossRef]
- Xu, L.; Liu, H.; Kilian, A.; Bhoite, R.; Liu, G.; Si, P.; Wang, J.; Zhou, W.; Yan, G. QTL Mapping Using a High-Density Genetic Map to Identify Candidate Genes Associated with Metribuzin Tolerance in Hexaploid Wheat (Triticum aestivum L.). Front. Plant Sci. 2020, 11, 1440. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bhoite, R.; Onyemaobi, I.; Si, P.; Siddique, K.H.M.; Yan, G. Identification and validation of QTL and their associated genes for pre-emergent metribuzin tolerance in hexaploid wheat (Triticum aestivum L.). BMC Genet. 2018, 19, 102. [Google Scholar] [CrossRef]
- Bhoite, R.; Si, P.; Liu, H.; Xu, L.; Siddique, K.H.M.; Yan, G. Inheritance of pre-emergent metribuzin tolerance and putative gene discovery through high-throughput SNP array in wheat (Triticum aestivum L.). BMC Plant Biol. 2019, 19, 457. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yu, Q.; Han, H.; Owen, M.J.; Powles, S.B. Metribuzin Resistance in a Wild Radish (Raphanus raphanistrum) Population via Both psbA Gene Mutation and Enhanced Metabolism. J. Agric. Food Chem. 2019, 67, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lu, H.; Han, H.; Yu, Q.; Powles, S. Metribuzin resistance via enhanced metabolism in a multiple herbicide resistant Lolium rigidum population. Pest Manag. Sci. 2020, 76, 3785–3791. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, P.; Pau, A.; Tambaro, S.; Asproni, B.; Ruiu, S.; Pinna, G.; Mastinu, A.; Curzu, M.M.; Reali, R.; Emilio Heiner Bottazzi, M.; et al. Synthesis and Pharmacological Evaluation of Novel 4-Alkyl-5-thien-2′-yl Pyrazole Carboxamides. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 254–276. [Google Scholar] [CrossRef]
- Zhila, N.; Murueva, A.; Shershneva, A.; Shishatskaya, E.; Volova, T. Herbicidal activity of slow-release herbicide formulations in wheat stands infested by weeds. J. Environ. Sci. Health. Part. B Pestic. Food Contam. Agric. Wastes 2017, 52, 729–735. [Google Scholar] [CrossRef][Green Version]
- Gupta, A.K.; Rather, M.A.; Jha, A.K.; Shashank, A.; Singhal, S.; Sharma, M.; Pathak, U.; Sharma, D.; Mastinu, A. Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. Flowers: New Sources of Bioactive Compounds. Plants 2020, 9, 1329. [Google Scholar] [CrossRef]
- Abate, G.; Zhang, L.L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dhua, S.; Sahu, P.P.; Abate, G.; Mishra, P.; Mastinu, A. Variation in Phytochemical, Antioxidant and Volatile Composition of Pomelo Fruit (Citrus grandis (L.) Osbeck) during Seasonal Growth and Development. Plants 2021, 10, 1941. [Google Scholar] [CrossRef]
- Khaleghnezhad, V.; Yousefi, A.R.; Tavakoli, A.; Farajmand, B.; Mastinu, A. Concentrations-dependent effect of exogenous abscisic acid on photosynthesis, growth and phenolic content of Dracocephalum moldavica L. under drought stress. Planta 2021, 253, 127. [Google Scholar] [CrossRef]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef]
- Mastinu, A.; Bonini, S.A.; Premoli, M.; Maccarinelli, G.; Mac Sweeney, E.; Zhang, L.L.; Lucini, L.; Memo, M. Protective Effects of Gynostemma pentaphyllum (var. Ginpent) against Lipopolysaccharide-Induced Inflammation and Motor Alteration in Mice. Molecules 2021, 26, 570. [Google Scholar] [CrossRef] [PubMed]
- Naservafaei, S.; Sohrabi, Y.; Moradi, P.; Mac Sweeney, E.; Mastinu, A. Biological Response of Lallemantia iberica to Brassinolide Treatment under Different Watering Conditions. Plants 2021, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Noryan, M.; Hervan, I.M.; Sabouri, H.; Kojouri, F.D.; Mastinu, A. Drought Resistance Loci in Recombinant Lines of Iranian Oryza sativa L. in Germination Stage. BioTech 2021, 10, 26. [Google Scholar] [CrossRef]
- Reza Yousefi, A.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Gianoncelli, A.; Bonini, S.A.; Bertuzzi, M.; Guarienti, M.; Vezzoli, S.; Kumar, R.; Delbarba, A.; Mastinu, A.; Sigala, S.; Spano, P.; et al. An Integrated Approach for a Structural and Functional Evaluation of Biosimilars: Implications for Erythropoietin. BioDrugs 2015, 29, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Umar, S.; Ahmad, A.; Iqbal, M.; Khan, N.A. Sulphur protects mustard (Brassica campestris L.) from cadmium toxicity by improving leaf ascorbate and glutathione. Plant Growth Regul. 2007, 54, 271–279. [Google Scholar] [CrossRef]
- Lazzari, P.; Sanna, A.; Mastinu, A.; Cabasino, S.; Manca, I.; Pani, L. Weight loss induced by rimonabant is associated with an altered leptin expression and hypothalamic leptin signaling in diet-induced obese mice. Behav. Brain Res. 2011, 217, 432–438. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Furutera, A.; Seki, K.; Toyoda, Y.; Tanaka, K.; Sugimoto, Y. Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiol. Biochem. 2008, 46, 786–793. [Google Scholar] [CrossRef]
- Almeida, A.C.; Gomes, T.; Langford, K.; Thomas, K.V.; Tollefsen, K.E. Oxidative stress potential of the herbicides bifenox and metribuzin in the microalgae Chlamydomonas reinhardtii. Aquat. Toxicol. 2019, 210, 117–128. [Google Scholar] [CrossRef]
- Husak, V.V.; Mosiichuk, N.M.; Maksymiv, I.V.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Oxidative stress responses in gills of goldfish, Carassius auratus, exposed to the metribuzin-containing herbicide Sencor. Environ. Toxicol. Pharmacol. 2016, 45, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Weisz, D.A.; Gross, M.L.; Pakrasi, H.B. Reactive oxygen species leave a damage trail that reveals water channels in Photosystem II. Sci. Adv. 2017, 3, eaao3013. [Google Scholar] [CrossRef] [PubMed]
- Pospisil, P. Production of Reactive Oxygen Species by Photosystem II as a Response to Light and Temperature Stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef] [PubMed]
- Demmig, B.; Winter, K.; Krüger, A.; Czygan, F.-C. Photoinhibition and Zeaxanthin Formation in Intact Leaves. Plant Physiol. 1987, 84, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Weiperth, A.; Hossain, M.S.; Kubec, J.; Grabicova, K.; Lozek, F.; Vesely, L.; Blaha, M.; Buric, M.; Kouba, A.; et al. The effects of the herbicides terbuthylazine and metazachlor at environmental concentration on the burrowing behaviour of red swamp crayfish. Chemosphere 2021, 270, 128656. [Google Scholar] [CrossRef]
- Shekoofa, A.; Brosnan, J.T.; Vargas, J.J.; Tuck, D.P.; Elmore, M.T. Environmental effects on efficacy of herbicides for postemergence goosegrass (Eleusine indica) control. Sci Rep. 2020, 10, 20579. [Google Scholar] [CrossRef]
- Xie, Y.L.; Zhao, Z.D.; Zhang, X.L.; Tang, L.L.; Zhang, Y.; Zhang, C.H. Simultaneous analysis of herbicide metribuzin and its transformation products in tomato using QuEChERS-based gas chromatography coupled to a triple quadrupole mass analyzer. Microchem. J. 2017, 133, 468–473. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Zhalnov, I.; Nedkov, N.K. Herbicides for Weed Control in Blessed Thistle (Silybum marianum). Weed Technol. 2017, 20, 1030–1034. [Google Scholar] [CrossRef]
- Jervekani, M.T.; Karimmojeni, H.; Razmjoo, J. Effects of light-dependent herbicides on growth and physiology of Salvia officinalis. Planta Daninha 2020, 38. [Google Scholar] [CrossRef]
| MS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SOV | df | LDW | RDW | RL | RV | LA | MDA | F0 | Fm | Fv/Fm |
| G | 3 | 40.35 ** | 140.83 ** | 856.51 ** | 1637 ** | 15,445,432 ** | 7.49 ** | 1131.2 ** | 3972 ** | 0.0246 ** |
| H | 4 | 2.78 ** | 15.01 ** | 49.79 ** | 236 ** | 1,082,277 ** | 30.43 ** | 9525.2 ** | 5140 ** | 0.0917 ** |
| G × H | 12 | 0.36 ** | 4.53 ** | 26.41 ** | 215 ** | 272,880.2 ** | 0.68 ** | 349.4 ** | 780 ** | 0.0134 ** |
| Error | 40 | 0.033 | 0.102 | 3 | 14.35 | 1638.48 | 0.151 | 37.51 | 42.57 | 0.00071 |
| CV | - | 8.65 | 5.90 | 6.42 | 15.20 | 3.20 | 6.45 | 5.54 | 2.68 | 4.91 |
| RDW | RL | RV | MDA | LA | Fv/Fm | F0 | Fm | |
|---|---|---|---|---|---|---|---|---|
| RDW | - | |||||||
| RL | 0.760 ** | - | ||||||
| RV | 0.888 ** | 0.702 ** | - | |||||
| MDA | −0.08 ns | 0.407 ** | −0.05 ns | - | ||||
| LA | 0.868 ** | 0.810 ** | 0.775 ** | 0.119 ns | - | |||
| Fv/Fm | −0.03 ns | −0.299 * | −0.04 ns | −0.47 ** | −0.03 ns | - | ||
| F0 | −0.01 ns | 0.377 ** | −0.03 ns | 0.766 ** | 0.05 ns | −0.88 ** | - | |
| Fm | −0.14 ns | 0.21 ns | −0.17 ns | 0.746 ** | 0.02 ns | −0.00 ns | 0.460 ** | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimmojeni, H.; Rezaei, M.; Tseng, T.-M.; Mastinu, A. Effects of Metribuzin Herbicide on Some Morpho-Physiological Characteristics of Two Echinacea Species. Horticulturae 2022, 8, 169. https://doi.org/10.3390/horticulturae8020169
Karimmojeni H, Rezaei M, Tseng T-M, Mastinu A. Effects of Metribuzin Herbicide on Some Morpho-Physiological Characteristics of Two Echinacea Species. Horticulturae. 2022; 8(2):169. https://doi.org/10.3390/horticulturae8020169
Chicago/Turabian StyleKarimmojeni, Hassan, Mohammad Rezaei, Te-Ming Tseng, and Andrea Mastinu. 2022. "Effects of Metribuzin Herbicide on Some Morpho-Physiological Characteristics of Two Echinacea Species" Horticulturae 8, no. 2: 169. https://doi.org/10.3390/horticulturae8020169
APA StyleKarimmojeni, H., Rezaei, M., Tseng, T.-M., & Mastinu, A. (2022). Effects of Metribuzin Herbicide on Some Morpho-Physiological Characteristics of Two Echinacea Species. Horticulturae, 8(2), 169. https://doi.org/10.3390/horticulturae8020169







