Comparison of Wild and Introduced Dracocephalum jacutense P.: Significant Differences of Multicomponent Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Fractional Maceration
2.4. Liquid Chromatography
2.5. Mass Spectrometry
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| № | Class of Compounds | Identified Compounds | Formula | Mass | Molecular Ion [M-H]- | Molecular Ion [M+H]+ | 2 Fragmentation MS/MS | 3 Fragmentation MS/MS | 4 Fragmentation MS/MS | References |
|---|---|---|---|---|---|---|---|---|---|---|
| POLYPHENOLS | ||||||||||
| 1 | Flavone | Formononetin [Biochanin B; Formononetol] * | C16H12O4 | 268.2641 | 269 | 213 | 170; 156; 129 | 141 | Astragali Radix [35,36,37]; Huolisu Oral Liquid [38] | |
| 2 | Flavone | Apigenin [5,7-Dixydroxy-2-(40Hydroxyphenyl)-4H-Chromen-4-One] | C15H10O5 | 270.2369 | 269 | 225 | 181 | 117 | Dracocephalum palmatum [8]; Dracocephalum [12]; Lonicera japonicum [31]; Andean blueberry [39] | |
| 3 | Flavone | Acacetin [Linarigenin; Buddleoflavonol] | C16H12O5 | 284.2635 | 285 | 268 | 211; 143 | Dracocephalum palmatum [8]; Dracocephalum [12]; Dracocephalum moldavica [22]; Wissadula periplocifolia [40] | ||
| 4 | Flavone | Calycosin [3′-Hydroxyformononetin] * | C16H12O5 | 284.2635 | 285 | 253; 242; 225; 200 | 235; 221; 209; 203 | Astragali Radix [35,36,37]; Huolisu Oral Liquid [38] | ||
| 5 | Flavone | Genkwanin [Gengkwanin; Puddumetin; Apigenin 7-Methyl Ether] | C16H12O5 | 284.2635 | 285 | 165 | Dracocephalum palmatum [8]; Rosmarinus officinalis [14]; Mentha [41] | |||
| 6 | Flavone | Luteolin | C15H10O6 | 286.2363 | 287 | 286; 153 | 171 | 153 | Dracocephalum palmatum [8]; Dracocephalum [12]; Lonicera japonicum [31] | |
| 7 | Flavone | Diosmetin [Luteolin 4′-Methyl Ether; Salinigricoflavonol] | C16H12O6 | 300.2629 | 301 | 286 | 258 | Dracocephalum [12]; Dracocephalum moldavica [22]; Lonicera japonicum [31]; Andean blueberry [39]; Mentha [41] | ||
| 8 | Flavone | Chrysoeriol [Chryseriol] | C16H12O6 | 300.2629 | 301 | 286; 167 | 258 | 203 | Dracocephalum palmatum [8]; Rhus coriaria [34]; Propolis [15] | |
| 9 | Flavone | Homoeriodictyol * | C16H14O6 | 302.2789 | 303 | 285; 177 | 163 | 145 | Mentha [41] | |
| 10 | Flavone | Cirsimaritin [Scrophulein; 4′,5-Dihydroxy-6,7-Dimethoxyflavone; 7-Methylcapillarisin] * | C17H14O6 | 314.2895 | 315 | 282 | 254 | 226; 119 | Rosmarinus officinalis [14]; Ocimum [42] | |
| 11 | Flavone | Dihydroxy-dimethoxy(iso)flavone * | C17H14O6 | 314.2895 | 315 | 300; 272 | 272 | 257; 243; 217; 201; 185; 167 | Rosmarinus officinalis [14]; Propolis [15]; Astragali radix [36] | |
| 12 | Flavone | 5,7-Dimethoxyluteolin * | C17H14O6 | 314.2895 | 313 | 285; 213; 185 | 185; 145 | Syzygium aromaticum [43]; Rosa rugosa [44] | ||
| 13 | Flavone | Myricetin * | C15H10O8 | 318.2351 | 319 | 291; 219; 143 | 191; 143 | 173 | Propolis [15]; Sanguisorba officinalis [24]; Andean blueberry [39]; millet grains [45] | |
| 14 | Flavone | Isothymusin | C17H14O7 | 330.2889 | 331 | 303; 203 | 203; 275 | 203 | Dracocephalum palmatum [8] | |
| 15 | Flavone | Cirsiliol * | C17H14O7 | 330.2889 | 331 | 316; 298; 233; 157 | 297; 187; 134 | Ocimum [42] | ||
| 16 | Flavone | Dimethoxy-trihydroxy(iso)flavone * | C17H14O7 | 330.2889 | 331 | 316; 226 | 298; 226 | 270; 226 | Propolis [15]; Jatropha [46] | |
| 17 | Flavone | Nevadensin | C18H16O7 | 344.3154 | 345 | 312; 241; 147 | 284; 269 | 269; 213; 135 | Dracocephalum [12]; Mentha [41]; Ocimum [42] | |
| 18 | Flavone | Gardenin B [Demethyltangeretin] * | C19H18O7 | 358.342 | 359 | 326; 298 | 298 | 270; 239; 162 | Mentha [41]; Ocimum [42]; Actinocarya tibetica [47] | |
| 19 | Flavone | Dihydroxy-tetramethoxy(iso)flavone * | C19H18O8 | 374.3414 | 375 | 342 | 313; 151 | 299; 151 | Propolis [15] | |
| 20 | Flavone | 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavone * | C20H20O8 | 388.3680 | 389 | 356 | 313 | 295; 221; 149 | Mentha [41] | |
| 21 | Flavone | Apigenin O-hexoside | C21H20O10 | 432.3775 | 431 | 269 | 269; 225; 149 | 224; 157 | Dracocephalum palmatum [8]; F. glaucescens; F. pottsii [25]; Chamaecrista nictitans [48] | |
| 22 | Flavone | Apigenin-7-O-glucoside [Apigetrin; Cosmosiin] | C21H20O10 | 432.3775 | 433 | 271 | 153 | Dracocephalum palmatum [8]; Mentha [23]; Dracocephalum [12] | ||
| 23 | Flavone | Aromadendrin 7-O-rhamnoside * | C21H22O10 | 434.3934 | 433 | 287; 259; 229 | 257; 227; 199; 157 | 199 | Eucalyptus [49]; Zostera marina [50] | |
| 24 | Flavone | Apigenin 7-O-glucuronide | C21H18O11 | 446.361 | 447 | 271 | 153 | 271; 171 | Dracocephalum [12]; Perilla frutescens [20]; Eucalyptus Globulus [51]; Bougainvillea [52] | |
| 25 | Flavone | Acacetin 7-O-glucoside [Tilianin] | C22H22O10 | 446.4041 | 447 | 285; 149 | 270 | 242 | Dracocephalum palmatum [8]; Dracocephalum [12]; Bougainvillea [52] | |
| 26 | Flavone | Luteolin 7-O-glucoside [Cynaroside; Luteoloside] | C21H20O11 | 448.3769 | 449 | 287; 199 | 153 | Dracocephalum [12]; Lonicera japonicum [31]; Passiflora incarnata [53] | ||
| 27 | Flavone | 3′-methoxyacacetin 7-O-beta-D-glucuronide | C22H20O12 | 476.3870 | 475 | 374; 347; 275 | 275; 247; 175 | 247; 175; 147 | Dracocephalum moldavica [22] | |
| 28 | Flavone | Acacetin 7-O-beta-D-glucuronide | C22H20O11 | 460.3876 | 461 | 270; 242; 153 | 242 | Dracocephalum [12]; Dracocephalum moldavica [22] | ||
| 29 | Flavone | 6,4′-Dimethoxyisoflavone-7-O-glucoside * | C23H24O10 | 460.4307 | 461 | 285 | 270; 242; 153 | 242 | Astragali radix [36] | |
| 30 | Flavone | Diosmetin-7-O-beta-glucoside | C22H22O11 | 462.4035 | 463 | 287 | 168 | 123 | Dracocephalum [12]; Dracocephalum moldavica [22]; Oxalis corniculata [54] | |
| 31 | Flavone | Apigenin-O-rhamnoside * | C22H22O11 | 462.4035 | 463 | 273; 153 | 153; 171 | 171 | Passion fruit [55] | |
| 32 | Flavone | Chrysoeriol-7-O-glucuronide * | C22H20O12 | 476.3870 | 477 | 301 | 286 | 258 | Propolis [15] | |
| 33 | Flavone | Acacetin 7-beta-O-(6”-acetyl)-glucoside | C24H24O11 | 488.4408 | 489 | 472; 354; 296; 223 | Dracocephalum moldavica [22] | |||
| 34 | Isoflavone | Apigenin 7-O-beta-D-(6”-O-malonyl)-glucoside | C24H22O13 | 518.4237 | 519 | 184; 500; 466; 371; 258 | 125 | Dracocephalum [12]; Dracocephalum moldavica [22]; Zostera marina [56] | ||
| 35 | Flavone | Acacetin 7-O-beta-D-(6”-O-malonylated)-glucoside | C25H24O13 | 532.4503 | 533 | 371; 285; 191; 165 | 353; 285; 191; 165 | 147 | Dracocephalum moldavica [22] | |
| 36 | Flavone | Diosmetin-7-O-beta-D-(6”-malonyl)-glucoside | C25H24O14 | 548.4497 | 549 | 387; 285 | 370; 272; 147 | 328; 250; 208; 147 | Dracocephalum moldavica [22] | |
| 37 | Flavone | Chrysoeriol O-hexoside C-hexoside * | C28H32O16 | 624.5441 | 625 | 445; 463; 377; 347 | 357; 217 | Triticum aestivum L. [57,58] | ||
| 38 | Flavone | Isovitexin 2”-O-glucoside-7-O-glucoside [Apigenin 6-C-glucoside 2”-O-glucoside-7-O-glucoside] * | C33H40O20 | 756.6587 | 757 | 595; 569; 464; 347; 273 | 577; 503; 431; 335; 242; 182 | Passiflora incarnata [53] | ||
| 39 | Flavonol | Kaempferol [3,5,7-Trihydroxy-2-(4-hydro- xyphenyl)-4H-chromen-4-one] | C15H10O6 | 286.2363 | 287 | 269; 202 | 233; 205 | 216 | Dracocephalum [12]; Rapeseed petals [18]; Lonicera japonicum [31]; Rhus coriaria (Sumac) [34]; Andean blueberry [39] | |
| 40 | Flavonol | Quercetin | C15H10O7 | 302.2357 | 303 | 285; 228; 165 | 229; 165 | 141 | Propolis [15]; Actinidia valvata [16]; Rhus coriaria [34]; Potato leaves [59] | |
| 41 | Flavonol | Herbacetin [3,5,7,8-Tetrahydroxy-2-(4-hydro- xyphenyl)-4H-chromen-4-one] * | C15H10O7 | 302.2357 | 303 | 203; 275 | 221 | Ocimum [42]; Rhodiola rosea [60,61] | ||
| 42 | Flavonol | Dihydroquercetin (Taxifolin; Taxifoliol) | C15H12O7 | 304.2516 | 305 | 287 | 286; 186 | 185 | Dracocephalum [12]; Andean blueberry [39]; Eucalyptus [49]; | |
| 43 | Flavonol | Isorhamnetin [Isorhamnetol; Quercetin 3′-Methyl ether; 3-Methylquercetin] * | C16H12O7 | 316.2623 | 317 | 299; 257; 214; 173 | 281; 188 | Embelia [13]; Rosmarinus officinalis [14]; Propolis [15]; Actinidia valvata [16]; Andean blueberry [39] | ||
| 44 | Flavonoid | 3,5—Diacetyltambulin * | C22H20O9 | 428.3888 | 427 | 381; 249 | 249; 161 | 161; 124 | A. cordifolia [25] | |
| 45 | Flavonol | Dihydrokaempferol-3-O-rhamnoside * | C21H22O10 | 434.3934 | 435 | 287; 261 | 259; 205 | 187 | Vitis vinifera [62,63] | |
| 46 | Flavonol | Astragalin [Kaempferol 3-O-glucoside; Kaempferol-3-Beta-Monoglucoside; Astragaline] | C21H20O11 | 448.3769 | 447 | 285; 327 | 241 | 199 | Dracocephalum [12]; Lonicera japonicum [31]; Mexican lupine species [64] | |
| 47 | Flavonol | Quercitrin [Quercetin 3-O- rhamnoside; Quercetrin] * | C21H20O11 | 448.3769 | 449 | 302 | 202; 174; 127 | 175 | Embelia [13]; Propolis [15]; Rhus coriaria [34]; Bryophyllum pinnatum [54]; Euphorbia hirta [65] | |
| 48 | Flavonol | Kaempferol-3-O-glucuronide | C21H18O12 | 462.3604 | 463 | 287 | 268; 169 | 241; 119 | Dracocephalum [12]; A. cordifolia; G. linguiforme [25]; Rhus coriaria [34]; Strawberry [55] | |
| 49 | Flavonol | Taxifolin-3-O-hexoside [Dihydroquercetin-3-O-hexoside] * | C21H22O12 | 466.3922 | 467 | 305; 259; 195; 153 | 259; 195; 153 | 231; 149 | Andean blueberry [39]; millet grains [45]; Euphorbia hirta [65] Actinidia deliciosa [66]; | |
| 50 | Flavonol | Kaempferol 3-O-rutinoside | C27H30O15 | 594.5181 | 595 | 287; 345; 389; 449 | 287; 245; 153 | 171 | Dracocephalum [12]; Lonicera japonicum [31]; Rhus coriaria [34]; | |
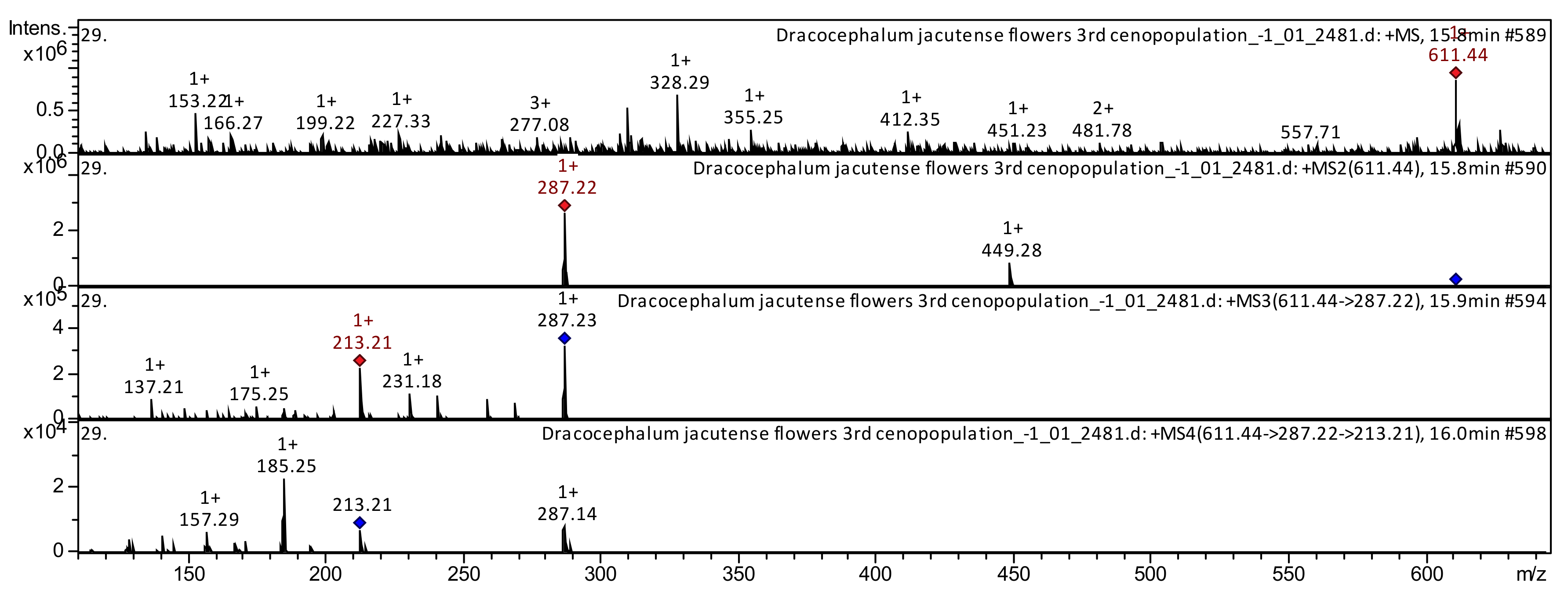
| 51 | Flavonol | Kaempferol-3,7-Di-O-glucoside * | C27H30O16 | 610.5175 | 611 | 287; 449 | 287; 213; 185; 137 | 185; 157 | Tomato [17]; Rapeseed petals [18]; Taraxacum officinale [19] | |
| 52 | Flavonol | Kaempferol dihexoside rhamnoside * | C33H40O20 | 756.6587 | 757 | 595; 287 | 287; 213; 137 | 185; 168 | C. edulis [25] | |
| 53 | Flavan-3-ol | (epi)Afzelechin * | C15H14O5 | 274.2687 | 275 | 228; 210; 175; 157; 132 | 212; 203; 183; 170 | 194 | A. cordifolia; F. glaucescens; F. herrerae [25]; Cassia granidis [67]; Cassia abbreviata [68] | |
| 54 | Flavan-3-ol | Catechin [D-Catechol] * | C15H14O6 | 290.2681 | 291 | 207; 123 | 123 | Vaccinium macrocarpon [69]; Vigna inguiculata [70]; Camellia kucha [71]; Actinidia [72] | ||
| 55 | Flavan-3-ol | (epi)catechin | C15H14O6 | 290.2681 | 291 | 273; 117 | 255; 145 | Dracocephalum [12]; C. edulis [25]; Andean blueberry [39] | ||
| 56 | Flavan-3-ol | Gallocatechin [+(-)Gallocatechin] | C15H14O7 | 306.2675 | 307 | 289 | 259 | Dracocephalum [12]; G. linguiforme [25]; Licania ridigna [73]; Rhodiola rosea [74] | ||
| 57 | Flavan-3-ol | (epi)Afzelechin derivative * | C18H16O10 | 392.3136 | 393 | 275; 179 | 191 | Zostera marina [50] | ||
| 58 | Flavan-3-ol | Catechin 3-O-gallate * | C22H18O10 | 442.3723 | 443 | 273; 205 | 263; 211; 171; 143 | Vitis vinifera [62]; Camellia kucha [71]; Terminalia arjuna [75] | ||
| 59 | Flavan-3-ol | Epigallocatechin-3-gallate * | C22H18O11 | 458.3717 | 459 | 290; 207 | 207; 123 | F. glaucescens [25]; Vitis vinifera [62]; Camellia kucha [71] | ||
| 60 | Flavanone | Naringenin [Naringetol; Naringenine] | C15H12O5 | 272.5228 | 273 | 153; 256 | 125 | Dracocephalum palmatum [8]; Dracocephalum [12] Rapeseed petals [18]; Andean blueberry [39] | ||
| 61 | Flavanone | Eriodictyol [3′,4′,5,7-tetrahydroxy-flavanone] | C15H12O6 | 288.2522 | 289 | 163; 271 | 145 | 117 | Dracocephalum palmatum [8]; Dracocephalum [12]; Mentha [23]; Andean blueberry [39] | |
| 62 | Isolavanone | Ferreirin | C16H14O6 | 302.2789 | 303 | 177; 285 | 163 | 135 | Mentha [23] | |
| 63 | Flavanone | Prunin [Naringenin-7-O-glucoside] | C21H22O10 | 434.3934 | 433 | 271; 151 | 269; 151 | Dracocephalum palmatum [8]; Dracocephalum [12]; Rapeseed petals [18] | ||
| 64 | Flavanone | Eriodictyol-7-O-glucoside [Pyracanthoside; Miscanthoside] | C21H22O11 | 450.3928 | 449 | 285; 151 | 243; 151 | Dracocephalum [12]; Mentha [23]; Dracocephalum palmatum [7,8] | ||
| 65 | Flavanone | Eriodictyol-7-O-glucuronide | C21H20O12 | 464.3763 | 463 | 285; 151 | 285; 243; 151 | Thymus vulgaris [76]; Mentha [77] | ||
| 66 | Hydroxybenzoic acid | Protocatechuic acid | C7H6O4 | 154.1201 | 155 | 127 | 117 | Lonicera japonicum [31]; Rhus coriaria [34]; Eucalyptus Globulus [51]; Vaccinium macrocarpon [69]; Actinidia [72] | ||
| 67 | Hydroxycinnamic acid | p-Coumaric acid [4-Hydroxycinnamic acid; P-Hydroxycinnamic acid; 4-Coumarate] | C9H8O3 | 164.1580 | 165 | 147 | 119 | Rapeseed petals [18]; F. pottsii [25]; Rhus coriaria [34]; Andean blueberry [39]; Brazilian propolis [78] | ||
| 68 | Hydroxycinnamic acid | Caffeic acid | C9H8O4 | 180.1574 | 181 | 135 | 119 | Dracocephalum palmatum [8]; Dracocephalum [12]; Eucalyptus [49] | ||
| 69 | Hydroxycinnamic acid | 3,4-Dihydroxyhydrocinnamic acid | C9H10O4 | 182.1733 | 183 | 137 | Eucalyptus Globulus [51] | |||
| 70 | Phenolic acid | 2,3,4,5-Tetrahydroxybenzoic acid [2-Hydroxygallussaure; 3,4,5-Trihydroxysalicylic acid] | C7H6O6 | 186.1189 | 187 | 144 | PubChem | |||
| 71 | Phenolic acid | Salvianic acid A [Danshensu] | C9H10O5 | 198.1727 | 197 | 179; 135 | 135 | Huolisu Oral Liquid [38]; Mentha [77]; Hedyotis diffusa [79] | ||
| 72 | Phenolic acid | 2,3-Dihydroxy-4-Mathoxycinnamic acid | C10H10O5 | 210.1834 | 211 | 192; 134 | 134; 174 | A. cordifolia [25] | ||
| 73 | Hydroxybenzoic acid | Ellagic acid [Benzoaric acid; Elagostasine; Lagistase; Eleagic acid] | C14H6O8 | 302.1926 | 301 | 284 | 221 | 112 | Dracocephalum [12]; Rhus coriaria [34]; Eucalyptus [49]; Eucalyptus Globulus [51] | |
| 74 | Phenolic acid | Protocatechuic acid-O-hexoside | C13H16O9 | 316.2607 | 315 | 153; 123 | 123 | Rhus coriaria [34]; Eucalyptus Globulus [51]; Euphorbia hirta [65] | ||
| 75 | Phenolic acid | Salvianolic acid G | C18H12O7 | 340.2837 | 341 | 296; 208 | 278; 208 | 235; 164 | Dracocephalum [12]; Mentha [41]; Salvia miltiorrhiza [80] | |
| 76 | Phenolic acid | Caffeic acid-4-O-beta-D-hexoside [Caffeoyl-O-hexoside] | C15H18O9 | 342.298 | 341 | 179; 119 | 143; 131 | Dracocephalum [12]; Cherimoya, papaya [55]; Sasa veitchii [81] | ||
| 77 | Phenolic acid | Chlorogenic acid [3-O-Caffeoylquinic acid] | C16H18O9 | 354.3087 | 355 | 179; 338; 227 | 127 | Dracocephalum palmatum [8]; Rapeseed petals [18]; Lonicera japonicum [31]; Rhus coriaria [34]; Andean blueberry [39] | ||
| 78 | Phenolic acid | Isochlorogenic acid | C16H18O9 | 354.3087 | 355 | 323; 269; 165 | 295; 208; 133 | 295; 249; 221 | Actinidia [72] | |
| 79 | Phenolic acid | Rosmarinic acid | C18H16O8 | 360.3148 | 359 | 161 | 133 | Dracocephalum palmatum [8]; Dracocephalum [12]; Mentha [41]; Zostera marina [56]; Salvia miltiorrhiza [80]; Lepechinia [82] | ||
| 80 | 3-Prenyl-4-(dihydrocinnamoyloxy)-cinnamic acid | C23H24O4 | 364.4343 | 365 | 261; 185 | 233; 179 | 179; 151 | Brazilian propolis [78] | ||
| 81 | Phenolic acid | Caffeic acid derivative | C16H18O9Na | 377.2985 | 377 | 341; 215 | 179 | Dracocephalum [12]; Bougainvillea [52] | ||
| 82 | Phenolic acid | 1/3/4/5-p-Coumaroylquinic acid +C2H2O | C18H20O9 | 380.3460 | 381 | 321; 275; 233 | 260; 218; 143 | Actinidia [72] | ||
| 83 | Phenolic acid | 8,8′-Aryl-Diferulic acid | C20H18O8 | 386.3521 | 385 | 193; 285 | 193; 161 | millet grains [45] | ||
| 84 | Phenolic acid | Caffeic acid hexoside dimer | C31H40O17 | 684.6391 | 683 | 341 | 179; 161 | 143 | Strawberry, Lemon, Cherimoya, Passion fruit [55] | |
| 85 | Phenolic acid | Didehydrosalvianolic acid B | C36H28O16 | 716.5979 | 717 | 574 | 319; 263; 187 | 299; 177 | Mentha [77] | |
| 86 | Phenolic acid | Salvianolic acid B [Danfensuan B] | C36H30O16 | 718.6138 | 717 | 519; 321 | 321; 279 | 279; 185 | Huolisu Oral Liquid [38]; Bougainvillea [52]; Mentha [77]; Salvia miltiorrhiza [80] | |
| 87 | Phenylpropanoic acid | Sagerinic acid | C36H32O16 | 720.6297 | 719 | 359 | 161; 197 | 133 | Dracocephalum palmatum [8]; Rosmarinus officinalis [14]; Perilla frutescens [20]; Huolisu Oral Liquid [38]; Mentha [41] | |
| 88 | Phenolic acid | Clerodendranoic acid H | C36H32O16 | 720.6297 | 719 | 359 | 161 | Lepechinia [82]; | ||
| 89 | Lignan | Phillygenin [Sylvatesmin; Phyllygenol; Forsythigenol] | C21H24O6 | 372.4117 | 371 | 163; 325 | 119 | Lignans [83] | ||
| 90 | Lignan | Medioresinol | C21H24O7 | 388.4111 | 387 | 207; 163; 119 | 163 | Rosmarinus officinalis [14]; Lignans [83]; Punica granatum [84] | ||
| 91 | Neolignan | Urolignoside + H2O | C25H34O11 | 510.5309 | 511 | 493; 451; 421; 349; 285 | 349; 254; 147 | 331; 289; 259 | Jasminum urophyllum [26]; Actinidia [72] | |
| 92 | Dihydrochalcone | Phloretin [Dihydronaringenin; Phloretol] | C15H14O5 | 274.2687 | 275 | 255; 229; 131 | 237; 209; 164 | G. linguiforme [25]; Rosa rugosa [44]; Punica granatum [84] | ||
| 93 | Hydroxycoumarin | Umbelliferone [Skimmetin; Hydragin] | C9H6O3 | 162.1421 | 163 | 145; 135; 117 | 117 | Sanguisorba officinalis [24]; F. glaucescens [25]; Zostera marina [50]; Actinidia [72] | ||
| 94 | Coumarin | Fraxetin [7,8-Dihydroxy-6-methoxycoumarin] | C10H8O5 | 208.1675 | 209 | 191; 149 | 149 | 147 | Embelia [13]; Jatropha [46]; Actinidia [72]; | |
| 95 | Hydroxycoumarin | Umbelliferone hexoside | C15H16O8 | 324.2827 | 325 | 307; 288; 271; 253; 241 | 127; 118 | G. linguiforme [25] | ||
| 96 | Coumarin glycoside | Fraxin (Fraxetin-8-O-glucoside) | C16H18O10 | 370.3081 | 371 | 209 | Rosa rugosa [44]; Actinidia [72] | |||
| 97 | Anthocyanidin | Petunidin | C16H13O7+ | 317.2702 | 318 | 166; 300 | 121 | Dracocephalum [12]; A. cordifolia; C. edulis [21] | ||
| 98 | Anthocyanidin | Pelargonidin-3-O-glucoside (callistephin) | C21H21O10 | 433.3854 | 433 | 271 | 153; 225 | 171 | Dracocephalum [12]; Triticum aestivum [85]; Rubus ulmifolius [86] | |
| 99 | Anthocyanidin | Cyanidin-3-O-glucoside [Cyanidin 3-O-beta-D-Glucoside; Kuromarin] | C21H21O11+ | 449.3848 | 449 | 287 | 153 | Dracocephalum [12]; Triticum aestivum [85]; Malpighia emarginata [87] | ||
| 100 | Anthocyanidin | Cyanidin 3,5-O-diglucoside | C27H31O16 | 611.5335 | 611 | 287; 449 | 287; 241; 213; 175; 149 | 213; 185; 172; 157; 145 | Rapeseed petals [18]; Muscadine pomace [88]; Berberis microphylla [89] | |
| 101 | Anthocyanidin | Peonidin-3,5-diglucoside [Peonin; Peonidin 3-Glucoside-5-Glucoside] | C28H33O16 | 625.5520 | 625 | 463; 374; 301 | 445; 373 | Triticum aestivum [85]; Muscadine pomace [88] | ||
| 102 | Anthocyanidin | Cyanidin-3-O-rutinoside-5-O-glucoside | C33H41O20 | 757.6666 | 757 | 287; 449; 595 | 287; 213; 137 | 185 | Camellia kucha [71]; Solanium nigrum [9] | |
| 103 | Anthocyanidin | Delphinidin 3-O-rutinoside-5-O-glucoside | C33H41O21 | 773.5769 | 773 | 303; 465; 611 | 257; 303; 229; 165 | 257; 229; 201; 116 | Berberis microphylla [89]; Solanium nigrum [90]; Iris dichotoma [91] | |
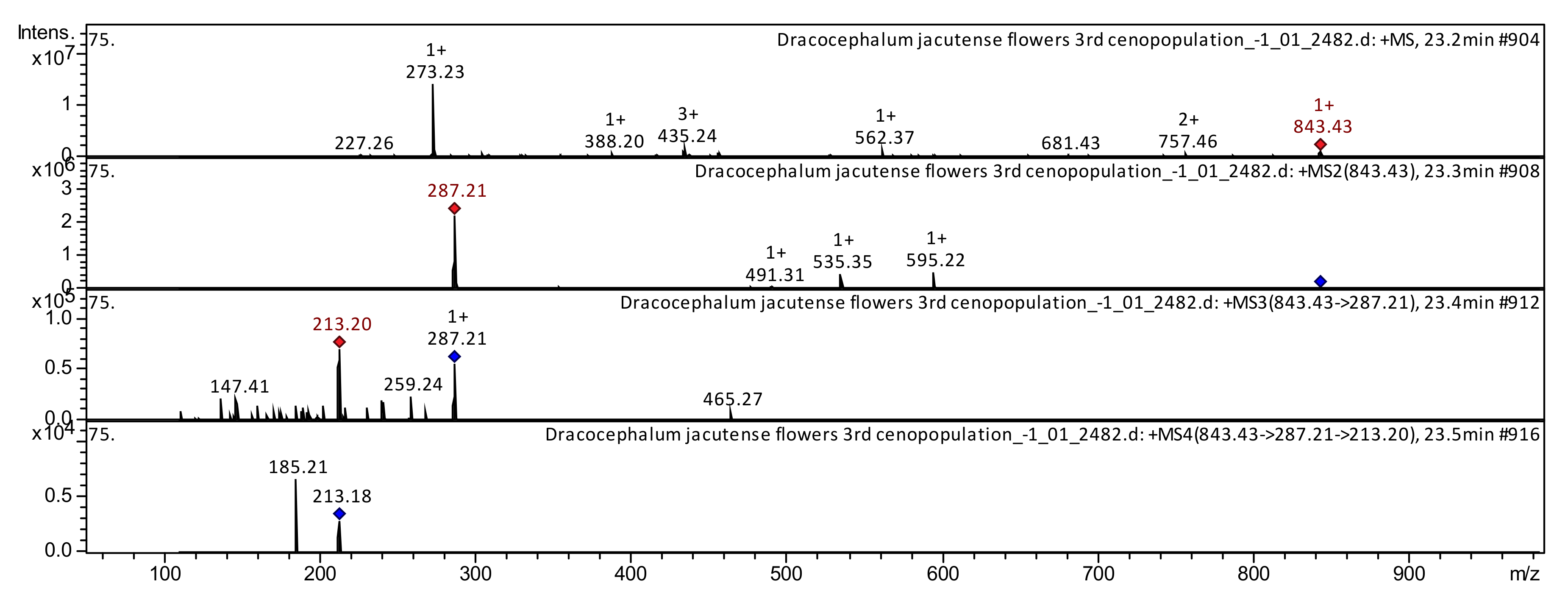
| 104 | Anthocyanidin | Malonyl-shisonin | C39H39O21+ | 843.7144 | 843 | 595; 535; 491; 287 | 287; 259; 213; 147 | 213; 185 | Perilla frutescens [20,21] | |
| OTHERS | ||||||||||
| 105 | Benzenediol | Catechol derivative | C6H6O3 | 126.1100 | 127 | 124; 118 | Embelia [13]; | |||
| 106 | Alkyl cinnamate | Methyl cinnamate [Methyl 3-Phenylacrilate] | C10H10O2 | 162.1852 | 164 | 144 | Strawberry [92] | |||
| 107 | Amino acid | Phenylalanine [L-Phenylalanine] | C9H11NO2 | 165.1891 | 166 | 147; 120 | Rapeseed petals [18]; Lonicera japonica [31]; Passiflora incarnata [53]; Potato leaves [59] | |||
| 108 | Amino acid | Tyrosine [(2S)-2-Amino-3-(4-Hydroxyphnyl)Propanoic acid] | C9H11NO3 | 181.1885 | 182 | 165; 150 | 113 | Euphorbia hirta [65]; Vigna unguiculata [93]; Hylocereus polyrhizus [94] | ||
| 109 | Monobasic carboxylic acid | Hydroxyphenyllactic acid | C9H10O4 | 182.1733 | 181 | 163; 135 | 119 | Mentha [95] | ||
| 110 | Amino acid | L-Tryptophan [Tryptophan; (S)-Tryptophan] | C11H12N2O2 | 204.2252 | 205 | 188 | 144 | 118 | Dracocephalum [12]; Rapeseed petals [18]; Huolisu Oral Liquid [38]; Rosa acicularis [44] | |
| 111 | Aminoalkylindole | 5-Methoxydimethyltryptamine | C13H18N2O | 218.2948 | 219 | 201 | 159; 118 | Dracocephalum [12]; Camellia kucha [71] | ||
| 112 | Omega-5 fatty acid | Myristoleic acid [Cis-9-Tetradecanoic acid] | C14H26O2 | 226.3550 | 227 | 209 | 139 | Dracocephalum [12]; F. glaucescens [25] | ||
| 113 | Germacranolide | Costunolide | C15H20O2 | 232.3181 | 233 | 187; 215 | 145 | 143 | Rosa davurica [44]; Weichang’an Pill [96] | |
| 114 | Medium-chain fatty acid | Hydroxy myristic acid [2S-Hydroxytetradecanoic acid; Alpha-Hydroxy Myristic acid] | C14H28O3 | 244.3703 | 246 | 228; 159 | 199; 172 | 144 | F. pottsii [25] | |
| 115 | Xanthone | Mangiferitin [Norathyriol; 1,3,6,7-Tetrahydroxyxanthone] | C13H8O6 | 260.1990 | 261 | 193; 135 | 179; 124 | 111 | Rhus coriaria [34] | |
| 116 | Aporphine alkaloid | Anonaine | C17H15NO2 | 265.3065 | 266 | 248; 171; 122 | 229; 182; 116 | 212; 182 | Rosa rugosa [44]; Magnolia [97] | |
| 117 | Ribonucleoside composite of adenine (purine) | Adenosine | C10H13N5O4 | 267.2413 | 268 | 136; 258 | Dracocephalum [12]; Lonicera japonica [31] | |||
| 118 | Omega 3-fatty acid | Stearidonic acid [6,9,12,15-Octadecatetraenoic acid; Moroctic acid] | C18H28O2 | 276.4137 | 277 | 177; 247 | 175 | G. linguiforme [25]; Rhus coriaria [34]; Jatropha [46]; Salvia Miltiorrhiza [80] | ||
| 119 | Omega 3-fatty acid | Linolenic acid (Alpha-Linolenic acid; Linolenate) | C18H30O2 | 278.4296 | 279 | 219; 259 | 159 | Jatropha [46]; Salvia Miltiorrhiza [80]; Pinus sylvestris [98] | ||
| 120 | Fatty amide | Linoleic acid amide | C18H33NO | 279.4607 | 280 | 262; 244 | 244; 234; 216; 196; 172 | 196; 168; 151 | Propolis [15]; Rhus coriaria [34] | |
| 121 | Fatty amide | Oleamide | C18H35NO | 281.4766 | 282 | 263; 246; 192 | 245; 228; 217; 197; 170 | Propolis [15] | ||
| 122 | Alkaloid | Mesembrenol | C17H23NO3 | 289.3694 | 290 | 242; 122 | 184; 149 | Dracocephalum [12]; Sceletium [29] | ||
| 123 | Diterpenoid naphthoquinone | Tanshinone IIA [Tanshinone II; Tanshinone B] | C19H18O3 | 294.3444 | 295 | 277; 259; 193; 149 | 259; 241; 199; 149 | 241; 147 | Huolisu Oral Liquid [38] | |
| 124 | Unsaturated hydroxy fatty acid | Hydroxyoctadecatrienoic acid | C18H30O3 | 294.4290 | 293 | 275; 235; 185; 172 | 231; 205; 177 | 231; 163 | Jatropha [46] | |
| 125 | Polyunsaturated fatty acid | Alpha-Kamlolenic Acid [18-Hydroxy-9Z,11E,13E- Octadecatrienoic Acid] | C18H30O3 | 294.4290 | 293 | 275; 231; 171 | 231; 177 | 231 | G. linguiforme; F. glaucescens; F. pottsii [25] | |
| 126 | Essential fatty acid | Hydroxyoctadecadienoic acid | C18H32O3 | 296.4449 | 295 | 277; 251; 195; 171; 152 | 233; 179; 155 | A. cordifolia; F. glaucescens; F. herrerae [25]; Jatropha [46] | ||
| 127 | Pterocarpan | 3-Hydroxy-9,10-dimethoxypterocarpan | C17H16O5 | 300.3059 | 301 | 286; 257; 229; 177; 153 | 163; 149 | 145 | Astragali radix [36]; Huolisu Oral Liquid [38] | |
| 128 | Diterpenoid | Tanshinone IIB [(S)-6-(Hydroxymethyl)-1,6-Dimethyl-6,7,8,9-Tetrahydrophenanthro [1,2-B]Furan-10,11-Dione] | C19H18O4 | 310.3438 | 311 | 283; 137 | 119 | Huolisu Oral Liquid [38]; Salvia Miltiorrhiza [80] | ||
| 129 | p-hydroxyphenacyl-β-D-glucopyranoside | C14H18O8 | 314.2879 | 313 | 161; 213 | 133; 161 | 133 | Rhodiola crenulata [99] | ||
| 130 | Long-chain fatty acid | Hydroxy eicosenoic acid | C20H38O3 | 326.5139 | 327 | 295; 268; 181; 125 | 268 | 237; 135 | A. cordifolia; F. pottsii [25] | |
| 131 | Fructose-phenylalanine | C15H21NO7 | 327.3297 | 328 | 310; 292 | 292; 264; 244; 216; 198; 178 | 244; 216; 198; 171; 156 | Potato leaves [59] | ||
| 132 | Oxylipins | 9,10-Dihydroxy-8-oxooctadec-12-enoic acid [oxo-DHODE; oxo-Dihydroxy-octadecenoic acid] | C18H32O5 | 328.4437 | 327 | 229 | 209 | 183 | Dracocephalum [12]; Phyllostachys nigra [81]; Bituminaria [100] | |
| 133 | Oxylipins | 13- Trihydroxy-Octadecenoic acid [THODE] | C18H34O5 | 330.4596 | 329 | 229; 293; 211; 171 | 211; 229; 155 | 183; 211 | Dracocephalum [12]; Sasa veitchii [81]; Bituminaria [100] | |
| 134 | Unsaturated essential fatty acid | Dihydroxy eicosatrienoic acid | C20H34O4 | 338.4816 | 339 | 321; 177; 145 | 145 | 117 | G. linguiforme; A. cordifolia; C. edulis [25] | |
| 135 | Diterpenoid | Komarovinone A | C21H28O4 | 344.4446 | 345 | 312; 240 | 284; 121 | 268; 135 | Dracocephalum komarovi [27] | |
| 136 | Triterpene | Dracocephalone A | C20H26O5 | 346.4174 | 347 | 319; 287; 219 | 219 | 191 | Dracocephalum komarovi [27] | |
| 137 | Unsaturated omega-3 fatty acid | Trihydroxy eicosatetraenoic acid | C20H32O5 | 352.4651 | 353 | 261; 293; 243; 207 | 243; 201; 159; 132 | 162 | F. glaucescens [25] | |
| 138 | Tetracyclic diterpenoid | Komaroviquinone | C21H28O5 | 360.4440 | 361 | 343; 302 | 310; 269; 218; 161 | 282 | Dracocephalum komarovi [27] | |
| 139 | Triterpene | Squalene (Trans-Squalene; Spinacene; Supraene) | C30H50 | 410.718 | 411 | 393; 36; 291; 244; 198 | Olive leaves [33]; squalene [101] | |||
| 140 | Sterol | Stigmasterol [Stigmasterin; Beta-Stigmasterol] | C29H48O | 412.6908 | 413 | 395; 301; 237; 189 | 189 | Dracocephalum [12]; A.cordifolia; F. pottsii [25]; Olive leaves [33]; Hedyotis diffusa [79] | ||
| 141 | Anabolic steroid; Androgen; Androgen ester | Vebonol | C30H44O3 | 452.6686 | 453 | 435; 336; 226 | 336 | 209 | Dracocephalum [12]; Rhus coriaria [34]; Hylocereus polyrhizus [94] | |
| 142 | Triterpenic acid | Betulonic acid [Betunolic acid; Liquidambaric acid] | C30H46O3 | 454.6844 | 455 | 436; 353; 313; 249 | 393; 336; 319; 282 | 154 | Rhus coriaria [34]; Rosa rugosa [44] | |
| 143 | Triterpenic acid | 1-Hydroxy-3-oxours-12-en-28-oic acid | C30H46O4 | 470.6838 | 471 | 453; 425; 407; 389 | 365; 335; 283; 205 | 177; 121 | Pear [102] | |
| 144 | Triterpenic acid | Pomolic acid | C30H48O4 | 472.6997 | 473 | 454; 371; 302; 144 | Sanguisorba officinalis [24]; Pear [102]; Malus domestica [103] | |||
| 145 | Triterpenic acid | Tormentic acid [Jacarandic acid; Tomentic acid] | C30H48O5 | 488.6991 | 487 | 470; 423; 372 | 403; 377 | Sanguisorba officinalis [24]; Actinidia [72]; Pear [102] | ||
| 146 | Monoterpene glycoside | Rhodioloside C [(2E,4R)-4-hydroxy-3,7-dimethyl-2,6-octadienyl β-D-glucopyranosyl(1-3)-β-D-glucopyranoside] | C22H38O12 | 494.5299 | 493 | 447; 329; 285 | 309; 285 | 294; 187 | Rhodiola crenulata [99]; Rhodiola rosea [104,105,106] | |
| 147 | Carotenoid | (all-E)-lutein 3′-O-myristate | C40H54O | 550.8562 | 551 | 533; 509; 429; 385; 355 | 133 | Rosa rugosa [30]; Carotenoids [107] | ||
| 148 | Indole sesquiterpene alkaloid | Sespendole | C33H45NO4 | 519.7147 | 520 | 184; 359 | 124 | Dracocephalum [12]; Rhus coriaria [34]; Hylocereus polyrhizus [94] | ||
| 149 | Carotenoid | Cryptoxanthin [Beta-cryptoxanthin] | C40H56O | 552.872 | 553 | 535; 325; 223 | 517 | Dracocephalum [12]; Sarsaparilla [28]; Carotenoids [107,108]; | ||
| 150 | Carotenoid | Zeaxanthin [All-Trans-Zexanthin; Anchovyxanthin] | C40H56O2 | 568.8714 | 569 | 553; 534; 471; 359 | 534; 486; 326; 262 | 516; 473; 308; 262 | Sarsaparilla [28]; Carotenoids [107]; orange juice [109] | |
| 151 | Product of chlorophyll breakdown | Pheophorbide a | C35H34N4O6 | 606.6677 | 607 | 547; 503; 461 | 461; 433 | 433 | Product of Chlorophylle breakdown [110] | |
| 152 | Cycloartanol | Cyclopassifloic acid glucoside | C37H62O12 | 698.8810 | 699 | 537 | 375; 331; 259; 185 | Passiflora incarnata [53] | ||
| 153 | Carotenoid | Carotenoid | C41H59O10 | 711.9012 | 712 | 695; 605; 543; 474; 456 | 412; 369; 200; 143 | Carotenoids [32] | ||
| 154 | Carotenoid | (all-E)-beta-cryptoxanthin laurate [Beta-Cryptoxanthin-Laurate] | C52H78O2 | 735.1745 | 735 | 323; 521; 277 | 295; 163 | 249; 173; 134 | Sarsaparilla [28]; Carotenoids [107]; Carica papaya [111] | |
| 155 | Product of chlorophyll degradation | Pheophytin A | C55H74N4O5 | 871.1999 | 593; 533 | 533; 461 | 461; 433 | Product of Chlorophylle breakdown [110]; Physalis peruviana [112]; Capsicum [113] |
References
- Zakharova, V.I.; Kuznetsova, L.V. Abstract of the Flora of Yakutia: Vascular Plants; Nauka Publishing House: Novosibirsk, Russia, 2012; p. 272. (In Russian) [Google Scholar]
- Danilova, N.S.V. 1: Rare and endangered species of plants and fungi. In Red Book of the Republic of Sakha (Yakutia); “Reart” Publishing House: Moscow, Russia, 2017; 412p. (In Russian) [Google Scholar]
- Peshkova, G.A. Dracocephalum L. Flora of Siberia; Nauka Publishing House: Novosibirsk, Russia, 1997; Volume 11, pp. 170–185. (In Russian) [Google Scholar]
- Egorova, P.S. To the introduction of Dracocephalum jacutense (Lamiaceae) in the Yakutsk Botanical Garden. Vestnik KrasSAU 2020, 5, 17–23. (In Russian) [Google Scholar]
- Budantsev, A.L.; Shavarda, A.L. Chemical composition and useful properties of Dracocephalum L. species of the USSR flora. Message 2. Plant Resour. 1987, 23, 287–295. (In Russian) [Google Scholar]
- Zeng, Q.; Jin, H.Z.; Qin, J.J.; Fu, J.J.; Hu, X.J.; Liu, J.H.; Yan, L.; Chen, M.; Zhang, W.D. Chemical constituents of plants from the genus Dracocephalum. Chem. Biodivers. 2010, 7, 1911–1929. [Google Scholar] [CrossRef] [PubMed]
- Komartin, R.S.; Stroescu, M.; Chira, N.; Stan, R.; Stoica-Guzun, A. Optimization of oil extraction from Lallemantia iberica seeds using ultrasound-assisted extraction. J. Food Meas. Charact. 2021, 15, 2010–2020. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Okhlopkova, Z.M.; Zulfugarov, I.S. Chemical Composition and Antioxidant Activity of Tánara Ótó (Dracocephalum palmatum Stephan), a Medicinal Plant Used by the North-Yakutian Nomads. Molecules 2013, 18, 14105. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.N.; Park, I.; Sivtseva, S.; Okhlopkova, Z.; Zulfugarov, I.S.; Kim, S.-W. Dracocephalum palmatum Stephan extract induces caspase- and mitochondria-dependent apoptosis via Myc inhibition in diffuse large B cell lymphoma. Oncol. Rep. 2020, 44, 2746–2756. [Google Scholar] [CrossRef]
- Lee, S.-E.; Okhlopkova, Z.; Lim, C.; Cho, S. Dracocephalum palmatum Stephan extract induces apoptosis in human prostate cancer cells via the caspase-8-mediated extrinsic pathway. Chin. J. Nat. Med. 2020, 18, 793–800. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Okhlopkova, Z.M.; Golokhvast, K.S. Research of Dracocephalum palmatum S. and Dracocephalum ruyschiana L. originating from Yakutia and identification of metabolites by tandem mass spectrometry. BIO Web Conf. 2022, 43, 01010. [Google Scholar] [CrossRef]
- Okhlopkova, Z.M.; Razgonova, M.P.; Pikula, K.S.; Zakharenko, A.M.; Piekoszewski, W.; Manakov, Y.A.; Ercisli, S.; Golokhvast, K.S. Dracocephalum palmatum S. and Dracocephalum ruyschiana L. originating from Yakutia: A high-resolution mass spectrometric approach for the comprehensive characterization of phenolic compounds. Appl. Sci. 2022, 12, 1766. [Google Scholar] [CrossRef]
- Vijayan, K.P.R.; Raghu, A.V. Tentative characterization of phenolic compounds in three species of the genus Embelia by liquid chromatography coupled with mass spectrometry analysis. Spectrosc. Lett. 2019, 52, 653–670. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef] [PubMed]
- Belmehdi, O.; Bouyahya, A.; Jekő, J.; Cziáky, Z.; Zengin, G.; Sotkó, G.; EL Baaboua, A.; Senhaji, N.S.; Abrini, J. Synergistic interaction between propolis extract, essential oils, and antibiotics against Staphylococcus epidermidis and methicillin resistant Staphylococcus aureus. Int. J. Second Metab. 2021, 8, 195–213. [Google Scholar] [CrossRef]
- Du, Q.-H.; Zhang, Q.-Y.; Han, T.; Jiang, Y.-P.; Peng, C.; Xin, H.-L. Dynamic changes of flavonoids in Actinidia valvata leaves at different growing stages measured by HPLC-MS/MS. Chin. J. Nat. Med. 2016, 14, 66–72. [Google Scholar] [PubMed]
- Le Gall, G.; DuPont, M.S.; Davis, A.L.; Collins, G.J.; Verhoeyen, M.E.; Colquhoun, I.J. Characterization and Content of Flavonoid Glycosides in Genetically Modified Tomato (Lycopersicum esculentum) Fruits. J. Agric. Food Chem. 2003, 51, 2438–2446. [Google Scholar] [CrossRef]
- Yin, N.-W.; Wang, S.-X.; Jia, L.-D.; Zhu, M.-C.; Yang, J.; Zhou, B.-J.; Yin, J.-M.; Lu, K.; Wang, R.; Li, J.-N.; et al. Identification and Characterization of Major Constituents in Different-Colored Rapeseed Petals by UPLC−HESI-MS/MS. J. Agric. Food Chem. 2019, 67, 11053–11065. [Google Scholar] [CrossRef]
- Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Mukhtar, H.; Raza, S.A.; Touqeer, T.; Saari, N. Anti-Obesity Attributes. UHPLC-QTOF-MS/MS-Based Metabolite Profiling and Molecular Docking Insights of Taraxacum officinale. Molecules 2020, 25, 4935. [Google Scholar] [CrossRef]
- Yamazaki, M.; Nakajima, J.-I.; Yamanashi, M.; Sugiyama, M.; Makita, Y.; Springob, K.; Awazuhara, M.; Saito, K. Metabolomics and differential gene expression in anthocyanin chemo-varietal forms of Perilla frutescens. Phytochemistry 2003, 62, 987–995. [Google Scholar] [CrossRef]
- He, Y.-K.; Yao, Y.-Y.; Chang, Y.-N. Characterization of Anthocyanins in Perilla frutescens var. acuta Extract by Advanced UPLC-ESI-IT-TOF-MSn Method and Their Anticancer Bioactivity. Molecules 2015, 20, 9155–9169. [Google Scholar]
- Martinez-Vazquez, M.; Estrada-Reyes, R.; Martinez-Laurrabaquio, A.; Lopez-Rubalcava, C.; Heinze, G. Neuropharmacological study of Dracocephalum moldavica L. (Lamiaceae) in mice: Sedative effect and chemical analysis of an aqueous extract. J. Ethnopharmacol. 2012, 141, 908–917. [Google Scholar] [CrossRef]
- Li, X.; Tian, T. Phytochemical Characterization of Mentha spicata L. Under Differential Dried-Conditions and Associated Nephrotoxicity Screening of Main Compound with Organ-on-a-Chip. Front. Pharmacol. 2018, 9, 1067. [Google Scholar] [CrossRef]
- Kim, S.; Oh, S.; Noh, H.B.; Ji, S.; Lee, S.H.; Koo, J.M.; Choi, C.W.; Jhun, H.P. In Vitro Antioxidant and Anti-Propionibacterium acnes Activities of Cold Water, Hot Water, and Methanol Extracts, and Their Respective Ethyl Acetate Fractions, from Sanguisorba officinalis L. Roots. Molecules 2018, 23, 3001. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.R.; El-Hawary, S.S.; Ibrahim, R.M.; Abdelmohsen, U.R.; El-Halawany, A.M. Identification of Chemopreventive Components from Halophytes Belonging to Aizoaceae and Cactaceae Through LC/MS –Bioassay Guided Approach. J. Chromatogr. Sci. 2021, 59, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C.; Hsieh, P.-W.; Kuo, Y.-H. Neolignan glucosides from Jasminum urophyllum. Phytochemistry 1998, 48, 719–723. [Google Scholar] [CrossRef]
- Uchiyama, N.; Kiuchi, F.; Ito, M.; Honda, G.; Takeda, Y.; Khodzhimatov, O.K.; Ashurmetov, O.A. New Icetexane and 20-Norabietane Diterpenes with Trypanocidal Activity from Dracocephalum komarovi. J. Nat. Prod. 2003, 66, 128–131. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Homero-Mendez, D. Identification and Quantitative Analysis of Carotenoids and Their Esters from Sarsaparilla (Smilax aspera L.) Berries. J. Chromatogr. A 2012, 60, 8225–8232. [Google Scholar] [CrossRef]
- Patnala, S.; Kanfer, I. Medicinal use of Sceletium: Characterization of Phytochemical Components of Sceletium Plant Species using HPLC with UV and Electrospray Ionization—Tandem Mass Spectroscopy. J. Pharm. Pharm. Sci. 2015, 18, 414–423. [Google Scholar] [CrossRef]
- Al-Yafeai, A.; Malarski, A.; Bohm, V. Characterization of carotenoids and vitamin E in R. rugosa and R. canina: Comparative analysis. Food Chem. 2018, 242, 435–442. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Zou, L.; Liu, X.; Chen, J.; Tan, M.; Mei, Y.; Wei, L. Comparison of Multiple Bioactive Constituents in the Flower and the Caulis of Lonicera japonica Based on UFLC-QTRAP-MS/MS Combined with Multivariate Statistical Analysis. Molecules 2019, 24, 1936. [Google Scholar] [CrossRef]
- Murador, D.C.; Salafia, F.; Zoccali, M.; Martins, P.L.G.; Ferreira, A.G.; Dugo, P.; Mondello, L.; de Rosso, V.V.; Giuffrida, D. Green Extraction Approaches for Carotenoids and Esters: Characterization of Native Composition from Orange Peel. Antioxidants 2019, 8, 613. [Google Scholar] [CrossRef]
- Suarez Montenegro, Z.J.; Alvarez-Rivera, G.; Mendiola, J.A.; Ibanez, E.; Cifuentes, A. Extraction and Mass Spectrometric Characterization of Terpenes Recovered from Olive Leaves Using a New Adsorbent-Assisted Supercritical CO2 Process. Foods 2021, 10, 1301. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arraes-Roman, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Song, F.; Liu, Z.; Liu, S. Studies on principal components and antioxidant activity of different Radix Astragali samples using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. Talanta 2009, 78, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, X.-J.; Xu, W.; Huang, J.; Zhu, D.; Qui, X.-H. Rapid Characterization and Identification of Flavonoids in Radix Astragali by Ultra-High-Pressure Liquid Chromatography Coupled with Linear Ion Trap-Orbitrap Mass Spectrometry. J. Chromatogr. Sci. 2015, 53, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, S.; Li, F.; Zhang, B.; Qu, Y.; Sun, T.; Luo, T.; Li, D. Investigation of Antioxidant interactions between Radix Astragali and Cimicifuga foetida and Identification of Synergistic Antioxidant Compounds. PLoS ONE 2014, 9, e87221. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, K.; Wei, L.; Chen, D.; Chen, Q.; Jiao, M.; Li, X.; Huang, J.; Gong, Z.; Kang, N.; et al. The Molecular Mechanism of Antioxidation of Huolisu Oral Liquid Based on Serum Analysis and Network Analysis. Front. Pharmacol. 2021, 12, 710976. [Google Scholar] [CrossRef]
- Aita, S.E.; Capriotti, A.L.; Cavaliere, C.; Cerrato, A.; Giannelli Moneta, B.; Montone, C.M.; Piovesana, S.; Lagana, A. Andean Blueberry of the Genus Disterigma: A High-Resolution Mass Spectrometric Approach for the Comprehensive Characterization of Phenolic Compounds. Separations 2021, 8, 58. [Google Scholar] [CrossRef]
- Teles, Y.C.E.; Rebello Horta, C.C.; de Fatima Agra, M.; Siheri, W.; Boyd, M.; Igoli, J.O.; Gray, A.I.; de Fatima Vanderlei de Souza, M. New Sulphated Flavonoids from Wissadula periplocifolia (L.) C. Presl. (Malvaceae). Molecules 2015, 20, 20161–20172. [Google Scholar] [CrossRef]
- Xu, L.L.; Xu, J.J.; Zhong, K.R.; Shang, Z.P.; Wang, F.; Wang, R.F.; Liu, B. Analysis of non-volatile chemical constituents of Menthae Haplocalycis herba by ultra-high performance liquid chromatography—High resolution mass spectrometry. Molecules 2017, 22, 1756. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, B. HPLC–QTOF–MS/MS-based rapid screening of phenolics and triterpenic acids in leaf extracts of Ocimum species and their interspecies variation. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 225–238. [Google Scholar] [CrossRef]
- Fanthoni, A.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.C.; Haib, J. Identification of Nonvolatile Compounds in Clove (Syzygium aromaticum) from Manado. In Proceedings of the International Symposium on Current Progress in Mathematics and Sciences 2016 (ISCPMS 2016), Depok, Indonesia, 24–25 August 2016; Volume 1862, pp. 030079-1–030079-10. [Google Scholar]
- Razgonova, M.P.; Bazhenova, B.B.; Zabalueva, Y.Y.; Burkhanova, A.G.; Zakharenko, A.M.; Kupriyanov, A.N.; Sabitov, A.S.; Ercisli, S.; Golokhvast, K.S. Rosa davurica Pall., Rosa rugosa Thumb. and Rosa acicularis Lindl. originating from Far Eastern Russia: Screening of 146 Chemical Constituents in Tree Species of the Genus Rosa. Appl. Sci. 2022, 12, 9401. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. J. Funct. Foods 2011, 3, 144–158. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.; Sinan, K.; Ak, G.; Etienne, O.; Sharmeen, J.; Brunetti, L.; Leone, S.; Di Simone, S.; Recinella, L.; et al. Chemical composition and biological properties of two Jatropha species: Different parts and different extraction methods. Antioxidants 2021, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Jain, S.K. Simultaneous Quantification of Five Bioactive Flavonoids in High Altitude Plant Actinocarya tibetica by LC-ESI-MS/MS. J. AOAC Int. 2015, 98, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.M.; Fuguet, E.; Jiménez-Ardón, A.; Herrero-Uribe, L.; Tamayo-Castillo, G.; Torres, J. Identification of polyphenols from antiviral Chamaecrista nictitans extract using high-resolution LC–ESI–MS/MS. Anal. Bioanal. Chem. 2014, 406, 5501–5506. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Freire, C.S.R.; Domingues, M.R.M.; Silvestre, A.J.D.; Neto, C.P. Characterization of Phenolic Components in Polar Extracts of Eucalyptus globulus Labill. Bark by High-Performance Liquid Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Tekutyeva, L.A.; Podvolotskaya, A.B.; Stepochkina, V.D.; Zakharenko, A.M.; Golokhvast, K.S. Zostera marina L. Supercritical CO2-Extraction and Mass Spectrometric Characterization of Chemical Constituents Recovered from Seagrass. Separations 2022, 9, 182. [Google Scholar] [CrossRef]
- Pan, M.; Lei, Q.; Zang, N.; Zhang, H. A Strategy Based on GC-MS/MS, UPLC-MS/MS and Virtual Molecular Docking for Analysis and Prediction of Bioactive Compounds in Eucalyptus Globulus Leaves. Int. J. Mol. Sci. 2019, 20, 3875. [Google Scholar] [CrossRef]
- El-Sayed, M.A.; Abbas, F.A.; Refaat, S.; El-Shafae, A.M.; Fikry, E. UPLC-ESI-MS/MS Profile of The Ethyl Acetate Fraction of Aerial Parts of Bougainvillea “Scarlett O’Hara” Cultivated in Egypt. Egypt. J. Chem. 2021, 64, 22. [Google Scholar] [CrossRef]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; de Siqueira, A.; Chaves, D.; Romaniuk, A.; Rybczynska, M.; Gryszczynska, A.; Sawikowska, A.; Kachlicki, P.; et al. Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Braz. J. Pharmacol. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- Pandey, B.P.; Pradhan, S.P.; Adhikari, K. LC-ESI-QTOF-MS for the Profiling of the Metabolites and in Vitro Enzymes Inhibition Activity of Bryophyllum pinnatum and Oxalis corniculata Collected from Ramechhap District of Nepal. Chem. Biodivers. 2020, 17, e2000155. [Google Scholar]
- Spinola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Enerstvedt, K.H.; Jordheim, M.; Andersen, O.M. Isolation and Identification of Flavonoids Found in Zostera marina Collected in Norwegian Coastal Waters. Am. J. Plant Sci. 2016, 7, 1163–1172. [Google Scholar] [CrossRef]
- Wojakowska, A.; Perkowski, J.; Góral, T.; Stobiecki, M. Structural characterization of flavonoid glycosides from leaves of wheat (Triticum aestivum L.) using LC/MS/MS profiling of the target compounds. J. Mass Spectrom. 2013, 48, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; Foglia, P.; Pastorini, E.; Samperi, R.; Laganà, A. Identification and mass spectrometric characterization of glycosylated flavonoids in Triticum durum plants by high-performance liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up Minute Res. Mass Spectrom. 2005, 19, 3143–3158. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, C.; Gomez-Caravaca, A.M.; Guerra-Hernandez, E.; Cerretani, L.; Garcia-Villanova, B.; Verardo, V. Comprehensive metabolite profiling of Solanum tuberosum L. (potato) leaves T by HPLC-ESI-QTOF-MS. Molecules 2018, 112, 390–399. [Google Scholar] [CrossRef]
- Zapesochnaya, G.G.; Kurkin, V.A.; Shchavlinskii, A.N. Flavonoids of the above-ground part of Rhodiola rosea. II. Structure of novel glycosides of herbacetin and gossypetin. Chem. Nat. Compd. 1985, 4, 496–507. [Google Scholar]
- Jeong, H.J.; Ryu, Y.B.; Park, S.J. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorganic Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. Phytochemical A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.) Roots, Woods, Canes, Stems, and Leaves. Antioxidants. 2020, 9, 398. [Google Scholar] [CrossRef]
- De Rosso, M.; Tonidandel, L.; Larcher, R.; Nicolini, G.; Dalla Vedova, A.; De Marchi, F.; Gardiman, M.; Giust, M.; Flamini, R. Identification of new flavonols in hybrid grapes by combined liquid chromatography-mass spectrometry approaches. Food Chem. 2014, 163, 244–251. [Google Scholar] [CrossRef]
- Wojakowska, A.; Piasecka, A.; Garcia-Lopez, P.M.; Zamora-Natera, F.; Krajewski, P.; Marczak, L.; Kachlicki, P.; Stobiecki, M. Structural analysis and profiling of phenolic secondary metabolites of Mexican lupine species using LC–MS techniques. Phytochemistry 2013, 92, 71–86. [Google Scholar] [CrossRef]
- Mekam, P.N.; Martini, S.; Nguefack, J.; Tagliazucchi, D.; Stefani, E. Phenolic compounds profile of water and ethanol extracts of Euphorbia hirta L. leaves showing antioxidant and antifungal properties. South Afr. J. Bot. 2019, 127, 319–332. [Google Scholar] [CrossRef]
- Negri, S.; Gambini, S.; Ceoldo, S.; Avesani, L.; Commisso, M.; Guzzo, F. Undifferentiated In Vitro Cultured Actinidia deliciosa as Cell Factory for the Production of Quercetin Glycosides. Plants 2021, 10, 2499. [Google Scholar] [CrossRef] [PubMed]
- Marcia Fuentes, J.A.; Lopez-Salas, L.; Borras-Linares, I.; Navarro-Alarcon, M.; Segura-Carretero, A.; Lozano-Sanchez, J. Development of an Innovative Pressurized Liquid Extraction Procedure by Response Surface Methodology to Recover Bioactive Compounds from Carao Tree Seeds. Foods 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.O.; Cheng, H.; El-Shazly, A.M.; Wink, M. A proanthocyanidin-rich extract from Cassia abbreviata exhibits antioxidant and hepatoprotective activities in vivo. J. Ethnopharmacol. 2018, 213, 38–47. [Google Scholar] [CrossRef]
- Abeywickrama, G.; Debnath, S.C.; Ambigaipalan, P.; Shahidi, F. Phenolics of selected cranberry genotypes (Vaccinium macrocarpon Ait.) and their antioxidant efficacy. J. Agric. Food Chem. 2016, 64, 9342–9351. [Google Scholar] [CrossRef]
- Ojwang, L.O.; Yang, L.; Dykes, L.; Awika, J. Proanthocyanidin profile of cowpea (Vigna unguiculata) reveals catechin-O-glucoside as the dominant compound. Food Chem. 2013, 130, 35–43. [Google Scholar] [CrossRef]
- Qin, D.; Wang, Q.; Li, H.; Jiang, X.; Fang, K.; Wang, Q.; Li, B.; Pan, C.; Wu, H. Identification of key metabolites based on non-targeted metabolomics and chemometrics analyses provides insights into bitterness in Kucha (Camellia kucha (Chang et Wang) Chang). Food Res. Int. 2020, 138, 109789. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, X.; Li, G.; He, X.; Yu, X.; Yu, X.; Xiao, Q.; Xiang, Z.; Wang, C. Chemical constituents of radix Actinidia chinensis planch by UPLC–QTOF–MS. Biomed. Chromatogr. 2021, 35, e5103. [Google Scholar] [CrossRef]
- De Freitas, M.A.; Silva Alves, A.I.; Andrade, J.C.; Leite-Andrade, M.C.; Lucas dos Santos, A.T.; de Oliveira, T.F.; dos Santos, F.; Silva Buonafina, M.D. Evaluation of the Antifungal Activity of the Licania Rigida Leaf Ethanolic Extract against Biofilms Formed by Candida Sp. Isolates in Acrylic Resin Discs. Antibiotics 2019, 8, 250. [Google Scholar] [CrossRef]
- Zakharenko, A.M.; Razgonova, M.P.; Pikula, K.S.; Golokhvast, K.S. Simultaneous determination of 78 compounds of Rhodiola rosea extract using supercritical CO2-extraction and HPLC-ESI-MS/MS spectrometry. HINDAWY. Biochem. Res. Int. 2021, 2021, 9957490. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumar, B. Profiling of Gallic and Ellagic Acid Derivatives in Different Plant Parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Justesen, U. Negative atmospheric pressure chemical ionisation low-energy collision activation mass spectrometry for the characterisation of flavonoids in extracts of fresh herbs. J. Chromatogr. A 2000, 902, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Bodalska, A.; Kowalczyk, A.; Włodarczyk, M.; Fecka, I. Analysis of Polyphenolic Composition of a Herbal Medicinal Product—Peppermint Tincture. Molecules 2020, 25, 69. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, B.; Wang, D.; Zhu, Y.; Miao, X.; Yang, W. The Chemical Composition of Brazilian Green Propolis and Its Protective Effects on Mouse Aortic Endothelial Cells against Inflammatory Injury. Molecules 2020, 25, 4612. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, P.; Liu, B.; Wei, L.; Xu, Y. Simultaneous determination of fourteen compounds of Hedyotis diffusa Willd extract in rats by UHPLC-MS/MS method: Application to pharmacokinetics and tissue distribution study. J. Pharm. Biomed. Anal. 2018, 159, 490–512. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.C.W.; Woo, K.-S.; Fung, K.-P. Chemistry and Biological Activities of Caffeic Acid Derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef]
- Van Hoyweghen, L.; De Bosscher, K.; Haegeman, G.; Deforce, D.; Heyerick, A. In Vitro Inhibition of the Transcription Factor NF-kB and Cyclooxygenase by Bamboo Extracts. Phytother. Res. 2014, 28, 224–230. [Google Scholar] [CrossRef]
- Serrano, C.A.; Villena, G.K.; Rodriguez, E.F. Phytochemical profile and rosmarinic acid purification from two Peruvian Lepechinia Willd. species (Salviinae, Mentheae, Lamiaceae). Sci. Rep. 2021, 11, 7260. [Google Scholar] [CrossRef]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.A.; Smeds, A.I.; Sjoholm, R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2008, 43, 97–107. [Google Scholar] [CrossRef]
- Bonzanini, F.; Bruni, R.; Palla, G.; Serlataite, N.; Caligiani, A. Identification and distribution of lignans in Punica granatum L. fruit endocarp, pulp, seeds, wood knots and commercial juices by GC–MS. Food Chem. 2009, 117, 745–749. [Google Scholar] [CrossRef]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; Saini, M.K.; et al. Transfer of grain colors to elite wheat cultivars and their characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Pereira, E.; Pires, T.C.S.P.; Alves, M.J.; Pereira, O.R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott fruits: A detailed study of its nutritional, chemical and bioactive properties. Food Res. Int. 2019, 119, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Vera de Rosso, V.; Hillebrand, S.; Cuevas Montilla, E.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of anthocyanins from acerola (Malpighia emarginata DC.) and ac-ai (Euterpe oleracea Mart.) by HPLC–PDA–MS/MS. J. Food Compos. Anal. 2008, 21, 291–299. [Google Scholar] [CrossRef]
- Anari, Z.; Mai, C.; Sengupta, A.; Howard, L.; Brownmiller, C.; Wickramasinghe, R. Combined Osmotic and Membrane Distillation for Concentration of Anthocyanin from Muscadine Pomace. J. Food Sci. 2019, 84, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfeld, A.; Obando, L.; Mardones, C. Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713. [Google Scholar] [CrossRef]
- Chhon, S.; Jeon, J.; Kim, J.; Park, S.Y. Accumulation of Anthocyanins through Overexpression of AtPAP1 in Solanum nigrum Lin. (Black Nightshade). Biomolecules 2020, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Luo, G.; Yu, F.; Jia, Q.; Zheng, Y.; Bi, X.; Lei, J. Characterization of anthocyanins in the hybrid progenies derived from Iris dichotoma and I. domestica by HPLC-DAD-ESI/MS analysis. Phytochemistry 2018, 150, 60–74. [Google Scholar] [CrossRef]
- Hanhineva, K.; Karenlampi, S.O.; Aharoni, A. Resent Advances in Strawberry Metabolomics. Genes Genomes Genom. 2011, 5, 65–75. [Google Scholar]
- Perchuk, I.; Shelenga, T.; Gurkina, M.; Miroshnichenko, E.; Burlyaeva, M. Composition of Primary and Secondary Metabolite Compounds in Seeds and Pods of Asparagus Bean (Vigna unguiculata (L.) Walp.) from China. Molecules 2020, 25, 3778. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; He, Y.; Shi, M.; Han, X.; Li, W.; Zhang, X.; Wen, X. Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation. Molecules 2019, 24, 1114. [Google Scholar] [CrossRef]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.A.; Nieman, K.M.; Dall’Asta, C.; Del Rio, D. Phenolic and volatile composition of a dry spearmint (Mentha spicata L.) extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, W.; Liu, Z.; Zhang, Z. Identification and Simultaneous Determination of Twelve Active Components in the Methanol Extract of Traditional Medicine Weichang’an Pill by HPLC-DAD-ESI-MS/MS. Iran. J. Pharm. Res. IJPR 2013, 12, 15–25. [Google Scholar] [PubMed]
- Guo, K.; Tong, C.; Fu, Q.; Xu, J.; Shi, S.; Xiao, Y. Identification of minor lignans, alkaloids, and phenylpropanoid glycosides in Magnolia officinalis by HPLC-DAD-QTOF-MS/MS. J. Pharm. Biomed. Anal. 2019, 170, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ekeberg, D.; Flate, P.-O.; Eikenes, M.; Fongen, M.; Naess-Andresen, C.F. Qualitative and quantitative determination of extractives in heartwood of Scots pine (Pinus sylvestris L.) by gas chromatography. J. Chromatogr. A 2006, 1109, 267–272. [Google Scholar] [CrossRef]
- Han, F.; Li, Y.; Ma, L.; Liu, T.; Wu, Y.; Xu, R.; Song, A.; Yin, R. A rapid and sensitive UHPLC-FT-ICR MS/MS method for identification of chemical constituents in Rhodiola crenulata extract, rat plasma and rat brain after oral administration. Talanta 2016, 160, 183–193. [Google Scholar] [CrossRef]
- Llorent-Martinez, E.J.; Spinola, V.; Gouveia, S.; Castilho, P.C. HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Ind. Crops Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Sun, S.; Gao, Y.; Ling, X.; Lou, H. The combination effects of phenolic compounds and fluconazole on the formation of ergosterol in Candida albicans determined by high-performance liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2005, 336, 39–45. [Google Scholar] [CrossRef]
- Sun, L.; Tao, S.; Zhang, S. Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS. Molecules 2019, 24, 159. [Google Scholar] [CrossRef]
- Sut, S.; Zengin, G.; Maggi, F.; Malagoli, M.; Dall’Acqua, S. Triterpene Acid and Phenolics from Ancient Apples of Friuli Venezia Giulia as Nutraceutical Ingredients: LC-MS Study and In Vitro Activities. Molecules 2019, 24, 1109. [Google Scholar] [CrossRef]
- Troshchenko, A.T.; Kutikova, G.A. Rhodioloside from Rhodiola rosea and R. quadrifida I. Chem. Nat. Compd. 1967, 3, 244–249. [Google Scholar] [CrossRef]
- Saratikov, A.S.; A Krasnov, E.; A Chnikina, L.; Duvidson, L.M.; I Sotova, M.; Marina, T.F.; Nechoda, M.F.; A Axenova, R.; Tscherdinzeff, S.G. Rhodiolosid, a new glycoside from Rhodiola rosea and its pharmacological properties. Pharmazie 1968, 23, 392–395. [Google Scholar]
- Van Diermen, D.; Marston, A.; Bravo, J.; Reist, M.; Carrupt, P.A.; Hostettmann, K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J. Ethnopharmacol. 2009, 122, 397–401. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Rodrigues, D.B.; Petry, F.C.; Barros Mariutti, L.R. Carotenoid esters in foods—A review and practical directions on analysis and occurrence. Food Res. Int. 2017, 99, 830–850. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, M.; Giuffrida, D.; Salafia, F.; Giofre, S.V.; Mondello, L. Carotenoids and apocarotenoids determination in intact human blood samples by online supercritical fluid extraction-supercritical fluid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2018, 1032, 40–47. [Google Scholar] [CrossRef]
- Dugo, P.; Herrero, M.; Giuffrida, D.; Ragonese, C.; Dugo, G.; Mindello, L. Analysis of native carotenoid composition in orange juice using C30 columns in tandem. J. Sep. Sci. 2008, 31, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Canjura, F.L.; Schwartz, S.J. Identification of Chlorophyll Derivatives by Mass Spectrometry. J. Agric. Food Chem. 1991, 39, 1452–1456. [Google Scholar] [CrossRef]
- Lara-Abia, S.; Lobo-Rodrigo, G.; Welti-Chanes, J.; Pilar Cano, M. Carotenoid and Carotenoid Ester Profile and Their Deposition in Plastids in Fruits of New Papaya (Carica papaya L.) Varieties from the Canary Islands. Foods 2021, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Etzbach, L.; Pfeiffer, A.; Weber, F.; Schieber, A. Characterization of carotenoid profiles in goldenberry (Physalis peruviana L.) fruits at various ripening stages and in different plant tissues by HPLC-DADAPCI-MSn. Food Chem. 2018, 245, 508–517. [Google Scholar] [CrossRef]
- Penagos-Calvete, D.; Guauque-Medina, J.; Villegas-Torres, M.F.; Guillermo, M. Analysis of triacylglycerides, carotenoids and capsaicinoids as disposable molecules from Capsicum agroindustry. Hortic. Environ. Biotechnol. 2019, 60, 227–238. [Google Scholar] [CrossRef]

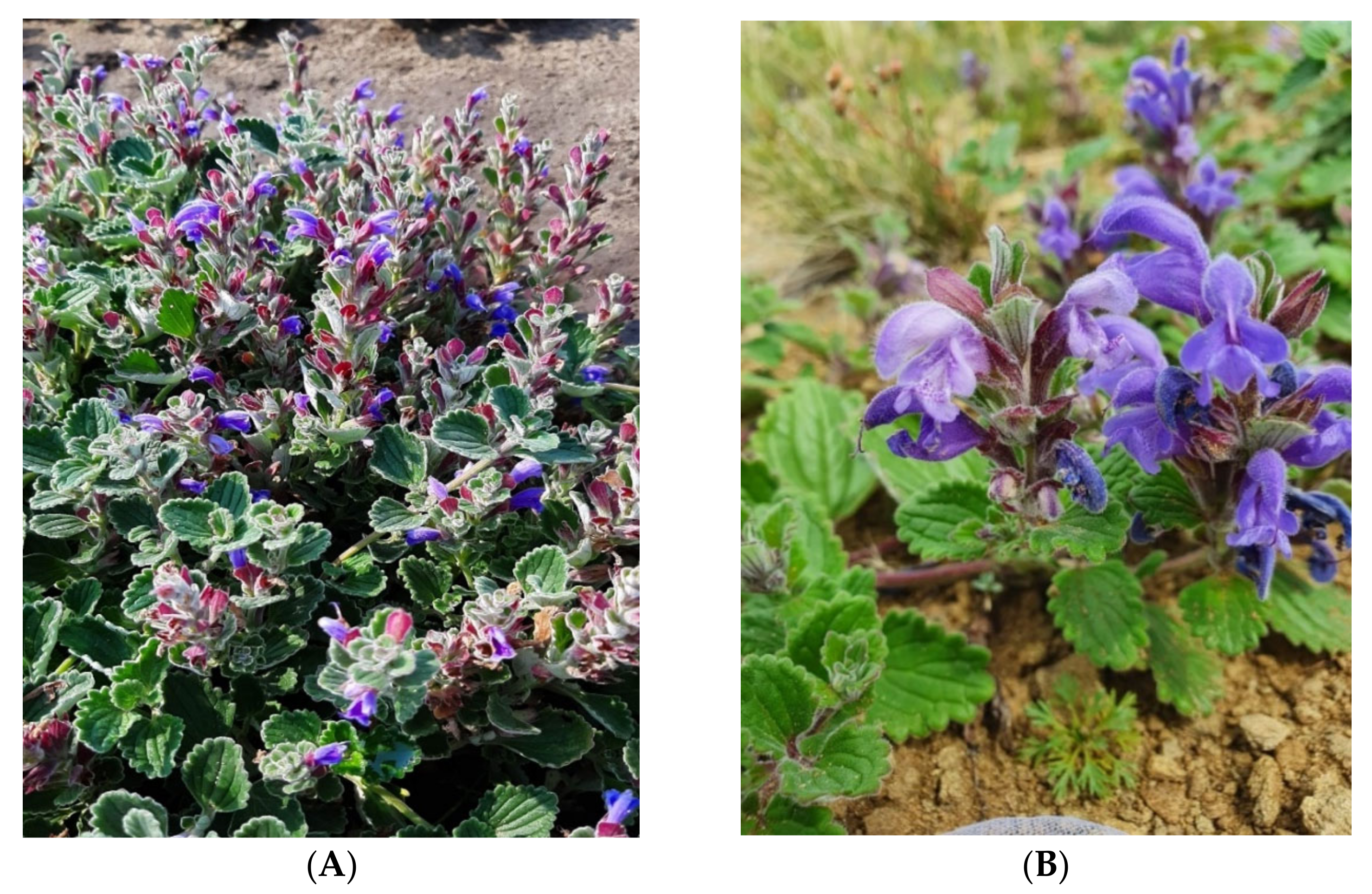

| № | Class of Compounds | Identified Compounds | Formula | Botanical Garden of Yakutsk | Wild Specimens |
|---|---|---|---|---|---|
| 1 | Flavone | Formononetin * | C16H12O4 | ||
| 2 | Flavone | Apigenin | C15H10O5 | ||
| 3 | Flavone | Acacetin | C16H12O5 | ||
| 4 | Flavone | Calycosin | C16H12O5 | ||
| 5 | Flavone | Gengkwanin | C16H12O5 | ||
| 6 | Flavone | Luteolin | C15H10O6 | ||
| 7 | Flavone | Diosmetin | C16H12O6 | ||
| 8 | Flavone | Chrysoeriol [Chryseriol] | C16H12O6 | ||
| 9 | Flavone | Homoeriodictyol * | C16H14O6 | ||
| 10 | Flavone | Cirsimaritin * | C17H14O6 | ||
| 11 | Flavone | Dihydroxy-dimethoxy(iso)flavone * | C17H14O6 | ||
| 12 | Flavone | 5,7-Dimethoxyluteolin * | C17H14O6 | ||
| 13 | Flavone | Myricetin * | C15H10O8 | ||
| 14 | Flavone | Isothymusin | C17H14O7 | ||
| 15 | Flavone | Cirsiliol * | C17H14O7 | ||
| 16 | Flavone | Dimethoxy-trihydroxy(iso)flavone * | C17H14O7 | ||
| 17 | Flavone | Nevadensin | C18H16O7 | ||
| 18 | Flavone | Gardenin B [Demethyltangeretin] * | C19H18O7 | ||
| 19 | Flavone | Dihydroxy-tetramethoxy(iso)flavone * | C19H18O8 | ||
| 20 | Flavone | 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavone * | C20H20O8 | ||
| 21 | Flavone | Apigenin O-hexoside | C21H20O10 | ||
| 22 | Flavone | Apigenin-7-O-glucoside | C21H20O10 | ||
| 23 | Flavone | Aromadendrin 7-O-rhamnoside * | C21H22O10 | ||
| 24 | Flavone | Apigenin 7-O-glucuronide | C21H18O11 | ||
| 25 | Flavone | Acacetin 7-O-glucoside [Tilianin] | C22H22O10 | ||
| 26 | Flavone | Luteolin 7-O-glucoside [Cynaroside; Luteoloside] | C21H20O11 | ||
| 27 | Flavone | 3′-methoxyacacetin 7-O-beta-D-glucuronide | C22H20O12 | ||
| 28 | Flavone | Acacetin 7-O-beta-D-glucuronide | C22H20O11 | ||
| 29 | Flavone | 6,4′-Dimethoxyisoflavone-7-O-glucoside * | C23H24O10 | ||
| 30 | Flavone | Diosmetin-7-O-beta-glucoside | C22H22O11 | ||
| 31 | Flavone | Apigenin-O-rhamnoside * | C22H22O11 | ||
| 32 | Flavone | Chrysoeriol-7-O-glucuronide * | C22H20O12 | ||
| 33 | Flavone | Acacetin 7-beta-O-(6”-acetyl)-glucoside | C24H24O11 | ||
| 34 | Isoflavone | Apigenin 7-O-beta-D-(6”-O-malonyl)-glucoside | C24H22O13 | ||
| 35 | Flavone | Acacetin 7-O-beta-D-(6”-O-malonylated)-glucoside | C25H24O13 | ||
| 36 | Flavone | Diosmetin-7-O-beta-D-(6”-malonyl)-glucoside | C25H24O14 | ||
| 37 | Flavone | Chrysoeriol O-hexoside C-hexoside * | C28H32O16 | ||
| 38 | Flavone | Isovitexin 2”-O-glucoside-7-O-glucoside * | C33H40O20 | ||
| 39 | Flavonol | Kaempferol | C15H10O6 | ||
| 40 | Flavonol | Quercetin | C15H10O7 | ||
| 41 | Flavonol | Herbacetin * | C15H10O7 | ||
| 42 | Flavonol | Dihydroquercetin (Taxifolin; Taxifoliol) | C15H12O7 | ||
| 43 | Flavonol | Isorhamnetin | C16H12O7 | ||
| 44 | Flavonoid | 3,5—Diacetyltambulin * | C22H20O9 | ||
| 45 | Flavonol | Dihydrokaempferol-3-O-rhamnoside * | C21H22O10 | ||
| 46 | Flavonol | Astragalin | C21H20O11 | ||
| 47 | Flavonol | Quercitrin [Quercetin 3-O- rhamnoside; Quercetrin] * | C21H20O11 | ||
| 48 | Flavonol | Kaempferol-3-O-glucuronide | C21H18O12 | ||
| 49 | Flavonol | Taxifolin-3-O-hexoside [Dihydroquercetin-3-O-hexoside] * | C21H22O12 | ||
| 50 | Flavonol | Kaempferol 3-O-rutinoside | C27H30O15 | ||
| 51 | Flavonol | Kaempferol-3,7-Di-O-glucoside * | C27H30O16 | ||
| 52 | Flavonol | Kaempferol dihexoside rhamnoside * | C33H40O20 | ||
| 53 | Flavan-3-ol | (epi)Afzelechin * | C15H14O5 | ||
| 54 | Flavan-3-ol | Catechin [D-Catechol] * | C15H14O6 | ||
| 55 | Flavan-3-ol | (epi)catechin | C15H14O6 | ||
| 56 | Flavan-3-ol | Gallocatechin * | C15H14O7 | ||
| 57 | Flavan-3-ol | (epi)Afzelechin derivative * | C18H16O10 | ||
| 58 | Flavan-3-ol | Catechin 3-O-gallate * | C22H18O10 | ||
| 59 | Flavan-3-ol | Epigallocatechin-3-gallate | C22H18O11 | ||
| 60 | Flavanone | Naringenin | C15H12O5 | ||
| 61 | Flavanone | Eriodictyol | C15H12O6 | ||
| 62 | Isoflavanone | Ferreirin * | C16H14O6 | ||
| 63 | Flavanone | Prunin | C21H22O10 | ||
| 64 | Flavanone | Eriodictyol-7-O-glucoside | C21H22O11 | ||
| 65 | Flavanone | Eriodictyol-7-O-glucuronide * | C21H20O12 | ||
| 66 | Hydroxybenzoic acid | Protocatechuic acid * | C7H6O4 | ||
| 67 | Hydroxycinnamic acid | p-Coumaric acid | C9H8O3 | ||
| 68 | Hydroxycinnamic acid | Caffeic acid | C9H8O4 | ||
| 69 | Hydroxycinnamic acid | 3,4-Dihydroxyhydrocinnamic acid * | C9H10O4 | ||
| 70 | Phenolic acid | 2,3,4,5-Tetrahydroxybenzoic acid | C7H6O6 | ||
| 71 | Phenolic acid | Salvianic acid A [Danshensu] * | C9H10O5 | ||
| 72 | Phenolic acid | 2,3-Dihydroxy-4-Mathoxycinnamic acid * | C10H10O5 | ||
| 73 | Hydroxybenzoic acid | Ellagic acid | C14H6O8 | ||
| 74 | Phenolic acid | Protocatechuic acid-O-hexoside * | C13H16O9 | ||
| 75 | Phenolic acid | Salvianolic acid G | C18H12O7 | ||
| 76 | Phenolic acid | Caffeic acid-4-O-beta-D-hexoside [Caffeoyl-O-hexoside] | C15H18O9 | ||
| 77 | Phenolic acid | Chlorogenic acid [3-O-Caffeoylquinic acid] | C16H18O9 | ||
| 78 | Phenolic acid | Isochlorogenic acid * | C16H18O9 | ||
| 79 | Phenolic acid | Rosmarinic acid | C18H16O8 | ||
| 80 | 3-Prenyl-4-(dihydrocinnamoyloxy)-cinnamic acid * | C23H24O4 | |||
| 81 | Phenolic acid | Caffeic acid derivative | C16H18O9Na | ||
| 82 | Phenolic acid | 1/3/4/5-p-Coumaroylquinic acid * + C2H2O | C18H20O9 | ||
| 83 | Phenolic acid | 8,8′-Aryl-Diferulic acid * | C20H18O8 | ||
| 84 | Phenolic acid | Caffeic acid hexoside dimer * | C31H40O17 | ||
| 85 | Phenolic acid | Didehydrosalvianolic acid B * | C36H28O16 | ||
| 86 | Phenolic acid | Salvianolic acid B [Danfensuan B] * | C36H30O16 | ||
| 87 | Phenylpropanoic acid | Sagerinic acid | C36H32O16 | ||
| 88 | Phenolic acid | Clerodendranoic acid H * | C36H32O16 | ||
| 89 | Lignan | Phillygenin [Sylvatesmin; Phyllygenol; Forsythigenol] * | C21H24O6 | ||
| 90 | Lignan | Medioresinol * | C21H24O7 | ||
| 91 | Neolignan | Urolignoside * + H2O | C25H34O11 | ||
| 92 | Dihydrochalcone | Phloretin * | C15H14O5 | ||
| 93 | Hydroxycoumarin | Umbelliferone * | C9H6O3 | ||
| 94 | Coumarin | Fraxetin * | C10H8O5 | ||
| 95 | Hydroxycoumarin | Umbelliferone hexoside * | C15H16O8 | ||
| 96 | Coumarin glycoside | Fraxin (Fraxetin-8-O-glucoside) | C16H18O10 | ||
| 97 | Anthocyanidin | Petunidin | C16H13O7+ | ||
| 98 | Anthocyanidin | Pelargonidin-3-O-glucoside (callistephin) | C21H21O10 | ||
| 99 | Anthocyanidin | Cyanidin-3-O-glucoside | C21H21O11+ | ||
| 100 | Anthocyanidin | Cyanidin 3,5-O-diglucoside * | C27H31O16 | ||
| 101 | Anthocyanidin | Peonidin-3,5-diglucoside * | C28H33O16 | ||
| 102 | Anthocyanidin | Cyanidin-3-O-rutinoside-5-O-glucoside * | C33H41O20 | ||
| 103 | Anthocyanidin | Delphinidin 3-O-rutinoside-5-O-glucoside * | C33H41O21 | ||
| 104 | Anthocyanidin | Malonyl-shisonin * | C39H39O21+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razgonova, M.P.; Okhlopkova, Z.M.; Rozhina, Z.G.; Egorova, P.S.; Ercisli, S.; Golokhvast, K.S. Comparison of Wild and Introduced Dracocephalum jacutense P.: Significant Differences of Multicomponent Composition. Horticulturae 2022, 8, 1211. https://doi.org/10.3390/horticulturae8121211
Razgonova MP, Okhlopkova ZM, Rozhina ZG, Egorova PS, Ercisli S, Golokhvast KS. Comparison of Wild and Introduced Dracocephalum jacutense P.: Significant Differences of Multicomponent Composition. Horticulturae. 2022; 8(12):1211. https://doi.org/10.3390/horticulturae8121211
Chicago/Turabian StyleRazgonova, Mayya P., Zhanna M. Okhlopkova, Zoya G. Rozhina, Polina S. Egorova, Sezai Ercisli, and Kirill S. Golokhvast. 2022. "Comparison of Wild and Introduced Dracocephalum jacutense P.: Significant Differences of Multicomponent Composition" Horticulturae 8, no. 12: 1211. https://doi.org/10.3390/horticulturae8121211
APA StyleRazgonova, M. P., Okhlopkova, Z. M., Rozhina, Z. G., Egorova, P. S., Ercisli, S., & Golokhvast, K. S. (2022). Comparison of Wild and Introduced Dracocephalum jacutense P.: Significant Differences of Multicomponent Composition. Horticulturae, 8(12), 1211. https://doi.org/10.3390/horticulturae8121211









