Liquid Leachate Produced from Vermicompost Effects on Some Agronomic Attributes and Secondary Metabolites of Sweet Basil (Ocimum basilicum L.) Exposed to Severe Water Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site, Plant Materials, Submitting Water Stress, and Harvest Time
2.2. Vermicompost Preparation and Physicochemical Properties of Liquid Leachate of Vermicompost
2.3. Agronomic Traits
2.4. Solid-Phase Micro-Extraction (SPME) of Essential Oils and GC-MS Conditions
2.5. Extraction and Quantification of Phenolics Using LC–MS/MS
2.6. Experimental Design and Statistical Analysis
3. Results
3.1. Agronomic Attributes
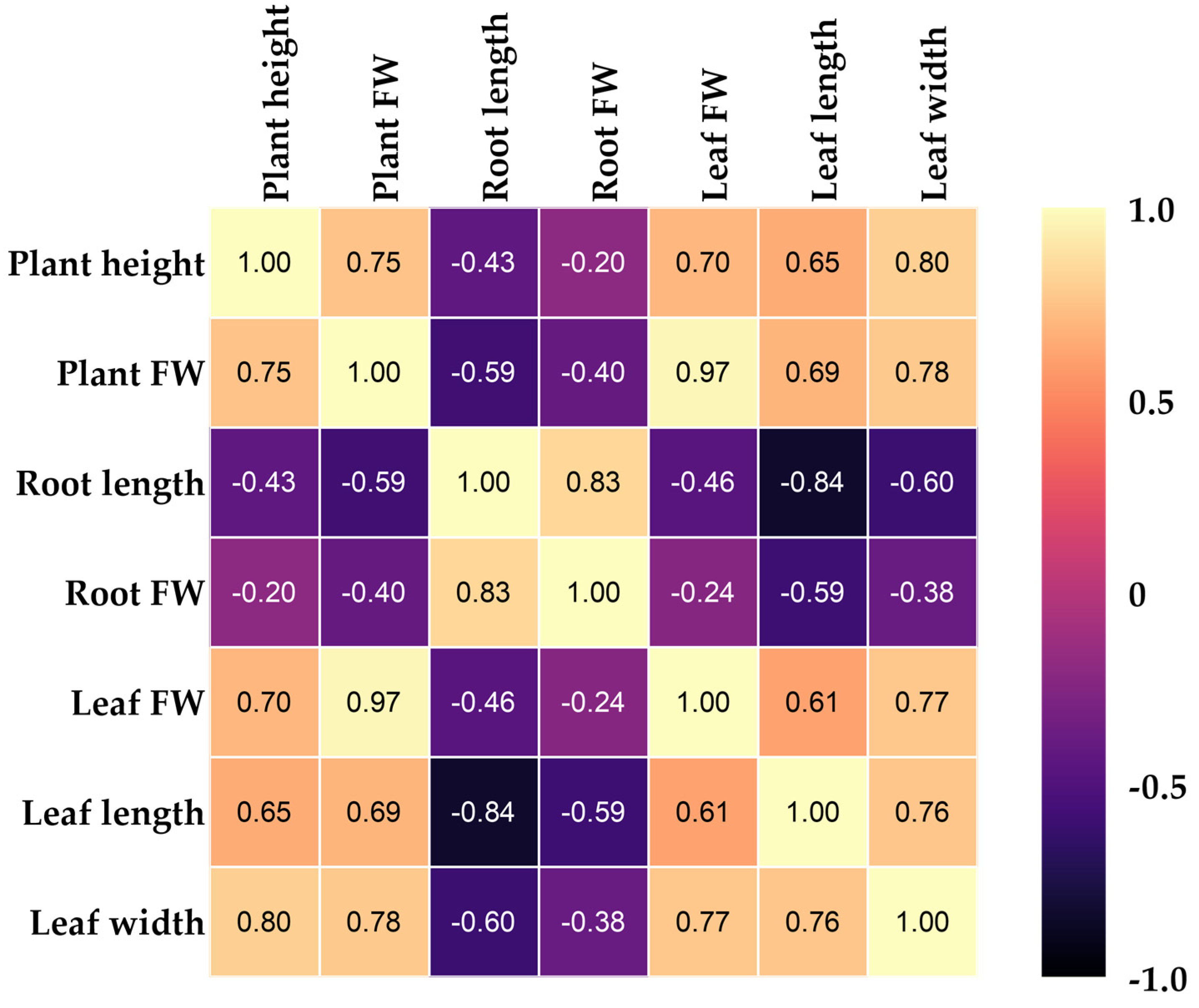
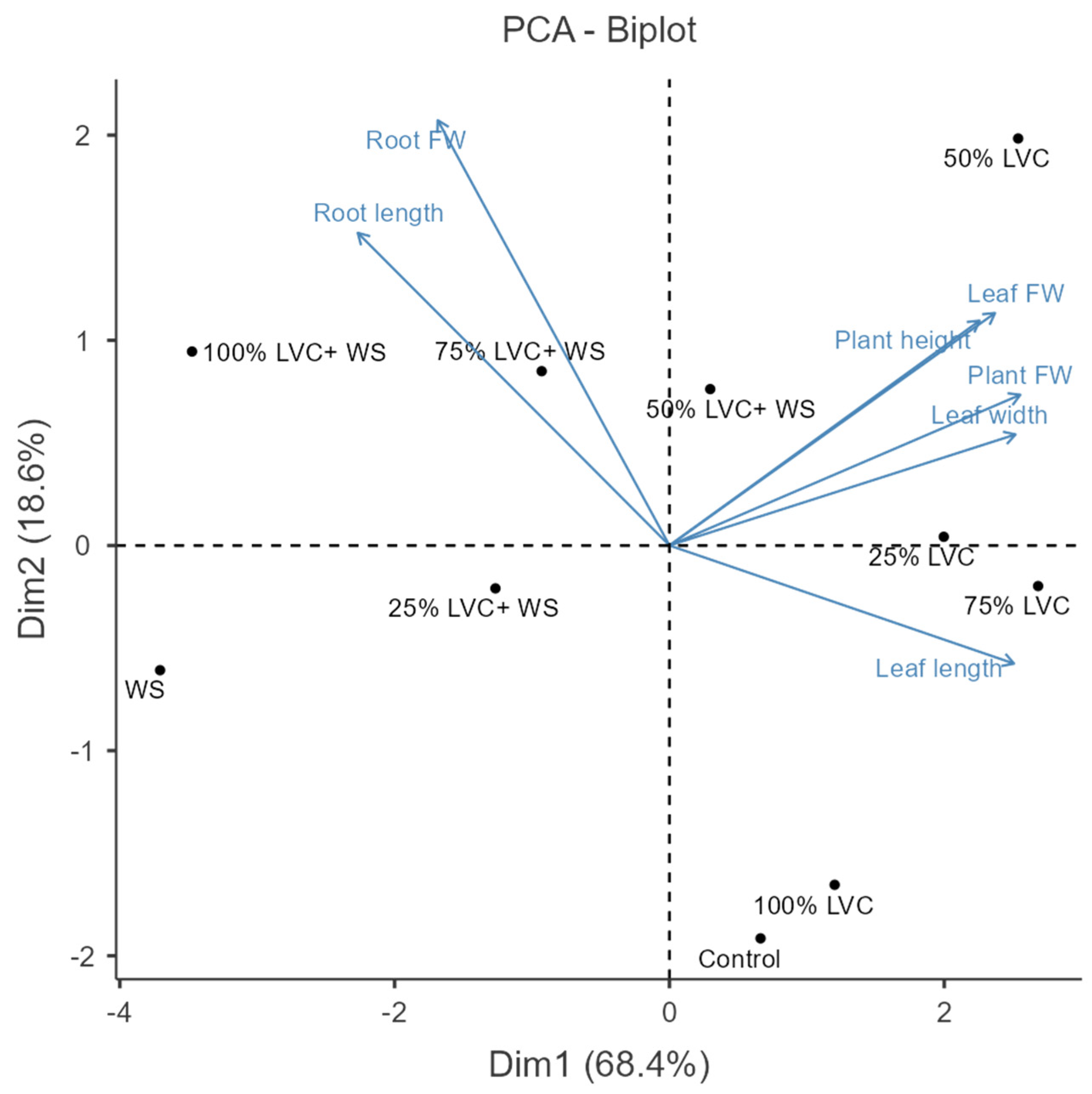
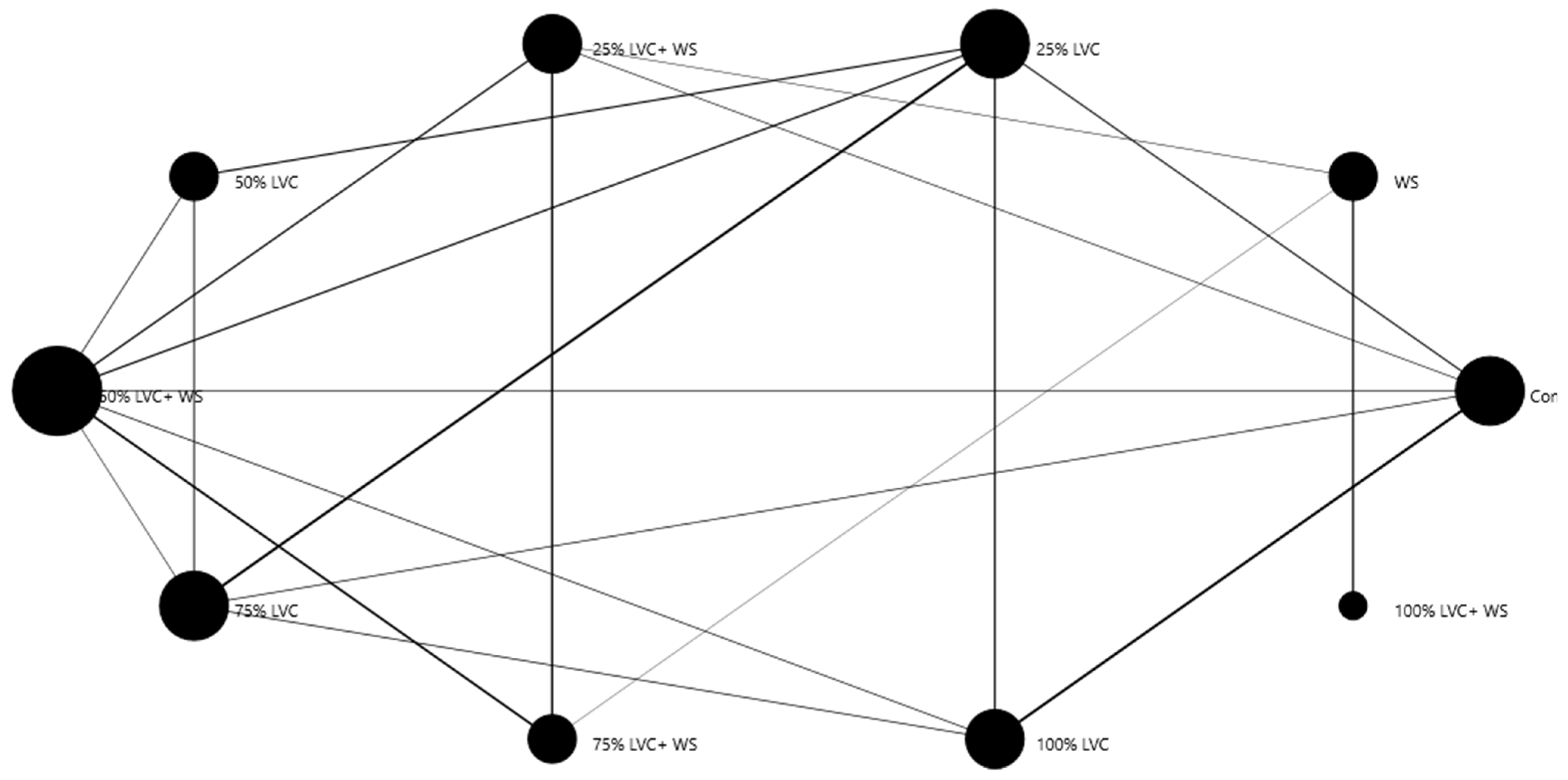
3.2. Heat Map Clustering, Correlation, Principal Component, and Network Plot Analyses of the Agronomic Attributes Corresponding to the Treatments
3.3. Essential Oil Compounds
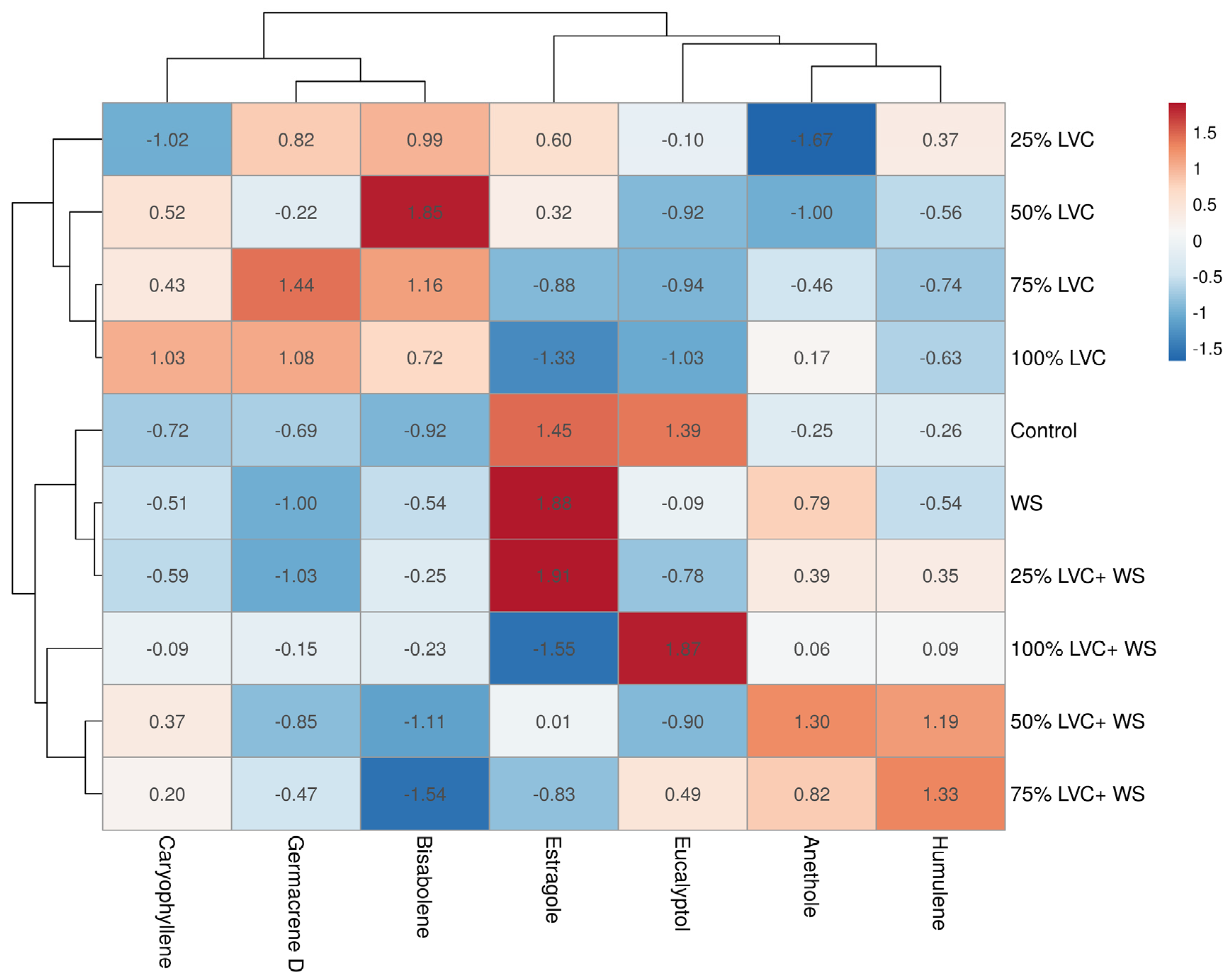
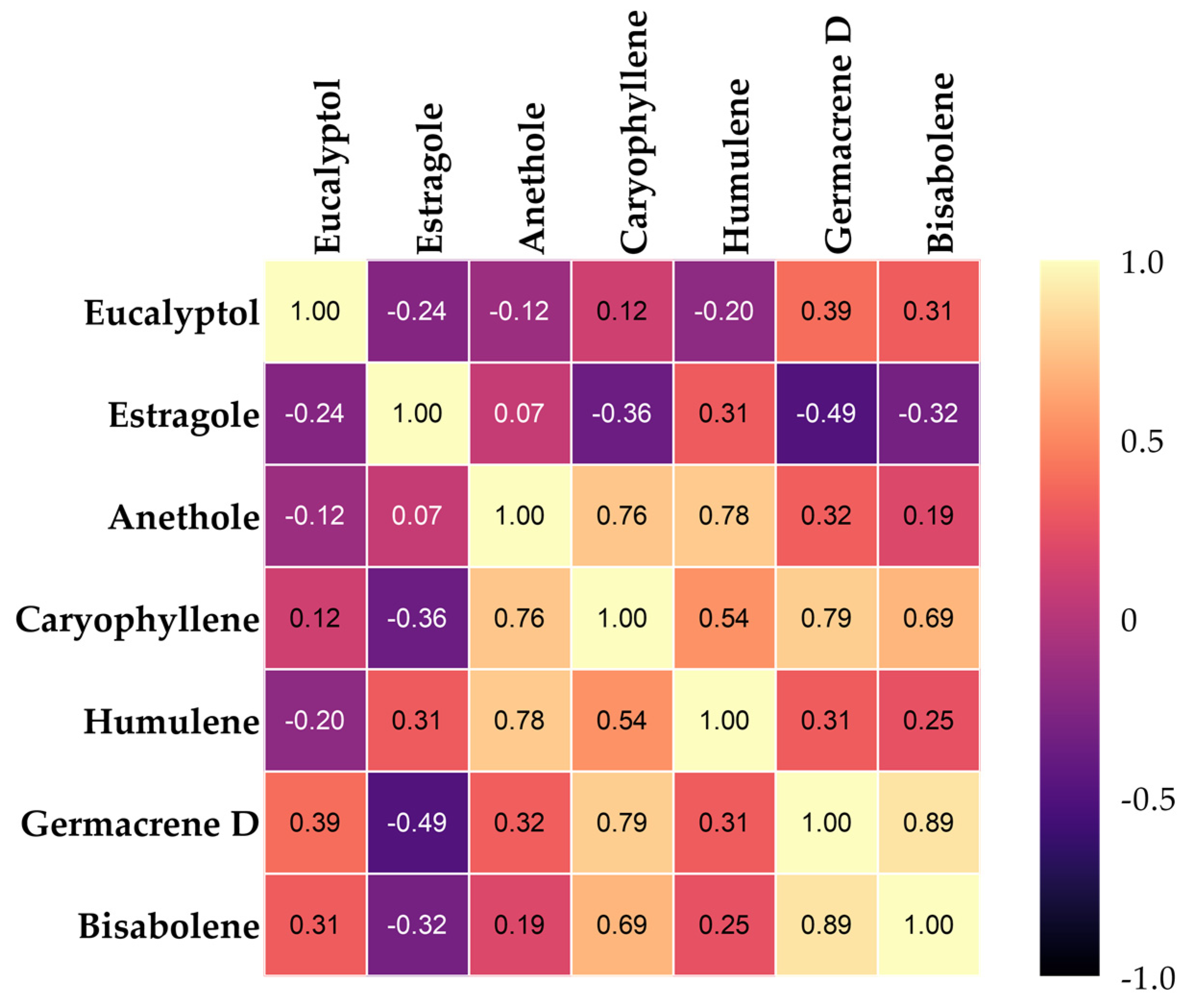
3.4. Heat Map Clustering, Correlation, Principal Component, and Network Plot Analyses of the Essential Oil Compound Corresponding to the Treatments
3.5. Phenolic Acids and Flavonoids
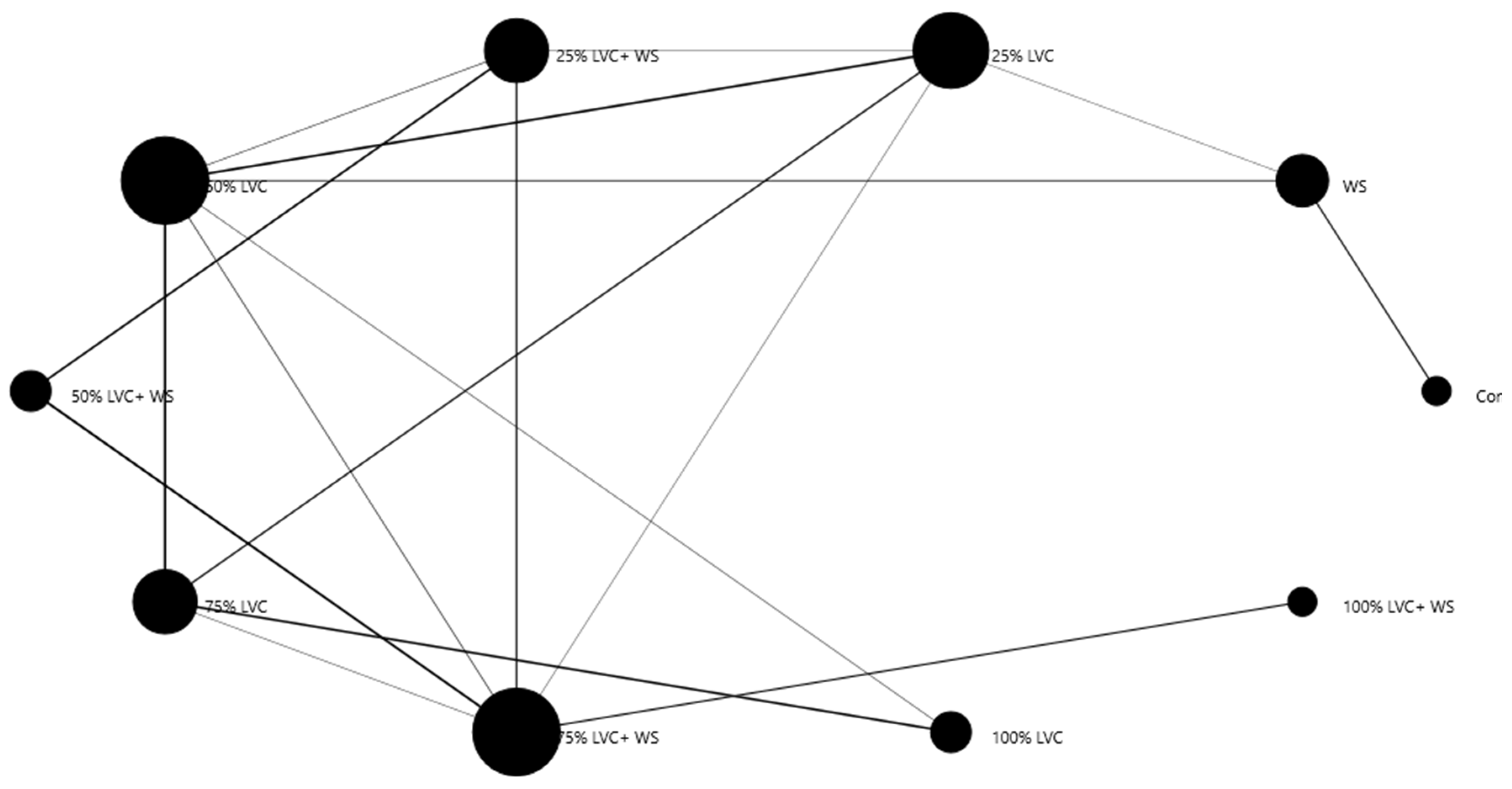
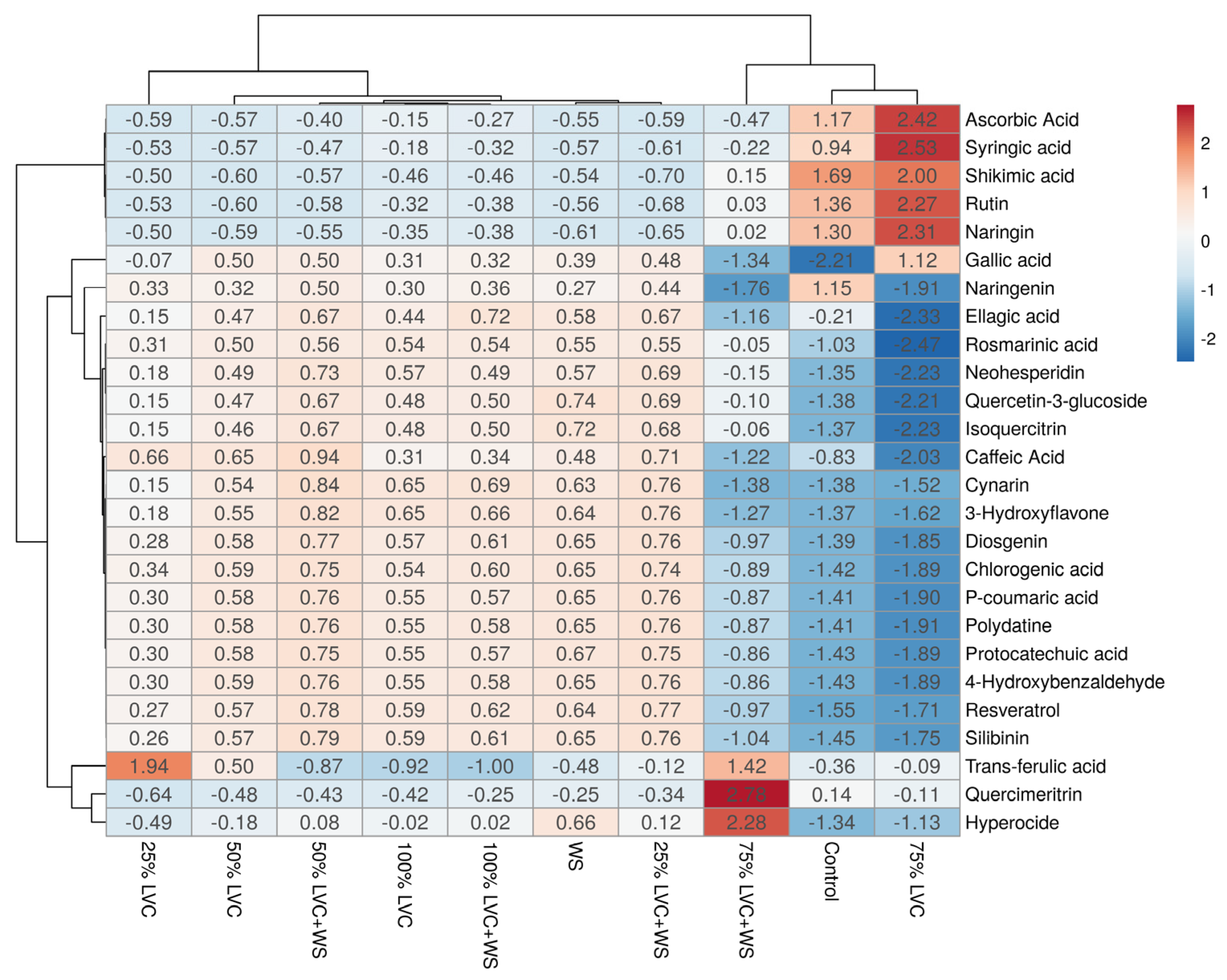
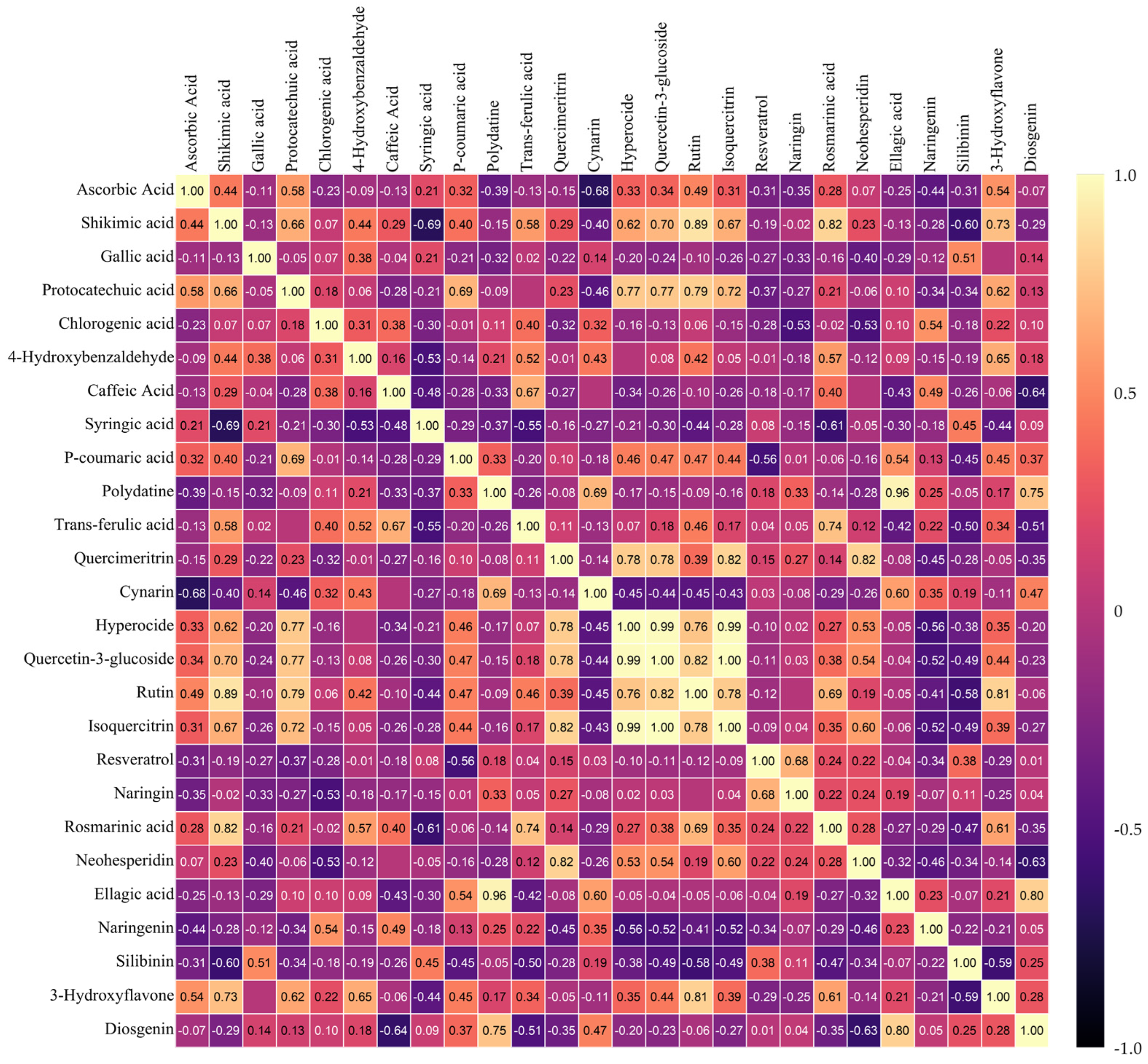
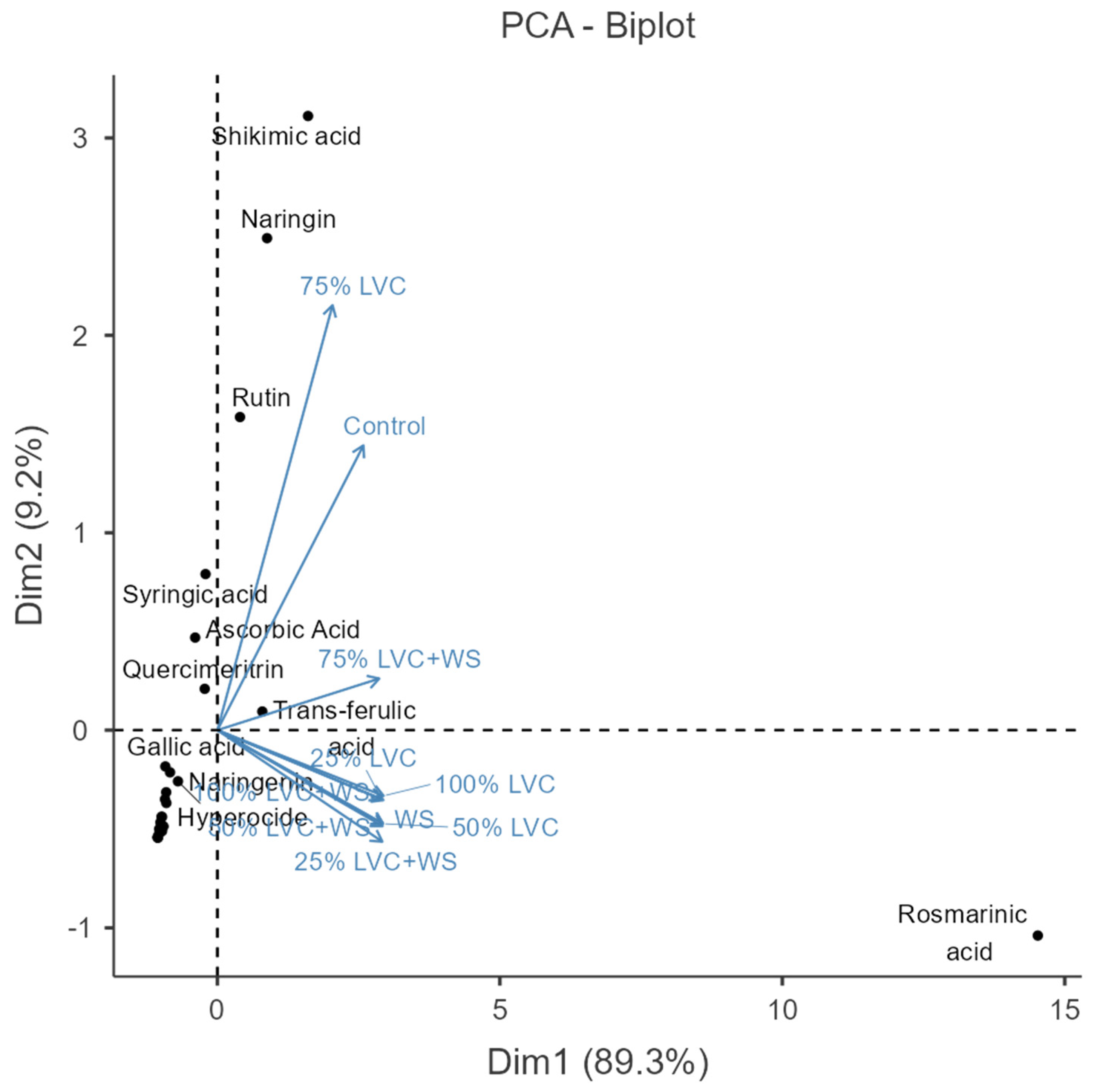
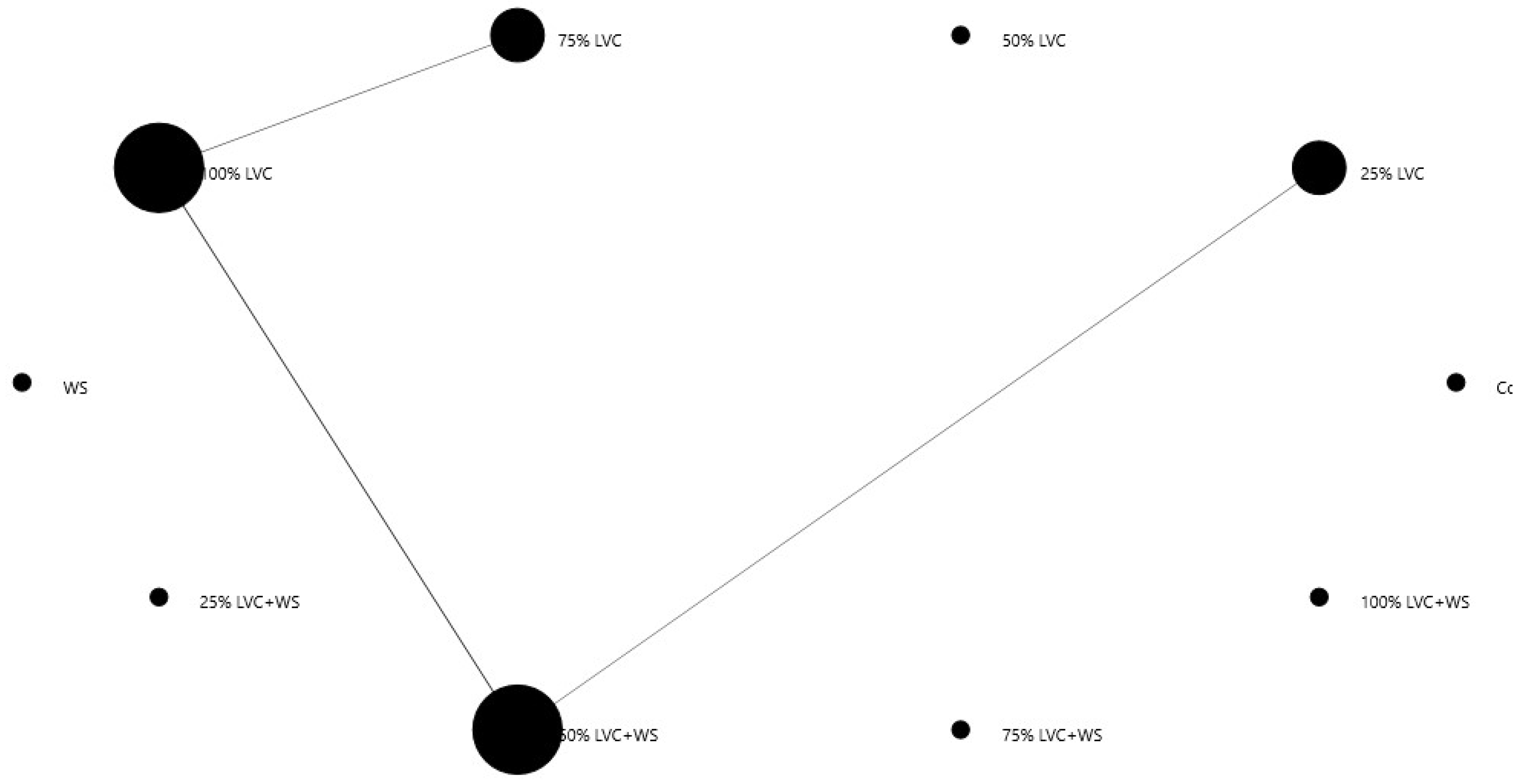
3.6. Heat Map Clustering, Correlation, Principal Component and Network Plot Analyses of the Phenolics and Flavonoids Corresponding to the Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, P.K.; Jha, B. Transcription Factors in Plants and ABA Dependent and Independent Abiotic Stress Signalling. Biol. Plant. 2010, 54, 201–212. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and Abiotic Stress Tolerance in Plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Daferera, D.; Polissiou, M.G.; Passam, H.C. The Effect of Water Deficit Stress on the Growth, Yield and Composition of Essential Oils of Parsley. Sci. Hortic. 2008, 115, 393–397. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Chen, H.Y.; Ruan, H. Response of Plants to Water Stress: A Meta-Analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar] [CrossRef]
- Heidari, M.; Golpayegani, A. Effects of Water Stress and Inoculation with Plant Growth Promoting Rhizobacteria (PGPR) on Antioxidant Status and Photosynthetic Pigments in Basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 2012, 11, 57–61. [Google Scholar] [CrossRef]
- Kalamartzis, I.; Dordas, C.; Georgiou, P.; Menexes, G. The Use of Appropriate Cultivar of Basil (Ocimum basilicum) Can Increase Water Use Efficiency under Water Stress. Agronomy 2020, 10, 70. [Google Scholar] [CrossRef]
- Kalamartzis, I.; Menexes, G.; Georgiou, P.; Dordas, C. Effect of Water Stress on the Physiological Characteristics of Five Basil (Ocimum basilicum L.) Cultivars. Agronomy 2020, 10, 1029. [Google Scholar] [CrossRef]
- Kulak, M.; Jorrín-Novo, J.V.; Romero-Rodriguez, M.C.; Yildirim, E.D.; Gul, F.; Karaman, S. Seed Priming with Salicylic Acid on Plant Growth and Essential Oil Composition in Basil (Ocimum basilicum L.) Plants Grown under Water Stress Conditions. Ind. Crops Prod. 2021, 161, 113235. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-Induced Responses of Photosynthesis and Antioxidant Metabolism in Higher Plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Rao, D.E.; Chaitanya, K.V. Photosynthesis and Antioxidative Defense Mechanisms in Deciphering Drought Stress Tolerance of Crop Plants. Biol. Plant. 2016, 60, 201–218. [Google Scholar] [CrossRef]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofi, N.R.; Salgotra, R.K. Impact of Drought on Photosynthesis: Molecular Perspective. Plant Gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Ding, L.; Lu, Z.; Gao, L.; Guo, S.; Shen, Q. Is Nitrogen a Key Determinant of Water Transport and Photosynthesis in Higher Plants upon Drought Stress? Front. Plant Sci. 2018, 9, 1143. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of Secondary Metabolites in Defense Mechanisms of Plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Zaynab, M.; Fatima, M.; Abbas, S.; Shari, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of Secondary Metabolites in Plant Defense against Pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Aguirre-Becerra, H.; Vazquez-Hernandez, M.C.; Saenz de la O, D.; Alvarado-Mariana, A.; Guevara-Gonzalez, R.G.; Garcia-Trejo, J.F.; Feregrino-Perez, A.A. Role of Stress and Defense in Plant Secondary Metabolites Production. In Bioactive Natural Products for Pharmaceutical Applications; Springer: Cham, Switzerland, 2021; pp. 151–195. [Google Scholar]
- Gulmez, C.; Kulak, M. New Insights to Enhance the Desired Anti-Diabetic Compounds in Medicinal and Aromatic Plants Exposed to Abiotic Stress Factors. In Biotechnology of Anti-Diabetic Medicinal Plants; Gantait, S., Verma, S.K., Sharangi, A.B., Eds.; Springer: Singapore, 2021; pp. 285–306. [Google Scholar]
- Karaman, S.; Kirecci, O.A.; Ilcim, A. Influence of Polyamines (Spermine, Spermidine and Putrescine) on the Essential Oil Composition of Basil (Ocimum basilicum L.). J. Essent. Oil Res. 2008, 20, 288–292. [Google Scholar] [CrossRef]
- Kahveci, H.; Bilginer, N.; Diraz-Yildirim, E.; Kulak, M.; Yazar, E.; Kocacinar, F.; Karaman, S. Priming with Salicylic Acid, β-Carotene and Tryptophan Modulates Growth, Phenolics and Essential Oil Components of Ocimum basilicum L. Grown under Salinity. Sci. Hortic. 2021, 281, 109964. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Prakash, O. The Impact of Chemical Fertilizers on Our Environment and Ecosystem. In Research Trends in Environmental Sciences, 2nd ed.; Sharma, P., Ed.; Akinik Publications: Khasmir, India, 2019; pp. 69–86. [Google Scholar]
- Büyük, G.; Kırpık, M.; Çelik, A.; Kuş, M.; Akça, E.; Zeydanlı, U. Adıyaman İlinin Hayvansal atik Kaynaklı Vermikompost üRetim Potansiyeli. Adyütayam 2017, 5, 31–37. (In Turkish) [Google Scholar]
- Domínguez, J.; Edwards, C.A. Relationships between Composting and Vermicomposting. In Vermiculture Technology: Earthworms, Organic Waste and Environmental Management, 2nd ed.; Sherman, Ed.; CRC Press.: Boca Raton, Florida, 2011; pp. 1–14. [Google Scholar]
- Yıldız, M.; Gürkan, M.O.; Turgut, C.; Kaya, Ü.; Ünal, G. Tarımsal Savaşımda Kullanılan Pestisitlerin Yol açtığı çevre Sorunları. VI. Türkiye Ziraat Mühendisliği Teknik Kongresi; TMMOB Ziraat Mühendisleri Odası: Ankara, Türkiye, 2005. (In Turkish) [Google Scholar]
- Demir, H.; Polat, E.; Sönmez, İ. Ülkemiz Için Yeni Bir Organik Gübre: Solucan Gübresi. Tarım Aktüel 2010, 14, 54–60. (In Turkish) [Google Scholar]
- Befrozfar, M.R.; Habibi, D.; Asgharzadeh, A.; Sadeghi-Shoae, M.; Tookallo, M.R. Vermicompost, Plant Growth Promoting Bacteria and Humic Acid Can Affect the Growth and Essence of Basil (Ocimum basilicum L.). Ann. Biol. Res. 2013, 4, 8–12. [Google Scholar]
- Esmaielpour, B.; Rahmanian, M.; Heidarpour, O.; Shahriari, M.H. Effect of Vermicompost and Spent Mushroom Compost on the Nutrient and Essential Oil Composition of Basil (Ocimum basilicum L.). J. Essent. Oil-Bear. Plants 2017, 20, 1283–1292. [Google Scholar] [CrossRef]
- Morelli, F.; Ferarrese, L.; Munhoz, C.L.; Alberton, O. Antimicrobial Activity of Essential Oil and Growth of Ocimum basilicum L. Inoculated with Mycorrhiza and Humic Substances Applied to Soil. Genet. Mol. Res. 2017, 16, 16039710. [Google Scholar] [CrossRef]
- Chelariu, E.L.; Ghiorghe, C.; Turcu, N. Vermicompost Influence on Production of Ocimum basilicum L. Seedlings. Lucr. Știinţifice USAMV Iași Ser. Hortic. 2018, 61, 203–206. [Google Scholar]
- Heidarpour, O.; Esmaielpour, B.; Soltani, A.A.; Khorramdel, S. Effect of Vermicompost on Essential Oil Composition of (Satureja hortensis L.) under Water Stress Condition. J. Essent. Oil-Bear. Plants 2019, 22, 484–492. [Google Scholar] [CrossRef]
- Celikcan, F.; Kocak, M.Z.; Kulak, M. Vermicompost Applications on Growth, Nutrition Uptake and Secondary Metabolites of Ocimum basilicum L. under Water Stress: A Comprehensive Analysis. Ind. Crops Prod. 2021, 171, 113973. [Google Scholar] [CrossRef]
- Reddy, A.R.; Teja, K.S.; Varghese, R.P.; Deepthi, M.; Sastry, K.P. Influence of Different Doses of Vermicompost and NPK on Growth of Herb Ocimum tenuiflorum var. CIM-Ayu. Res. J. Pharm. Technol. 2018, 11, 1713–1717. [Google Scholar] [CrossRef]
- Rahmanian, M.; Esmaielpour, B.; Hadian, J.; Shahriari, M.H.; Fatemi, H. The Effect of Organic Fertilizers on Morphological Traits, Essential Oil Content and Components of Basil (Ocimum basilicum L.). J. Agric. Sci. Sustain. Prod. 2017, 27, 103–118. [Google Scholar]
- Ayastuy, M.E.; Muscolino, C.; Fernandez, J.A.; Belladonna, D.; Rodriguez, R.A.; Caro, L.; Hernandez, L.F. Effect of Organic Substrate and Aqueous Extract of Vermicompost on Nursery Basil Growth. Acta Hortic. 2020, 71–76. [Google Scholar] [CrossRef]
- Reyes Araujo, D.Y.; Mora Herrera, M.E.; Lugo, J. Estabilización Por Vermicomposteo de Lodos Residuales Aplicados en la Productividad de Albahaca (Ocimum basilicum L.). Rev. Int. De Contam. Ambient. 2020, 36, 371–381. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Amani Machiani, M.; Javanmard, A.; Mahdavikia, H.; Maggi, F.; Morshedloo, M.R. Vermicompost Application in Different Intercropping Patterns Improves the Mineral Nutrient Uptake and Essential Oil Compositions of Sweet Basil (Ocimum basilicum L.). J. Soil Sci. Plant Nutr. 2021, 21, 450–466. [Google Scholar] [CrossRef]
- Aras, A.; Türkan, F.; Yildiko, U.; Atalar, M.N.; Kılıç, O.; Alma, M.H.; Bursal, E. Biochemical Constituent, Enzyme Inhibitory Activity, and Molecular Docking Analysis of an Endemic Plant Species, Thymus migricus. Chem Pap. 2021, 75, 1133–1146. [Google Scholar] [CrossRef]
- Yilmaz, M.A. Simultaneous Quantitative Screening of 53 Phytochemicals in 33 Species of Medicinal and Aromatic Plants: A Detailed, Robust and Comprehensive LC–MS/MS Method Validation. Ind. Crop Prod. 2020, 149, 112347. [Google Scholar] [CrossRef]
- Farzaneh, A.; Ghani, A.; Azizi, M. The Effect of Water Stress on Morphological Characteristic and Essential Oil Content of Improved Sweet Basil (Ocimum basilicum L.). J. Plant. Prod. 2010, 17, 103–111. [Google Scholar]
- Radácsi, P.; Inotai, K.; Sárosi, S.; Czövek, P.; Bernath, J.; Nemeth, E. Effect of Water Supply on the Physiological Characteristic and Production of Basil (Ocimum basilicum L.). Eur. J. Hortic. Sci. 2010, 75, 193. [Google Scholar]
- Eriyagama, N.; Smakhtin, V.Y.; Gamage, N. Mapping Drought Patterns and Impacts: A Global Perspective; International Water Management Institute (IWMI): Colombo, Sri Lank, 2009. [Google Scholar]
- Kulak, M.; Ozkan, A.; Bindak, R. A Bibliometric Analysis of the Essential Oil-Bearing Plants Exposed to the Water Stress: How Long Way We Have Come and How Much Further? Sci. Hortic. 2019, 246, 418–436. [Google Scholar] [CrossRef]
- Kandpal, G. Review on Impact of Chemical Fertilizers on Environment. Int. J. Mod. Agric. 2021, 10, 758–763. [Google Scholar]
- Alfarisy, M.Y.; Yassi, A.; Mustari, K. Increasing Productivity and Biomass of Corn Plants toward Grant Organic Fertilizer and Liquid Organic Fertilizer. ENDLESS Int. J. Future Stud. 2021, 4, 236–248. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhowmik, A.; Mallick, R.; Debsarcar, A.; Mukherjee, J. Application of Recycled Slaughterhouse Wastes as an Organic Fertilizer for Successive Cultivations of Bell Pepper and Amaranth. Sci. Hortic. 2021, 280, 109927. [Google Scholar] [CrossRef]
- Leno, N.; Sudharmaidevi, C.R. Physicochemical and Nutrient Release Characteristics of a Thermochemical Organic Fertilizer Produced from Degradable Solid Waste and Its Effect on Productivity of Banana. Commun. Soil Sci. Plant Anal. 2021, 52, 2562–2577. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.; Ali, O.A.; Hafez, E.M.; El Shamey, E.A.; Zhou, Z.; Seleiman, M.F. A Vermicompost and Deep Tillage System to Improve Saline-Sodic Soil Quality and Wheat Productivity. J. Environ. Manag. 2021, 277, 111388. [Google Scholar] [CrossRef]
- Feizabadi, A.; Noormohammadi, G.; Fatehi, F. Changes in Growth, Physiology, and Fatty Acid Profile of Rapeseed Cultivars Treated with Vermicompost under Drought Stress. J. Soil Sci. Plant Nutr. 2021, 21, 200–208. [Google Scholar] [CrossRef]
- Greco, C.; Comparetti, A.; Fascella, G.; Febo, P.; La Placa, G.; Saiano, F.; Laudicina, V.A. Effects of Vermicompost, Compost and Digestate as Commercial Alternative Peat-Based Substrates on Qualitative Parameters of Salvia officinalis. Agronomy 2021, 11, 98. [Google Scholar] [CrossRef]
- Hafez, E.M.; Omara, A.E.D.; Alhumaydhi, F.A.; El-Esawi, M.A. Minimizing Hazard Impacts of Soil Salinity and Water Stress on Wheat Plants by Soil Application of Vermicompost and Biochar. Physiol. Plant. 2021, 172, 587–602. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Okorokova-Façanha, A.L.; Façanha, A.R. Humic Acids Isolated from Earthworm Compost Enhance Root Elongation, Lateral Root Emergence, and Plasma Membrane H+-ATPase Activity in Maize Roots. Plant Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef]
- Suthar, S. Evidence of Plant Hormone Like Substances in Vermiwash: An Ecologically Safe Option of Synthetic Chemicals for Sustainable Farming. Ecol. Eng. 2010, 36, 1089–1092. [Google Scholar] [CrossRef]
- Theunissen, J.; Ndakidemi, P.A.; Laubscher, C.P. Potential of Vermicompost Produced from Plant Waste on the Growth and Nutrient Status in Vegetable Production. Int. J. Phys. Sci. 2010, 5, 1964–1973. [Google Scholar]
- Pant, A.P.; Radovich, T.J.; Hue, N.V.; Talcott, S.T.; Krenek, K.A. Vermicompost Extracts Influence Growth, Mineral Nutrients, Phytonutrients and Antioxidant Activity in Pak Choi (Brassica rapa cv. Bonsai, Chinensis Group) Grown under Vermicompost and Chemical Fertiliser. J. Sci. Food Agric. 2009, 89, 2383–2392. [Google Scholar] [CrossRef]
- Singh, R.; Agarwal, S.K. Growth and Yield of Wheat (Triticum aestivum) as Influenced by Levels of Farmyard Manure and Nitrogen. Indian J. Agron. 2001, 46, 462–467. [Google Scholar]
- Guzmán-Albores, J.M.; Montes-Molina, J.A.; Castañón-González, J.H.; Abud Archila, M.; Gutiérrez-Miceli, F.A.; Ruiz-Valdiviezo, V.M. Effect of Different Vermicompost Doses and Water Stress Conditions on Plant Growth and Biochemical Profile in Medicinal Plant. Moringa Oleifera Lam. J. Environ. Biol. 2020, 41, 240–246. [Google Scholar] [CrossRef]
- Bidabadi, S.S.; Dehghanipoodeh, S.; Wright, G.C. Vermicompost Leachate Reduces Some Negative Effects of Salt Stress in Pomegranate. Int. J. Recycl. Org. Waste Agric. 2017, 6, 255–263. [Google Scholar] [CrossRef]
- Arthur, G.D.; Jäger, A.K.; Van Staden, J. The Release of Cytokinin-Like Compounds from Gingko Biloba Leaf Material during Composting. Environ. Exp. Bot. 2001, 45, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Martinez, I.G.; Sosa, F.C.; Saavedra, A.L.; Hernandez, M.S. Extraction of Auxin-Like Substances from Compost. Crop Res. Hisar 2002, 24, 323–327. [Google Scholar]
- Khalid, K.A. Influence of Water Stress on Growth, Essential Oil, and Chemical Composition of Herbs (Ocimum sp.). Int. Agrophysics 2006, 20, 289–296. [Google Scholar]
- Mandoulakani, B.A.; Eyvazpour, E.; Ghadimzadeh, M. The Effect of Drought Stress on the Expression of Key Genes Involved in the Biosynthesis of Phenylpropanoids and Essential Oil Components in Basil (Ocimum basilicum L.). Phytochemistry 2017, 139, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mota, I.; Sánchez-Sánchez, J.; Pedro, L.G.; Sousa, M.J. Composition Variation of the Essential Oil from Ocimum basilicum L. cv. Genovese Gigante in Response to Glomus Intraradices and Mild Water Stress at Different Stages of Growth. Biochem. Syst. Ecol. 2020, 90, 104021. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Shitan, N. Secondary Metabolites in Plants: Transport and Self-Tolerance Mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental Stress and Secondary Metabolites in Plants: An Overview. In Plant Metabolites and Regulation under Environmental Stress; Parvaiz, A., Mohammad, A.A., Vijay, P.S., Durgesh, K.T., Pravej, A., Mohammed, N.A., Eds.; Academic Press: London, UK, 2018; pp. 153–167. [Google Scholar]
- Szabó, K.; Zubay, P.; Németh-Zámboriné, É. What Shapes Our Knowledge of the Relationship between Water Deficiency Stress and Plant Volatiles? Acta Physiol. Plant. 2020, 42, 130. [Google Scholar] [CrossRef]
- Sánchez, E.; Soto, J.M.; García, P.C.; López-Lefebre, L.R.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Phenolic Compounds and Oxidative Metabolism in Green Bean Plants under Nitrogen Toxicity. Funct. Plant Biol. 2000, 27, 973–978. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic Compounds and Their Antioxidant Activity in Plants Growing under Heavy Metal Stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Chalker-Scott, L.; Fuchigami, L.H. The Role of Phenolic Compounds in Plant Stress Responses. In Low Temperature Stress Physiology in Crops; CRC Press: Boca Raton, FL, USA, 2018; pp. 67–80. [Google Scholar]
- Bahcesular, B.; Yildirim, E.D.; Karaçocuk, M.; Kulak, M.; Karaman, S. Seed Priming with Melatonin Effects on Growth, Essential Oil Compounds and Antioxidant Activity of Basil (Ocimum basilicum L.) under Salinity Stress. Ind. Crops Prod. 2020, 146, 112165. [Google Scholar] [CrossRef]
- Fletcher, R.S.; Slimmon, T.; McAuley, C.Y.; Kott, L.S. Heat Stress Reduces the Accumulation of Rosmarinic Acid and the Total Antioxidant Capacity in Spearmint (Mentha spicata L.). J. Sci. Food Agric. 2005, 85, 2429–2436. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Fe2O3 Nanoparticles Induced Biochemical Responses and Expression of Genes Involved in Rosmarinic Acid Biosynthesis Pathway in Moldavian Balm under Salinity Stress. Physiol. Plant. 2020, 169, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz Mirzamohammadi, H.; Modarres-Sanavy, S.A.M.; Sefidkon, F.; MokhtassiBidgoli, A.; Mirjalili, M.H. Irrigation and Fertilizer Treatments Affecting Rosmarinic Acid Accumulation, Total Phenolic Content, Antioxidant Potential and Correlation between Them in Peppermint (Mentha piperita L.). Irrig. Sci. 2021, 39, 671–683. [Google Scholar] [CrossRef]
- Sereme, A.; Dabire, C.; Koala, M.; Somda, M.K.; Traore, A.S. Influence of Organic and Mineral Fertilizers on the Antioxidants and Total Phenolic Compounds Level in Tomato (Solanum lycopersicum) var. Mongal F1. J. Exp. Biol. Agric. Sci. 2016, 4, 414–420. [Google Scholar] [CrossRef]


| Acronym | Vermicompost Treatments | Irrigation Level |
|---|---|---|
| Control | Leachate amended | Well-watered plants |
| Water Stress (WS) * | Non-leachate amended | Severe water stressed plants |
| 25% LVC ** | Leachate amended (25% LVC/75% distilled water, v/v) | Well-watered plants |
| 25% LVC + WS | Leachate amended (25% LVC/75% distilled water, v/v) *** | Severe water stressed plants |
| 50% LVC | Leachate amended (50% LVC/50% distilled water, v/v) | Well-watered plants |
| 50% LVC + WS | Leachate amended (50% LVC/50% distilled water, v/v) | Severe water stressed plants |
| 75% LVC | Leachate amended (75% LVC/25% distilled water, v/v) | Well-watered plants |
| 75% LVC + WS | Leachate amended (75% LVC/25% distilled water, v/v) | Severe water stressed plants |
| 100% LVC | Leachate amended (100% LVC/0% distilled water, v/v) | Well-watered plants |
| 100% LVC + WS | Leachate amended (100% LVC/0% distilled water, v/v) | Severe water stressed plants |
| Treatments | Plant Height (cm) | Plant FW (g) | Root Length (cm) | Root FW (g) | Leaf FW (g) | Leaf Length (cm) | Leaf Width (cm) |
|---|---|---|---|---|---|---|---|
| Control | 13.480 ± 1.000 cde | 3.900 ± 0.229 d | 14.460 ± 0.841 c | 0.353 ± 0.045 fg | 1.096 ± 0.110 c | 4.020 ± 0.453 b | 1.610 ± 0.079 bcd |
| WS * | 8.333 ± 0.666 f | 2.683 ± 0.480 e | 24.847 ± 1.264 a | 1.024 ± 0.132 bc | 0.520 ± 0.076 d | 2.820 ± 0.072 c | 1.320 ± 0.092 d |
| 25% LVC ** | 16.700 ± 1.410 b | 4.767 ± 0.737 abc | 14.933 ± 0.306 c | 0.747 ± 0.095 cde | 1.550 ± 0.132 b | 4.203 ± 0.300 ab | 1.870 ± 0.066 ab |
| 25% LVC+ WS | 12.517 ± 0.797 de | 4.203 ± 0.211 cd | 22.277 ± 0.751 b | 0.653 ± 0.115 de | 1.253 ± 0.105 c | 2.808 ± 0.357 c | 1.338 ± 0.078 d |
| 50% LVC | 18.933 ± 1.504 a | 5.227 ± 0.261 a | 21.350 ± 1.103 b | 0.910 ± 0.168 bcd | 2.033 ± 0.260 a | 4.230 ± 0.305 ab | 2.080 ± 0.203 a |
| 50% LVC+ WS | 15.333 ± 0.950 bc | 4.457 ± 0.172 bcd | 20.550 ± 0.853 b | 0.790 ± 0.236 b–e | 1.543 ± 0.081 b | 2.967 ± 0.153 c | 1.787 ± 0.220 ab |
| 75% LVC | 15.200 ± 0.900 bc | 5.060 ± 0.333 ab | 14.767 ± 0.751 c | 0.597 ± 0.015 ef | 1.990 ± 0.105 a | 4.660 ± 0.295 a | 1.867 ± 0.090 ab |
| 75% LVC+ WS | 11.333 ± 1.258 e | 4.120 ± 0.209 cd | 22.083 ± 1.551 b | 1.034 ± 0.070 b | 1.597 ± 0.257 b | 2.877 ± 0.125 c | 1.679 ± 0.427 bc |
| 100% LVC | 14.200 ± 2.138 cd | 3.867 ± 0.252 d | 15.703 ± 0.754 c | 0.260 ± 0.036 g | 1.093 ± 0.110 c | 3.947 ± 0.311 b | 1.940 ± 0.052 ab |
| 100% LVC+ WS | 13.450 ± 0.606 cde | 2.573 ± 0.459 e | 26.363 ± 1.061 a | 1.343 ± 0.316 a | 0.557 ± 0.067 d | 2.633 ± 0.153 c | 1.383 ± 0.104 cd |
| LVC: 0.000 | LVC: 0.000 | LVC: 0.000 | LVC: 0.283 | LVC: 0.000 | LVC: 0.070 | LVC: 0.003 | |
| p-value | WS: 0.000 | WS: 0.000 | WS: 0.000 | WS: 0.000 | WS: 0.000 | WS: 0.000 | WS: 0.000 |
| LVCxWS: 0.054 | LVCxWS: 0.433 | LVCxWS: 0.000 | LVCxWS: 0.000 | VCxWS: 0.485 | LVCxWS: 0.411 | LVCxWS: 0.321 |
| Treatments | Eucalyptol | Estragole | Anethole | Caryophyllene | Humulene | Germacrene D | Bisabolene |
|---|---|---|---|---|---|---|---|
| Control | 2.37 ± 0.08 b | 89.12 ± 2.10 b | 0.32 ± 0.06 d | 0.51 ± 0.08 e | 0.23 ± 0.04 e | 0.20 ± 0.04 g | 0.33 ± 0.05 e |
| WS * | 1.75 ± 0.18 ef | 87.77 ± 0.44 bc | 0.44 ± 0.06 c | 0.64 ± 0.06 de | 0.26 ± 0.03 e | 0.29 ± 0.01 f | 0.54 ± 0.06 de |
| 25% LVC ** | 2.04 ± 0.05c d | 88.33 ± 0.72 b | 0.31 ± 0.03 d | 0.69 ± 0.05 d | 0.53 ± 0.07 c | 0.69 ± 0.01 c | 0.94 ± 0.22 ab |
| 25% LVC +WS | 2.20 ± 0.12 bc | 91.28 ± 0.85 a | 0.65 ± 0.05 b | 0.91 ± 0.05 bc | 0.68 ± 0.01 b | 0.60 ± 0.02 d | 0.92 ± 0.02 abc |
| 50% LVC | 1.86 ± 0.04 de | 87.58 ± 0.65 bc | 0.41 ± 0.01 c | 0.86 ± 0.04 c | 0.40 ± 0.01 d | 0.52 ± 0.04 e | 0.96 ± 0.05 ab |
| 50% LVC +WS | 2.11 ± 0.15 c | 89.06 ± 0.16 b | 0.77 ± 0.04 a | 1.03 ± 0.05 ab | 0.83 ± 0.04 a | 0.59 ± 0.02 d | 0.76 ± 0.10 bcd |
| 75% LVC | 1.83 ± 0.06 def | 86.29 ± 0.59 cd | 0.46 ± 0.01 c | 0.91 ± 0.02 bc | 0.38 ± 0.02 cd | 0.82 ± 0.03 b | 1.00 ± 0.14 ab |
| 75% LVC +WS | 2.37 ± 0.05 b | 87.49 ± 0.46 bc | 0.64 ± 0.04 b | 0.94 ± 0.03 bc | 0.73 ± 0.04 b | 0.61 ± 0.02 d | 0.69 ± 0.14 cd |
| 100% LVC | 1.63 ± 0.08 f | 84.42 ± 0.74 d | 0.56 ± 0.04 b | 1.11 ± 0.15 a | 0.35 ± 0.03 d | 0.90 ± 0.01 a | 1.03 ± 0.05 a |
| 100% LVC +WS | 2.96 ± 0.05 a | 85.20 ± 0.32 d | 0.57 ± 0.04 b | 0.89 ± 0.05 bc | 0.58 ± 0.02 c | 0.62 ± 0.02 d | 0.82 ± 0.06 abc |
| LVC: <0.004 | LVC: <0.001 | LVC: <0.001 | LVC: <0.001 | LVC: <0.001 | LVC: <0.001 | LVC: <0.001 | |
| p-value | WS: <0.001 | WS: <0.017 | WS: <0.001 | WS: <0.046 | WS: <0.001 | WS: <0.001 | WS: <0.032 |
| LVCxWS: <0.001 | LVCxWS: 0.035 | LVCxWS: <0.001 | LVCxWS: <0.001 | LVCxWS: <0.001 | LVCxWS: <0.001 | LVCxWS: <0.001 |
| Compounds | Control | 25% LVC ** | 50% LVC | 75% LVC | 10% LVC | WS * | 25% LVC + WS | 50% LVC + WS | 75% LVC + WS | 100% LVC + WS |
|---|---|---|---|---|---|---|---|---|---|---|
| Ascorbic acid | 105.61 ± 4.26 | 104.58 ± 5.38 | 107.06 ± 3.06 | 104.79 ± 5.13 | 114.23 ± 9.18 | 115.05 ± 10.61 | 107.03 ± 2.68 | 112.13 ± 7.60 | 103.86 ± 0.98 | 103.13 ± 2.77 |
| Shikimic acid | 451.56 ± 76.13 | 461.57 ± 76.95 | 565.73 ± 103.00 | 353.15 ± 47.74 | 356.80 ± 40.00 | 816.95 ± 333.28 | 756.45 ± 150.81 | 561.68 ± 83.68 | 442.51 ± 97.31 | 391.64 ± 77.30 |
| Gallic acid | 0.00 ± 0.00 | 0.00 ± 0.00 | 60.30 ± 19.71 | 53.40 ± 11.34 | 8.62 ± 0.84 | 12.73 ± 1.42 | 0.99 ± 0.20 | 3.74 ± 2.30 | 7.34 ± 0.32 | 7.88 ± 2.06 |
| Protocatechuic acid | 0.00 ± 0.00 | 0.34 ± 0.59 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.06 ± 0.92 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Chlorogenic acid | 1.91 ± 0.53 | 10.40 ± 5.18 | 6.62 ± 0.74 | 1.32 ± 0.14 | 1.02 ± 0.16 | 5.00 ± 0.72 | 1.32 ± 0.25 | 1.20 ± 0.39 | 1.13 ± 2.53 | 5.91 ± 0.18 |
| 4-Hydroxybenzaldehyde | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.96 ± 0.85 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.33 ± 0.30 | 0.65 ± 0.57 | 0.06 ± 0.11 | 0.00 ± 0.00 | 0.62 ± 0.54 |
| Caffeic acid | 29.65 ± 3.47 | 87.91 ± 6.59 | 67.15 ± 20.07 | 14.39 ± 0.00 | 0.00 ± 0.82 | 8.12 ± 2.30 | 62.79 ± 12.62 | 73.92 ± 7.85 | 20.53 ± 0.00 | 0.00 ± 1.56 |
| Syringic acid | 125.91 ± 8.97 | 129.82 ± 9.16 | 127.10 ± 3.15 | 143.32 ± 1.21 | 154.89 ± 7.16 | 125.34 ± 7.88 | 117.19 ± 2.03 | 128.50 ± 1.72 | 136.23 ± 6.73 | 127.63 ± 2.70 |
| P-coumaric acid | 0.82 ± 0.76 | 0.00 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.86 ± 0.76 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.0 0 | 0.00 ± 0.00 |
| Polydatine | 0.92 ± 0.27 | 0.07 ± 0.12 | 0.03 ± 0.06 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.16 ± 0.27 | 0.26 ± 0.31 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.16 ± 2.01 |
| Trans-ferulic acid | 103.48 ± 54.08 | 1722.96 ± 176.75 | 1614.05 ± 242.85 | 93.27 ± 8.62 | 68.85 ± 20.43 | 716.28 ± 70.83 | 2447.94 ± 475.02 | 151.43 ± 8.06 | 506.89 ± 98.17 | 34.65 ± 8.59 |
| Quercimeritrin | 87.16 ± 39.27 | 9.90 ± 1.41 | 48.59 ± 4.05 | 58.18 ± 7.49 | 41.38 ± 2.51 | 238.27 ± 14.54 | 245.87 ± 69.37 | 27.00 ± 5.29 | 497.84 ± 153.88 | 102.78 ± 8.98 |
| Cynarin | 24.54 ± 3.30 | 23.70 ± 1.49 | 24.87 ± 1.19 | 23.38 ± 1.68 | 22.82 ± 0.80 | 22.48 ± 1.52 | 23.20 ± 0.73 | 23.48 ± 1.88 | 23.89 ± 1.29 | 25.89 ± 1.83 |
| Hyperocide | 24.17 ± 11.03 | 5.16 ± 1.52 | 10.05 ± 7.35 | 27.74 ± 1.22 | 28.16 ± 7.99 | 295.74 ± 15.51 | 113.92 ± 4.30 | 32.17 ± 3.01 | 205.73 ± 5.84 | 34.52 ± 10.24 |
| Quercetin-3-glucoside | 10.73 ± 5.09 | 5.22 ± 2.88 | 8.66 ± 2.50 | 2.89 ± 0.41 | 9.31 ± 2.73 | 80.59 ± 12.89 | 39.86 ± 3.85 | 10.85 ± 1.26 | 53.93 ± 18.63 | 10.55 ± 3.49 |
| Rutin | 229.90 ± 12.22 | 231.50 ± 11.82 | 258.32 ± 13.01 | 218.27 ± 7.17 | 243.24 ± 10.60 | 354.55 ± 102.51y | 311.79 ± 70.89 | 223.30 ± 1.48 | 240.65 ± 5.30 | 235.38 ± 4.72 |
| Isoquercitrin | 11.01 ± 5.21 | 6.38 ± 1.69 | 8.05 ± 2.47 | 2.65 ± 0.45 | 10.29 ± 0.68 | 73.27 ± 17.35 | 38.05 ± 2.37 | 10.61 ± 0.71 | 56.19 ± 19.92 | 10.25 ± 3.41 |
| Resveratrol | 5.32 ± 1.14 | 7.32 ± 2.36 | 4.33 ± 2.34 | 9.15 ± 2.35 | 8.22 ± 2.14 | 5.32 ± 1.00 | 10.94 ± 1.26 | 7.39 ± 0.99 | 7.93 ± 0.70 | 10.42 ± 1.19 |
| Naringin | 313.50 ± 18.35 | 296.67 ± 12.64 | 283.66 ± 5.77 | 313.50 ± 5.41 | 299.47 ± 14.59 | 294.55 ± 5.98 | 325.34 ± 9.34 | 297.84 ± 11.80 | 304.97 ± 15.94 | 305.51 ± 11.09 |
| Rosmarinic acid | 682.88 ± 285.372 | 5950.13 ± 110.82 | 11251.46 ± 1230.71 | 352.23 ± 75.79 | 4690.59 ± 276.48 | 13904.23 ± 2.460.64 | 29879.68 ± 600.89 | 11319.82 ± 1149.15 | 1798.03 ± 446.64 | 5192.40 ± 329.97 |
| Neohesperidin | 8.68 ± 1.51 | 2.98 ± 0.83 | 6.20 ± 0.95 | 0.00 ± 0.00 | 17.39 ± 4.04 | 13.34 ± 1.02 | 31.12 ± 1.83 | 23.06 ± 2.58 | 48.92 ± 10.52 | 5.36 ± 0.77 |
| Ellagic acid | 34.67 ± 8.81 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0. 00 | 0.00 ± 0.00 | 12.82 ± 2.33 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 35.63 ± 8.11 |
| Naringenin | 64.78 ± 6.840484 | 72.23 ± 11.57 | 38.51 ± 17.38 | 20.28 ± 1.71 | 18.05 ± 2.98 | 6.41 ± 0.93 | 18.19 ± 1.21 | 17.65 ± 6.71 | 14.67 ± 1.27 | 22.38 ± 9.79 |
| Silibinin | 10.53 ± 0.60 | 10.59 ± 0.58 | 10.62 ± 0.55 | 11.06 ± 1.06 | 10.63 ± 0.32 | 10.50 ± 0.66 | 10.50 ± 0.62 | 10.74 ± 0.46 | 10.63 ± 0.37 | 10.80 ± 0.38 |
| 3-Hydroxyflavone | 21.20 ± 1.83 | 20.50 ± 1.72 | 22.48 ± 2.15 | 19.69 ± 1.29 | 21.57 ± 3.00 | 23.55 ± 2.12 | 22.31 ± 3.56 | 20.79 ± 1.22 | 19.73 ± 3.27 | 21.63 ± 2.07 |
| Diosgenin | 5.15 ± 2.91 | 2.29 ± 1.05 | 3.49 ± 0.39 | 4.21 ± 0.86 | 4.10 ± 0.62 | 4.17 ± 0.31 | 2.23 ± 0.72 | 2.08 ± 0.27 | 1.60 ± 0.72 | 6.40 ± 1.80 |
| Compounds | Water Stress | Vermicompost | Water Stress × Vermicompost |
|---|---|---|---|
| Ascorbic acid | 0.654 ns | 0.373 ns | 0.071 ns |
| Shikimic acid | 0.005 | 0.007 | 0.105 ns |
| Gallic acid | 0.000 | 0.000 | 0.000 |
| Protocatechuic acid | 0.000 | 0.000 | 0.000 |
| Chlorogenic acid | 0.063 ns | 0.006 | 0.000 |
| 4-Hydroxybenzaldehyde | 0.326 ns | 0.226 ns | 0.012 |
| Caffeic acid | 0.037 | 0.000 | 0.005 |
| Syringic acid | 0.000 | 0.000 | 0.000 |
| P-coumaric acid | 0.946 ns | 0.001 | 1.000 ns |
| Polydatine | 0.645 ns | 0.383 ns | 0.199 ns |
| Trans-ferulic acid | 0.454 ns | 0.000 | 0.000 |
| Quercimeritrin | 0.000 | 0.000 | 0.000 |
| Cynarin | 0.906 ns | 0.844 ns | 0.126 ns |
| Hyperocide | 0.000 | 0.000 | 0.000 |
| Quercetin-3-glucoside | 0.000 | 0.000 | 0.000 |
| Rutin | 0.021 | 0.071 ns | 0.016 |
| Isoquercitrin | 0.000 | 0.000 | 0.000 |
| Resveratrol | 0.021 | 0.001 | 0.095 ns |
| Naringin | 0.335 ns | 0.061 ns | 0.020 |
| Rosmarinic acid | 0.000 | 0.000 | 0.000 |
| Neohesperidin | 0.000 | 0.000 | 0.000 |
| Ellagic acid | 0.065 ns | 0.000 | 0.000 |
| Naringenin | 0.000 | 0.000 | 0.000 |
| Silibinin | 0.820 ns | 0.872 ns | 0.918 ns |
| 3-Hydroxyflavone | 0.558 ns | 0.404 ns | 0.599 ns |
| Diosgenin | 0.229 ns | 0.001 | 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosem, H.; Kocak, M.Z.; Kaysim, M.G.; Celikcan, F.; Kulak, M. Liquid Leachate Produced from Vermicompost Effects on Some Agronomic Attributes and Secondary Metabolites of Sweet Basil (Ocimum basilicum L.) Exposed to Severe Water Stress Conditions. Horticulturae 2022, 8, 1190. https://doi.org/10.3390/horticulturae8121190
Kosem H, Kocak MZ, Kaysim MG, Celikcan F, Kulak M. Liquid Leachate Produced from Vermicompost Effects on Some Agronomic Attributes and Secondary Metabolites of Sweet Basil (Ocimum basilicum L.) Exposed to Severe Water Stress Conditions. Horticulturae. 2022; 8(12):1190. https://doi.org/10.3390/horticulturae8121190
Chicago/Turabian StyleKosem, Hatice, Mehmet Zeki Kocak, Mustafa Guven Kaysim, Ferdi Celikcan, and Muhittin Kulak. 2022. "Liquid Leachate Produced from Vermicompost Effects on Some Agronomic Attributes and Secondary Metabolites of Sweet Basil (Ocimum basilicum L.) Exposed to Severe Water Stress Conditions" Horticulturae 8, no. 12: 1190. https://doi.org/10.3390/horticulturae8121190
APA StyleKosem, H., Kocak, M. Z., Kaysim, M. G., Celikcan, F., & Kulak, M. (2022). Liquid Leachate Produced from Vermicompost Effects on Some Agronomic Attributes and Secondary Metabolites of Sweet Basil (Ocimum basilicum L.) Exposed to Severe Water Stress Conditions. Horticulturae, 8(12), 1190. https://doi.org/10.3390/horticulturae8121190









