Understanding the Priming Effect and the Routes and Stocks of C in Incubated Soil with Residue Inputs
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Characterization
2.2. C and N Stocks in Soil
2.3. Data Processing and Statistical Analysis
3. Results
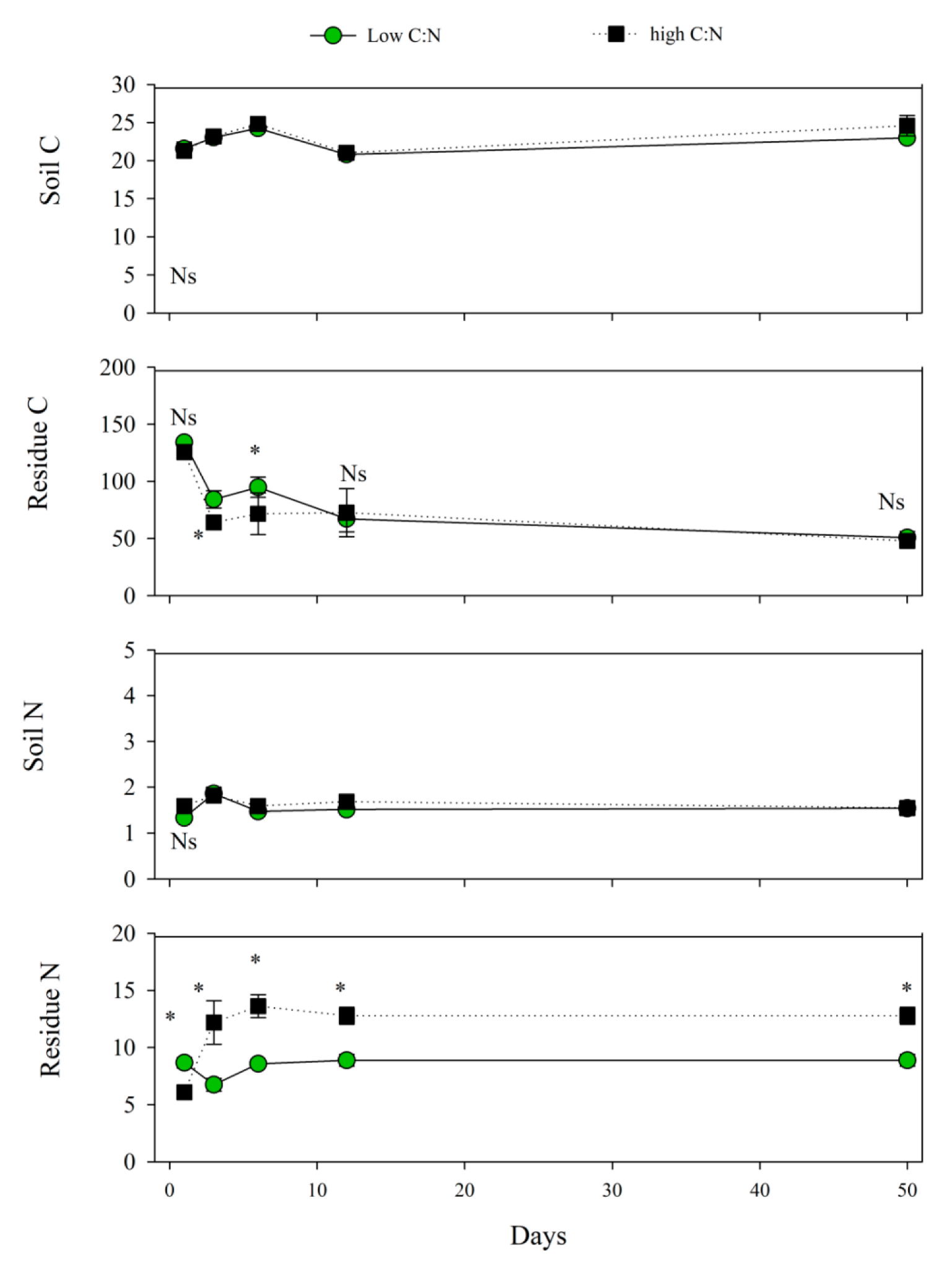
3.1. Daily C and N in Soil and Residues
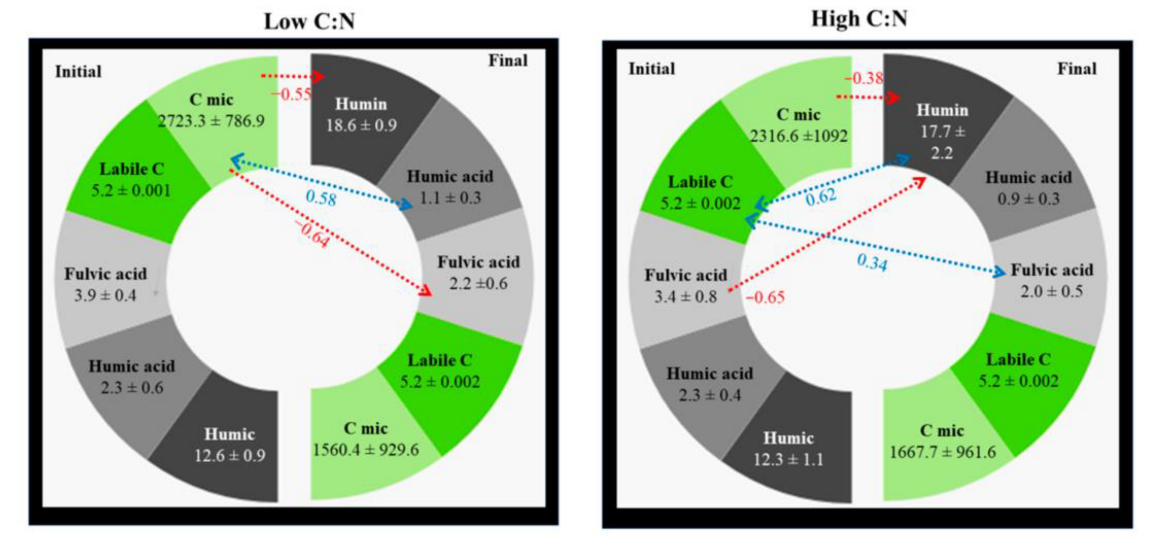
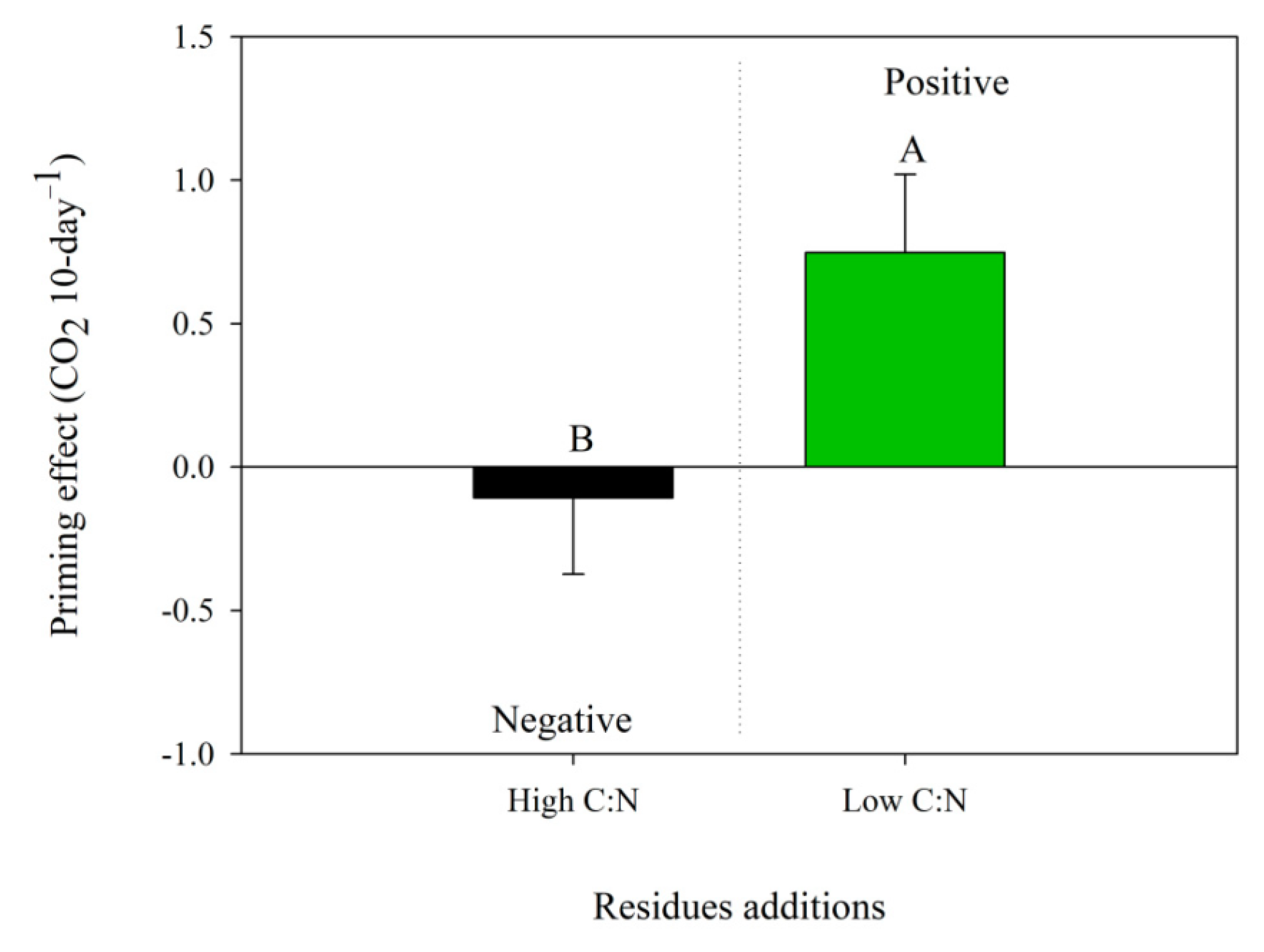
3.2. Priming Effect and Route of C Stocks
4. Discussion
4.1. Daily C and N in Soil and Residues
4.2. Priming Effect and Route of C Stocks
5. Conclusions
Funding
Conflicts of Interest
References
- Calegari, A.; Araújo, A.G.; de Costa, A.; Lanillo, R.F.; Casão Junior, R.; Santos, D.R.d. Conservation agriculture in Brazil. In Conservation Agriculture: Global Prospects and Challenges; CABI: Wallingford, UK, 2013; pp. 54–88. [Google Scholar]
- Marcelo, A.V.; Corá, J.E.; Fernandes, C.; Martins, M.d.R.; Jorge, R.F. Crop sequences in no-tillage system: Effects on soil fertility and soybean, maize and rice yield. Rev. Bras. Ciênc. Solo 2009, 33, 417–428. [Google Scholar] [CrossRef]
- De Freitas, P.L.; Landers, J.N. The Transformation of Agriculture in Brazil Through Development and Adoption of Zero Tillage Conservation Agriculture. Int. Soil Water Conserv. Res. 2014, 2, 35–46. [Google Scholar] [CrossRef]
- FBPDI Federação Brasileira de Plantio Direto e Irrigação. Available online: https://febrapdp.org.br/publicacoes-em-pd (accessed on 17 June 2020).
- Kassam, A.; Friedrich, T.; Derpsch, R.; Kienzle, J. Overview of the Worldwide Spread of Conservation Agriculture. Available online: https://journals.openedition.org/factsreports/3966 (accessed on 17 June 2020).
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Yong, R. The circular economy in China. J. Mater. Cycles Waste Manag. 2007, 9, 121–129. [Google Scholar] [CrossRef]
- Carvalho, J.L.N.; Otto, R.; Franco, H.C.J.; Trivelin, P.C.O. Input of sugarcane post-harvest residues into the soil. Sci. Agric. 2013, 70, 336–344. [Google Scholar] [CrossRef]
- De Landel, M.G.A.; Scarpari, M.S.; Xavier, M.A.; dos Anjos, I.A.; Baptista, A.S.; de Aguiar, C.L.; da Silva, D.N.; Bidóia, M.A.P.; Brancalião, S.R.; Bressiani, J.A.; et al. Residual biomass potential of commercial and pre-commercial sugarcane cultivars. Sci. Agric. 2013, 70, 299–304. [Google Scholar] [CrossRef]
- Cavalli, E.; Lange, A.; Cavalli, C.; Behling, M. Decomposition and release of nutrients from crop residues on soybean-maize cropping systems. Rev. Bras. Ciênc. Solo 2018, 13, 1–8. [Google Scholar] [CrossRef]
- Torres, J.L.R.; Pereira, M.G.; Rodrigues Junior, D.J.; Loss, A. Efeitos de práticas de descompactação do solo em área sob sistema plantio direto. Rev. Cienc. Agron. 2015, 46, 451–459. [Google Scholar] [CrossRef]
- Gunnarsson, S.; Marstorp, H.; Dahlin, A.S.; Witter, E. Influence of non-cellulose structural carbohydrate composition on plant material decomposition in soil. Biol. Fertil. Soils 2008, 45, 27–36. [Google Scholar] [CrossRef]
- Wang, W.J.; Baldock, J.A.; Dalal, R.C.; Moody, P.W. Decomposition dynamics of plant materials in relation to nitrogen availability and biochemistry determined by NMR and wet-chemical analysis. Soil Biol. Biochem. 2004, 36, 2045–2058. [Google Scholar] [CrossRef]
- Trinsoutrot, I.; Recous, S.; Mary, B.; Nicolardot, B. C and N fluxes of decomposing 13C and 15N Brassica napus L.: Effects of residue composition and N content. Soil Biol. Biochem. 2000, 32, 1717–1730. [Google Scholar] [CrossRef]
- Moritsuka, N.; Yanai, J.; Mori, K.; Kosaki, T. Biotic and abiotic processes of nitrogen immobilization in the soil-residue interface. Soil Biol. Biochem. 2004, 36, 1141–1148. [Google Scholar] [CrossRef]
- De Almeida, R.F.; Silveira, C.H.; Mota, R.P.; Moitinho, M.; Arruda, E.M.; Mendonça, E.D.S.; La Scala, N.; Wendling, B. For how long does the quality and quantity of residues in the soil affect the carbon compartments and CO2-C emissions? J. Soils Sediments 2016, 16, 2354–2364. [Google Scholar] [CrossRef]
- Franchini, J.C.; Debiasi, H.; Balbinot Junior, A.A.; Tonon, B.C.; Farias, J.R.B.; de Oliveira, M.C.N.; Torres, E. Evolution of crop yields in different tillage and cropping systems over two decades in southern Brazil. Field Crop. Res. 2012, 137, 178–185. [Google Scholar] [CrossRef]
- Nascente, A.S.; Li, Y.; Crusciol, C.A.C. Agregação do solo, concentração de carbono orgânico e densidade do solo em razão de espécies de plantas de cobertura no sistema de plantio direto. Rev. Bras. Ciênc. Solo 2015, 39, 871–879. [Google Scholar] [CrossRef]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Srinivasa Rao, C.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Soil organic carbon dynamics: Impact of land use changes and management practices: A review. In Advances in Agronomy; Academic Press Inc.: Cambridge, MA, USA, 2019; Volume 156, pp. 1–107. ISBN 9780128175989. [Google Scholar]
- Jat, H.S.; Datta, A.; Choudhary, M.; Yadav, A.K.; Choudhary, V.; Sharma, P.C.; Gathala, M.K.; Jat, M.L.; McDonald, A. Effects of tillage, crop establishment and diversification on soil organic carbon, aggregation, aggregate associated carbon and productivity in cereal systems of semi-arid Northwest India. Soil Tillage Res. 2019, 190, 128–138. [Google Scholar] [CrossRef]
- Almeida, R.F.; Spokas, K.A.; de Bortoli Teixeira, D.; Júnior, N.L.S. Biochar insights from laboratory incubations monitoring O2 consumption and CO2 production. Biochar 2019, 1, 249–258. [Google Scholar] [CrossRef]
- Paustian, K.; Larson, E.; Kent, J.; Marx, E.; Swan, A. Soil C Sequestration as a Biological Negative Emission Strategy. Front. Clim. 2019, 1, 8. [Google Scholar] [CrossRef]
- Lal, R. Sequestration of atmospheric CO2 in global carbon pools. Energy Environ. Sci. 2008, 1, 86–100. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Wendling, B.; Jucksch, I.; de Sá Mendonça, E.; de Almeida, R.F.; Alvarenga, R.C. Simulação dos estoques de Carbono e Nitrogênio pelo Modelo Century em Latossolos, no Cerrado Brasileiro. Rev. Cienc. Agron. 2014, 45, 238–248. [Google Scholar] [CrossRef][Green Version]
- Almeida, R.F.; Mikhael, J.E.R.; Franco, F.O.; Santana, L.M.F.; Wendling, B. Measuring the labile and recalcitrant pools of carbon and nitrogen in forested and agricultural soils: A study under tropical conditions. Forests 2019, 10, 544. [Google Scholar] [CrossRef]
- Sayer, E.J.; Lopez-Sangil, L.; Crawford, J.A.; Bréchet, L.M.; Birkett, A.J.; Baxendale, C.; Castro, B.; Rodtassana, C.; Garnett, M.H.; Weiss, L.; et al. Tropical forest soil carbon stocks do not increase despite 15 years of doubled litter inputs. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Loureiro, D.C.; de-Polli, H.; Ceddia, M.B.; de Aquino, A.M. Variabilidade espacial da biomassa microbiana e compartimentos lábeis da matéria orgânica de um planossolo háplico. Bragantia 2010, 69, 85–95. [Google Scholar] [CrossRef]
- Almeida, R.F.; Nave, E.R.; Mota, R.P. Soil quality: Enzymatic activity of soil β-glucosidase. Glob. Sci. Res. J. 2015, 3, 146–150. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 1st ed.; John Wiley & Sons,: New York, NY, USA, 1994. [Google Scholar]
- Almeida, R.F.; Haddad Silveira, C.; Mikhael, J.E.R.; Franco, F.O.; Ribeiro, B.T.; de Bruno Siqueira, A.; de Sa Mendonca, E.; Wendling, B. CO2 emissions from soil incubated with sugarcane straw and nitrogen fertilizer. Afr. J. Biotechnol. 2014, 13, 3376–3384. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, F.; Wei, D.; Zhou, B.; Cai, S.; Li, Y.; Kuang, E.; Sun, L.; Li, L.J. Impacts of Long-term Fertilization on the Molecular Structure of Humic Acid and Organic Carbon Content in Soil Aggregates in Black Soil. Sci. Rep. 2019, 9, 11908. [Google Scholar] [CrossRef]
- Mikhael, J.E.R.; Almeida, R.F.; Franco, F.d.O.; Camargo, R.O.; Wendling, B. Recalcitrant carbon and nitrogen in agriculture soils with residue accumulation and fertilization under tropical conditions. Biosci. J. 2019, 35, 732–740. [Google Scholar] [CrossRef]
- Dou, S.; Shan, J.; Song, X.; Cao, R.; Wu, M.; Li, C.; Guan, S. Are humic substances soil microbial residues or unique synthesized compounds? A perspective on their distinctiveness. Pedosphere 2020, 30, 159–167. [Google Scholar] [CrossRef]
- Bae, H.C.; Cota-Robles, E.H.; Casida, L.E. Microflora of Soil as Viewed by Transmission Electron Microscopy1. Appl. Microbiol. 1972, 23, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Joergensen, R.G.; Anderson, T.H.; Wolters, V. Carbon and nitrogen relationships in the microbial biomass of soils in beech (Fagus sylvatica L.) forests. Biol. Fertil. Soils 1995, 19, 141–147. [Google Scholar] [CrossRef]
- Diniz, L.T.; Ramos, M.L.G.; Junior, W.Q.R.; Cruz, A.F.; de Franca, L.V.; Diniz, B.T.; Amabile, R.F. Effect of nitrogen fertilization on soil microbial biomass in an Oxisol cultivated with irrigated barley in the Brazilian Cerrado. Acta Agron. 2016, 65, 137–143. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, T.; Guo, J. Warming and nitrogen addition change the soil and soil microbial biomass C:N:P stoichiometry of a meadow steppe. Int. J. Environ. Res. Public Health 2019, 16, 2705. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.M.; Silva, A.M.M.; Estrada-Bonilla, G.A.; Ferraz-Almeida, R.; Vieira, J.L.V.; Otto, R.; Vitti, G.C.; Cardoso, E.J.B.N. Improving the fertilizer value of sugarcane wastes through phosphate rock amendment and phosphate-solubilizing bacteria inoculation. J. Clean. Prod. 2021, 298, 126821. [Google Scholar] [CrossRef]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Evaluation of humic substances during co-composting of food waste, sawdust and Chinese medicinal herbal residues. Bioresour. Technol. 2014, 168, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Qiao, N.; Xu, X.; Hu, Y.; Blagodatskaya, E.; Liu, Y.; Schaefer, D.; Kuzyakov, Y. Carbon and nitrogen additions induce distinct priming effects along an organic-matter decay continuum. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Melillo, J.M.; Butler, S.; Johnson, J.; Mohan, J.; Steudler, P.; Lux, H.; Burrows, E.; Bowles, F.; Smith, R.; Scott, L.; et al. Soil warming, carbon-nitrogen interactions, and forest carbon budgets. Proc. Natl. Acad. Sci. USA 2011, 108, 9508–9512. [Google Scholar] [CrossRef]
- EMBRAPA, Empresa Brasileira de Pesquisa Agropecuária. Manual de Métodos de Análise de Solo; Centro Nacional de Pesquisa de Solos: Rio Janeiro, Brazil, 1997. [Google Scholar]
- Gee, G.W.; Orr, D. Particle-size analysis. In Methods of Soil Analysis; Dane, J., Topp, G., Eds.; Soils Science Society of America: Madison, WI, USA, 2002; pp. 255–293. [Google Scholar]
- Embrapa. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Rio Janeiro, Brazil, 2018. [Google Scholar]
- Soil Survey Staff Soil Survey. NRCS Soils. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/main/soils/survey/ (accessed on 29 May 2020).
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil1. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Guo, L.; Nishimura, T.; Imoto, H.; Sun, Z. Applicability of soil column incubation experiments to measure CO2 efflux**. Int. Agrophys. 2015, 29, 413–421. [Google Scholar] [CrossRef][Green Version]
- Swift, R. Organic matter characterization. In Methods of Soil Analysis: Chemical Methods, Part 3; Sparks, D., Page, A., Helmke, P., Loeppert, R., Soltanpour, P., Tabatabai, M., Johnston, C., Sumner, M., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 1011–1020. [Google Scholar]
- Mendonça, E.; Matos, E. Matéria Orgânica do Solo: Métodos de Análises; D&M Gráfica e Editora Ltd.a,: Ponte Nova, Brazil, 2005. [Google Scholar]
- Islam, K.R.; Weil, R.R. Microwave irradiation of soil for routine measurement of microbial biomass carbon. Biol. Fertil. Soils 1998, 27, 408–416. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Rahman, M.M. Carbon Dioxide Emission from Soil. Agric. Res. 2013, 2, 132–139. [Google Scholar] [CrossRef]
- Li, X.S.; Han, H.F.; Ning, T.Y.; Lal, R. CO 2-C evolution rate in an incubation study with straw input to soil managed by different tillage systems. RSC Adv. 2018, 8, 12588–12596. [Google Scholar] [CrossRef]
- Vargas, V.P.; Cantarella, H.; Martins, A.A.; Soares, J.R.; do Carmo, J.B.; de Andrade, C.A. Sugarcane Crop Residue Increases N2O and CO2 Emissions Under High Soil Moisture Conditions. Sugar Tech. 2014, 16, 174–179. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, X. Soil Respiration and the Environment; Elsevier Inc.: Amsterdam, The Netherlands, 2006; ISBN 9780120887828. [Google Scholar]
- Ferraz-Almeida, R.; de Bortoli Teixeira, D.; Montanari, R.; Bolonhezi, A.C.; Teixeira, E.B.; Moitinho, M.R.; Panosso, A.R.; Spokas, K.A.; La Scala Júnior, N. Ratio of CO2 and O2 as index for categorising soil biological activity in sugarcane areas under contrasting straw management regimes. Soil Res. 2018, 56, 373. [Google Scholar] [CrossRef]
- Tavares, R.L.M.; Spokas, K.; Hall, K.; Colosky, E.; de Souza, Z.M.; La Scala, N. Sugarcane residue management impact soil greenhouse gas. Ciênc. e Agrotecnol. 2018, 42, 195–203. [Google Scholar] [CrossRef]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.H.; dos Santos, C.A.; Alves, P.R.L.; de Paula, A.M.; Nakatani, A.S.; Pereira, J. de M.; Nogueira, M.A. Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agric. 2013, 70, 274–289. [Google Scholar] [CrossRef]
- Uchida, Y.; Akiyama, H. Soil Science and Plant Nutrition Mitigation of postharvest nitrous oxide emissions from soybean ecosystems: A review Mitigation of postharvest nitrous oxide emissions from soybean ecosystems: A review. Soil Sci. Plant Nutr. 2013, 59, 477–487. [Google Scholar] [CrossRef]
- Ferraz-Almeida, R.; Da Silva, N.; Wendling, B. How Does N Mineral Fertilizer Influence the Crop Residue N Credit? Nitrogen 2020, 1, 99–110. [Google Scholar] [CrossRef]
- Danga, B.O.; Ouma, J.P.; Wakindiki, I.I.C.; Bar-Tal, A. Chapter 5 Legume-Wheat Rotation Effects on Residual Soil Moisture, Nitrogen and Wheat Yield in Tropical Regions. Adv. Agron. 2009, 101, 315–349. [Google Scholar]
- Uzoh, I.M.; Igwe, C.A.; Okebalama, C.B.; Babalola, O.O. Legume-maize rotation effect on maize productivity and soil fertility parameters under selected agronomic practices in a sandy loam soil. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Bacanamwo, M.; Harper, J.E. Regulation of nitrogenase activity in Bradyrhizobium japonicum/soybean symbiosis by plant N status as determined by shoot C:N ratio. Physiol. Plant. 1996, 98, 529–538. [Google Scholar] [CrossRef]
- Fontana, A.; Pereira, M.G.; Loss, A.; Cunha, T.J.F.; Salton, J.C. Atributos de fertilidade e frações húmicas de um Latossolo Vermelho no Cerrado. Pesqui. Agropecu. Bras. 2006, 41, 847–853. [Google Scholar] [CrossRef]
- Ebeling, A.G.; dos Anjos, L.H.C.; Pereira, M.G.; Pinheiro, É.F.M.; Valladares, G.S. Substâncias húmicas e relação com atributos edáficos. Bragantia 2011, 70, 157–165. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Wang, H.; Boutton, T.W.; Xu, W.; Hu, G.; Jiang, P.; Bai, E. Quality of fresh organic matter affects priming of soil organic matter and substrate utilization patterns of microbes. Sci. Rep. 2015, 5, 10102. [Google Scholar] [CrossRef]



| Stocks of C and N | - | Residues | |
|---|---|---|---|
| Control | Low C:N | High C:N | |
| Humin, g kg−1, Ns | 14.2 ± 0.2 | 15.0 ± 0.2 | 14.5 ± 0.2 |
| Humic acid, g kg−1, Ns | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.1 |
| Fulvic acid, g kg−1 | 3.0 ± 0.1 | 3.2 ± 0.1 A | 2.9 ± 0.1B |
| Labile carbon, g kg−1, Ns | 5.2 ± 0.0 | 5.2 ± 0.0 | 5.2 ± 0.0 |
| C mic, μg g−1 ha−1, Ns | 1718.0 ± 50.3 | 2048.9 ± 132.1 | 2057.1 ± 97.5 |
| CO2 emission, g CO2 m−2 h−1 | 1.0 ± 0.1 | 9.4 ± 1.0A | 5.7 ± 0.4 B |
| Soil C, g kg−1, Ns | - | 22.9 ± 1.0 | 22.5 ± 0.6 |
| Residue C, g kg−1 | - | 76.3 ± 16.1 B | 86.2 ± 11.1 A |
| Soil N, g kg−1, Ns | - | 1.6 ± 0.1 | 1.5 ± 0.09 |
| Residue N, g kg−1 | - | 11.5 ± 1.5 A | 8.3 ± 0.5 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz-Almeida, R. Understanding the Priming Effect and the Routes and Stocks of C in Incubated Soil with Residue Inputs. Horticulturae 2022, 8, 154. https://doi.org/10.3390/horticulturae8020154
Ferraz-Almeida R. Understanding the Priming Effect and the Routes and Stocks of C in Incubated Soil with Residue Inputs. Horticulturae. 2022; 8(2):154. https://doi.org/10.3390/horticulturae8020154
Chicago/Turabian StyleFerraz-Almeida, Risely. 2022. "Understanding the Priming Effect and the Routes and Stocks of C in Incubated Soil with Residue Inputs" Horticulturae 8, no. 2: 154. https://doi.org/10.3390/horticulturae8020154
APA StyleFerraz-Almeida, R. (2022). Understanding the Priming Effect and the Routes and Stocks of C in Incubated Soil with Residue Inputs. Horticulturae, 8(2), 154. https://doi.org/10.3390/horticulturae8020154






